Abstract
Abstract
The microcircuitry of cerebellar cortex and, in particular, the physiology of its main element, the Purkinje neuron, has been extensively investigated and described. However, activity in Purkinje neurons, either as single cells or populations, does not directly mediate the cerebellar effects on the motor effector systems. Rather, the result of the entire cerebellar cortical computation is passed to the relatively small cerebellar nuclei that act as the final, integrative processing unit in the cerebellar circuitry. The nuclei ultimately control the temporal and spatial features of the cerebellar output. Given this key role, it is striking that the internal organization and the connectivity with afferent and efferent pathways in the cerebellar nuclei are rather poorly known. In the present review, we discuss some of the many critical shortcomings in the understanding of cerebellar nuclei microcircuitry: the extent of convergence and divergence of the cerebellar cortical pathway to the various cerebellar nuclei neurons and subareas, the possible (lack of) conservation of the finely-divided topographical organization in the cerebellar cortex at the level of the nuclei, as well as the absence of knowledge of the synaptic circuitry within the cerebellar nuclei. All these issues are important for predicting the pattern-extraction and encoding capabilities of the cerebellar nuclei and, until resolved, theories and models of cerebellar motor control and learning may err considerably.
Marylka ‘Yoe’ Uusisaari received her PhD from Helsinki University, Finland, in 2003, having specialized in the network phenomena related to pathological activity in epileptic brains. Since 2004, she has worked in RIKEN BSI, and OIST, Japan, with others founding the morphological and physiological classification of the cerebellar nuclear neurons. Erik De Schutter studied medicine in Belgium where he received his MD in 1984 at the University of Antwerp and subsequently specialized as a neuropsychiatrist. In 1990 he became a research fellow at the California Institute of Science and Technology where he developed his famous Purkinje cell model. In 1993 he started the Theoretical Neurobiology group at the University of Antwerp, and in 2007 he became a principal investigator at OIST where he leads the Computational Neuroscience Unit. Erik De Schutter is the president of the Organization for Computational Neurosciences and a member of the governing board of the International Neuroinformatics Coordinating Facility.
 |
Introduction
The role played by the olivo-cerebellar system in motor control has been the subject of intensive research; it is thought to be involved in motor learning (Kawato, 1999; Ohyama et al. 2003) and/or in the timing of motor execution (Welsh et al. 1995; Welsh, 2002; Ivry & Spencer, 2004). Several decades of anatomical work have resulted in detailed knowledge of the connectivity within the cerebellar cortex as well as the organization of the afferent and efferent pathways connecting the cerebellum to the rest of the brain (Fig. 1A).
Figure 1. Overview of cerebellar circuitry.
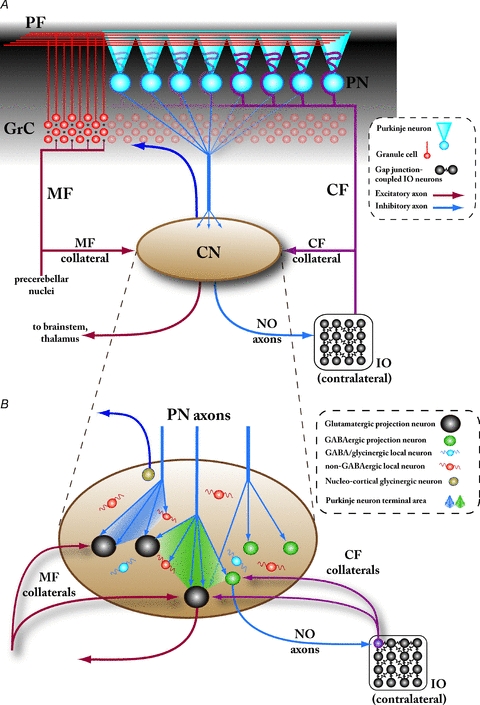
A, schematic representation of the gross circuit organization of the cerebellum. The cerebellum receives afferent input via two excitatory pathways, the mossy fibres (MF) originating in various precerebellar nuclei and climbing fibres (CF) that arise solely from the inferior olivary nucleus (IO) in the contralateral brainstem. These pathways converge in the cerebellar cortex (black shaded area), with the CFs exciting the Purkinje neurons (PN) directly and MFs indirectly by first diverging onto a large number of excitatory cerebellar granule cells (GrC). Each GrC axon gives rise to a parallel fibre (PF) that extends up to several millimetres, coursing medio-laterally in the molecular layer of cerebellar cortex and contacting numerous PNs. (Note that the several classes of cortical inhibitory interneurons are omitted in this drawing for clarity.) The PNs are the sole output from the cerebellar cortex, and the cerebellar nuclei (CN) are their main target; the CN, on the other hand, are the sole output structure of the cerebellum as well as a second location for MF- and CF-pathway convergence via their respective collateral axons. The two main cerebellar efferents formed by axons of the CN neurons can be roughly divided into the inhibitory nucleo-olivary (NO) projection, and the excitatory projection diverging to various relay nuclei in the brainstem and thalamus. The properties and physiological significance of the nucleo-cortical projection (dark blue arrow reaching the granule cell layer) are unknown. Note that in this and other figures, the CN are drawn schematically without differentiating between the various subnuclei (fastigial, anterior and posterior interpositus, and lateral nuclei). B, currently known neuronal components of the cerebellar nuclei. At least six different neuron types can be distinguished within the CN based on morphological and electrophysiological features as well as molecular markers, five of which are presented here: large, glutamatergic projection neurons (black), small GABAergic projection neurons targeting the IO (nucleo-olivary neurons; green), medium-sized, putatively glycinergic projection neurons targeting the cerebellar cortex (probably the Golgi cells within the granule cell layer; brown), and at least two types of interneurons, some of which are GABAergic (or mixed GABA/glycinergic; blue) and some are non-GABAergic, putatively glutamatergic (orange). The majority of synapses within the CN are formed by PN axon terminals, which typically branch into dense, partially overlapping conical terminal fields (depicted as shaded areas between the PN axons). PN synapses have been unequivocally demonstrated on the glutamatergic and GABAergic nucleo-olivary neurons, and indirectly on the non-GABAergic interneurons; at present, it is unclear whether they target the GABAergic interneurons or the glycinergic projection neurons. Terminals of CF collaterals from the contralateral IO have been shown on the glutamatergic projection neurons, as well as on the NO neurons that are thought to project back to the origin of the same CFs (depicted as the purple IO neuron). Conversely, collaterals of the MFs are known to target glutamatergic projection neurons but the MF innervation of other CN cell types remains to be clarified. Abbreviations: PF, parallel fibres; PN, Purkinje neuron; GrC, cerebellar granule cell; CF, climbing fibre; MF, mossy fibre; IO, inferior olive; NO, nucleo-olivary.
A cornerstone of cerebellar anatomy is the concept of topographic organization on several levels (Apps & Hawkes, 2009). It is based on the observation that the afferent fibres (‘climbing fibres’, CFs) from the inferior olive (IO) are organized in strictly parasagittal bands in the cerebellar cortex, so that nearby neurons in the IO target Purkinje neurons (PNs) localized in narrow, rostrocaudal bands. This organization is complemented by another topographically arranged afferent input, the mossy fibre (MF)–parallel fibre (PF) pathway. Together, the CF and MF–PF pathways are thought to subdivide the otherwise quite homogeneous cerebellar cortex into numerous areas (sometimes called ‘zones’ or ‘patches’; Apps & Hawkes, 2009), each dedicated to processing signals originating from a certain body area or sensory modality. However, as will be discussed later, it is not at all clear that this proposed segregation into independent processing units would extend to the cerebellar output pathways.
The anatomical organization combined with physiological and behavioural studies have given rise to several models of the cerebellar role in motor learning, the most widely believed of which defines the input arriving on climbing fibres as ‘error’ or ‘teacher’ signals that lead to depression of the parallel fibre–PN synapses and to reduced PN output (Ito, 2001). However, the complex dynamic behaviour of the cerebello-olivary neuronal networks being revealed suggests that, at least, the effects of plasticity are more subtle (Steuber et al. 2007) and that many more modes and loci of plasticity are involved in learning (Hansel et al. 2001; Jörntell & Hansel, 2006).
Regardless of the debate on how the cerebellar cortex contributes to motor learning, it is clear that the signals must be finally integrated in the PNs as they are its only output. The result of cerebellar computation – encoded in a still-debated manner in the sequence of simple and complex spikes (Welsh, 2002; Jacobson et al. 2008; De Schutter & Steuber, 2009) – is conveyed by the GABAergic PN axons mainly to cerebellar nuclei (CN) neurons via projections roughly following the anatomical organization of the cerebellar cortex. A supposedly analogous but weaker connection from the floccular cerebellum also targets the vestibular nuclei (VN) and contributes to the vestibulo-ocular reflex (De Zeeuw et al. 1994; Sekirnjak et al. 2003). The vestibular nuclei will not, however, be discussed in this review.
In classic theory, the role of CN has been thought to be limited to assigning opposite ‘signs’ to the cerebellar output: inhibitory GABAergic signalling to the IO (the nucleo-olivary pathway, NO; Fredette & Mugnaini, 1991), excitatory glutamatergic signalling to the forebrain motor areas, with the cerebellar subnuclei targeting different systems. More recently, the typical physiology of the CN neurons, with strong rebound spikes following release from inhibition (Llinás & Mühlethaler, 1988; Tadayonnejad et al. 2010), has led to theories proposing that the rebound spike transmits a timing signal (Kistler et al. 2000; De Schutter & Steuber, 2009; Steuber et al. 2011). Nevertheless, all these theories are limited by poor knowledge of CN neuron and synapse physiology.
Importantly for the current thoughts on CN function, the cortico-nuclear pathway as well as the nucleo-olivary (NO) projection by GABAergic CN projection neurons have been shown, as will be discussed in more detail later, to preserve the topographical organization of the cerebellar cortex to some degree, leading to the suggestion of closed feedback loops within each cerebellar functional unit (Kenyon et al. 1998; Bengtsson & Hesslow, 2006). Furthermore, there is evidence for conserved projection arrangements from the CN to the motor system (Dum & Strick, 2003) even though the precise organization of cerebellar output to the motor areas is comparatively poorly known.
