Abstract
Abstract
Small-conductance calcium-activated K+ channels (SK channels) regulate the excitability of neurons and their responsiveness to synaptic input patterns. SK channels contribute to the afterhyperpolarization (AHP) following action potential bursts, and curtail excitatory postsynaptic potentials (EPSPs) in neuronal dendrites. Here we review evidence that SK2 channels are expressed in rat cerebellar Purkinje cells during development and throughout adulthood, and play a key role in diverse cellular processes such as the regulation of the spike firing frequency and the modulation of calcium transients in dendritic spines. In Purkinje cells as well as in other types of neurons, SK2 channel plasticity seems to provide an important mechanism allowing these cells to adjust their intrinsic excitability and to alter the probabilities for the induction of synaptic learning correlates, such as long-term potentiation (LTP).
Christian Hansel is a neurophysiologist with expertise in synaptic transmission, synaptic plasticity, calcium signalling and cerebellar physiology. In 2008 he moved his laboratory to the University of Chicago. He performed his PhD work in the laboratory of Wolf Singer at the Max-Planck-Institute for Brain Research in Frankfurt, and trained as a postdoc in the laboratory of David Linden at Johns Hopkins University. From 2000 to 2008 he was leading a research group at the Erasmus University Medical Center in Rotterdam. His work focuses on synaptic and non-synaptic mechanisms of information storage and learning in cerebellar circuits.
 |
Introduction
Small-conductance calcium-activated K+ channels (SK channels) are strictly voltage-independent K+ channels that are exclusively activated by intracellular calcium ions (Köhler et al. 1996; Xia et al. 1998). SK channels are tetrameric assemblies of four α-subunits that form the channel pore and bind to the calcium sensor calmodulin. Calcium binding to calmodulin triggers conformational changes in the channel complex and subsequent channel gating (Xia et al. 1998; Lujan et al. 2009). Three types of SK channel subunits, SK1–3, have been described in the CNS, which show very similar calcium sensitivities (EC50 = 0.3–0.5 μm) and rely on a calmodulin-dependent gating mechanism (Köhler et al. 1996; Xia et al. 1998). SK channels open K+ conductances in response to calcium transients that result from membrane depolarization, and thus contribute to the repolarization of neurons. This effect is most evident in the participation of SK conductances in the afterhyperpolarization (AHP) following bursts of action potentials (Sah, 1996; Stocker et al. 1999; Pedarzani et al. 2001; Edgerton & Reinhart, 2003). SK channels thus provide a negative feedback loop that regulates the excitability of neurons. SK channel gating is controlled by complex protein kinase and phosphatase interactions. Each channel subunit constitutively binds casein kinase 2 (CK2) and protein phosphatase 2A (PP2A). CK2 is a constitutively active kinase that phosphorylates calmodulin bound to SK subunits and reduces the calcium sensitivity of SK channels (Bildl et al. 2004). PP2A binds to SK channels as well and provides a phosphorylation switch with CK2 (Allen et al. 2007). While the molecular composition of the SK channel multiprotein complex has been studied in detail (Lujan et al. 2009), it remains to be determined under what physiological conditions the calcium sensitivity of SK channels is modified by CK2 and PP2A.
Voltage-clamp recordings reveal distinct kinetic phases of the AHP current: a fast component (IfAHP) that is characterized by time constants on the order of 50 ms (Lancaster & Adams, 1986), a medium component (ImAHP), which is reduced by apamin, a selective SK channel blocker, and has a time constant on the order of 200 ms (Stocker et al. 1999), and a slow component (IsAHP), which decays over seconds (Stocker et al. 1999). Knock-out studies show that SK2 channels mediate ImAHP, and that none of the three types of SK channel subunits, SK1–3, is involved in the slow IsAHP (Bond et al. 2004). The contribution of SK channels to an AHP current with a duration of tens to hundreds of milliseconds corresponds well with the observation that SK channel blockade by apamin enhances the spike frequency of neurons within bursts of action potentials, and the number of action potentials evoked by current injection (Stocker et al. 1999). SK conductances also mediate the repolarization of dendritic plateau potentials (Cai et al. 2004) and regulate calcium transients in dendritic spines (Ngo-Anh et al. 2005; Belmeguenai et al. 2010), which shows that SK channels control dendritic integration properties. Together, these data suggest that the main function of SK channels is to put a brake on neuronal firing and dendritic excitability in response to even moderate rises in the cytosolic calcium concentration.
