Abstract
The Wilms tumor suppressor WT1 encodes a zinc finger transcription factor that is expressed in glomerular podocytes during a narrow window in kidney development. By immunoprecipitation and protein microsequencing analysis, we have identified a major cellular protein associated with endogenous WT1 to be the inducible chaperone Hsp70. WT1 and Hsp70 are physically associated in embryonic rat kidney cells, in primary Wilms tumor specimens and in cultured cells with inducible expression of WT1. Colocalization of WT1 and Hsp70 is evident within podocytes of the developing kidney, and Hsp70 is recruited to the characteristic subnuclear clusters that contain WT1. The amino-terminal transactivation domain of WT1 is required for binding to Hsp70, and expression of that domain itself is sufficient to induce expression of Hsp70 through the heat shock element (HSE). Substitution of a heterologous Hsp70-binding domain derived from human DNAJ is sufficient to restore the functional properties of a WT1 protein with an amino-terminal deletion, an effect that is abrogated by a point mutation in DNAJ that reduces binding to Hsp70. These observations indicate that Hsp70 is an important cofactor for the function of WT1, and suggest a potential role for this chaperone during kidney differentiation.
Keywords: WT1, hsp70, p21, Wilms tumor, renal development, cell cycle arrest
WT1 encodes a tumor suppressor that is expressed in precursor cells of the kidney glomerulus (for review, see Haber and Housman 1992; Hastie 1994). Its developmental role is best demonstrated in WT1-null mice, which show widespread apoptosis of renal stem cells and failure of renal mesenchyme to respond to normal inductive signals (Kreidberg et al. 1993). In humans, heterozygous germ-line WT1 mutations are associated with genito-urinary malformations and confer genetic predisposition to Wilms tumor, a pediatric cancer originating from renal precursor cells (Huff et al. 1991; Pelletier et al. 1991a,b). WT1 mutations are found infrequently in sporadic Wilms tumors, which typically express high levels of wild-type gene product, consistent with a developmental arrest at an early stage of renal differentiation (Haber et al. 1990; Little et al. 1992; Varanisi et al. 1994). Transfection of wild-type WT1 into a Wilms tumor cell line with an aberrant endogenous transcript results in growth suppression, consistent with its function as a tumor suppressor (Haber et al. 1993). However, the functional properties of WT1 are complex and dependent on experimental conditions as well as a number of physiological alternative splice variants.
The WT1 isoform denoted (−KTS) binds to multiple GC and TC-rich promoters through four zinc finger domains, mediating transcriptional repression in promoter-reporter assays (for review, see Rauscher III 1993). Inducible expression of this isoform in a WT1-responsive osteosarcoma cell model leads to cell cycle arrest followed by apoptosis, associated respectively with the induction of the cyclin-dependent kinase inhibitor p21 and repression of the epidermal growth factor receptor (EGFR) (Englert et al. 1995a, 1997). When expressed in these cells at levels below those required to inhibit cellular proliferation, WT1(−KTS) inhibits apoptosis triggered by p53 and DNA damage response pathways (Maheswaran et al. 1995), consistent with the observation of widespread cell death in the developing kidneys of WT1-null mice (Kreidberg et al. 1993). The most prevalent WT1 splicing variant has an insertion of three amino acids disrupting the spacing between zinc fingers 3 and 4 (Haber et al. 1991), resulting in reduced DNA binding activity and diminished ability to inhibit cellular proliferation (Rauscher III et al. 1990; Englert et al. 1995a). Unlike the diffuse nuclear expression pattern observed with WT1(−KTS), the WT1(+KTS) isoform is localized to discrete subnuclear clusters (Larsson et al. 1995). Binding to subnuclear clusters, which requires the amino terminus of WT1, is markedly enhanced in dominant-negative mutants with a disrupted DNA binding domain (Englert et al. 1995b). Although the functional interactions between WT1 isoforms are unknown, the evolutionary conservation of these splicing variants (Kent et al. 1995), their consistent coexpression in WT1-expressing cells (Haber et al. 1991), and the presence of WT1-associated subnuclear clusters in cells of the differentiating kidney (Mundlos et al. 1993) suggest a physiologically important relationship.
Heat shock proteins are a highly conserved family of molecular chaperones, with diverse functions, including mediating protein folding and degradation, transport across cellular membranes, and assembly into macromolecular structures (Creighton 1991; Schlesinger 1991; Johnson and Craig 1997; Hartl 1996). Those migrating at 65–70 kD include the constitutive Hsc70, an abundant protein known to bind abnormally folded proteins, including mutant p53 (Pinhasi-Kimhi 1986; Finlay et al. 1988; Hainaut and Milner 1992), and the inducible Hsp70 proteins, which are expressed at low levels physiologically but are rapidly induced following growth of cells at 40°C (Lindquist and Craig 1988; Hightower 1991). During growth at a physiological temperature, Hsp70 expression is tightly regulated during cell cycle progression (Milarski and Morimoto 1986) and it is induced by stimuli that induce cellular proliferation, including serum (Wu and Morimoto 1985), expression of c-myc (Kingston et al. 1984), and the viral oncoproteins adenovirus E1A (Kao and Nevins 1983; Wu et al. 1986) and large T from SV 40 and polyoma (Khandjian and Turler 1983). Induction of Hsp70 by viral oncoproteins is associated with disruption of an inhibitory complex containing p53 and CCAAT Binding Factor (CBF), that targets the CCAAT site within the hsp70 promoter (Jones et al. 1987; Lum et al. 1992; Agoff et al. 1993). In contrast, both the heat shock response and the cell cycle regulation of Hsp70 expression are linked to activation of heat shock factor (HSF) family members binding the heat shock element (HSE) within the promoter (for review, see Sorger 1991; Wu 1995). Although less well characterized than its induction by proliferation signals, Hsp70 is also induced in models of hematopoietic differentiation (Sistonen et al. 1992; Garcia-Bermejo et al. 1995; Teshima et al. 1996; Leppa et al. 1997). However, the function of hsp70 in these physiological pathways is unknown.
In searching for endogenous cellular proteins interacting with WT1, we observed that WT1 and Hsp70 are physically associated in cells of the developing kidney, in primary Wilms tumor specimens, and in cultured cells expressing WT1. Expression of WT1 induces hsp70 through the HSE regulatory element, and the two proteins products show precise subnuclear colocalization. Of particular importance in defining the functional significance of this protein interaction, deletion of the amino-terminal domain required for binding to Hsp70 abrogates the ability of WT1 to inhibit cellular proliferation, whereas substitution of a heterologous Hsp70 binding sequence derived from human DNAJ restores its function. By modulating the functional properties of WT1, Hsp70 may play an important role in normal kidney differentiation and in the functional property of this tumor suppressor.
Results
Coimmunoprecipitation of Hsp70 with WT1
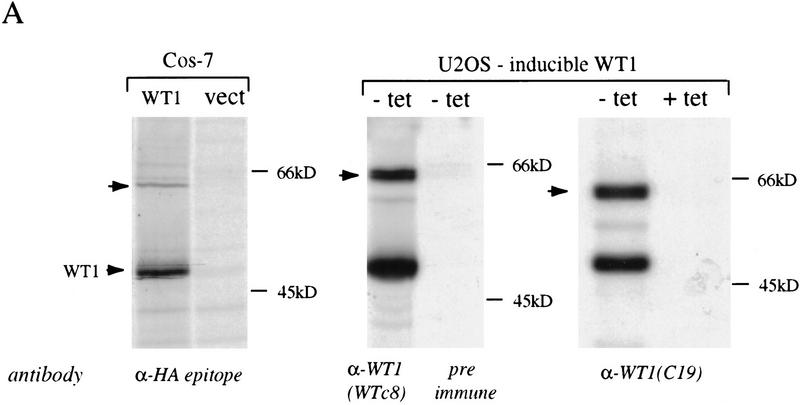
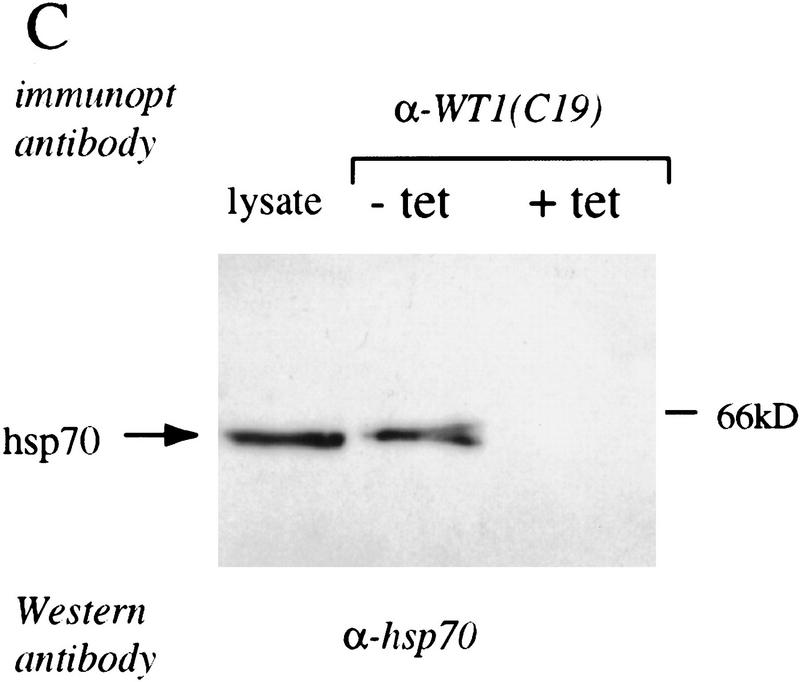
To gain insight into the functional properties of WT1, we undertook to identify associated cellular proteins. Because WT1 is normally expressed transiently during glomerular differentiation and is not readily detectable in cultured cells, we established osteosarcoma cell lines with inducible, tetracycline-regulated WT1. Metabolic labeling of these cells, followed by immunoprecipitation analysis using antibody against WT1 revealed a coprecipitating protein migrating around 65 kD (Fig. 1A). Coprecipitation of the 65 kD band was observed by use of antibodies raised against both the amino terminus (WTc8) and the carboxyl terminus (C19) of WT1, and it was also observed following transfection of HA epitope-tagged WT1 into Cos-7 cells, indicating that it did not result from cross reactivity with a specific antibody. WT1 immunoprecipitation analysis of unlabeled extracts from neonatal rat kidneys, followed by detection of proteins with silver staining was used to confirm coprecipitation of the 65 kD protein with endogenous WT1 (data not shown). To obtain sufficient material for microsequencing analysis, we isolated extracts from U2OS cells with inducible WT1–delZ, a carboxy-terminal deletion mutant that fails to induce cell death, but has unaltered association with the 65 kD protein. Extracts from ∼109 cells were immunoprecipitated by use of antibody against WT1 that had been covalently crosslinked to protein A–Sepharose, followed by preparative gel electrophoresis, immunoblotting, and excision of the 65-kD band. Microsequence analysis of several peptides obtained following proteolytic digestion, revealed the identity of the protein as either Hsp70.1 or Hsp70.2, two members of the inducible subset of heat shock proteins with virtually identical protein sequence (Fig. 1B) (Hunt and Morimoto 1985; Wu et al. 1985; Milner and Campbell 1990; Bonnycastle et al. 1994). To confirm the identification of the WT1-associated protein as Hsp70, WT1 immunoprecipitates from cells with inducible WT1 were analyzed by Western blot by use of antibody raised against the inducible subset of Hsp70 proteins (antibody K20; Santa Cruz), demonstrating coprecipitation of these two proteins (Fig. 1C).