In her seminal work, Chan-Palay (1973c, 1977) described six different neuronal populations within the CN, at least two of which were local interneurons, together with diverse patterns of innervation by PN and local neuron axons as well as by collaterals of the same CF and MF axons that provide input to the cerebellar cortex. Chan-Palay's anatomical examination did not, however, lead to incorporation of the intra-CN network into the conceptual models of cerebellar function, as difficulties in distinguishing the neuronal types (Czubayko et al. 2001; Aizenman et al. 2003; Sultan et al. 2003) prevented their functional investigation.
More recently, electrophysiological examination of identified CN neuronal subgroups have been made possible by the advent of genetically targeted fluorescent labelling, and at the time of this review, at least six different CN neuronal groups have been identified in terms of neurotransmitter type, morphology and intrinsic properties (Fig. 1B; Uusisaari et al. 2007; Bagnall et al. 2009; Uusisaari & Knöpfel, 2009). In addition to the previously mentioned glutamatergic and GABAergic projection neurons, these groups comprise at least two separate interneuronal types, one of which is GABAergic or mixed GABA/glycinergic and the other (probably) glutamatergic as well as two glycinergic projection neurons: a nucleo-cortical glycinergic neuron found in the lateral CN (Uusisaari & Knöpfel, 2009), and a group of glycinergic neurons in the fastigial CN projecting into the vestibular nuclei (Bagnall et al. 2009).
Despite this progress in knowledge of single neuron function in the CN, our understanding of its role in cerebellar function remains confounded by an almost complete lack of knowledge about the internal neuronal and synaptic organization of the CN. As in any complex neuronal network, the properties and activity of the interneuronal network is likely to contribute significantly to modulating cerebellar output.
Recently, known aspects of the CN circuitry have been reviewed (Uusisaari & Knöpfel, 2010). In what follows, we will outline what in our opinion are some of the most critical remaining shortcomings in our understanding of the cerebellar nuclei, with specific focus on the anatomical arrangement of elements in the olivo-cortico-nucleo-olivary (OCNO) pathway and of the neuronal circuits forming the output channels of the CN. First, we start by discussing the current views (and their shortcomings) on synaptic pathways between the cerebellar cortex and the nuclei as well as the consistency of the OCNO pathways across different subnuclei. Next, we will look at the extent and significance of afferent and intrinsic synaptic connectivity among the local and projecting CN neurons, and finally consider the limitations this organization imposes on cerebellar efferent signalling. We hope that this overview will inspire research into these crucial issues that will complement the anatomical knowledge with the necessary electrophysiological understanding of this structure.
Properties of the corticonuclear connection
The connection between Purkinje neurons and cerebellar nuclei has been keenly studied in terms of anatomy, axonal and synaptic physiology and plasticity (Chan-Palay, 1973b; De Zeeuw & Berrebi, 1995; Sastry et al. 1997; Aizenman et al. 1998, 2000; Teune et al. 1998; Ouardouz & Sastry, 2000; Telgkamp & Raman, 2002; Pedroarena & Schwartz, 2003; Telgkamp et al. 2004; Monsivais et al. 2005; Pugh & Raman, 2005; Sugihara et al. 2009). It is characterized by preferential targeting of cell somata rather than dendrites, and functional features of their synapses (multiple release sites allowing spillover-mediated synaptic transmission, low release probability and fast synaptic depression) that make them well-suited for reliable transmission of changes – rather than average activity rates – in high-frequency PN spiking. Unfortunately, due to the known difficulties with electrophysiological examination of the CN neurons in vitro as well as the (until recently) ill-characterized CN neuronal classes, most of the work has focused solely on one CN neuronal type, the large glutamatergic projection neuron in juvenile animals. Moreover, because it is very difficult to prepare cerebellar slices that contain an entire PN axon, it has not been possible to characterize the synaptic contact by paired PN–CN recordings, limiting the experiments to fibre tract stimulation. This is further complicated by a similar lack of anatomical knowledge at the single-cell level. This is unfortunate as the exact distribution of PN–CN connections will have a large influence on how information coded in the PN activity patterns (simple and complex spikes; see Hong & De Schutter, 2008; De Schutter & Steuber, 2009) is transmitted by CN neurons (Fig. 2).
Figure 2. Corticonuclear convergence and divergence.
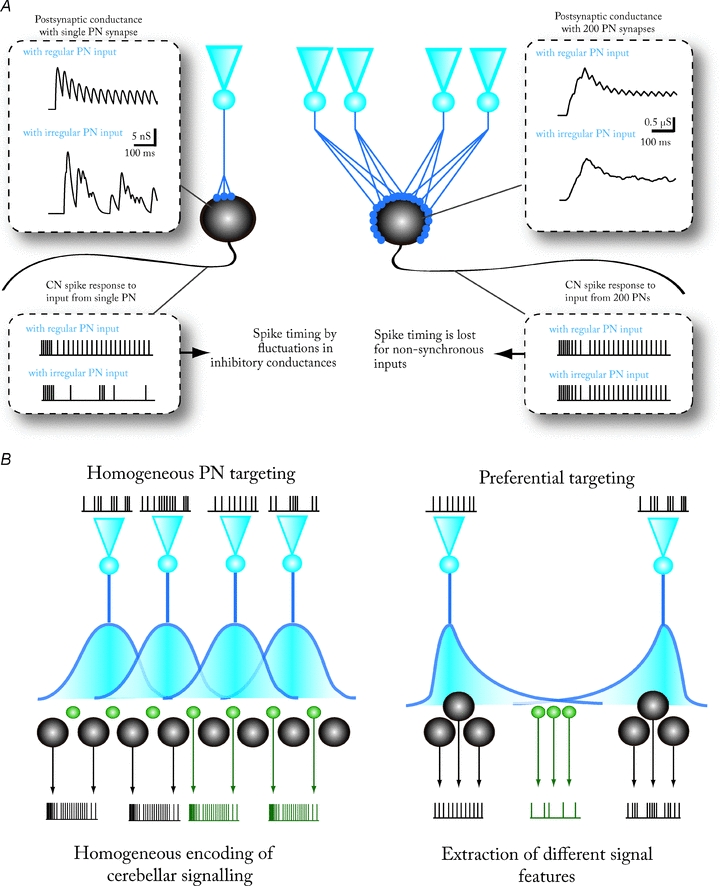
A, conceptual drawing, based on simulation of synaptic conductance fluctuations, showing the effect of two extreme cases of PN-to-CN convergence ratios on CN spike timing. Left: 1-to-1 connection between a PN and a CN neuron results in large inhibitory current fluctuations that can precisely time CN spikes. Different firing patterns in PNs (in this case, either regular or irregular firing) lead to easily distinguishable CN firing patterns. Right: at higher (200-to-1) convergence ratios, the individual spikes cannot be resolved in postsynaptic current fluctuations, and the CN neuron only responds to changes in the average firing rate of the pool of presynaptic PNs. Notably, the CN output is quasi-identical whether the presynaptic PNs fire regularly or not. See Shin et al. (2007) for further information on the synaptic conductance model used. B, heterogeneity of PN–CN divergence determines the diversity of the spiking responses of CN neurons to a cortical activity pattern. Left: homogeneous divergence and convergence of PNs over all CN neurons leads to the CN acting as a homogeneous feature extractor. All CN neurons in a given cortical target zone will encode and transmit a single property of the cerebellar computation. If all CN neurons are equally innervated by a large number of converging PNs, and each PN contacts CN cells of all neuronal subtypes, the extracted feature is the average firing rate of PNs. Right: in the opposite case, PNs target preferably certain CN neuron populations (black) that are nearly exclusively contacted by many terminals from single PNs (or a small group of PNs with similar firing patterns). Other CN populations (green) receive synaptic input from overlapping groups of PNs via fewer synaptic terminals. In this case, the ‘black’ CN neurons are able to convey information about individual PN spike timing, whereas the ‘green’ CN neurons will encode average cerebellar spike rates in their own output. Note that reliable transmission of either feature to the extracerebellar targets requires that a group of CN neurons receives exactly the same cortical signal.
Convergence of PN axons on CN cells
Based on anatomical considerations, and the fact there are many more PNs than CN neurons (PN-to-CN neuron count ratio 26:1, Palkovits et al. 1977 (cat); approx. 200,000 Purkinje neurons in mouse, e.g. Zanjani et al. 1996; approx 30,000 neurons in mouse CN, Sultan et al. 2002), it seems clear that there must be substantial convergence of the cortico-nuclear pathways (Fig. 2). If the convergence was equal over all CN and CN cell types, and each PN would be assumed to innervate only a single postsynaptic target, one would expect in the order of 20 PNs converging on a single CN neuron. A PN axon is, however, known to branch extensively within their conical target fields, within which several tens of large CN neuron cell bodies or dendritic segments are estimated to fit. A detailed study by Palkovits and colleagues (1977), that takes into account the physical spread of the PN axonal field, number of PN terminals in a volume and the extent of CN dendritic trees predicted a high convergence ratio (1:860, cat).
However, as readily admitted by Palkovits et al., numerical treatments based on average synapse densities can only give limiting values as they do not address the possibility that the strength of PN connection between individual postsynaptic neurons would be variable. Indeed, it is known that a single PN axon can form as many as 50 on some and as few as one synaptic terminal on other target neurons (Palkovits et al. 1977), suggesting that even though a CN cell could be contacted by a large number of individual presynaptic PNs, most of the synaptic terminals could be formed by axonal branches from just a few (3–4) presynaptic PNs with tens of presynaptic terminals each. In such a case or with a very low PN–CN convergence ratio the inhibitory input to CN would be expected to fluctuate heavily, with the conductance changes dominated by the activity of a few PNs (Fig. 2A).