This review focuses on SK channel function in cerebellar Purkinje cells. These cells differ from many other types of neurons, including hippocampal and neocortical pyramidal cells, in their spike firing properties as well as in synaptic and non-synaptic plasticity mechanisms used for information storage (Hansel et al. 2001; Jörntell & Hansel, 2006). We will discuss how SK channel modulation specifically regulates these processes in Purkinje cells.
SK channel expression in cerebellar Purkinje cells
Of the three types of SK channel subunits, only SK2 channels are expressed in rat Purkinje cells (Cingolani et al. 2002). Therefore, apamin sensitivity in Purkinje cells can be exclusively assigned to SK2 channels (see Grunnet et al. 2001). It has been reported that in rat Purkinje cells the level of SK2 channel expression significantly decreases during the first 3 weeks after birth, at both the mRNA and protein levels, with weak expression remaining in scattered Purkinje cells at P60 (Cingolani et al. 2002). This decrease in SK2 channel expression was reflected in the observation that in adult Purkinje cells apamin failed to induce spontaneous spike firing (Cingolani et al. 2002). In contrast to rats, mice express SK2 channels in the adult Purkinje cell layer (Sailer et al. 2004). While these results could reflect a species difference in SK2 channel expression, an electrophysiological study questions the functional downregulation of SK2 channels in adult rat Purkinje cells: apamin increased the spike firing rate of Purkinje cells obtained from rats ≥3 months old to a similar degree as observed in young rats (Womack & Khodakhah, 2003). In this latter study, a requirement for apamin to work in slices prepared from adult rats was to perform the recordings at near-physiological temperature in cells that showed spontaneous activity even in the absence of apamin. When the recordings were performed under similar conditions as in Cingolani et al. (2002), at room temperature and from silent neurons, apamin did not trigger spontaneous firing (Womack & Khodakhah, 2003). Together, these data (supported by observations from our laboratory; see below) suggest that SK2 channel function is not downregulated in adult rat Purkinje cells under more physiological recording conditions.
We performed SK2 channel immunohistochemistry to take up the question whether adult rat Purkinje cells express SK2 channels, and to obtain more detailed information on the cellular distribution of SK2 channels in these cells. Immunostainings were performed using antibodies directed against the C-terminal domain of the SK2 channel subunit, and the Purkinje cell-specific marker calbindin. The specificity of the anti-SK2 antibody was confirmed by the absence of staining in slices prepared from SK2−/− mice (Fig. 1; SK2−/− mice were kindly provided by J. P. Adelman). Cerebellar sections were obtained from 2-week-old rats, 4-week-old rats, and adult (3–4 months) rats. In all three age groups, SK2 channel subunits were detected, and their expression overlapped with the Purkinje cell-specific calbindin staining (Fig. 1). Particularly strong expression levels were seen in Purkinje cell dendrites. At all ages, additional SK2 channel staining was found in cellular elements in the molecular layer, which was clearly distinct from the Purkinje cell staining. Our immunostaining data are not well-suited to detect subtle to moderate differences in the expression levels of SK2 channels. Nevertheless, these stainings show that SK2 channels are expressed in rat Purkinje cells during development and throughout adulthood, and are thus in line with the electrophysiological data presented by Womack & Khodakhah (2003).
Figure 1. SK2 channel immunostaining reveals expression of SK2 channels in developing and adult rat Purkinje cells.