Figure 1.
Coimmunoprecipitation of WT1 and Hsp70. (A) (Left) Immunoprecipitation of metabolically labeled cellular lysates from Cos-7 cells transfected with an HA-epitope tagged WT1 construct or vector, with the 12-CA-5 monoclonal antibody directed against the HA-epitope. (Middle) Immunoprecipitation of labeled lysates from U2OS cells with tetracycline-regulated expression of WT1. WT1 expression was induced by withdrawal of tetracycline and extracts were immunoprecipitated with the polyclonal antibody WTc8, directed against the amino terminus of WT1, or its preimmune serum. (Right) U2OS cells were grown in the presence or absence of tetracycline, followed by metabolic labeling and immunoprecipitation with the monoclonal antibody C19, directed against the carboxyl terminus of WT1. The arrow denotes the migration position of the coprecipitated band of ∼65 kD. (B) Amino acid sequence of two peptides derived from microsequencing of proteolytic products, showing identity with Hsp70.1 and Hsp70.2. Parentheses denote probable, but not definitive residues. (C) hsp70–Western blot of anti-WT1 immunoprecipitates, derived from U2OS cells with inducible WT1 grown in the presence or absence of tetracycline. Cellular lysate (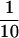 the amount immunoprecipitated) was analyzed directly to demonstrate the migration position of native Hsp70.
the amount immunoprecipitated) was analyzed directly to demonstrate the migration position of native Hsp70.
Physical association of endogenous WT1 and Hsp70 in rat kidney cells and Wilms tumor specimens
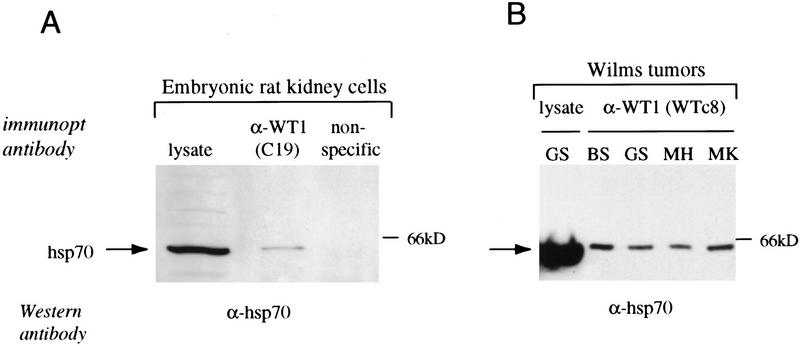
Given the known binding of heat shock family members to poorly folded proteins, we were concerned that the physical association between WT1 and Hsp70 might simply result from its overexpression in cells with an inducible promoter. To determine whether endogenous WT1 was associated with Hsp70, we first analyzed an immortalized cell line derived from embryonic day 13 rat kidney and known to express native WT1. Immuno-precipitation of proteins from cellular extracts with the carboxy-terminal anti-WT1 antibody C19, followed by immunoblotting with antibody against Hsp70 demonstrated coprecipitation of these two proteins (Fig. 2A). As a control, Hsp70 was not identified following immunoprecipitation with an irrelevant antibody (against c-Rel). We then analyzed four specimens of sporadic Wilms tumor known to express wild-type WT1. In all four cases, immunoprecipitation-Western analysis, this time with the amino-terminal anti-WT1 antibody WTc8, demonstrated coprecipitation of WT1 and Hsp70. Therefore, we concluded that the physical association between WT1 and Hsp70 was present in kidney-derived cells, expressing endogenous WT1 protein.
Figure 2.
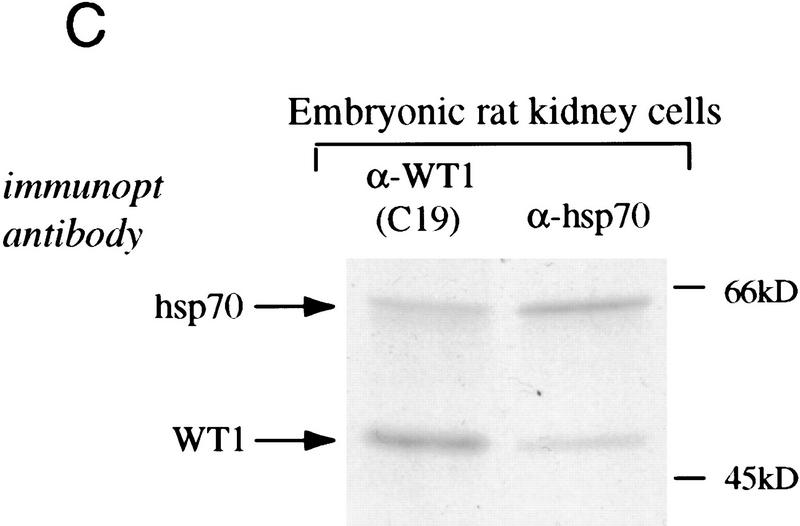
Physical association of endogenous WT1 and Hsp70. (A) Immunoprecipitation-Western analysis of extracts from embryonic rat kidney cells that express endogenous WT1. Equal amounts of cellular lysates were immunoprecipitated with either anti-WT1 antibody C19 or a nonspecific control antibody (against c-rel), followed by immunoblotting analysis with anti-Hsp70 antibody. Cellular lysate (1/20 the amount immunoprecipitated) was analyzed directly to show the migration position of native Hsp70. (B) Immunoprecipitation–Western analysis of lysates from sporadic Wilms tumor specimens. The tumors, denoted by initials, are known to express wild-type WT1. Equal amounts of cellular lysates were immunoprecipitated with anti-WT1 antibody WTc8, followed by immunoblotting with antibody against Hsp70. Cellular lysate from tumor GS (1/20 the amount immunoprecipitated) was analyzed directly by immunoblotting. (C) Immunoprecipitation of radiolabeled lysates from embryonic rat kidney cells to demonstrate the relative proportion of WT1 and Hsp70 that are coimmunoprecipitated with each other. Equal amounts of cellular lysates were immunoprecipitated with either anti-WT1 antibody C19 or anti-Hsp70 antibody. The amount of total cellular WT1 directly immunoprecipitated with C19 was compared with the amount coimmunoprecipitated with Hsp70 antibody; the amount of total cellular Hsp70 immunoprecipitated directly was compared with the amount coimmunoprecipitated by use of anti-WT1 antibody C19.
To address the stoichiometry of the WT1–Hsp70 interaction in cells expressing endogenous levels of these proteins, we analyzed metabolically labeled extracts from embryonic rat kidney cells by immunoprecipitation with either anti-WT1 or anti-Hsp70 antibody. Immunoprecipitation with anti-Hsp70 antibody resulted in the coprecipitation of ∼25% of the total cellular WT1 that was directly immunoprecipitated with anti-WT1 antibody (Fig. 2B). Similarly, ∼25% of the total cellular Hsp70 that was immunoprecipitated with anti-Hsp70 antibody was coprecipitated by use of anti-WT1 antibody. We conclude that, in embryonic kidney cells expressing native WT1 and Hsp70, a significant fraction of both proteins is present within a physical complex.
Colocalization of WT1 and Hsp70
The distinct subnuclear localization patterns exhibited by WT1 made it possible to use confocal microscopy to confirm the association between WT1 and Hsp70 in vivo. Immunofluorescence analysis of U2OS cells with tetracycline-regulated WT1 constructs demonstrated the absence of detectable baseline expression of either WT1 or Hsp70 when cells were grown in the presence of tetracycline (Fig. 3A). Withdrawal of tetracycline and induction of WT1(−KTS) led to the expected diffuse nuclear expression pattern, with sparing of nucleoli: Staining for Hsp70 demonstrated an increase in the level of Hsp70 expression (see below), sharing the diffuse nuclear distribution of WT1. In contrast, induction of WT1–delZ, which is expressed in a speckled subnuclear distribution, resulted in a similar speckled localization pattern for Hsp70 (Fig. 3A). Staining of these cells with fluorescein-conjugated anti-WT1 antibody and rhodamine-conjugated anti-Hsp70 antibody, followed by analysis by use of confocal imaging, showed precise colocalization of these two proteins within subnuclear clusters (Fig. 3B). Thus, Hsp70 is recruited to the subnuclear compartment containing WT1. Inducible expression of EWS–WT1, a tumor-associated chromosomal translocation product in which the amino-terminal domain of the Ewing sarcoma gene (EWS) is fused with the carboxy-terminal zinc finger domain of WT1 (Ladanyi and Gerald 1994) did not show detectable nuclear Hsp70 expression (Fig. 3A), indicating that association with Hsp70 is not the consequence of overexpressing zinc finger transcription factors. Induction of Hsp70 expression and its colocalization with WT1 specifically requires the amino terminus of WT1 (see below), which is present in the WT1–delZ mutant and lacking in the EWS–WT1 chimera.