Even so, physiological evidence seems to speak against such a scenario: in vivo, large individual IPSPs are rare, at least in the presumed large glutamatergic projection neurons (Bengtsson et al. 2008). This lack of significant fluctuations suggests that large projection neurons are the target of a large number of equally contributing, for the most time non-synchronized PNs that jointly evoke thousands of inhibitory postsynaptic events per second. The PN–CN connection seems to be optimized to deliver a rather steady inhibitory current as the strong depression combines with regular simple spike firing of PNs (Fig. 2A, Telgkamp & Raman, 2002; Pedroarena & Schwartz, 2003; Shin et al. 2007), greatly diminishing the influence of individual, unsynchronized single spikes on action potential frequency and precision in the target CN cells (Gauck & Jaeger, 2000; Shin et al. 2007; Jaeger, 2011; Lang & Blekinsop, 2011). Transient synchronization of simple spikes (Jaeger, 2003; Shin & De Schutter, 2006; Medina & Lisberger, 2007; de Solages et al. 2008; Wise et al. 2010) and of the lower-frequency complex spikes (Welsh et al. 1995; Ozden et al. 2009, 2010; Schultz et al. 2009) may cause transiently synchronized PN output.
Importantly, synchronous pauses of PN firing may result in a temporary lift of the tonic inhibition of CN target neurons (Shin & De Schutter, 2006; De Schutter & Steuber, 2009) which can play a critical role in the generation of rebound activity in CN neurons, a hotly debated phenomenon (Alviña et al. 2008; Zheng & Raman, 2009; Hoebeek et al. 2010; Tadayonnejad et al. 2010). Rebound spikes could potentially endow the CN projection neurons with a capability to precisely encode both rate- and time-related signals (De Schutter & Steuber, 2009). Even though rebound firing is clearly within the physiological capabilities of CN neurons under certain conditions, elucidation of the functional significance of rebound activity will require knowledge not only of the PN–CN connection but also of the convergence/divergence of the CN efferents to extracerebellar structures.
In conclusion, the question of PN–CN convergence and thereby, the signal transduction capability of the pathway is far from being settled. Moreover, the PN axon termination areas and distribution of synaptic swellings differ significantly between different CN neuron types (see below) and among the CN subnuclei (Sugihara et al. 2009), suggesting that CN neuron subpopulations could be tuned for extracting different features of PN spike patterns (Fig. 2B). Finally, it remains to be verified to what extent the observed projection patterns are conserved between species.
Divergence of PN axons on different CN cell types
Even though most of the current knowledge about the PN–CN connection is based on examinations of a single class of CN neurons (the large projection neurons), the PNs target CN neurons of various sizes and molecular identities, including GABAergic, glycinergic and glutamatergic neurons (Chan-Palay, 1977; De Zeeuw et al. 1994; De Zeeuw & Berrebi, 1995; Teune et al. 1998). Specifically, a single PN has been shown to contact both a large glutamatergic projection neuron and a small, GABAergic, IO-projecting neuron (Teune et al. 1998). Because of this demonstrated existence of PN terminals on various CN cell types it is often assumed that all CN neurons are targeted by PN axons; however, this is not necessarily so.
In fact, indirect morphological (Chan-Palay, 1977) and electrophysiological (Uusisaari & Knöpfel, 2008) evidence suggests that the GABAergic (possibly mixed GABAergic/glycinergic) interneuron cell bodies are not targeted by PN axons. The presence of PN terminals on their distal dendrites could, however, not be excluded. Furthermore, 14% of the larger, possibly efferent neurons in the lateral CN do not have Purkinje neuron terminals on their cell bodies (Chan-Palay, 1973b). The identity of the latter neurons is not known, but it could be speculated that these large CN neurons lacking somatic PN innervation correspond to the recently described putatively glycinergic (GlyT2-positive) neurons that project to the cerebellar granule cell layer (Gly-I; Uusisaari & Knöpfel, 2009) since the other, large neuronal type of the CN (the glutamatergic projection neuron) is heavily innervated by axosomatic PN terminals (Chan-Palay, 1977; De Zeeuw & Berrebi, 1995; Uusisaari & Knöpfel, 2008).
In conclusion, it seems that the assumption of uniform PN innervation of CN neurons is unwarranted and requires further attention. Specifically, it should be noted that there is no knowledge about the amount of PN convergence on the NO neurons and thus it is not clear if the NO neurons are likely to be sensitive to the same features of cortical activity as the large glutamatergic projection neurons.
Are the closed olivo-cerebellar loops homogeneous and ubiquitous?
A cornerstone of many theories of cerebellar function is a strictly conserved topographical organization of the olivo-cortico-nucleo-olivary (OCNO) pathway, an idea which is supported by the remarkably neat arrangement especially between the floccular PN, the flocculus-related areas in the inferior olive (ventrolateral outgrowth, and dorsal cap of Kooy) and GABAergic nucleo-olivary (NO) neurons in the ventral part of lateral CN (De Zeeuw et al. 1994 (rabbit); Teune et al. 1998 (rat); Schonewille et al. 2006 (mouse)). Specifically, NO neurons are known to project to IO areas that provide CFs for PNs targeting the same NO neurons (Fig. 3Aa). This inhibitory nucleo-olivary pathway is proposed to suppress CF input from the IO to the related area in the cerebellar cortex (Ruigrok & Voogd, 1990, 2000; Bengtsson & Hesslow, 2006; Marshall & Lang, 2009) when a correct response to a mossy fibre (MF) activity pattern is learned either in the cerebellar cortex or within the CN (McCormick & Thompson, 1984; Kim et al. 1998; Kenyon et al. 1998; Bao et al. 2002; Ito, 2006).
Figure 3. Circuit integrity and uniformity of the olivo-cortico-nucleo-olivary (OCNO) loop.
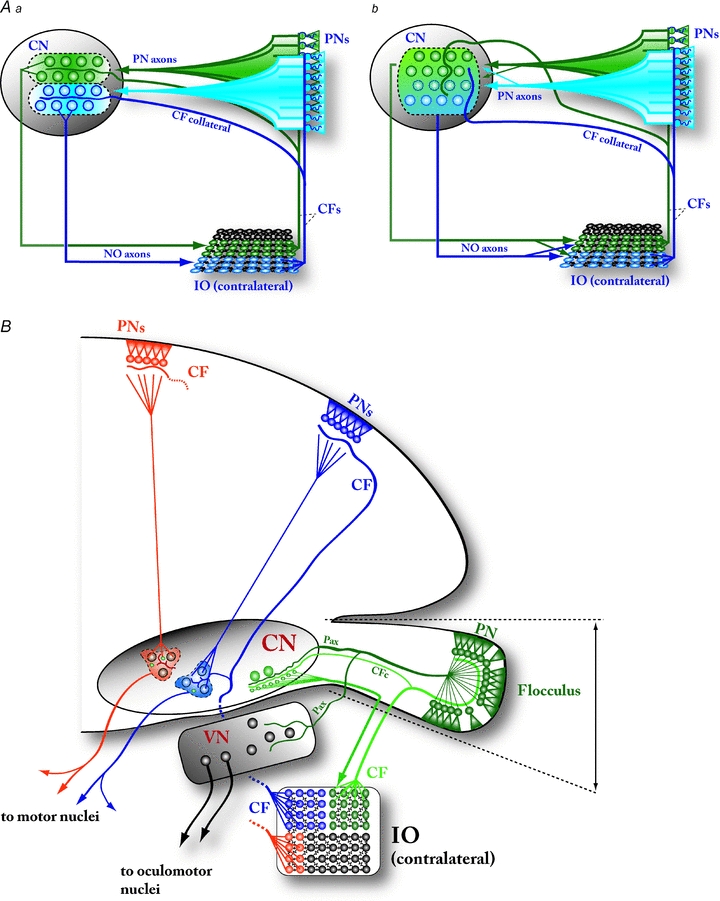
A, the concept of cerebellar microzones is partly built on the anatomical conservation of dedicated OCNO loops. Aa, according to the OCNO conservation model, single climbing fibres (CFs) diverge onto 5–10 PNs that are strictly localized in narrow parasagittal bands in the cerebellar cortex, and CFs originating from same inferior olivary (IO) areas target PNs in the same bands (‘blue’ IO neurons targeting PNs in the blue band, and ‘green’ IO neurons to the green band). This division into specialized bands is thought to extend into the CN by way of a precise topographic cortico-nuclear connection (the PN axons), that targets specific groups of nucleo-olivary (NO) neurons projecting to the IO region belonging to the same ‘loop’ (schematized as ‘blue’ or ‘green’ areas). This organization is further accentuated by collaterals of the CFs that are known to target NO neurons (and possibly other CN neurons) again within the same band. Strict conservation of OCNO bands allows the cerebellar cortex to respond to olivary signals (complex spikes) in each cortical zone independently. Ab, even though the strict zonal arrangement of CFs between IO and PNs, as well as a relatively clear topographical organization of cortico-nuclear PN axons, are well supported by anatomical evidence, the possibility of inter-zonal crosstalk at the level of CN is not excluded. First, the strict restriction of the olivo-nuclear projection via CF collaterals to CN neurons receiving input from PNs within the same parasagittal band has not been demonstrated, even though the majority of CF collaterals target CN neurons within the coarser anatomical groups (‘A–D2’, Voogd & Glickstein, 1998; ‘I–V’, Sugihara, 2010). Second, the role of the nucleo-olivary connection as a modulator of interneuronal coupling and synchronization among clusters of IO neurons suggests a more diffuse targeting. ‘Fuzzy’ OCNO loops allow activity in one cerebellar receptive field to influence IO-originating complex spikes (and the resulting effects on PN firing and PF–PN plasticity) across other, possibly not directly related cerebellar zones. B, schematic drawing of the cerebellar circuitry in a frontal view. The floccular cerebellum (FC; far right) and the related behavioural function (vestibulo-ocular reflex) have been the focus of intense study, and features described in this system (such as conservation of OCNO pathway on a single-cell level) have been thought to generalize to the entire cerebellum. However, the connections of the vestibulocerebellum (incorporating the floccular cerebellar cortex, the ventralmost part of the lateral CN, and specific regions of the contralateral IO, the ventrolateral olive and dorsal cap of Kooy) to the CN exhibit many structural differences compared to those from the cerebellar hemispheres. Most strikingly, the olivo-nuclear CF collaterals and PN axons in the FC target an area in the CN that has significantly fewer large glutamatergic projection neurons than the rest of the CN, and part of the FC signalling to its extracerebellar targets (the oculomotor nuclei) is conveyed via the projection neurons within the vestibular nuclei (VN) that are outside the OCNO loop. The areas in CN targeted by PNs from the cerebellar hemispheres have, on the other hand, fewer NO neurons compared to glutamatergic projection neurons. Whether the CF collaterals in these areas conform to the strict OCNO arrangement is not known. Abbreviations: CN, cerebellar nuclei; PNs, Purkinje neurons; CFs, climbing fibres; IO, inferior olive; NO, nucleo-olivary; CFc, climbing fibre collateral; Pax, Purkinje neuron axon; VN, vestibular nuclei.