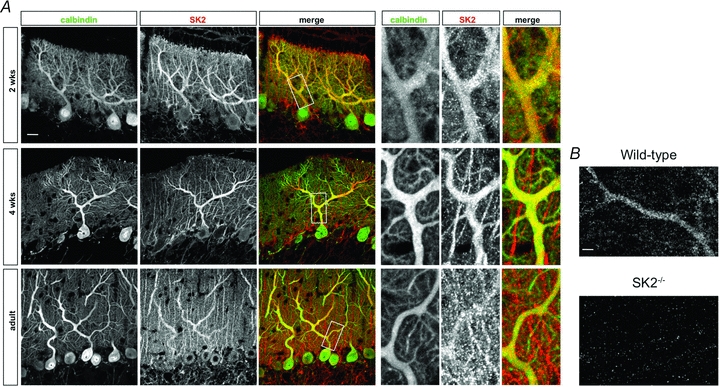
A, anti-SK2-channel (red) and anti-calbindin (green) antibody stainings of cerebellar sections from 2-week-old (top row), 4-week-old (middle row), and 3- to 4-month-old (bottom row) rats show SK2 staining in Purkinje cell dendrites. In the cerebellar cortex, calbindin is exclusively expressed in Purkinje cells. Scale bar (top left): 20 μm. Right side: Enlarged views taken from the areas indicated by white boxes on the left. B, specificity control of the anti-SK2 antibody. Top, in slices obtained from wild-type mice (P70), the anti-SK2 antibody stains Purkinje cell dendrites. These immunostainings closely resemble the stainings obtained from rat cerebellar slices (A). Bottom, no staining was observed in slices obtained from SK2−/− mice (P70). The antibody dilution (1:500) was the same in all stainings. Scale bar: 5 μm. SK2−/− mice were kindly provided by J. P. Adelman. This figure was modified from Belmeguenai et al. (2010) with permission from the Society for Neuroscience.
SK channel function in cerebellar Purkinje cells
Bath-application of apamin results in an increase in Purkinje cell excitability. In patch-clamp recordings from rat Purkinje cells, injection of depolarizing current steps evokes spike firing. In the presence of apamin (3 nm), the number of evoked spikes was significantly enhanced (Fig. 2A). The blockade of SK2 channels by apamin did not affect the waveform of individual action potentials (Fig. 2A), but increased the rate of depolarization toward spike threshold after each action potential, resulting in a higher spike frequency and spike count. We observed apamin effects on Purkinje cell excitability in young adult rats (P17–21; Fig. 2A), but also in P51–52 rats (Fig. 2B; we did not perform recordings from older rats). Thus, we do not find any evidence for a developmental downregulation of SK2 channels at 3 weeks after birth.
Figure 2. The SK channel blocker apamin enhances Purkinje cell excitability and occludes intrinsic plasticity.
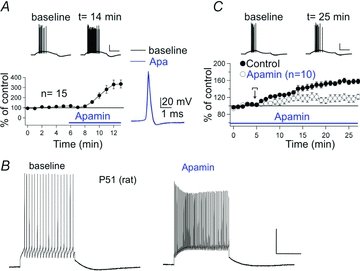
A, bath-application of apamin (3 nm) increases the excitability of rat Purkinje cells (P17–21; n = 15), without affecting the action potential waveform. The excitability was measured as the spike count in response to a depolarizing current step (∼100–200 pA). B, apamin (10 nm) also enhances Purkinje cell excitability in slices obtained from adult rats. The traces were obtained from a P51 rat Purkinje cell. We observed the same apamin effect in slices obtained from two P52 rats (data not shown). C, apamin bath-application partially occludes excitability increases triggered by repeated injection of depolarizing current steps (100 ms pulses applied at 5 Hz for 3–4 s; control: n = 15; apamin: n = 10). Scale bars: 20 mV and 200 ms. The recordings shown in A and C were performed at near-physiological temperature (34–35°C). The recordings shown in (B) were performed at room temperature. Error bars indicate SEM. Panels A and C of this figure were modified from Belmeguenai et al. (2010) with permission from the Society for Neuroscience.