Figure 3.

Colocalization of WT1 and Hsp70 in cultured cells. (A) Immunofluorescence analysis of U2OS cells with tetracycline-regulated expression of the wild-type isoform WT1(−KTS), a truncated mutant lacking the carboxy-terminal zinc finger domain (WT1–del Z), and the characteristic chromosomal translocation product identified in Desmoplastic Small Round Cell Tumor (the EWS–WT1(−KTS) chimera, comprised of the amino-terminal domain of the EWS fused to the carboxy-terminal zinc finger domain of WT1). Cells were grown in the presence or absence of tetracycline and stained by use of antibodies against the amino terminus of WT1 (WT1 (+ and − KTS), the HA epitope tag (EWS–WT1), or against Hsp70. (B) Confocal imaging of U2OS cells with inducible expression of WT1–delZ, following staining with rhodamine-conjugated anti-WT1 and fluorescein-conjugated anti-Hsp70 antibodies. The yellow signal in the merged image identifies precise overlap between red and green signals. Bar, 10 μm.
Expression of Hsp70 in the developing glomerulus
To determine whether the colocalization of WT1 and Hsp70 was also evident in cells of the developing kidney that express endogenous WT1, we analyzed histological sections of human fetal kidney. WT1 expression in the kidney is remarkable for its precise developmental regulation, with a sharp peak of expression during the differentiation of glomeruli, followed by a dramatic decline in the mature kidney (Pritchard-Jones et al. 1990). By immunohistochemistry, WT1 is detectable in the podocyte layer of the developing glomerulus, in which the presence of both (−KTS) and (+KTS) alternative splicing variants leads to a speckled nuclear expression pattern (Mundlos et al. 1993). As expected, immunohistochemical analysis of a 13 week human kidney showed speckled nuclear staining restricted to glomerular podocytes (Fig. 4A). Remarkably, staining for Hsp70 also revealed high levels of expression restricted to glomerular podocytes of the fetal kidney. Furthermore, in contrast to the cytoplasmic Hsp70 staining pattern in other tissues, immature podocytes showed a speckled nuclear appearance for Hsp70, similar to that observed with WT1. Rare kidney tubular cells, which do not express WT1, showed low levels of cytoplasmic Hsp70 expression. Expression of both WT1 and Hsp70 was greatly reduced in the adult kidney (data not shown). The striking parallel pattern of expression of WT1 and Hsp70 in the embryonic kidney, both in terms of their restriction to a specific cell type and their characteristic subcellular localization, is consistent with a developmentally regulated interaction.
Figure 4.
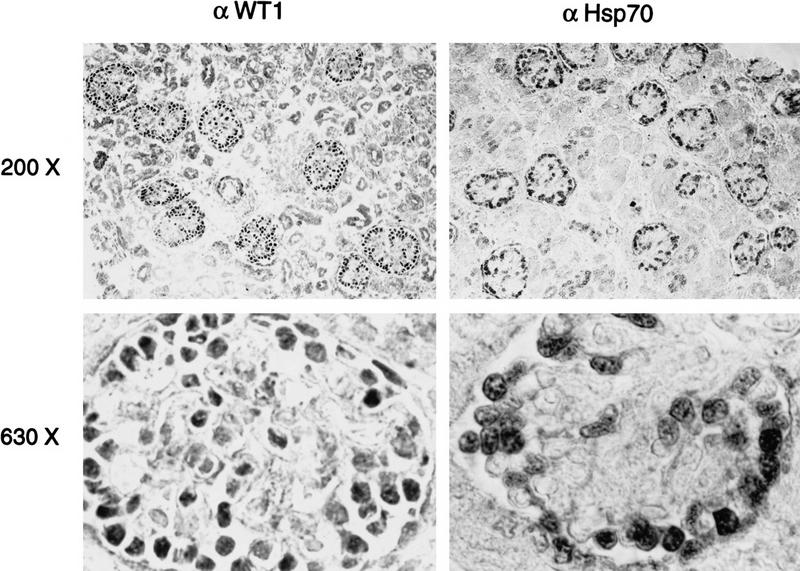
Colocalization of WT1 and Hsp70 in developing glomerular podocytes. Immunohistochemical analysis of sections from a 13-week human kidney, by use of antibodies against WT1 and Hsp70. Low power (200×) reveals developing glomeruli, in which podocytes are seen as a ring of peripheral cells that stain intensely for both WT1 and Hsp70. The mesangial cells, renal tubular cells, and stroma are negative. At higher power (630X), staining for both WT1 and Hsp70 is seen to be restricted to the nuclei of podocytes, and exhibit a speckled pattern.
Induction of hsp70 expression by WT1
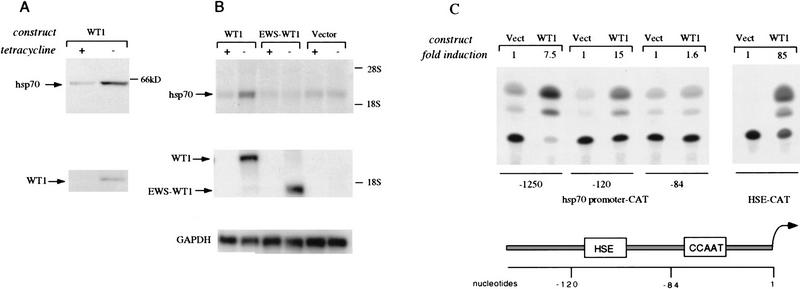
Immunofluorescence analysis suggested that inducible expression of WT1 leads to induction of Hsp70 (Fig. 3A). Quantitation of cellular hsp70 by Western blotting demonstrated low baseline expression that was increased by 5- to 10-fold following induction of WT1 expression (Fig. 5A). Northern blot analysis, by use of a gene-specific cDNA probe derived from the 3′ untranslated sequence of hsp70.1, showed a fivefold increase in endogenous Hsp70.1 mRNA (Fig. 5B). Consistent with immunofluorescence experiments (Fig. 3), no increase in hsp70 mRNA was observed following inducible expression of the EWS–WT1 chimera, lacking the amino-terminal domain of WT1, or with the tetracycline transactivator alone (Fig. 5B).
Figure 5.
Transcriptional activation of hsp70 by WT1. (A) Induction of Hsp70 protein following expression of WT1. U2OS cells with inducible expression of WT1 were grown in the presence or absence (24 hr) of tetracycline, and equal amounts of cellular lysates were analyzed by immunoblotting with antibody against Hsp70. Induction of WT1 is shown at bottom. (B) Induction of hsp70.1 mRNA by WT1. Northern blot analysis of U2OS cells with inducible WT1, EWS–WT1, or empty vector, following growth in the presence or absence (24 hr) of tetracycline. A gene-specific probe was derived from the 3′ untranslated region of human hsp70.1. (Middle) Reprobing of the blot with a WT1 cDNA to confirm inducible expression of WT1 and the EWS–WT1 chimera; (bottom) a GAPDH loading control. (C) Transcriptional activation of the hsp70 promoter by WT1. U2OS cells were transfected with CMV-driven WT1(−KTS) or empty vector, along with reporter constructs, followed by determination of CAT activity. The respective fragments of the hsp70 promoter reporter are shown (bottom), including the primary HSE and CCAAT regulatory elements. The HSE–CAT reporter contains multimerized HSE sites. The fold induction of CAT activity was determined by scintillation counting.
The hsp70 promoter has been extensively characterized as a model for transcriptional regulation, leading to the identification of two primary regulatory elements: a CCAAT box between nucleotides 1 and −84, that is targeted by CBF, and an HSE site between nucleotides −84 and −120, bound by HSF family members (Rabindran et al. 1991; Sarge et al. 1991; Schuetz et al. 1991). Transient transfection of an hsp70 promoter–CAT construct into U2OS cells showed ∼10-fold transcriptional activation following cotransfection of WT1. Analysis of reporter constructs with progressive deletions of the hsp70 promoter identified the WT1-responsive element between nucleotides −84 and −120, containing the HSE site and excluding the CCAAT box (Fig. 5B). Requirement for the HSE site was confirmed by use of a minimal reporter construct containing multimeric HSE sites, which showed 85-fold transcriptional activation following cotransfection of WT1 (Fig. 5B). Transcriptional activation of hsp70 by WT1 was observed following transfection of wild-type WT1 as well as the carboxy-terminal deletion mutant WT1–delZ, demonstrating that this effect was independent of its DNA binding activity (data not shown). Gel retardation assays by use of the HSE sequence also showed no evidence of direct binding by WT1 (data not shown). These observations are consistent with current models of HSE-dependent hsp70 induction (Abravaya et al. 1992; Mosser et al. 1993; Cotto et al. 1996), suggesting that binding of the WT1 amino-terminal domain to Hsp70 induces release of HSF from its complex with Hsp70, resulting in HSF-mediated induction of hsp70 expression.