Despite its appeal, this picture is certainly oversimplified, as NO neurons in certain subregions of the CN are known to project to both contra- and ipsilateral IO (Ruigrok & Voogd, 1990; Teune et al. 2000), thereby breaking the strict reciprocity of the nucleo-olivary connections as the olivo-nuclear axons are entirely contralateral (Ruigrok & Voogd, 1990).
Classic theories of cerebellar function do not necessarily require strict conservation of the topography, but several other features specific to CN – most saliently, known organizational differences among the CN subnuclei – suggest that conserved OCNO loops may be more the exception than the rule, or be limited in precision to the level of anatomical subregions of the CN (Sugihara, 2010), thereby casting at least some doubts on the validity of the microzone concept.
The anatomy of the OCNO loop
A critical question in assessing this theory is, whether the OCNO loop is equally well conserved over all of the cerebellum (Fig. 3B). In other words, do all NO neurons receive cortical inhibition exclusively from PNs driven by IO neurons that the same NO neurons target or do they integrate converging input from a wider range of PNs? Conversely, do all cerebellar cortical areas control NO neurons equally or are some of them specialized for this function?
In general, precise connectivity between individual Purkinje neurons and NO neurons is not known well enough to form strong opinions on the above, as the evidence regarding the projection arrangements between the cerebellar cortex and nuclei are mostly on the level of gross anatomical modules. For instance, almost the entire lateral nucleus is considered a single cortical target ‘region’ (Sugihara, 2010). Furthermore, some 5% of the PN axonal terminals diverge into CN areas outside the ‘correct’ OCNO region (Sugihara et al. 2009) suggesting the existence of at least some cross-talk between OCNO regions. Even though the morphological features of NO neurons have not been rigorously studied, they are likely to be small and have fewer and shorter dendrites than the glutamatergic projection neurons (Chan-Palay, 1977), possibly meaning that the convergence or divergence of PN innervation on these cells would differ from the larger projection neurons. This difference would also suggest that the simple sign-reversed (GABAergic vs. glutamatergic projection) ‘mirror image’ of the nucleo-thalamic or nucleo-rubral pathway's function for the nucleo-olivary pathway cannot be taken for granted.
Moreover, even though the cerebellum and its nuclei are commonly thought to be relatively homogeneous in terms of neuron types and afferent distribution, the most ventral region of the lateral and interposed CN, targeted by PNs in floccular, nodular and most lateral parts of the hemispheric cerebellum (Schonewille et al. 2006; Sugihara, 2010) appears to differ. This area contains a higher density of NO neurons (Chan-Palay, 1973c; Giaquinta et al. 1999), and the arrangement of the PN axons that target this ventrolateral CN area differs from those terminating in medial and dorsal CN in terms of terminal spread (Chan-Palay, 1977; Sugihara, 2010) as well as by their extension into the VN (Fig. 3B; De Zeeuw et al. 1994; Teune et al. 2000; Schonewille et al. 2006; Voogd & Barmack, 2006; Bagnall et al. 2007; Sugihara et al. 2009). Even though the VN are often thought to be ‘analogous’ to the CN, the VN neurons targeted by PNs do not inhibit the IO (Sekirnjak et al. 2003), clearly placing them outside of the OCNO loops. Furthermore, the neurons in the related region of the IO are characterized by electrophysiological and morphological features that clearly set them apart from those providing climbing fibres to other hemispheric regions of the cerebellum (Urbano et al. 2006). Consequently, despite this connection being intensely studied due to its relation to the relatively well-understood cerebellar role in oculomotor control, it is misleading to generalize knowledge obtained from the flocculo-nuclear cerebellum to the rest of the cerebellar circuitry until there is more evidence in support of such a view.
Finally, even if on single neuron level the topography of OCNO would seem to be conserved in the floccular cerebellum, the functional significance of such organization will depend on intrinsic connectivity between neurons and subregions of the CN.
Nuclear desynchronization effect on IO: when and how?
The GABAergic NO neurons discussed above are, like the majority of CN neurons, spontaneously active in slice (Uusisaari & Knöpfel, 2010). Their axonal terminals in IO glomeruli (De Zeeuw et al. 1998) show remarkably asynchronous and sustained release properties (Best & Regehr, 2009). This probably results in a steady postsynaptic conductance in target IO neurons that carries little information about precise timing of individual spikes in the CN. The localization of GABAergic NO terminals in IO glomeruli, near the gap junctions forming the only intra-olivary communication pathway (Devor & Yarom, 2002) suggests that the NO input decreases gap junction-mediated synchronization of either input (Kistler & De Zeeuw, 2005) or output (Llinás et al. 2002; Lang, 2002; Jacobson et al. 2008, 2009) of the connected IO cells. This nucleo-olivary pathway is thus thought to desynchronize or decouple the IO network. The effects of physiologically relevant nucleo-olivary activity patterns on complex spikes in the cerebellar cortex are still unknown, but the reported movement- or sensory stimulus-related changes in synchronization of complex spikes (Welsh et al. 1995; Ozden et al. 2009, 2010; Schultz et al. 2009) are thought to depend on nucleo-olivary modulation of strength and spatial features of the gap junctional coupling between IO neurons (Lang et al. 1996; De Zeeuw et al. 1998; Devor & Yarom, 2000; Svensson et al. 2005; Blekinsop & Lang, 2006; Bengtsson & Hesslow, 2006; see also Garifoli et al. 2001; Ozden et al. 2009).
An important question in this respect is, whether the NO neurons fire spontaneously under ‘resting’ PN activity (as is the case in the large glutamatergic projection neurons), or instead are silenced by it until the presynaptic PN activity stops synchronously. Such pauses, proposed to occur after complex spikes, could initiate rebound burst firing (note that the GABAergic CN neurons seem to exhibit a somewhat stronger bursting phenotype than the glutamatergic CN neurons; Uusisaari et al. 2007). In other words, is the level of electric coupling in IO smoothly modulated by ongoing PN firing frequency or is the IO decoupling an all-or-none gating mechanism? Unfortunately, even though the presence of PN terminals on NO neurons is unquestionable, there are no published reports of inhibitory effects of PN input to these cells that would allow the formation of a clear hypothesis on how PN spike patterns influence NO neuron activity. Moreover, even though every olivary spine (with gap junctions) is contacted by NO terminals, part of the inhibitory NO synapses are located outside the glomeruli and their gap junctions (De Zeeuw et al. 1998), suggesting that the importance of ‘classic’ inhibition by the CN may have been overlooked. Finally, even though the NO projection conforms to the gross anatomical organization of the olivo-cortico-nuclear system (Ruigrok, 1997), very little is known of the extent of nucleo-olivary innervation within the IO in terms of divergence or convergence or the possible differences between glomerular and non-glomerular synapses.
Additional loops?
In addition to being modulated by the inhibitory PN synapses, the firing of NO neurons is also likely to be modulated by the excitatory and at least partly reciprocally arranged olivary climbing fibre collaterals (Fig. 3) (De Zeeuw et al. 1997). This pathway may form a negative feedback loop for synchronous IO activity. However, since nothing is known of the physiology of the olivo-nuclear pathway – synaptic responses to IO stimulation in NO neurons have not even been shown – this proposal remains speculative. Anatomically it is known that 90% of the CFs collateralize into the CN in a localized manner (Sugihara et al. 2009) and that the density of CF collateral terminals is significantly higher in the ventral part of the lateral CN functionally related to the floccular cerebellar cortex (Van der Want et al. 1989). Thus, even though the CF collateral innervation in CN has been generally considered as ‘sparse’ (as compared with other afferent pathways; Chan-Palay, 1977), the CF collateral feedback may have a stronger role in cerebellar computations related to vestibulo-ocular functions.
Integration of afferent excitation from olivary and precerebellar sources
The CF collateral pathway is not the only afferent input to the CN; a significant portion of the cerebellar mossy fibres (MFs) collateralize into the CN. The extent of possible complementary MF collateral innervation of NO neurons is presently unclear. The terminal targets of MFs in both cerebellar cortex and CN seem to be roughly linked to the topographically conserved corticonuclear projection (Shinoda et al. 2000), and thus convergence of cortical and nuclear MF pathways on single NO neurons would be anatomically possible. Currently the existence of MF collateral synapses on NO neurons remains to be demonstrated.
In contrast to the NO projecting neurons, the afferent excitatory input (Fig. 4A) to the large glutamatergic projection neurons has been studied extensively. Properties of the extrinsic glutamatergic synapses on these cells have been examined in detail and they have been shown to be modifiable in a synapse-specific manner (Zhang & Linden, 2006; Pugh & Raman, 2006, 2008, 2009; Person & Raman, 2010; Zheng & Raman, 2010), providing support for the idea that part of cerebellar learning is stored in the CN in addition to the cortex (McCormick & Thompson, 1984; Bao et al. 2002). Still, the role of afferent excitation on the glutamatergic projection neuron spike generation needs clarification.