SK channels and large-conductance calcium-activated BK-type channels, respectively, assume specific roles in membrane potential repolarization following spike activity. While BK channels are involved in the repolarization following individual action potentials, and thus shape the action potential waveform, SK channels mediate slower AHP currents in response to repetitive action potential firing (Edgerton & Reinhart, 2003). A modulation of SK conductances will thus affect spike firing within trains of action potentials, and can adjust the spike frequency by determining the rate at which the membrane potential reaches the spike threshold. Under physiological conditions, Purkinje cells do not show uninterrupted tonic spike firing, but often display trains of spontaneous bursts. SK channels play a role in setting both the interspike and interburst intervals, suggesting that SK channel-mediated control of intrinsic excitability can adjust the Purkinje cell output (Womack & Khodakhah, 2004).
In Purkinje cells, the calcium for the activation of both BK and SK channels is provided by P/Q-type calcium channels (Edgerton & Reinhard, 2003; Womack et al. 2004). The tight coupling between these conductances explains why both P/Q-type channels and SK channels have been implicated in cerebellar ataxia. Episodic ataxia type-2 (EA2) is a channelopathy resulting from a mutation in CACNA1A, the gene coding for the α-1a subunit of P/Q-type calcium channels (Ophoff et al. 1996). In leaner (tgla) mice, which suffer from a spontaneous mutation in the CACNA1A gene, and thus provide a useful mouse model for EA2, irregular pacemaking has been observed in Purkinje cells, resulting in motor performance deficits. Suprisingly, in tottering mice (tg), which also carry a mutation in the α-1a subunit, motor performance was improved by the SK channel activator 1-ethyl-2-benzimidazolinone (EBIO) (Walter et al. 2006). This observation shows that EA2 is caused by a mutation in the gene coding for the α-1a subunit of P/Q-type calcium channels, but ultimately results from a suboptimal activation of SK channels. It should be noted that application of 4-aminopyridine (4-AP), an antagonist of some types of voltage-gated K+ channels, also restores the regularity of pacemaking in tg/tg Purkinje cells (Alvina & Khodakhah, 2010). A possible explanation is that blockade of 4-AP sensitive K+ conductances facilitates the activation of SK channels. Voltage-gated calcium channels are also involved in the induction of long-term depression (LTD) and long-term potentiation (LTP) at parallel fibre (PF) synapses onto Purkinje cells (Jörntell & Hansel, 2006). However, basic motor coordination is not grossly affected in some types of genetically altered mice that show deficits in LTD or LTP induction. It seems that irregularities in spike firing patterns, rather than synaptic plasticity deficits, cause EA2 (Rinaldo & Hansel, 2010). Thus, SK2 channels are crucial to maintain regularity and precision in Purkinje cell spike patterns, and to enable cerebellar circuits to generate well-timed electrical output.
SK channel modulation and plasticity
SK channels regulate the excitability of neurons and determine their spike firing properties. Thus, cellular mechanisms that affect SK channel function or membrane expression will have a strong impact on neuronal signalling. In layer V pyramidal cells, as well as in Purkinje cells, forms of intrinsic plasticity (changes in intrinsic excitability) have been described that are activity dependent and are, at least partially, mediated by a downregulation of SK channels (Sourdet et al. 2003; Belmeguenai et al. 2010; see Fig. 2C). In both types of neurons, the enhanced excitability was evident by a reduction in the amplitude of burst-evoked AHPs, as well as an increase in the number of spikes evoked by injection of depolarizing current steps (Sourdet et al. 2003; Belmeguenai et al. 2010). In Purkinje cells, this form of SK channel-mediated intrinsic plasticity can be co-induced with LTP following PF tetanization and, like LTP, depends on the activation of protein phosphatases 1, 2A and 2B (Belmeguenai & Hansel, 2005; Belmeguenai et al. 2010; Schonewille et al. 2010). In addition, Purkinje cell intrinsic plasticity requires activity of protein kinase A (PKA) and CK2 (Belmeguenai et al. 2010), which have been implicated in the surface expression of SK2 channels (Ren et al. 2006), and regulation of their calcium sensitivity (Allen et al. 2007), respectively. In CA1 hippocampal pyramidal cells, a similar phenomenon has been observed: LTP at Schaffer collateral synapses is accompanied by a PKA-dependent endocytosis of SK2 channels (Ren et al. 2006; Lin et al. 2008, 2010). PKA-dependent trafficking of SK2 channels has also been described in the amygdala, where activation of β adrenoceptors triggers SK2 removal from the synapses (Faber et al. 2008).