Substitution of a heterologous Hsp70 binding domain from DNAJ for the amino terminus of WT1
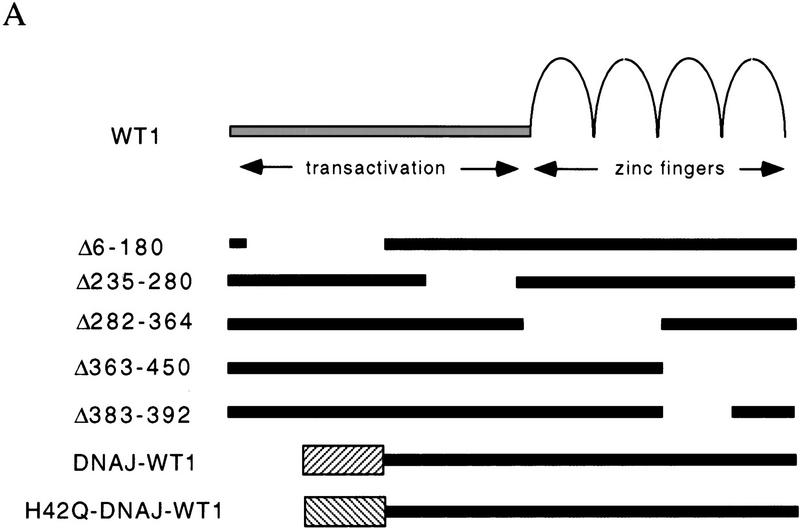
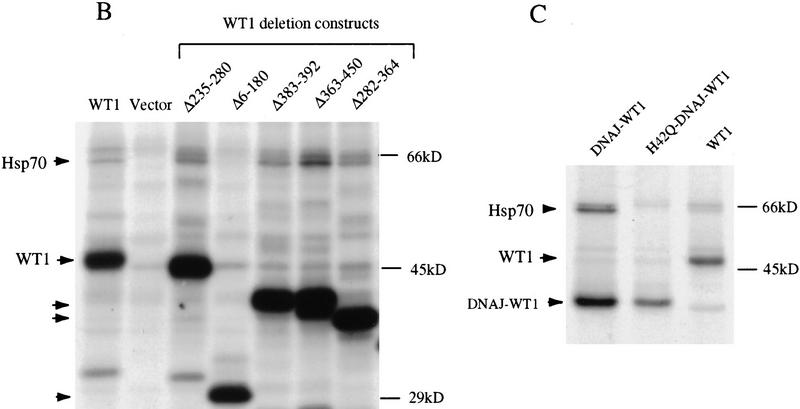
To determine the functional significance of the WT1–Hsp70 complex, we sought to compare the properties of WT1 in the presence or absence of this protein interaction. Because expression of Hsp70 is ubiquitous, and WT1 itself induces Hsp70 expression, we were unable to choose a cell type lacking this protein association. Therefore, we chose to identify the minimal WT1 domain required for association with Hsp70, determine whether it is required for WT1 function and then test whether it can be replaced with a heterologous Hsp70-binding domain derived from the known Hsp70 cofactor, DNAJ (Langer et al. 1992; Silver and Way 1993; Liberek et al. 1995). We determined previously that the amino terminus of WT1, which is retained in the WT1–delZ mutant, was required for interaction with Hsp70, whereas the zinc finger domain, present in the EWS–WT1 fusion protein, was not involved in this interaction. To refine the domain of interaction, we transiently transfected a panel of truncated, HA-epitope tagged WT1 constructs into Cos-7 cells, followed by metabolic labeling and immunoprecipitation analysis. These experiments indicated that the extreme amino terminus of WT1 (amino acids 6–180) was required for coimmunoprecipitation of Hsp70 (Fig. 6A). Therefore, we used WT1(Δ6–180) to generate chimeric constructs, in which the deleted extreme amino terminus of WT1 was replaced by the J domain of HSJ1, a human DNAJ homolog (Cheetham et al. 1992). Two chimerae were tested, one encoding the wild-type J domain (DNAJ–WT1) and one in which an H to Q point mutation in the conserved HPDK motif (Campbell et al. 1997; Stubdal et al. 1997) abrogates Hsp70 binding (H42Q–DNAJ–WT1). As predicted, transfection of constructs encoding DNAJ–WT1 into Cos-7 cells followed by immunoprecipitation with antibody to the carboxyl terminus of WT1 resulted in coprecipitation of Hsp70, which was greatly reduced following transfection of H42Q–DNAJ–WT1 (Fig. 6B) As a control for the DNA binding properties of DNAJ–WT1, we also generated a chimeric construct with a disruption of the WT1 zinc finger domain, DNAJ–WT1–delZ.
Figure 6.
Identification of amino-terminal WT1 domain required for association with Hsp70. (A) Schematic representation of WT1 deletion constructs. The chimeric constructs DNAJ–WT1 encodes the 78 amino acid J domain of human DNAJ (HSJ1). H42Q–DNAJ–WT1 contains a substitution of glutamine for histidine within the critical HPD residues required for association with Hsp70 (Wall et al. 1994; Tsai and Douglas 1996). (B) Coimmunoprecipitation of Hsp70 with truncated WT1 proteins. Cos-7 cells were transfected with CMV-driven constructs, followed by immunoprecipitation of radiolabeled lysates with antibody 12-CA-5 against the HA epitope. Comparable expression of the WT1 constructs is demonstrated, along with coimmunoprecipitation of Hsp70 with all WT1 deletion constructs except Δ6-180. (C) Coimmunoprecipitation of Hsp70 with DNAJ–WT1, but not H42Q–DNAJ–WT1. Cos-7 cells were transfected with CMV driven constructs encoding wild-type WT1 or the WT1–DNAJ chimerae, followed by coimmunoprecipitation with antibody 12-CA-5.
WT1-mediated growth inhibition requires binding to Hsp70
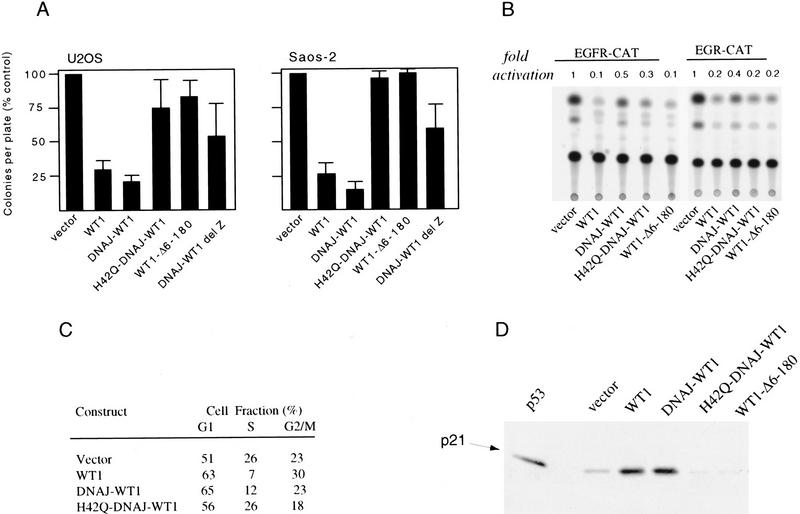
Although the precise functional properties of WT1 remain uncertain, its ability to inhibit cellular proliferation appears most consistent with its characteristic as a tumor suppressor gene. We have shown previously that WT1 inhibits colony formation in two osteosarcoma cell lines, U2OS and Saos-2. To test the functional consequences of the WT1–Hsp70 interaction, we transfected these cells with constructs encoding WT1, WT1 (Δ6–180), DNAJ–WT1, H42Q–DNAJ–WT1, and DNAJ–WT1–delZ, along with a hygromycin-resistance plasmid. As predicted, WT1 suppressed colony formation in both U2OS and Saos-2 cells, an effect that was lost following deletion of the amino-terminal domain WT1(Δ6–180). Remarkably, the DNAJ–WT1 chimera was able to inhibit colony formation to the same extent as wild-type WT1, whereas H42Q–DNAJ–WT1 was inactive (Fig. 7A). DNAJ–WT1–delZ had no effect on colony formation, indicating that this effect required DNA binding by the chimeric protein and was not simply the result of directing nuclear expression of a wild-type DNAJ domain. Similarly, fusion of DNAJ to another nuclear protein, SV40 T antigen, does not by itself inhibit cellular proliferation (Campbell et al. 1997; Stubdal et al. 1997). Thus, the addition of a heterologous Hsp70-binding domain to an amino-terminal-deleted WT1 construct was sufficient to restore its ability to inhibit cellular proliferation, suggesting that interaction with Hsp70 is required for growth inhibition by WT1.
Figure 7.
Inhibition of cellular proliferation by WT1 requires association with Hsp70. (A) Inhibition of colony formation in U2OS and Saos-2 cells following transfection of wild-type WT1, the amino-terminal truncation WT1–Δ6–180, DNAJ–WT1, H42Q–DNAJ–WT1 (encoding a point mutation within the DNAJ domain), and DNAJ–WT1–delZ (encoding a truncation of the WT1 DNA binding domain). All WT1 constructs (except DNAJ–WT1–delZ) encoded the (−KTS) splicing variant, with an uninterrupted DNA binding domain, which has been linked to inhibition of cellular proliferation in these osteosarcoma cells. Cells were cotransfected with a construct encoding puromycin resistance and drug resistant colonies were counted after 14 days (U2OS) or 21 days (Saos-2) in culture. Standard deviations are given. (B) Absence of correlation between transcriptional repression or promoter-reporter constructs and inhibition of colony formation by WT1 variants. U2OS cells were transfected with WT1 and DNAJ–WT1 constructs, along with either the GC-rich EGR1–CAT or the TC-rich EGFR–CAT promoter reporters. Representative experiments are shown, with transcriptional repression quantitated by scintillation counting. (C) G1 phase cell cycle arrest induced by wild-type WT1 and DNAJ–WT1. Saos-2 cells were transiently transfected with WT1 and DNAJ–WT1 constructs, along with a construct encoding the cell surface marker CD20. CD20-expressing transfectants were identified by FACS analysis, and their cell cycle distribution was determined by staining with propidium iodide. A representative experiment is shown. (D) Induction of p21 by wild-type WT1 and DNAJ–WT1. Saos-2 cells were transiently transfected with constructs encoding WT1 and DNAJ–WT1 variants. Cellular extracts were isolated 24 hr after transfection, and equal amounts of lysates were analyzed by immunoblotting by use of antibody against p21. Saos-2 cells have a deletion of endogenous p53 and express low levels of endogenous p21; their high transfection efficiency makes it possible to analyze the induction of the native p21 gene following transient transfection of expression constructs. As a control, induction of p21 is shown following transient transfection of CMV-driven p53 (1 μg).