Figure 4. Unknown synaptic organization of the CN within and between PN target areas.
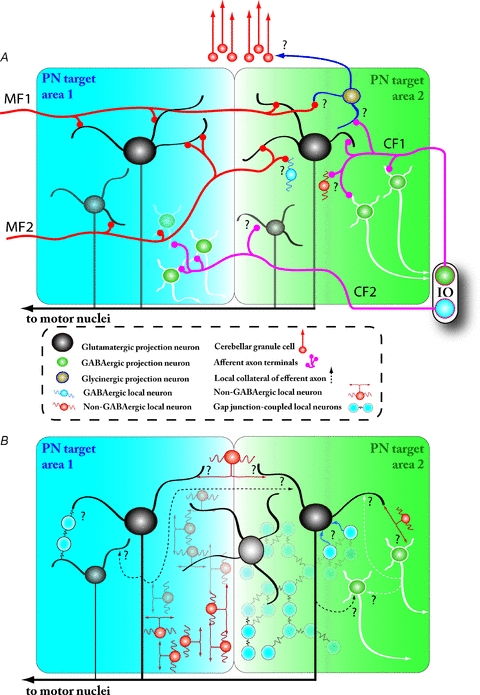
A, afferent innervation in the CN by mossy fibre (MF; red) and climbing fibre (CF; pink) collaterals may differ in terms of target neuron types and divergence across PN target areas (represented by blue and green shaded areas). In general, the MF collaterals innervate CN neurons in much more widely spread regions of the CN than the CF collaterals; the MFs are explicitly known to cross several PN target fields and form synapses on at least glutamatergic (black) and GABAergic (green with white outline) projection neuron dendrites. CF collateral terminals have also been shown on both neuronal types. The afferent innervation of the various CN interneurons (blue, orange) or the glycinergic projection neurons (brown) projecting to presently unknown targets in the cerebellar granule cell layer remains unstudied. Also, it is not clear to what extent the MF and CF pathways converge on individual CN neurons. Question marks denote synaptic connections that have not been demonstrated. Intrinsic connectivity within the CN determines how neuronal ensembles are organized in this structure. All CN projection neuron types have local collaterals (dashed-line arrows), but the extent of their targets (whether they extend to neighbouring PN target areas or even to more distant CN areas) and the identities of their postsynaptic neurons are unknown. Synaptic communication between CN neurons and distinct CN areas may also be mediated via dedicated ‘bridging interneurons’ described by Chan-Palay (1973; orange interneuron at the top of the drawing) and other locally projecting neurons, some of which are known to be connected by gap junctions (blue cells on the right). The existence of interneuronal networks connected via chemical (orange cells in the background) or electrical (blue cells in the background) synapses may support the rapid spread of activity across CN and make region-wide synchronization possible.
The MFs originate from the excitatory neurons in the pontine nuclei, nucleus reticularis tegmenti pontis and lateral reticular nucleus (Shinoda et al. 2000). In contrast to the CF collaterals that terminate in relatively tightly localized areas in the contralateral CN (Sugihara et al. 1999), the MF collateral innervation of CN is bilateral and more widely spread even though it does follow the gross topographical ordering of the cerebellar modules (Wu et al. 1999; Parenti et al. 2002; Voogd et al. 2003). Because of this imbalance of innervation by CF and MF collaterals in CN (Chan-Palay, 1973a), the results obtained in slice studies examining excitatory efferents to CN are usually assumed to pertain mainly to the MF collateral input to the CN. However, since the CF collaterals are also known to synapse upon the large glutamatergic neurons (Chan-Palay, 1973b; De Zeeuw et al. 1997) it has not been possible to distinguish whether the synaptic responses in slice originate from MF or CF stimulation. The possible convergence of CFs and MFs on single CN projection neurons needs to be clarified, for instance using optogenetic approaches, before any clear understanding of the functional interaction between excitation and inhibition (Gauck & Jaeger, 2000) on CN neurons becomes possible.
The existence and properties of afferent excitation on the other CN neuron types, especially the enigmatic, silent glycinergic neurons projecting to the cerebellar cortex (Uusisaari & Knöpfel, 2009) also requires further work.
Intrinsic CN network
The MF and CF collaterals and PN axons are not the only sources of synaptic inputs to the CN neurons. As in any non-trivial neuronal network, the intrinsic connections, both those formed by local collaterals of the projection neurons as well as by local interneurons, are bound to play a critical role in shaping and controlling signal processing of the CN (Fig. 4).
Chan-Palay's seminal work (1973a,b,c, 1977) as well as more recent examinations of the neuronal populations using genetic labelling have resulted in at least six different neuronal groups being defined in the lateral and fastigial CN (Bagnall et al. 2009; Uusisaari & Knöpfel, 2010), some of which are local interneurons of GABA/glycinergic and possibly glutamatergic type. Drawing analogies with other brain structures, it is likely that the interneuron classification especially is oversimplified and further studies will result in the discovery of additional types. However, virtually all physiological examinations of the CN have focused exclusively on the large glutamatergic projection neuron, and little besides basic intrinsic behaviour is known about the other neuronal types. Even less is known about the functional properties of the intrinsic synaptic connections and there have been no published reports of successful paired CN neuron recordings. At present the only available information is that the preferred localization of intrinsic synapses is on dendrites rather than on somata.
It could be argued that the massive amount of converging axosomatic inhibitory input from constantly active PNs would dwarf local, possibly dendritic synaptic influence, reducing the significance of intrinsic CN synapses to homeostatic control of neuronal excitability. However, their localization on dendritic branches potentially allows them to play a critical role in processes related to synaptic plasticity. Moreover, because some of the CN neurons have very large and complex dendritic arborizations that may span large regions or nearly an entire CN subnucleus (Chan-Palay, 1977; Sultan et al. 2003), complex dendritic interactions between synaptic inputs originating from MF, CF and local interneurons can be assumed.
As almost all the CN neurons are known to be spontaneously active (in vitro: Raman et al. 2000; Uusisaari & Knöpfel, 2010; in vivo: Thach, 1968 (monkey); LeDoux et al. 1998 (rat)), the effect of dendritic local synapses may be more related to synchronization of oscillatory behaviour (Ermentrout & Kopell, 1998; Maex & De Schutter, 2005; Netoff et al. 2005; Schultheiss et al. 2010) than to a (possibly weak) influence on firing frequency. The occurrence of neural synchronization within the CN has not been examined much (but see Soteropoulos & Baker, 2006). However, the presence of CN interneurons arranged in a bridge-like manner between putative columns of CN projection neurons (Chan-Palay, 1977) as well as of connexin 36 (Cx36)-based gap junctions (Degen et al. 2004; Van der Giessen et al. 2006) invites further investigation.
What does the CN code?
In contrast to the hundreds or thousands of proposed separate processing units in the cerebellar cortex, each dedicated to a specific body area or sensory modality, the CN seem to be rather coarsely organized. A handful of CN target areas (Voogd & Glickstein, 1998; Sugihara, 2010) each receive input from PNs converging in both mediolateral (Apps & Garwicz, 2000; Pantòet al. 2001) and rostrocaudal (Trott et al. 1998a,b; Pantòet al. 2001; Sugihara et al. 2009) axes with significant terminal field overlaps. The fine cerebellar topology is further confounded by the nucleo-cortical projections, both excitatory and inhibitory, that show at least some degree of divergence into cerebellar cortical areas related to various body parts (Provini et al. 1998; Trott et al. 1998a,b;). It could thus be that the remarkably well-organized cerebellar modules do not extend in equally definite terms into the CN, thereby calling into question the existence of specific somatotopic output channels in the cerebellum (see also Cerminara & Apps, 2010).
Considering the full cerebellum, it is striking that the entire output of its vast cortex with numerous input-specific patches is funnelled through a very small number of CN projection neurons. The mouse CN have no more than ∼12,000 glutamatergic neurons (‘pure’ glutamatergic; Sultan et al. 2002), not all of which are projection neurons, conveying the information produced by more than 200,000 Purkinje neurons. To ensure reliable transmission a group of these efferent neurons must partake in propagating each signal, resulting in a rather low number of possible cerebellar output channels.
These considerations are in line with evidence showing that, unlike the cerebellar cortical neurons (Bower & Woolston, 1983; Ekerot & Jörntell, 2003; Jörntell & Ekerot, 2011), the CN neurons show very wide receptive fields and little specificity to the modality of incoming signals (Giaquinta et al. 1999; Rowland & Jaeger, 2005, 2008). This probably results from convergence and divergence of both PN and MF axons within the CN and especially in the case of MF collaterals, across individual PN axonal terminal fields (Chan-Palay, 1977). Furthermore, single efferent axons of CN neurons have been shown to branch and project into various extracerebellar structures, such as medullary and spinal targets (Kakei et al. 1995). Taken together, this suggests that the output of CN is probably related to broader aspects of behaviour (planning, execution; Middleton & Strick, 1997) or to timing (Ivry & Spencer, 2004; De Schutter & Steuber, 2009) rather than being specific for modality or topography like most of the cerebellar input.
The properties and dynamics of the CN local circuitry will ultimately define both the integrative possibilities of individual projection neurons as well as delineate the boundaries (if such exist) of cerebellar output channels. Most theories of cerebellar function have been based on the circuitry within the cerebellar cortex (Marr, 1969), emphasizing the specific anatomical features of the cortical connectivity and the signal-processing capabilities of the PNs. It should be clear from this review that a complete understanding of how the cerebellum affects animal behaviour requires a deeper investigation of the anatomy and physiology of the CN, taking into account its different neuronal identities and complex synaptic organization.
Acknowledgments
This work was supported by OISTPC and by Fonds Wetenschappelijk Onderzoek (Flanders). We thank Rodrigo Publio for running the simulations that inspired Fig. 2A and Bernd Kuhn, Mario Negrello, Shiwei Huang and Dimitar Dimitrov for reading and commenting on an earlier version of this paper.