SK channel regulation, in turn, affects the probability for LTP induction. At the level of individual spines in CA1 pyramidal cells, SK channel activity reduces the amplitude of calcium transients evoked by NMDA receptor activation. Blockade of SK channels by apamin thus enhances spine calcium transients and facilitates the induction of LTP (Ngo-Anh et al. 2005; Lin et al. 2008). In Purkinje cells, SK2 channel downregulation mediates an increase in excitability. This form of intrinsic plasticity is associated with an increase in spine calcium transients (Fig. 3) that, in contrast to the observations made in pyramidal cells, results in a reduced probability for LTP induction (Belmeguenai et al. 2010). It currently remains unclear how SK2 channel regulation affects NMDA receptor signalling in Purkinje cells. Functional NMDA receptors have recently been described in adult Purkinje cells (Piochon et al. 2007; Renzi et al. 2007). Activity of Purkinje cell NMDA receptors is required for LTD, but not LTP induction at PF synapses (Piochon et al. 2010). Since cerebellar LTD requires larger calcium transients than LTP (Coesmans et al. 2004), it is thus conceivable that in Purkinje cells SK2 channel downregulation enhances calcium signalling through NMDA receptors, thus shifting the balance of induction probabilities from LTP toward LTD (Fig. 4). These data show that SK channels provide a negative feedback loop, which acts as a brake on dendritic calcium transients and synaptic responses. Recent studies show that synaptic modulators affect dendritic integration by adjusting the gain of this feedback loop. M1 muscarinic receptors amplify calcium transients and synaptic potentials (Buchanan et al. 2010; Giessel & Sabatini, 2010), and facilitate hippocampal LTP induction by inhibiting SK channels (Buchanan et al. 2010). These examples illustrate how crucial SK channels are for regulating the excitability of neurons, as well as for determining the probability of synaptic gain changes.
Figure 3. Intrinsic plasticity enhances spine calcium transients.
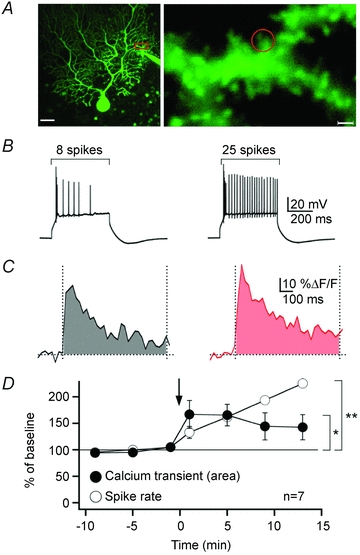
A, left, Purkinje cell filled with Oregon Green BAPTA-2 (200 μm). Scale bar: 20 μm. Right, enhanced view of the area marked by the red box in the left image. The red circle indicates the region of interest. Scale bar: 2 μm. B, after repeated injection of depolarizing current steps (5 Hz, 3 s), the spike count was upregulated. The traces show spikes evoked by constant current injection before (left) and after tetanization (right). C, calcium transients evoked at corresponding time points by brief 100 Hz PF stimulation. The area under the curve was monitored between 0 and 800 ms after stimulation and is highlighted by the shaded area below the traces. D, time course of changes in the spike rate (open circles) and the spine calcium transients (filled circles). The arrow indicates the time point of tetanization. Statistical significance was tested for the last two data points (**P = 0.002; *P = 0.025; Student's paired t test). Error bars indicate SEM. This figure is taken from Belmeguenai et al. (2010) with permission from the Society for Neuroscience.
Figure 4. SK2 channels modulate NMDA receptor-mediated calcium signalling and synaptic plasticity.