To examine the mechanism underlying the inhibition of colony formation by DNAJ–WT1, we first tested its transactivational activity using transient transfection assays. WT1(−KTS) has been shown to bind to multiple GC- and TC-rich motifs mediating transcriptional repression, although the specificity of this effect has been questioned. As expected, we found that two prototype WT1-responsive promoters, the GC-rich Early Growth Response (EGR1) promoter (Rauscher et al. 1990) and the TC-rich EGFR promoter (Englert et al. 1995a) were effectively repressed following cotransfection of full length WT1. However, these reporters were also repressed by the amino-terminal deleted construct WT1Δ6–180, which is inactive in mediating growth suppression, as well as by both DNAJ–WT1 and H42Q–DNAJ–WT1 (Fig. 7B). Hence, we did not observe a correlation between transcriptional repression of these reporter constructs in transient transfection assays and the ability of WT1 variants to inhibit cellular proliferation.
Inducible expression of WT1(−KTS) in Saos-2 cells is also associated with induction of the cyclin dependent kinase inhibitor p21 and G1-phase cell cycle arrest (Englert et al. 1997). To test whether this functional property of WT1 was more closely correlated with their ability to inhibit cellular proliferation, Saos-2 cells were transiently transfected with constructs encoding the variants of WT1 and DNAJ–WT1, along with a plasmid encoding the cell surface marker CD20. Analysis of CD20-positive transfectants by FACS for DNA content demonstrated a 10%–15% increase in G1 phase following cotransfection with WT1, but not with the truncated WT1–Δ6–180. Cells cotransfected with DNAJ–WT1 also showed a 10%–15% increased G1 phase fraction, which was not observed following cotransfection with H42Q–DNAJ–WT1. Because G1 arrest mediated by WT1 is correlated with induction of p21, we analyzed endogenous p21 levels by immunoblotting, following transfection of WT1 variants into Saos-2 cells. Transient transfection is highly efficient in these cells, which have a deletion of p53 and low levels of native p21 expression, making it possible to determine the effect of transfected constructs on endogenous p21 levels. Transfection of WT1 induced a 5- to 10-fold increase in endogenous p21 expression, whereas WT1–Δ6–180 had no effect. Transfection of DNAJ–WT1 induced an increase in p21 levels comparable with wild-type WT1, but H42Q–DNAJ–WT1 was inactive. Thus, recruitment of Hsp70 to WT1 appears to be required for its ability to induce expression of p21 and mediate a G1 phase cell cycle arrest, functional properties that are well correlated with the ability of WT1 variants to inhibit cellular proliferation in these cells.
Discussion
By coimmunoprecipitation and protein microsequencing analysis, we have identified a major cellular protein partner of WT1 as Hsp70. A number of observations indicate that this protein interaction is physiologically significant: (1) Coimmunoprecipitation of WT1 and Hsp70 is observed in kidney-derived cells expressing endogenous WT1, namely embryonic rat kidney cells and primary Wilms tumor specimens, (2) precise subnuclear colocalization of WT1 and Hsp70 is evident in cultured cells and a striking coexpression pattern is present in glomerular podocytes of the developing kidney, (3) WT1 itself is a potent inducer of Hsp70 expression, an effect that is mediated through the HSE site of the hsp70 promoter and, hence, may involve the HSF family of transcription factors, and (4) functional studies demonstrate that the extreme amino terminus of WT1, which binds Hsp70 and is required for growth inhibition by WT1, can be replaced with a heterologous Hsp70-binding domain derived from a human DNAJ homolog. The reconstituted Hsp70-binding chimeric WT1 protein has restored ability to inhibit colony formation, induce p21 and mediate G1 phase cell cycle arrest, whereas a chimera with a point mutation disrupting the Hsp70-binding domain is inactive. These observations suggest that binding to Hsp70 is important to the function of WT1 as an inhibitor of cellular proliferation.
Binding of denatured or misfolded cellular proteins to members of the heat shock family is a well known phenomenon, which complicates attempts to distinguish specific physiological pathways in which these chaperones may play an integral part. Analysis of cells with endogenous WT1 ensures that the association between this protein and Hsp70 is not the result of aberrant overexpression associated with tetracycline-regulated promoters. In embryonic rat kidney cells that express physiologically relevant levels of WT1, ∼25% of the cellular WT1 is associated with Hsp70 and a similar proportion of cellular Hsp70 is bound to WT1, indicating that an important fraction of these cellular proteins are associated with each other. Similarly, the mapping of this interaction to the extreme amino-terminal domain of WT1 indicates that Hsp70 recognizes a specific epitope within the transactivation domain. Like a number of other transcription factors (Mitchell and Tijan 1989), the extreme amino terminus of WT1 contains long stretches of prolines, which suggests that Hsp70 binding may allow proper folding of this rigid domain. It is of interest that the amino-terminal domain of WT1 is poorly soluble when overproduced in bateria and in insect cells, pointing to the need for correct molecular folding. However, binding of Hsp70 is unlikely to simply mediate stabilization of WT1 protein in vivo, because pulsed chase analysis did not demonstrate different turnover for WT1 variants capable of binding Hsp70 (wild-type WT1, DNAJ–WT1), compared with a protein lacking this interaction domain (H42Q–DNAJ–WT1) (data not shown). By analogy with its presumed function in other model systems (Creighton 1991; Schlesinger 1991; Hartl 1996; Johnson and Craig 1997), binding to Hsp70 may therefore ensure correct folding of the amino terminus of WT1, enhancing its functional properties and its potential interactions with other proteins (see below).
Expression of the WT1 amino-terminal domain is itself sufficient to induce expression of hsp70 through the HSE site. This observation distinguishes the effect of WT1 from that noted for p53, which targets the CCAAT box in the hsp70 promoter through an interaction with CBF (Agoff et al. 1993), and it implicates members of the HSF family in WT1-mediated induction of Hsp70. The heat shock response is thought to involve the trimerization and phosphorylation of HSF1, which binds the HSE within the hsp70 promoter (Abravaya et al. 1992; Mosser et al. 1993; Cotto et al. 1996). Recently, a second family member, HSF2, has been implicated in the induction of Hsp70 during hematopoietic differentiation (Sistonen et al. 1992; Leppa et al. 1997), and a chicken homolog, HSF3, appears to bind directly to the product of c-myb and mediate cell cycle regulation of Hsp70 expression (Kanei-Ishii et al. 1997). Although we observed increased binding to the HSE element following induction of WT1, we were unable to distinguish between the known family members HSF1 and HSF2 using supershift analysis (data not shown). We did not observe the characteristic phosphorylation of HSF1 following induction of WT1, suggesting that WT1-associated transactivation of hsp70 may be mediated through other members of the HSF family. DNA binding by WT1 itself is not required for the induction of Hsp70, consistent with the model of HSF-regulated expression of Hsp70, which suggests that binding of Hsp70 to its target protein leads to its release of free transcriptionally active HSF (Abravaya et al. 1992; Mosser et al. 1993; Cotto et al. 1996).
The potent induction of Hsp70 by WT1 and the physical association between these two proteins suggests a role for Hsp70 in a cellular differentiation pathway. This is consistent with its apparent contribution to steroid hormone response pathways and hematopoietic differentiation. Both Hsp70 and Hsp90 are components of the steroid hormone receptor complex: Hsp90 appears to facillitate binding of the estrogen receptor to its ligand, whereas Hsp70 enhances ligand-independent DNA binding activity (Schowalter et al. 1991; Smith and Toft 1993; Landel et al. 1994). In this context, high levels of Hsp70 expression are noted in the ovary during the natural regression of the corpus luteum, a relative estrogen withdrawal state that follows ovulation (Khanna et al. 1995). Dramatic induction of Hsp70 expression is also evident during hematopoietic differentiation induced by sodium butyrate, hemin, or macrophage-colony stimulating factor (M-CSF) (Sistonen et al. 1992; Garcia-Bermejo et al. 1995; Teshima et al. 1996; Leppa et al. 1997). Whereas induction of HSF2 during hematopoietic differentiation has been reported, the underlying mechanism and the consequences of increased Hsp70 expression are unknown. WT1 is remarkable for its highly restricted expression pattern during glomerular differentiation in the developing kidney (Pritchard-Jones et al. 1990). The coimmunoprecipitation of WT1 and Hsp70 from embryonic rat kidney cells and their striking colocalization within the differentiating kidney therefore points to a developmentally regulated interaction. Although they provide dramatic evidence supporting in vivo colocalization, the functional properties of WT1-associated subnuclear clusters are unknown. Colocalization of WT1 alternative splicing variants with snRNPs has led to a postulated role in pre-mRNA splicing (Larsson et al. 1995), whereas the enhanced speckled distribution of proteins encoded by dominant negative WT1 mutants suggests that these structure might be involved in the sequestration of wild-type proein (Englert et al. 1995b). It is of interest to note that the induction of Hsp70 by c-Myc or v-Myc is also associated with colocalization of these proteins within subnuclear clusters, an effect that is not observed with the closely related protein N-Myc (Koskinen et al. 1991). Further understanding of the identity of these subnuclear structures will be required to determine whether the recruitment of Hsp70 contributes to their functional properties.