Glossary
Abbreviations
- CF
climbing fibre
- CN
cerebellar nuclei
- IO
inferior olive
- MF
mossy fibre
- NO
nucleo-olivary
- OCNO
olivo-cortico-nucleo-olivary
- PN
Purkinje neuron
- VN
vestibular nuclei
References
- Aizenman CD, Huang EJ, Linden DJ. Morphological correlates of intrinsic electrical excitability in neurons of the deep cerebellar nuclei. J Neurophysiol. 2003;89:1738–1747. doi: 10.1152/jn.01043.2002. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Huang EJ, Manis PB, Linden DJ. Use-dependent changes in synaptic strength at the Purkinje cell to deep nuclear synapse. In: Gerrits N, Ruigrok T, De Zeeuw CI, editors. Cerebellar Modules: Molecules, Morphology and Function. Elsevier; 2000. pp. 257–273. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Manis PB, Linden DJ. Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron. 1998;21:827–835. doi: 10.1016/s0896-6273(00)80598-x. [DOI] [PubMed] [Google Scholar]
- Alviña K, Walter J, Kohn A, Ellis-Davies G, Khodakhah K. Questioning the role of rebound firing in the cerebellum. Nat Neurosci. 2008;11:1256–1258. doi: 10.1038/nn.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps R, Garwicz M. Precise matching of olivo-cortical divergence and cortico-nuclear convergence between somatotopically corresponding areas in the medial c1 and medial c3 zones of the paravermal cerebellum. Eur J Neurosci. 2000;12:205–214. doi: 10.1046/j.1460-9568.2000.00897.x. [DOI] [PubMed] [Google Scholar]
- Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci. 2009;10:670–681. doi: 10.1038/nrn2698. [DOI] [PubMed] [Google Scholar]
- Bagnall MW, Stevens RJ, du Lac S. Transgenic mouse lines subdivide medial vestibular nucleus neurons into discrete, neurochemically distinct populations. J Neurosci. 2007;27:2318–2330. doi: 10.1523/JNEUROSCI.4322-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnall MW, Zingg B, Sakatos A, Moghadam SH, Zeilhofer HU, du Lac S. Glycinergic projection neurons of the cerebellum. J Neurosci. 2009;29:10104–10110. doi: 10.1523/JNEUROSCI.2087-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chen L, Kim JJ, Thompson RF. Cerebellar cortical inhibition and classical eyeblink conditioning. Proc Natl Acad Sci U S A. 2002;99:1592–1597. doi: 10.1073/pnas.032655399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson F, Ekerot C, Jörntell H. Washington, DC, USA: Society for Neuroscience; 2008. Convergence of Purkinje cell synaptic input to deep cerebellar nuclear neurons in vivo. Program No. 777.12 2008 Neuroscience Meeting Planner. [Google Scholar]
- Bengtsson F, Hesslow G. Cerebellar control of the inferior olive. Cerebellum. 2006;5:7–14. doi: 10.1080/14734220500462757. [DOI] [PubMed] [Google Scholar]
- Best AR, Regehr WG. Inhibitory regulation of electrically coupled neurons in the inferior olive is mediated by asynchronous release of GABA. Neuron. 2009;62:555–565. doi: 10.1016/j.neuron.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenkinsop TA, Lang EJ. Block of inferior olive gap junctional coupling decreases Purkinje cell complex spike synchrony and rhythmicity. J Neurosci. 2006;26:1739–1748. doi: 10.1523/JNEUROSCI.3677-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JM, Woolston DC. Congruence of spatial organization of tactile projections to granule cell and Purkinje cell layers of cerebellar hemispheres of the albino rat: vertical organization of cerebellar cortex. J Neurophysiol. 1983;49:745–766. doi: 10.1152/jn.1983.49.3.745. [DOI] [PubMed] [Google Scholar]
- Cerminara NL, Apps R. Behavioural significance of cerebellar modules. Cerebellum. 2010 doi: 10.1007/s12311-010-0209-2. DOI: 10.1007/s12311-010-0209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V. Afferent axons and their relations with neurons in the nucleus lateralis of the cerebellum: a light microscopic study. Z Anat Entwicklungsgesch. 1973a;142:1–21. doi: 10.1007/BF00519873. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V. On the identification of the afferent axon terminals in the nucleus lateralis of the cerebellum: an electron microscope study. Z Anat Entwicklungsgesch. 1973b;142:149–186. doi: 10.1007/BF00519720. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V. The cytology of neurons and their dendrites in the simple mammalian nucleus lateralis: an electron microscope study. Z Anat Entwicklungsgesch. 1973c;141:289–317. doi: 10.1007/BF00519049. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V. Cerebellar Dentate Nucleus: Organization, Cytology and Transmitters. Berlin: Springer-Verlag; 1977. [Google Scholar]
- Czubayko U, Sultan F, Thier P, Schwarz C. Two types of neurons in the rat cerebellar nuclei as distinguished by membrane potentials and intracellular fillings. J Neurophysiol. 2001;85:2017–2029. doi: 10.1152/jn.2001.85.5.2017. [DOI] [PubMed] [Google Scholar]
- Degen J, Meier C, Van Der Giessen RS, Söhl G, Petrasch-Parwez E, Urschel S, Dermietzel R, Schilling K, De Zeeuw CI, Willecke K. Expression pattern of lacZ reporter gene representing connexin36 in transgenic mice. J Comp Neurol. 2004;473:511–525. doi: 10.1002/cne.20085. [DOI] [PubMed] [Google Scholar]
- De Schutter E, Steuber V. Patterns and pauses in Purkinje cell simple spike trains: experiments, modeling and theory. Neuroscience. 2009;162:816–826. doi: 10.1016/j.neuroscience.2009.02.040. [DOI] [PubMed] [Google Scholar]
- de Solages C, Szapiro G, Brunel N, Hakim V, Isope P, Buisseret P, Rousseau C, Barbour B, Léna C. High-frequency organization and synchrony of activity in the Purkinje cell layer of the cerebellum. Neuron. 2008;58:775–788. doi: 10.1016/j.neuron.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Devor A, Yarom Y. GABAergic modulation of olivary oscillations. In: Gerrits N, Ruigrok T, De Zeeuw CI, editors. Cerebellar Modules: Molecules, Morphology and Function. Elsevier; 2000. pp. 213–220. [Google Scholar]
- Devor A, Yarom Y. Electrotonic coupling in the inferior olivary nucleus revealed by simultaneous double patch recordings. J Neurophysiol. 2002;87:3048–3058. doi: 10.1152/jn.2002.87.6.3048. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Berrebi AS. Postsynaptic targets of Purkinje cell terminals in the cerebellar and vestibular nuclei of the rat. Eur J Neurosci. 1995;7:2322–2333. doi: 10.1111/j.1460-9568.1995.tb00653.x. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Hoogenraad CC, Koekkoek SKE, Ruigrok TJH, Galjart N, Simpson JI. Microcircuitry and function of the inferior olive. Trends Neurosci. 1998;21:391–400. doi: 10.1016/s0166-2236(98)01310-1. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Van Alphen AM, Hawkins RK, Ruigrok TJ. Climbing fibre collaterals contact neurons in the cerebellar nuclei that provide a GABAergic feedback to the inferior olive. Neuroscience. 1997;80:981–986. doi: 10.1016/s0306-4522(97)00249-2. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Wylie DR, DiGiorgi PL, Simpson JI. Projections of individual Purkinje cells of identified zones in the flocculus to the vestibular and cerebellar nuclei in the rabbit. J Comp Neurol. 1994;349:428–447. doi: 10.1002/cne.903490308. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. 2003;89:634–639. doi: 10.1152/jn.00626.2002. [DOI] [PubMed] [Google Scholar]
- Ekerot C, Jörntell H. Parallel fiber receptive fields: a key to understanding cerebellar operation and learning. Cerebellum. 2003;2:101–109. doi: 10.1080/14734220309411. [DOI] [PubMed] [Google Scholar]
- Ermentrout GB, Kopell N. Fine structure of neural spiking and synchronization in the presence of conduction delays. Proc Natl Acad Sci U S A. 1998;95:1259–1264. doi: 10.1073/pnas.95.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredette BJ, Mugnaini E. The GABAergic cerebello-olivary projection in the rat. Anat Embryol (Berl) 1991;184:225–243. doi: 10.1007/BF01673258. [DOI] [PubMed] [Google Scholar]
- Garifoli A, Scardilli G, Perciavalle V. Effects of cerebellar dentate nucleus GABAergic cells on rat inferior olivary neurons. Neuroreport. 2001;12:3709–3713. doi: 10.1097/00001756-200112040-00021. [DOI] [PubMed] [Google Scholar]
- Gauck V, Jaeger D. The control of rate and timing of spikes in the deep cerebellar nuclei by inhibition. J Neurosci. 2000;20:3006–3016. doi: 10.1523/JNEUROSCI.20-08-03006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta G, Casabona A, Smecca G, Bosco G, Perciavalle V. Cortical control of cerebellar dentato-rubral and dentato-olivary neurons. Neuroreport. 1999;10:3009–3013. doi: 10.1097/00001756-199909290-00025. [DOI] [PubMed] [Google Scholar]
- Hansel C, Linden DJ, D'Angelo E. Beyond parallel fiber LTD: The diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat Neurosci. 2001;4:467–475. doi: 10.1038/87419. [DOI] [PubMed] [Google Scholar]