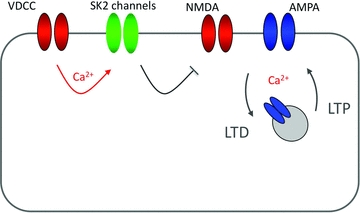
SK2 channels are activated by calcium influx through voltage-gated calcium channels (VDCCs; P/Q-type channels in cerebellar Purkinje cells). SK conductances reduce calcium influx through NMDA receptor-associated channels, thus influencing the LTD/LTP balance. SK channel blockade thus promotes the induction of LTP in hippocampal pyramidal cells. It is conceivable that in cerebellar Purkinje cells SK channel blockade/amplification of NMDA receptor-mediated calcium transients would facilitate the induction of LTD instead. Calcium sources (VDCCs/NMDA receptors) are shown in red.
Future directions
SK channels provide an important feedback loop in neurons, which links depolarization-triggered calcium influx to the activation of hyperpolarizing currents. This feature allows SK channels to contribute to the AHP following bursts of action potentials, and to curtail dendritic potentials and calcium transients. In cerebellar Purkinje cells, it has been shown that the expression of SK2 channels is downregulated during the first three weeks after birth (Cingolani et al. 2002). Recent electrophysiological (Womack & Khodakhah, 2003; Fig. 2B) and immunohistochemical studies (Belmeguenai et al. 2010; Fig. 1), however, provide evidence for SK2 channel expression in adult rat Purkinje cells. As these techniques are not well-suited for quantitative approaches, it remains possible that SK2 expression levels decrease, but remain sufficiently high during adulthood to mediate apamin-sensitivity of the neurons (Womack & Khoadakhah, 2003; Fig. 2B) and to cause significant immunohistochemical staining (Belmeguenai et al. 2010). The notion that SK2 channels are present in adult neurons is important, because of the key role of SK2 channels in the regulation of neuronal excitability and in adjusting the probability for subsequent induction of LTP (Ngo-Anh et al. 2005; Lin et al. 2008; Belmeguenai et al. 2010).
Future research efforts will have to further examine the role of SK2 channel regulation in information storage, and the way SK2 channel plasticity complements long-term synaptic plasticity. It will be important to determine whether excitability changes mediated by SK2 channel regulation occur throughout the entire dendrite, or whether these changes can be specific to individual dendritic branches. These data will allow us to draw conclusions about the function of excitability alterations: while cell-wide modifications might reflect general gain adjustment, such as in homeostatic plasticity, localized changes point toward a more direct involvement in information storage. Finally, it will be important to examine whether BK conductances, which also regulate Purkinje cell excitability (Sausbier et al. 2004; Womack & Khodakhah, 2004; Rancz & Häusser, 2006, 2010) and are reduced in a form of intrinsic plasticity in vestibular nucleus neurons (Nelson et al. 2005), can play a similar or complementing role in adjusting dendritic responsiveness and LTP/LTD induction probabilities.
Acknowledgments
This study was supported by a grant from the National Institute of Neurological Disorders and Stroke NS-62771 (C.H.).
Author's present address
E.Teuling: Department of Genetics, University of Groningen, The Netherlands.