In contrast to the WT1(+KTS) isoform that is associated with subnuclear clusters and whose functional properties are uncertain, the WT1(−KTS) variant is expressed diffusely in the nucleus and appears to mediate transactivation and inhibit cellular proliferation. Characterizing the contribution of Hsp70 to these functional properties thus provides insight into the consequences of this protein interaction, as well as into the critical properties of WT1 itself. Because Hsp70 is ubiquitous and its expression is induced by WT1 itself, we addressed this question by constructing chimeric proteins, in which the amino-terminal WT1 domain required for association with Hsp70 is replaced with a heterologous J domain derived from the known Hsp70 cofactor DNAJ (Langer et al. 1992; Silver and Way 1993; Liberek et al. 1995). The use of such chimeric DNAJ–WT1 constructs, including either the wild-type J domain or a point mutant (H42Q) disrupting its interaction with Hsp70, has been used effectively to dissect the contribution of heat shock binding to the functional properties of SV40 T antigen (Campbell et al. 1997; Stubdal et al. 1997). Our observations indicate that the extreme amino terminus of WT1 is required for the inhibition by WT1 of colony formation in U2OS and Saos-2 cells, a correlate of its function as a tumor suppressor (Englert et al. 1995a). The fact that a heterologous Hsp70-binding domain can restore function to an amino-terminal truncated WT1 construct, whereas the H42Q mutant is inactive, provides strong evidence that Hsp70 binding is important to the inhibition of cellular proliferation by WT1.
Contrary to expectations, the ability of WT1 and DNAJ–WT1 variants to inhibit cellular proliferation was not linked to their activity in repressing transcription from prototype WT1-responsive promoter-reporter constructs. Both DNAJ–WT1 and H42Q–DNAJ–WT1, as well as an amino-terminal deleted WT1 construct, demonstrated comparable transcriptional repression, by use of both GC-rich and TC-rich target sequences. Transcriptional repression of such reporters by WT1 has been shown to be modulated by promoter and cellular context, and even by the choice of expression vector (Maheswaran et al. 1993; Wang et al. 1993; Reddy et al. 1995), raising concern about its physiological significance (Reddy and Licht 1996). In contrast, a distinct functional property of WT1, its ability to induce a G1 phase arrest, associated with induction of the endogenous p21 gene (Kudoh et al. 1995; Englert et al. 1997), appears to be well correlated with its growth suppressive properties. Cell cycle arrest and induction of native p21 were observed following transfection of both wild-type WT1(−KTS) and DNAJ–WT1, but not mutant H42Q–DNAJ–WT1 and an amino-terminal WT1 truncation mutant. These observations, therefore, provide a framework for assessing potential physiological properties of WT1 and they suggest that Hsp70 is an important cofactor in the function of this tumor suppressor.
Materials and methods
Cell culture and expression constructs
U2OS and Saos-2 osteosarcoma cells with tetracycline-regulated expression of WT1 variants have been described previously (Englert et al. 1995a; Maheswaran et al. 1995). In these cells, withdrawal of tetracycline results in induction of WT1 expression. Rat kidney cells, isolated from day 13 embryos and immortalized with polyoma middle T antigen were kindly provided by D. Herzlinger (Cornell Medical School, New York, NY). Sporadic Wilms tumor specimens expressing wild-type WT1 have been described elsewhere (Haber et al. 1990). CMV-driven expression constructs (pcDNA3-1; Invitrogen), encoding wild-type splicing variants of WT1 and internal in-frame deletions have been described previously (Maheswaran et al. 1995). Plasmids containing the J domain from the human DNAJ homolog HSJ1 (Cheetham et al. 1992) were derived from SV40 T antigen chimerae (Campbell et al. 1997; Stubdal et al. 1997). The H to Q mutation (H42Q) within the conserved HPDK motif abrogates binding to Hsp70. The mutation is at residue 42 in the SV40 T antigen chimera, corresponding to residue 31 in the native HSJ1 sequence. Constructs in which the amino-terminal domain of WT1 (amino acids 6–186) is replaced by the 78 amino acid J domain derived from human HSJ1 were generated by PCR amplification, with an NcoI restriction site within the WT1 cDNA. The DNAJ–WT1–delZ chimeric construct, lacking the zinc finger domain of WT1, was generated by truncating DNAJ–WT1 with the RsrII restriction site within WT1. Transient transfection of plasmids into Saos-2 and U2OS cells were done by calcium phosphate DNA precipitation and into Cos-7 cells by electroporation. For Northern blot analysis, total cellular RNA was isolated by the LiCl/urea method, analyzed by electrophoresis by use of 0.8% agarose/formaldehyde gels, and transferred to Genescreen Plus (NEN), followed by hybridization by use of standard procedures. A 200-bp probe specific for the 3′ untranslated sequence of hsp70.1 was derived by PCR amplification by use of primers (sense) GGGGCCTTTCCAAGATTGCTG and (antisense) CAACTTAAAAAATGGCCTGAGTTAAGT.
Preparative protein isolation and microsequencing analysis
Cellular lysates were extracted from 109 U2OS cells with inducible expression of WT1–delZ, a carboxy-terminal truncation that does not inhibit cellular proliferation but retains the domain required for coprecipitation of the 65-kD band. Lysates were extracted with 50 mm Tris, at pH 8.0, 400 mm NaCl, 1% NP-40 and 1 mm EDTA, precleared by use of protein A Sepharose and incubated with rabbit polyclonal anti-WT1 antibody WT1c8, which had been covalently crosslinked to protein A Sepharose (Harlow and Lane 1988). Immunoprecipitated proteins were resolved by preparative gel electrophoresis and transferred to nitrocellulose. The coprecipitated 65-kD band was visualized by staining with Ponceau, excised and subjected to protein microsequencing analysis (Harvard University Protein Microsequencing Facility).
Antibodies and immunological methods
The WTc8 rabbit polyclonal antibody, directed against the amino-terminal domain of WT1, has been described previously, and the carboxy-terminal monoclonal antibody was purchased from Santa Cruz. The goat polyclonal antibody K20 (Santa Cruz) was used to detect hsp70. For immunoprecipitation and for Western blot analyses, lysates were extracted by use of 50 mm Tris, at pH 8.0, 400 mm NaCl, 1% NP-40 and 1mm EDTA, and Western and immunoprecipitation analyses were performed according to standard protocol. For immunofluorescence analysis, cells were fixed with 4% paraformaldehyde, permeabilized with 1% Nonidet P-40/10 mm glycine, preadsorbed with 3% bovine serum albumin (BSA), and exposed to either rabbit anti-WT1 antibody WTc8 or mouse anti-Hsp70 antibody W27, followed by either rhodamine-conjugated goat anti-rabbit antibody or fluorescein-conjugated goat anti-mouse antibody (Jackson ImmunoResearch). The mouse monoclonal antibody 12-CA-5 against the HA epitope was used to detect the tagged EWS–WT1 chimeric product. Samples were analyzed by a laser confocal microscope (Bio-Rad MRC600 imaging system with Zeiss axiovert microscope) by use of X63 and X100 planeofluar objectives. For WT1 immunohistochemical analysis, best results were obtained with frozen sections from 13-week human kidney that were fixed with acetone, hydrated with 10% goat serum in PBS, and incubated with the mouse monoclonal antibody mWT12 (10 μg/ml), followed by the secondary goat anti-mouse antibody conjugated to horseradish peroxidase (Jackson ImmunoResearch). For Hsp70 immunohistochemistry, optimal results were obtained by use of formalin-fixed, paraffin-embedded sections of 13-week kidney. Sections were deparaffinized with xylene, hydrated with graded alcohol, microwaved for 10 min at 100°C, blocked with 0.1 mg/ml avidin-D (Vector, Burlingame, CA) containing 1% goat serum, and incubated with the mouse monoclonal anti-Hsp70 antibody W27, followed by biotin-labeled rabbit anti-mouse antibody, followed by avidin-biotinylated peroxidase complex (Vector) as described (Bhan 1995).
CAT assays and FACS analysis
To analyze transcriptional activation of the hsp70 promoter by WT1 variants, U2OS were transiently transfected with CMV-driven constructs, along with reporters encoding hsp70 regulatory sequences upstream of chloramphenicol acetyl transferase (CAT) (kindly provided by R. Kingston, MGH, Boston, MA). Experiments were undertaken in triplicate, and the amount of transfected CMV sequences was equalized by addition of empty vector. Transfection efficiency was standardized by cotransfection of a human growth hormone reporter (Nichols Institute), CAT activity was determined by use of standard procedures, and quantitated by scintillation counting, following chromatography on TLC plates. To analyze the transactivational properties of WT1 and DNAJ–WT1 variants with prototype WT1responsive promoters, CMV-driven expression constructs were cotransfected into U2OS cells either with the Early Growth Response 1 reporter (EGR1–CAT), which contains the classical GC-rich WT1 binding sites, or with the reporter (EGFR–CAT), which has TC-rich sites that are also recognized by WT1. To measure the ability of WT1 and DNAJ–WT1 variants to inhibit cellular proliferation, U2OS and Saos-2 cells were transfected with CMV-driven constructs along with a hygromycin-resistance plasmid. Cultures were selected for growth in hygromycin for 2–3 weeks, stained, and drug-resistant colonies were counted. To analyze the effect of WT1 and DNAJ–WT1 variants on cell cycle progression, Saos-2 cells in logarrhythmic growth phase were transiently transfected with these constructs, along with a plasmid encoding the cell surface marker CD20 as described by van den Heuvel and Harlow (1993). Cells were fixed 36 hr after transfection and stained for CD20 expression and for DNA content by use of propidium iodide. The cell cycle distribution of CD20 positive cells was determined by FACS analysis. To determine the effect of WT1 andDNAJ–WT1 variants on expression of endogenous p21, cells were transiently transfected as above, and transfection efficiency was quantitated by use of a growth hormone reporter. Equal amounts of cellular lysates from cultures with comparable transfection efficiencies were analyzed for p21 expression by Western blotting analysis, by use of antibody CP36 (kindly provided by E. Harlow). The high transfection efficiency of Saos-2 cells and the low baseline expression of p21 in these p53-null cells made it possible to detect induction of the endogenous p21 gene following transient transfection of expression constructs.