- Hoebeek FE, Witter L, Ruigrok TJH, De Zeeuw CI. Differential olivo-cerebellar cortical control of rebound activity in the cerebellar nuclei. Proc Natl Acad Sci U S A. 2010;107:8410–8415. doi: 10.1073/pnas.0907118107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, De Schutter E. Purkinje neurons: What is the signal for complex spikes? Curr Biol. 2008;18:R969–R971. doi: 10.1016/j.cub.2008.08.056. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Spencer RM. The neural representation of time. Curr Opin Neurobiol. 2004;14:225–232. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Jacobson GA, Lev I, Yarom Y, Cohen D. Invariant phase structure of olivo-cerebellar oscillations and its putative role in temporal pattern generation. Proc Natl Acad Sci U S A. 2009;106:3579–3584. doi: 10.1073/pnas.0806661106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson GA, Rokni D, Yarom Y. A model of the olivo-cerebellar system as a temporal pattern generator. Trends Neurosci. 2008;31:617–625. doi: 10.1016/j.tins.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Jaeger D. No parallel fiber volleys in the cerebellar cortex: evidence from cross-correlation analysis between Purkinje cells in a computer model and in recordings from anesthetized rats. J Comput Neurosci. 2003;14:311–327. doi: 10.1023/a:1023217111784. [DOI] [PubMed] [Google Scholar]
- Jaeger D. Mini-review: synaptic integration in the cerebellar nuclei –perspectives from dynamic clamp and computer simulation studies. Cerebellum. 2011 doi: 10.1007/s12311-011-0248-3. DOI: 10.1007/s12311-011-0248-3. [DOI] [PubMed] [Google Scholar]
- Jörntell H, Ekerot C-F. Receptive field remodeling induced by skin stimulation in cerebellar neurons in vivo. Front Neural Circuits. 2011;5:3. doi: 10.3389/fncir.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron. 2006;52:227–238. doi: 10.1016/j.neuron.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Kakei S, Yagi J, Wannier T, Na J, Shinoda Y. Cerebellar and cerebral inputs to corticocortical and corticofugal neurons in areas 5 and 7 in the cat. J Neurophysiol. 1995;74:400–412. doi: 10.1152/jn.1995.74.1.400. [DOI] [PubMed] [Google Scholar]
- Kawato M. Internal models for motor control and trajectory planning. Curr Opin Neurobiol. 1999;9:718–727. doi: 10.1016/s0959-4388(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Kenyon GT, Medina JF, Mauk MD. A mathematical model of the cerebellar-olivary system I: self-regulating equilibrium of climbing fiber activity. J Comput Neurosci. 1998;5:17–33. doi: 10.1023/a:1008874209991. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Krupa DJ, Thompson RF. Inhibitory cerebello-olivary projections and blocking effect in classical conditioning. Science. 1998;279:570–573. doi: 10.1126/science.279.5350.570. [DOI] [PubMed] [Google Scholar]
- Kistler W, De Zeeuw C. Gap junctions synchronize synaptic input rather than spike output of olivary neurons. In: De Zeeuw CI, Cicirata F, editors. Creating Coordination in the Cerebellum. Elsevier; 2005. pp. 189–197. [DOI] [PubMed] [Google Scholar]
- Kistler WM, van Hemmen JL, De Zeeuw CI. Time window control: a model for cerebellar function based on synchronization, reverberation, and time slicing. Prog Brain Res. 2000;124:275–297. doi: 10.1016/S0079-6123(00)24023-5. [DOI] [PubMed] [Google Scholar]
- Lang EJ. GABAergic and glutamatergic modulation of spontaneous and motor-cortex-evoked complex spike activity. J Neurophysiol. 2002;87:1993–2008. doi: 10.1152/jn.00477.2001. [DOI] [PubMed] [Google Scholar]
- Lang EJ, Blenkinsop TA. Control of cerebellar nuclear cells: a direct role for complex spikes? Cerebellum. 2011 doi: 10.1007/s12311-011-0261-6. DOI: 10.1007/s12311-011-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang EJ, Sugihara I, Llinás R. GABAergic modulation of complex spike activity by the cerebellar nucleoolivary pathway in rat. J Neurophysiol. 1996;76:255–275. doi: 10.1152/jn.1996.76.1.255. [DOI] [PubMed] [Google Scholar]
- LeDoux MS, Hurst DC, Lorden JF. Single-unit activity of cerebellar nuclear cells in the awake genetically dystonic rat. Neuroscience. 1998;86:533–545. doi: 10.1016/s0306-4522(98)00007-4. [DOI] [PubMed] [Google Scholar]
- Llinás R, Mühlethaler M. Electrophysiology of guinea-pig cerebellar nuclear cells in the in vitro brain stem–cerebellar preparation. J Physiol. 1988;404:241–258. doi: 10.1113/jphysiol.1988.sp017288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Leznik E, Makarenko VI. On the amazing olivocerebellar system. Annals N Y Acad Sci. 2002;978:258–272. doi: 10.1111/j.1749-6632.2002.tb07573.x. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned eyelid response. Science. 1984;223:296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- Maex R, De Schutter E. Oscillations in the cerebellar cortex: a prediction of their frequency bands. In: De Zeeuw CI, Cicirata F, editors. Creating Coordination in the Cerebellum. Elsevier; 2005. pp. 181–188. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SP, Lang EJ. Local changes in the excitability of the cerebellar cortex produce spatially restricted changes in complex spike synchrony. J Neurosci. 2009;29:14352–14362. doi: 10.1523/JNEUROSCI.3498-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Lisberger SG. Variation, signal, and noise in cerebellar sensory-motor processing for smooth-pursuit eye movements. J Neurosci. 2007;27:6832–6842. doi: 10.1523/JNEUROSCI.1323-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Dentate output channels: motor and cognitive components. In: De Zeeuw CI, Strata P, Voogd J, editors. The Cerebellum: From Structure to Control. Elsevier; 1997. pp. 553–566. [DOI] [PubMed] [Google Scholar]
- Monsivais P, Clark BA, Roth A, Hausser M. Determinants of action potential propagation in cerebellar Purkinje cell axons. J Neurosci. 2005;25:464–472. doi: 10.1523/JNEUROSCI.3871-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netoff TI, Banks MI, Dorval AD, Acker CD, Haas JS, Kopell N, White JA. Synchronization in hybrid neuronal networks of the hippocampal formation. J Neurophysiol. 2005;93:1197–1208. doi: 10.1152/jn.00982.2004. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Nores WL, Murphy M, Mauk MD. What the cerebellum computes. Trends Neurosci. 2003;26:222–227. doi: 10.1016/S0166-2236(03)00054-7. [DOI] [PubMed] [Google Scholar]
- Ouardouz M, Sastry BR. Mechanisms underlying LTP of inhibitory synaptic transmission in the deep cerebellar nuclei. J Neurophysiol. 2000;84:1414–1421. doi: 10.1152/jn.2000.84.3.1414. [DOI] [PubMed] [Google Scholar]
- Ozden I, Dombeck D, Hoogland T, Tank D, Wang SS. San Diego, CA, USA: Society for Neuroscience; 2010. Co-regulation of climbing fiber and granule cell encoding in the cerebella of locomoting mice. Program No. 785.13 2010 Neuroscience Meeting Planner. [Google Scholar]
- Ozden I, Sullivan MR, Lee HM, Wang SS. Reliable coding emerges from coactivation of climbing fibers in microbands of cerebellar Purkinje neurons. J Neurosci. 2009;29:10463–10473. doi: 10.1523/JNEUROSCI.0967-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M, Mezey É, Hámori J, Szentágothai J. Quantitative histological analysis of the cerebellar nuclei in the cat. I. numerical data on cells and on synapses. Exp Brain Res. 1977;28:189–209. doi: 10.1007/BF00237096. [DOI] [PubMed] [Google Scholar]
- Pantò MR, Zappalà A, Parenti R, Serapide MF, Cicirata F. Corticonuclear projections of the cerebellum preserve both anteroposterior and mediolateral pairing patterns. Eur J Neurosci. 2001;13:694–708. doi: 10.1046/j.0953-816x.2000.01442.x. [DOI] [PubMed] [Google Scholar]
- Parenti R, Zappalà A, Serapide MF, Pantò MR, Cicirata F. Projections of the basilar pontine nuclei and nucleus reticularis tegmenti pontis to the cerebellar nuclei of the rat. J Comp Neurol. 2002;452:115–127. doi: 10.1002/cne.10316. [DOI] [PubMed] [Google Scholar]
- Pedroarena CM, Schwarz C. Efficacy and short-term plasticity at GABAergic synapses between Purkinje and cerebellar nuclei neurons. J Neurophysiol. 2003;89:704–715. doi: 10.1152/jn.00558.2002. [DOI] [PubMed] [Google Scholar]
- Person AL, Raman IM. Deactivation of L-type Ca current by inhibition controls LTP at excitatory synapses in the cerebellar nuclei. Neuron. 2010;66:550–559. doi: 10.1016/j.neuron.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provini L, Marcotti W, Morara S, Rosina A. Somatotopic nucleocortical projections to the multiple somatosensory cerebellar maps. Neuroscience. 1998;83:1085–1104. doi: 10.1016/s0306-4522(97)00477-6. [DOI] [PubMed] [Google Scholar]
- Pugh JR, Raman IM. GABAA receptor kinetics in the cerebellar nuclei: evidence for detection of transmitter from distant release sites. Biophys J. 2005;88:1740–1754. doi: 10.1529/biophysj.104.055814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh JR, Raman IM. Potentiation of mossy fiber EPSCs in the cerebellar nuclei by NMDA receptor activation followed by postinhibitory rebound current. Neuron. 2006;51:113–123. doi: 10.1016/j.neuron.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Pugh JR, Raman IM. Mechanisms of potentiation of mossy fiber EPSCs in the cerebellar nuclei by coincident synaptic excitation and inhibition. J Neurosci. 2008;28:10549–10560. doi: 10.1523/JNEUROSCI.2061-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh JR, Raman IM. Nothing can be coincidence: synaptic inhibition and plasticity in the cerebellar nuclei. Trends Neurosci. 2009;32:170–177. doi: 10.1016/j.tins.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Gustafson AE, Padgett D. Ionic currents and spontaneous firing in neurons isolated from the cerebellar nuclei. J Neurosci. 2000;20:9004–9016. doi: 10.1523/JNEUROSCI.20-24-09004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland NC, Jaeger D. Coding of tactile response properties in the rat deep cerebellar nuclei. J Neurophysiol. 2005;94:1236–1251. doi: 10.1152/jn.00285.2005. [DOI] [PubMed] [Google Scholar]