References
- Allen D, Fakler B, Maylie J, Adelman JP. Organization and regulation of small conductance Ca2+-activated K+ channel multiprotein complexes. J Neurosci. 2007;27:2369–2376. doi: 10.1523/JNEUROSCI.3565-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvina K, Khodakhah K. The therapeutic mode of action of 4-aminopyridine in cerebellar ataxia. J Neurosci. 2010;30:7258–7268. doi: 10.1523/JNEUROSCI.3582-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmeguenai A, Hansel C. A role for protein phosphatases 1, 2A, and 2B in cerebellar long-term potentiation. J Neurosci. 2005;25:10768–10772. doi: 10.1523/JNEUROSCI.2876-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmeguenai A, Hosy E, Bengtsson F, Pedroarena CM, Piochon C, Teuling E, He Q, Ohtsuki G, De Jeu MT, Elgersma Y, De Zeeuw CI, Jörntell H, Hansel C. Intrinsic plasticity complements long-term potentiation in parallel fiber input gain control in cerebellar Purkinje cells. J Neurosci. 2010;30:13630–13643. doi: 10.1523/JNEUROSCI.3226-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bildl W, Strassmeier T, Thurm H, Andersen J, Eble S, Oliver D, Knipper M, Mann M, Schulte U, Adelman JP, Fakler B. Protein kinase CK2 is coassembled with small conductance Ca2+-activated K+ channels and regulates channel gating. Neuron. 2004;43:847–858. doi: 10.1016/j.neuron.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, Adelman JP. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci. 2004;24:5301–5306. doi: 10.1523/JNEUROSCI.0182-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan KA, Petrovic MM, Chamberlain SE, Marrion NV, Mellor JR. Facilitation of long-term potentiation by muscarinic M1 receptors is mediated by inhibition of SK channels. Neuron. 2010;68:948–963. doi: 10.1016/j.neuron.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Liang CW, Muralidharan S, Kao JP, Tang CM, Thompson SM. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron. 2004;44:351–364. doi: 10.1016/j.neuron.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Cingolani LA, Gymnopoulos M, Boccaccio A, Stocker M, Pedarzani P. Developmental regulation of small-conductance Ca2+-activated K+ channel expression and function in rat Purkinje neurons. J Neurosci. 2002;22:4456–4467. doi: 10.1523/JNEUROSCI.22-11-04456.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron. 2004;44:691–700. doi: 10.1016/j.neuron.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Edgerton JR, Reinhart PH. Distinct contributions of small and large conductance Ca2+-activated K+ channels to rat Purkinje neuron function. J Physiol. 2003;548:53–69. doi: 10.1113/jphysiol.2002.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ESL, Delaney AJ, Power JM, Sedlak PL, Crane JW, Sah P. Modulation of SK channel trafficking by β adrenoceptors enhances excitatory transmission and plasticity in the amygdala. J Neurosci. 2008;28:10803–10813. doi: 10.1523/JNEUROSCI.1796-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giessel AJ, Sabatini BL. M1 muscarinic receptors boost synaptic potentials and calcium influx in dendritic spines by inhibiting postsynaptic SK channels. Neuron. 2010;68:936–947. doi: 10.1016/j.neuron.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunnet M, Jensen BS, Olesen SP, Klaerke DA. Apamin interacts with all subtypes of cloned small-conductance Ca2+-activated K+ channels. Pflugers Arch. 2001;441:544–550. doi: 10.1007/s004240000447. [DOI] [PubMed] [Google Scholar]
- Hansel C, Linden DJ, D'Angelo E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat Neurosci. 2001;4:467–475. doi: 10.1038/87419. [DOI] [PubMed] [Google Scholar]
- Jörntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron. 2006;52:227–238. doi: 10.1016/j.neuron.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Köhler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- Lancaster B, Adams PR. Calcium-dependent current generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol. 1986;55:1268–1282. doi: 10.1152/jn.1986.55.6.1268. [DOI] [PubMed] [Google Scholar]
- Lin MT, Lujan R, Watanabe M, Adelman JP, Maylie J. SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat Neurosci. 2008;11:170–177. doi: 10.1038/nn2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Lujan R, Watanabe M, Frerking M, Maylie J, Adelman JP. Coupled activity-dependent trafficking of synaptic SK2 channels and AMPA receptors. J Neurosci. 2010;30:11726–11734. doi: 10.1523/JNEUROSCI.1411-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Maylie J, Adelman JP. New sites of action for GIRK and SK channels. Nat Rev Neurosci. 2009;10:475–480. doi: 10.1038/nrn2668. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Gittis AH, du Lac S. Decreases in CaMKII activity trigger persistent potentiation of intrinsic excitability in spontaneously firing vestibular nucleus neurons. Neuron. 2005;46:623–631. doi: 10.1016/j.neuron.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci. 2005;8:642–649. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, Lamerdin JE, Mohrenweiser HW, Bulman DE, Ferrari M, Haan J, Lindhout D, van Ommen GJ, Hofker MH, Ferrari MD, Frants RR. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543–552. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Mosbacher J, Rivard A, Cingolani LA, Oliver D, Stocker M, Adelman JP, Fakler B. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J Biol Chem. 2001;276:9762–9769. doi: 10.1074/jbc.M010001200. [DOI] [PubMed] [Google Scholar]