Acknowledgments
We are grateful to R. Kingston for valuable advice and for providing hsp70 promoter constructs and to R. Morimoto for the gift of antibodies to HSF1 and 2. We are also indebted to L. Ellisen and J. Settleman for critically reviewing this manuscript. This work was supported by National Institutes of Health grant CA 58596 (D.A.H.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL haber@helix.mgh.harvard.edu; FAX (617) 726-5637.
References
- Abravaya K, Myers M, Murphy S, Morimoto R. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes & Dev. 1992;6:1153–1164. doi: 10.1101/gad.6.7.1153. [DOI] [PubMed] [Google Scholar]
- Agoff SN, Hou J, Linzer DI, Wu B. Regulation of the human hsp70 promoter by p53. Science. 1993;259:84–87. doi: 10.1126/science.8418500. [DOI] [PubMed] [Google Scholar]
- Bhan AK. Immunoperoxidase. In: Colvin RB, Bhan AK, McCluskey RT, editors. Diagnostic immunopathology. 2nd edition. NY: Raven Press; 1995. pp. 711–723. [Google Scholar]
- Bonnycastle LL, Yu CE, Hunt CR, Trask BJ, Clancy KP, Weber JL, Patterson D, Schellenberg GD. Cloning, sequencing, and mapping of the human chromosome 14 heat shock protein gene (HSPA2) Genomics. 1994;23:85–93. doi: 10.1006/geno.1994.1462. [DOI] [PubMed] [Google Scholar]
- Campbell K, Mullane K, Aksoy I, Stubdal H, Zalvide J, Pipas J, Silver P, Roberts T, Schaffhausen B, DeCaprio J. DnaJ/hsp40 chaperone domain of SV40 large T antigen promotes efficient viral DNA replication. Genes & Dev. 1997;11:1098–1110. doi: 10.1101/gad.11.9.1098. [DOI] [PubMed] [Google Scholar]
- Cheetham ME, Brion JP, Anderton BH. Human homologues of the bacterial heat-shock protein DnaJ are preferntially expressed in neurons. Biochem J. 1992;284:469–476. doi: 10.1042/bj2840469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotto J, Kline M, Morimoto R. Activation of heat shock factor 1 DNA binding precedes stress-induced serine phosphorylation. Evidence for a multistep pathway of regulation. J Biol Chem. 1996;271:3355–3358. doi: 10.1074/jbc.271.7.3355. [DOI] [PubMed] [Google Scholar]
- Creighton T. Unfolding protein folding. Nature. 1991;352:17–18. doi: 10.1038/352017a0. [DOI] [PubMed] [Google Scholar]
- Englert C, Hou X, Maheswaran S, Bennett P, Ngwu C, Re G, Garvin A, Rosner M, Haber D. WT1 suppresses synthesis of the epidermal growth factor receptor and induces apoptosis. EMBO J. 1995a;14:4662–4675. doi: 10.1002/j.1460-2075.1995.tb00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englert C, Vidal M, Maheswaran S, Ge Y, Ezzell R, Isselbacher K, Haber D. Truncated WT1 mutants alter the subnuclear localization of the wild-type protein. Proc Natl Acad Sci. 1995b;92:11960–11964. doi: 10.1073/pnas.92.26.11960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englert C, Maheswaran S, Garvin AJ, Kreidberg J, Haber DA. Induction of p21 by the Wilms’ tumor suppressor gene WT1. Cancer Res. 1997;57:1429–1434. [PubMed] [Google Scholar]
- Finlay C, Hinds P, Tan T, Eliyahu D, Oren M, Levine A. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988;8:531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bermejo L, Vilaboa N, Perez C, deBlas E, Calle C, Aller P. Modulation of hsp70 and hsp27 gene expression by the differentiation inducer sodium butyrate in U-937 human promonocytic leukemia cells. Leuk Res. 1995;19:713–718. doi: 10.1016/0145-2126(95)00045-p. [DOI] [PubMed] [Google Scholar]
- Haber D, Housman D. The genetics of Wilms’ tumor. Adv Cancer Res. 1992;59:41–68. doi: 10.1016/s0065-230x(08)60302-4. [DOI] [PubMed] [Google Scholar]
- Haber D, Buckler A, Glaser T, Call K, Pelletier J, Sohn R, Douglass E, Housman D. An internal deletion within an 11p13 zinc finger gene contributes to the development of Wilms’ tumor. Cell. 1990;61:1257–1269. doi: 10.1016/0092-8674(90)90690-g. [DOI] [PubMed] [Google Scholar]
- Haber D, Sohn R, Buckler A, Pelletier J, Call K, Housman D. Alternative splicing and genomic structure of the Wilms tumor gene WT1. Proc Natl Acad Sci. 1991;88:9618–9622. doi: 10.1073/pnas.88.21.9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber D, Park S, Maheswaran S, Englert C, Re G, Hazen-Martin D, Sens D, Garvin A. WT1-mediated growth suppression of Wilms tumor cells expressing a WT1 splicing variant. Science. 1993;262:2057–2059. doi: 10.1126/science.8266105. [DOI] [PubMed] [Google Scholar]
- Hainaut P, Milner J. Interaction of heat-shock protein 70 with p53 translated in vitro: Evidence for interaction with dimeric p53 and for a role in the regulation of p53 conformation. EMBO J. 1992;11:3513–3520. doi: 10.1002/j.1460-2075.1992.tb05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- Hartl F. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Hastie N. The genetics of Wilms’ tumor—a case of disrupted development. Annu Rev Genet. 1994;28:523–558. doi: 10.1146/annurev.ge.28.120194.002515. [DOI] [PubMed] [Google Scholar]
- Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Huff V, Miwa H, Haber D, Call K, Housman D, Strong L, Saunders G. Evidence for WT1 as a Wilms tumor (WT) gene: Intragenic germinal deletion in bilateral WT. Am J Hum Genet. 1991;48:997–1003. [PMC free article] [PubMed] [Google Scholar]
- Hunt C, Morimoto R. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci. 1985;82:6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Craig E. Protein folding in vivo: Unraveling complex pathways. Cell. 1997;90:201–204. doi: 10.1016/s0092-8674(00)80327-x. [DOI] [PubMed] [Google Scholar]
- Jones K, Kadonaga J, Rosenfeld P, Kelly T, Tijan R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987;48:79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Kanei-Ishii C, Tanikawa J, Nakai A, Morimoto R, Ishii S. Activation of heat shock transcription factor 3 by c-myb in the absence of cellular stress. Science. 1997;277:246–248. doi: 10.1126/science.277.5323.246. [DOI] [PubMed] [Google Scholar]
- Kao HT, Nevins JR. Transcriptional activation and subsequent control of the human heat shock gene during adenovirus infection. Mol Cell Biol. 1983;3:2058–2065. doi: 10.1128/mcb.3.11.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent J, Coriat AM, Sharpe PT, Hastie ND, van Heyningen V. The evolution of WT1 sequence and expression pattern in the vertebrates. Oncogene. 1995;11:1781–1792. [PubMed] [Google Scholar]
- Khandjian EW, Turler H. Simian virus 40 and polyoma virus induce synthesis of heat shock proteins in permissive cells. Mol Cell Biol. 1983;3:1–8. doi: 10.1128/mcb.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna A, Aten R, Behrman H. Heat shock protein-70 induction mediates luteal regression in the rat. Mol Endocrinol. 1995;9:1431–1440. doi: 10.1210/mend.9.11.8584020. [DOI] [PubMed] [Google Scholar]
- Kingston RE, Baldwin AS, Sharp PA. Regulation of heat shock protein 70 gene expression by c-myc. Nature. 1984;312:280–282. doi: 10.1038/312280a0. [DOI] [PubMed] [Google Scholar]
- Koskinen PJ, Sistonen L, Evan G, Morimoto R, Alitalo K. Nuclear colocalization of cellular and viral myc proteins with hsp70 inmyc-overexpressing cells. J Virol. 1991;65:842–851. doi: 10.1128/jvi.65.2.842-851.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg J, Sariola H, Loring J, Maeda M, Pelletier J, Housman D, Jaenisch R. WT1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- Kudoh T, Ishidate T, Moriyama M, Toyoshima K, Akiyama T. G1 phase arrest induced by Wilms’ tumor protein WT1 is abrogated by cyclin/CDK complexes. Proc Natl Acad Sci. 1995;92:4517–4521. doi: 10.1073/pnas.92.10.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladanyi M, Gerald W. Fusion of the EWS and WT1 genes in the desmoplastic small round cell tumor. Cancer Res. 1994;54:2837–2840. [PubMed] [Google Scholar]
- Landel C, Kushner P, Greene G. The interaction of human estrogen receptor with DNA is modulated by receptor-associated proteins. Mol Endocrinol. 1994;8:1407–1419. doi: 10.1210/mend.8.10.7854357. [DOI] [PubMed] [Google Scholar]
- Langer T, Lu C, Echols H, Flanagan J, Hayer M, Hartl F. Successive action of DnaK, DnaJ, and GroEL along the pathway of chaperone-mediated protein folding. Nature. 1992;356:683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- Larsson S, Charlieu J, Miyagawa K, Engelkamp D, Rassoutzadegan M, Ross A, Cuzin F, vanHeyningen V, Hastie N. Subnuclear localization of WT1 in splicing or transcription factor domains is regulated by alternative splicing. Cell. 1995;81:391–401. doi: 10.1016/0092-8674(95)90392-5. [DOI] [PubMed] [Google Scholar]
- Leppa S, Pirkkala L, Saarento H, Sarge K, Sistonen L. Overexpression of HSF2-b inhibits hemin-induced heat shock gene expression and erythroid differentiation in K562 cells. J Biol Chem. 1997;272:15293–15298. doi: 10.1074/jbc.272.24.15293. [DOI] [PubMed] [Google Scholar]