- Piochon C, Irinopoulou T, Brusciano D, Bailly Y, Mariani J, Levenes C. NMDA receptor contribution to the climbing fiber response in the adult mouse Purkinje cell. J Neurosci. 2007;27:10797–10809. doi: 10.1523/JNEUROSCI.2422-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piochon C, Levenes C, Ohtsuki G, Hansel C. Purkinje cell NMDA receptors assume a key role in synaptic gain control in the mature cerebellum. J Neurosci. 2010;30:15330–15335. doi: 10.1523/JNEUROSCI.4344-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancz EA, Häusser M. Dendritic calcium spikes are tunable triggers of cannabinoid release and short-term synaptic plasticity in cerebellar Purkinje neurons. J Neurosci. 2006;26:5428–5437. doi: 10.1523/JNEUROSCI.5284-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancz EA, Häusser M. Dendritic spikes mediate negative synaptic gain control in cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 2010;107:22284–22289. doi: 10.1073/pnas.1008605107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Barnwell LF, Alexander JC, Lubin FD, Adelman JP, Pfaffinger PJ, Schrader LA, Anderson AE. Regulation of surface localization of the small conductance Ca2+-activated potassium channel, SK2, through direct phosphorylation by cAMP-dependent protein kinase. J Biol Chem. 2006;281:11 769–11 779. doi: 10.1074/jbc.M513125200. [DOI] [PubMed] [Google Scholar]
- Renzi M, Farrant M, Cull-Candy SG. Climbing-fibre activation of NMDA receptors in Purkinje cells of adult mice. J Physiol. 2007;585:91–101. doi: 10.1113/jphysiol.2007.141531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo L, Hansel C. Ataxias and cerebellar dysfunction: involvement of synaptic plasticity deficits? Funct Neurol. 2010;25:135–139. [PMC free article] [PubMed] [Google Scholar]
- Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- Sailer CA, Kaufmann WA, Marksteiner J, Knaus HG. Comparative immunohistochemical distribution of three small-conductance Ca2+-activated potassium channel subunits, SK1, SK2, and SK3 in mouse brain. Mol Cell Neurosci. 2004;26:458–469. doi: 10.1016/j.mcn.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Sausbier M, Hu H, Arntz C, Feil S, Kamm S, Adelsberger H, Sausbier U, Sailer CA, Feil R, Hofmann F, Korth M, Shipston MJ, Knaus HG, Wolfer DP, Pedroarena CM, Storm JF, Ruth P. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci U S A. 2004;101:9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonewille M, Belmeguenai A, Koekkoek SK, Houtman SH, Boele HJ, van Beugen BJ, Gao Z, Badura A, Ohtsuki G, Amerika WE, Hosy E, Hoebeek FE, Elgersma Y, Hansel C, De Zeeuw CI. Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning. Neuron. 2010;67:618–628. doi: 10.1016/j.neuron.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourdet V, Russier M, Daoudal G, Ankri N, Debanne D. Long-term enhancement of neuronal excitability and temporal fidelity mediated by metabotropic glutamate receptor subtype 5. J Neurosci. 2003;23:10238–10248. doi: 10.1523/JNEUROSCI.23-32-10238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 1999;96:4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter JT, Alvina K, Womack MD, Chevez C, Khodakhah K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat Neurosci. 2006;9:389–396. doi: 10.1038/nn1648. [DOI] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Somatic and dendritic small-conductance calcium-activated potassium channels regulate the output of cerebellar Purkinje neurons. J Neurosci. 2003;23:2600–2607. doi: 10.1523/JNEUROSCI.23-07-02600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Dendritic control of spontaneous bursting in cerebellar Purkinje cells. J Neurosci. 2004;24:3511–3521. doi: 10.1523/JNEUROSCI.0290-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Chevez C, Khodakhah K. Calcium-activated potassium channels are selectively coupled to P/Q-type calcium channels in cerebellar Purkinje neurons. J Neurosci. 2004;24:8818–8822. doi: 10.1523/JNEUROSCI.2915-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hischberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]