- Liberek K, Wall D, Georgopoulos C. The DnaJ chaperone catalytically activates the DnaK chaperone to preferentially bind the sigma 32 heat shock transcriptional regulator. Proc Natl Acad Sci. 1995;92:6224–6228. doi: 10.1073/pnas.92.14.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat shock response. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Little M, Prosser J, Condie A, Smith P, van Heyningen V, Hastie N. Zinc finger point mutations within the WT1 gene in Wilms tumor patients. Proc Natl Acad Sci. 1992;89:4791–4795. doi: 10.1073/pnas.89.11.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum LS, Hsu S, Vaewhongs M, Wu B. The hsp70 gene CCAAT-binding factor mediates transcriptional activation by the adenovirus E1a protein. Mol Cell Biol. 1992;12:2599–2605. doi: 10.1128/mcb.12.6.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheswaran S, Park S, Bernard A, Morris J, Rauscher III F, Hill D, Haber D. Physical and functional interction between WT1 and p53 proteins. Proc Natl Acad Sci. 1993;90:5100–5104. doi: 10.1073/pnas.90.11.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheswaran S, Englert C, Bennett P, Heinrich G, Haber D. The WT1 gene product stabilizes p53 and inhibits p53-mediated apoptosis. Genes & Dev. 1995;9:2143–2156. doi: 10.1101/gad.9.17.2143. [DOI] [PubMed] [Google Scholar]
- Milarski K, Morimoto R. Expression of human hsp70 during the synthetic phase of the cell cycle. Proc Natl Acad Sci. 1986;83:9517–9521. doi: 10.1073/pnas.83.24.9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner CM, Campbell RD. Structure and expression of three MHC-linked HSP70 genes. Immunogenetics. 1990;32:242–251. doi: 10.1007/BF00187095. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, Tijan R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989;245:371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Mosser D, Duchaine J, Massie B. The DNA-binding activity of the human heat shock transcription factor 1 is regulated in vivo by hsp70. Mol Cell Biol. 1993;13:5427–5438. doi: 10.1128/mcb.13.9.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundlos S, Pelletier J, Darveau A, Bachmann M, Winterpacht A, Zabel B. Nuclear localization of the protein encoded by the Wilms’ tumor gene WT1 in embryonic and adult tissues. Development. 1993;119:1329–1341. doi: 10.1242/dev.119.4.1329. [DOI] [PubMed] [Google Scholar]
- Pelletier J, Bruening W, KAshtan C, Mauer S, Manivel J, Striegel J, Houghton D, Junien C, Habib R, Fouser L, Fine R, Silverman B, Haber D, Housman D. Germline mutations in the Wilms’ tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell. 1991a;67:437–447. doi: 10.1016/0092-8674(91)90194-4. [DOI] [PubMed] [Google Scholar]
- Pelletier J, Bruening W, Li F, Haber D, Glaser T, Housman D. WT1 mutations contribute to abnormal genital system development and hereditary Wilms’ tumour. Nature. 1991b;353:431–434. doi: 10.1038/353431a0. [DOI] [PubMed] [Google Scholar]
- Pinhasi-Kimshi O, Michalovitz D, Ben-Zeev A, Oren M. Specific interaction between the p53 cellular tumour antigen and major heat shock proteins. Nature. 1986;320:182–185. doi: 10.1038/320182a0. [DOI] [PubMed] [Google Scholar]
- Pritchard-Jones K, Fleming S, Davidson D, Bickmore W, Porteous D, Gosden C, Bard J, Buckler A, Pelletier J, Housman D, Heyningen V, Hastie N. The candidate Wilms’ tumour gene is involved in genitourinary development. Nature. 1990;346:194–197. doi: 10.1038/346194a0. [DOI] [PubMed] [Google Scholar]
- Rabindran SK, Giorgi G, Clos J, Wu C. Molecular cloning and expression of a human heat shock factor, HSF1. Proc Natl Acad Sci. 1991;88:6906–6910. doi: 10.1073/pnas.88.16.6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauscher III F. The WT1 Wilms tumor gene product: A developmentally regulated transcription factor in the kidney that functions as a tumor suppressor. FASEB J. 1993;7:896–903. [PubMed] [Google Scholar]
- Rauscher III F, Morris J, Tournay O, Cook D, Curran T. Binding of the Wilms’ tumor locus zinc finger protein to the EGR-1 consensus sequence. Science. 1990;250:1259–1262. doi: 10.1126/science.2244209. [DOI] [PubMed] [Google Scholar]
- Reddy J, Licht J. The WT1 Wilms tumor suppressor gene: How much do we really know? Biochim Biophys Acta. 1996;1287:1–28. doi: 10.1016/0304-419x(95)00014-7. [DOI] [PubMed] [Google Scholar]
- Reddy J, Hosono S, Licht J. The transcriptional effect of WT1 is modulated by choice of expression vector. J Biol Chem. 1995;270:29976–29982. doi: 10.1074/jbc.270.50.29976. [DOI] [PubMed] [Google Scholar]
- Sarge KD, Zimarino V, Holm K, Wu C, Morimoto RI. Cloning and characterization of two mouse heat shock factors with distinct inducible and constitutive DNA-binding ability. Genes & Dev. 1991;5:1902–1922. doi: 10.1101/gad.5.10.1902. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. Heat shock proteins. J Biol Chem. 1991;265:12111–12114. [PubMed] [Google Scholar]
- Schowalter D, Sullivan W, Maihle N, Dobson A, Connely O, O’Malley B, Toft D. Characterization of progesterone receptor binding to the 90 and 70 kDa heat shock proteins. J Biol Chem. 1991;166:21165–21173. [PubMed] [Google Scholar]
- Schuetz T, Gallo G, Sheldon L, Tempst P, Kingston R. Isolation of a cDNA for HSF2: Evidence for two heat shock factor genes in humans. Proc Natl Acad Sci. 1991;88:6911–6915. doi: 10.1073/pnas.88.16.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver P, Way J. Eukaryotic DnaJ homologs and the specificity of hsp70 activity. Cell. 1993;74:5–6. doi: 10.1016/0092-8674(93)90287-z. [DOI] [PubMed] [Google Scholar]
- Sistonen L, Sarge K, Phillips B, Abravaya K, Morimoto R. Activation of heat shock factor 2 during hemin-induced differentiation of human erythroleukemia cells. Mol Cell Biol. 1992;12:4104–4111. doi: 10.1128/mcb.12.9.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D, Toft D. Steroid receptors and their associated proteins. Mol Endocrinol. 1993;7:4–11. doi: 10.1210/mend.7.1.8446107. [DOI] [PubMed] [Google Scholar]
- Sorger P. Heat shock factor and the heat shock response. Cell. 1991;65:363–366. doi: 10.1016/0092-8674(91)90452-5. [DOI] [PubMed] [Google Scholar]
- Stubdal H, Zalvide J, Campbell K, Schweitzer C, Roberts T, DeCaprio J. Inactivation of pRB-related proteins p130 and p107 mediated by the J domain of simian virus 40 large T antigen. Mol Cell Biol. 1997;17:4979–4990. doi: 10.1128/mcb.17.9.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshima S, Rokutan K, Takahashi M, Nikawa T, Kishi K. Induction of heat shock proteins and their possible roles in macrophages during activation by macrophage colony-stimulating factor. Biochem J. 1996;315:497–504. doi: 10.1042/bj3150497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J, Douglas M. A conserved HPD sequence of the J-domain is necessary for YDJ1 stimulation of hsp70 ATPase activity at a site distinct from substrate binding. J Biol Chem. 1996;271:9347–9354. doi: 10.1074/jbc.271.16.9347. [DOI] [PubMed] [Google Scholar]
- Varanasi R, Bardeesy N, Gharemani M, Petruzzi M-J, Nowak N, Adam M, Grundy P, Shows T, Pelletier J. Fine structure analysis of the WT1 gene in sporadic Wilms tumors. Proc Natl Acad Sci. 1994;91:3554–3558. doi: 10.1073/pnas.91.9.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science. 1993;262:2050–2054. doi: 10.1126/science.8266103. [DOI] [PubMed] [Google Scholar]
- Wall D, Zylick M, Georgopoulos C. The NH2-terminal 108 amino acids of the Escherichia coli DnaJ protein stimulate the ATPase activity of DnaK and are sufficient for l replication. J Biol Chem. 1994;269:5446–5451. [PubMed] [Google Scholar]
- Wang Z, Qiu Q, Enger K, Deuel T. A second transcriptionally active DNA-binding site for the Wilms tumor gene product, WT1. Proc Natl Acad Sci. 1993;90:8896–8900. doi: 10.1073/pnas.90.19.8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu BJ, Morimoto RI. Transcription of the human hsp70 gene is induced by serum stimulation. Proc Natl Acad Sci. 1985;82:6070–6074. doi: 10.1073/pnas.82.18.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Hunt C, Morimoto R. Structure and expression of the human gene encoding major heat shock protein hsp70. Mol Cell Biol. 1985;5:330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu BJ, Hurst HC, Jones NC, Morimoto RI. The E1A 13S product of adenovirus 5 activates transcription of the cellular human hsp70 gene. Mol Cell Biol. 1986;6:2994–2999. doi: 10.1128/mcb.6.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. Heat shock transcription factors: Structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]