- Rowland NC, Jaeger D. Responses to tactile stimulation in deep cerebellar nucleus neurons result from recurrent activation in multiple pathways. J Neurophysiol. 2008;99:704–717. doi: 10.1152/jn.01100.2007. [DOI] [PubMed] [Google Scholar]
- Ruigrok TJ. Cerebellar nuclei: the olivary connection. In: Gerrits N, Ruigrok T, De Zeeuw CI, editors. The Cerebellum: From Structure to Control. Elsevier; 1997. pp. 167–192. [DOI] [PubMed] [Google Scholar]
- Ruigrok TJ, Voogd J. Cerebellar nucleo-olivary projections in the rat: an anterograde tracing study with Phaseolus vulgaris-leucoagglutinin (PHA-L) J Comp Neurol. 1990;298:315–333. doi: 10.1002/cne.902980305. [DOI] [PubMed] [Google Scholar]
- Ruigrok TJH, Voogd J. Organization of projections from the inferior olive to the cerebellar nuclei in the rat. J Comp Neurol. 2000;426:209–228. doi: 10.1002/1096-9861(20001016)426:2<209::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Sastry BR, Morishita W, Yip S, Shew T. GABAergic transmission in deep cerebellar nuclei. Progr Neurobiol. 1997;53:259–271. doi: 10.1016/s0301-0082(97)00033-6. [DOI] [PubMed] [Google Scholar]
- Schonewille M, Luo C, Ruigrok TJH, Voogd J, Schmolesky MT, Rutteman M, Hoebeek FE, De Jeu MTG, De Zeeuw CI. Zonal organization of the mouse flocculus: physiology, input, and output. J Comp Neurol. 2006;497:670–682. doi: 10.1002/cne.21036. [DOI] [PubMed] [Google Scholar]
- Schultheiss NW, Edgerton JR, Jaeger D. Phase response curve analysis of a full morphological globus pallidus neuron model reveals distinct perisomatic and dendritic modes of synaptic integration. J Neurosci. 2010;30:2767–2782. doi: 10.1523/JNEUROSCI.3959-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz SR, Kitamura K, Post-Uiterweer A, Krupic J, Häusser M. Spatial pattern coding of sensory information by climbing fiber-evoked calcium signals in networks of neighboring cerebellar Purkinje cells. J Neurosci. 2009;29:8005–8015. doi: 10.1523/JNEUROSCI.4919-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirnjak C, Vissel B, Bollinger J, Faulstich M, du Lac S. Purkinje cell synapses target physiologically unique brainstem neurons. J Neurosci. 2003;23:6392–6398. doi: 10.1523/JNEUROSCI.23-15-06392.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, De Schutter E. Dynamic synchronization of Purkinje cell simple spikes. J Neurophysiol. 2006;96:3485–3491. doi: 10.1152/jn.00570.2006. [DOI] [PubMed] [Google Scholar]
- Shin S, Hoebeek FE, Schonewille M, De Zeeuw CI, Aertsen A, De Schutter E. Regular patterns in cerebellar Purkinje cell simple spike trains. PLoS ONE. 2007;2:e485. doi: 10.1371/journal.pone.0000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda Y, Sugihara I, Wu HS, Sugiuchi Y. The entire trajectory of single climbing and mossy fibers in the cerebellar nuclei and cortex. In: Gerrits N, Ruigrok T, De Zeeuw C, editors. Cerebellar Modules: Molecules, Morphology and Function. Elsevier; 2000. pp. 173–186. [DOI] [PubMed] [Google Scholar]
- Soteropoulos DS, Baker SN. Cortico-cerebellar coherence during a precision grip task in the monkey. J Neurophysiol. 2006;95:1194–1206. doi: 10.1152/jn.00935.2005. [DOI] [PubMed] [Google Scholar]
- Steuber V, Mittmann W, Hoebeek FE, Silver RA, De Zeeuw CI, Häusser M, De Schutter E. Cerebellar LTD and pattern recognition by Purkinje cells. Neuron. 2007;54:121–136. doi: 10.1016/j.neuron.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuber V, Schultheiss NW, Silver RA, Schutter E, Jaeger D. Determinants of synaptic integration and heterogeneity in rebound firing explored with data-driven models of deep cerebellar nucleus cells. J Comput Neurosci. 2011;30:633–658. doi: 10.1007/s10827-010-0282-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara I. Compartmentalization of the deep cerebellar nuclei based on afferent projections and aldolase C expression. Cerebellum. 2010 doi: 10.1007/s12311-010-0226-1. DOI: 10.1007/s12311-010-0226-1. [DOI] [PubMed] [Google Scholar]
- Sugihara I, Fujita H, Na J, Quy PN, Li B, Ikeda D. Projection of reconstructed single Purkinje cell axons in relation to the cortical and nuclear aldolase C compartments of the rat cerebellum. J Comp Neurol. 2009;512:282–304. doi: 10.1002/cne.21889. [DOI] [PubMed] [Google Scholar]
- Sugihara I, Wu H, Shinoda Y. Morphology of single olivocerebellar axons labeled with biotinylated dextran amine in the rat. J Comp Neurol. 1999;414:131–148. [PubMed] [Google Scholar]
- Sultan F, Czubayko U, Thier P. Morphological classification of the rat lateral cerebellar nuclear neurons by principal component analysis. J Comp Neurol. 2003;455:139–155. doi: 10.1002/cne.10443. [DOI] [PubMed] [Google Scholar]
- Sultan F, König T, Möck M, Thier P. Quantitative organization of neurotransmitters in the deep cerebellar nuclei of the lurcher mutant. J Comp Neurol. 2002;452:311–323. doi: 10.1002/cne.10365. [DOI] [PubMed] [Google Scholar]
- Svensson P, Bengtsson F, Hesslow G. Cerebellar inhibition of inferior olivary transmission in the decerebrate ferret. Exp Brain Res. 2005;168:241–253. doi: 10.1007/s00221-005-0086-y. [DOI] [PubMed] [Google Scholar]
- Tadayonnejad R, Anderson D, Molineux ML, Mehaffey WH, Jayasuriya K, Turner RW. Rebound discharge in deep cerebellar nuclear neurons in vitro. Cerebellum. 2010;9:352–374. doi: 10.1007/s12311-010-0168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telgkamp P, Padgett DE, Ledoux VA, Woolley CS, Raman IM. Maintenance of high-frequency transmission at Purkinje to cerebellar nuclear synapses by spillover from boutons with multiple release sites. Neuron. 2004;41:113–126. doi: 10.1016/s0896-6273(03)00802-x. [DOI] [PubMed] [Google Scholar]
- Telgkamp P, Raman IM. Depression of inhibitory synaptic transmission between Purkinje cells and neurons of the cerebellar nuclei. J Neurosci. 2002;22:8447–8457. doi: 10.1523/JNEUROSCI.22-19-08447.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teune TM, van der Burg J, de Zeeuw CI, Voogd J, Ruigrok TJ. Single Purkinje cell can innervate multiple classes of projection neurons in the cerebellar nuclei of the rat: a light microscopic and ultrastructural triple-tracer study in the rat. J Comp Neurol. 1998;392:164–178. doi: 10.1002/(sici)1096-9861(19980309)392:2<164::aid-cne2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Teune TM, van der Burg J, van der Moer J, Voogd J, Ruigrok T. Topography of cerebellar nuclear projections to the brain stem in the rat. In: Gerrits N, Ruigrok TJ, De Zeeuw CI, editors. Cerebellar Modules: Molecules, Morphology and Function. Elsevier; 2000. pp. 141–172. [DOI] [PubMed] [Google Scholar]
- Thach WT. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J Neurophysiol. 1968;31:785–797. doi: 10.1152/jn.1968.31.5.785. [DOI] [PubMed] [Google Scholar]
- Trott JR, Apps R, Armstrong DM. Zonal organization of cortico-nuclear and nucleo-cortical projections of the paramedian lobule of the cat cerebellum. 1. The C1 zone. Exp Brain Res. 1998a;118:298–315. doi: 10.1007/s002210050285. [DOI] [PubMed] [Google Scholar]
- Trott JR, Apps R, Armstrong DM. Zonal organization of cortico-nuclear and nucleo-cortical projections of the paramedian lobule of the cat cerebellum. 2. The C2 zone. Exp Brain Res. 1998b;118:316–330. doi: 10.1007/s002210050286. [DOI] [PubMed] [Google Scholar]
- Urbano FJ, Simpson JI, Llinás RR. Somatomotor and oculomotor inferior olivary neurons have distinct electrophysiological phenotypes. Proc Natl Acad Sci U S A. 2006;103:16550–16555. doi: 10.1073/pnas.0607888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusisaari M, Knöpfel T. GABAergic synaptic communication in the GABAergic and non-GABAergic cells in the deep cerebellar nuclei. Neuroscience. 2008;156:537–549. doi: 10.1016/j.neuroscience.2008.07.060. [DOI] [PubMed] [Google Scholar]
- Uusisaari M, Knöpfel T. GlyT2+ neurons in the lateral cerebellar nucleus. Cerebellum. 2009;9:42–55. doi: 10.1007/s12311-009-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusisaari M, Knöpfel T. Functional classification of neurons in the mouse lateral cerebellar nuclei. Cerebellum. 2010 doi: 10.1007/s12311-010-0240-3. DOI: 10.1007/s12311-010-0240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusisaari M, Obata K, Knöpfel T. Morphological and electrophysiological properties of GABAergic and non-GABAergic cells in the deep cerebellar nuclei. J Neurophysiol. 2007;97:901–911. doi: 10.1152/jn.00974.2006. [DOI] [PubMed] [Google Scholar]
- Van Der Giessen RS, Maxeiner S, French PJ, Willecke K, De Zeeuw CI. Spatiotemporal distribution of connexin45 in the olivocerebellar system. J Comp Neurol. 2006;495:173–184. doi: 10.1002/cne.20873. [DOI] [PubMed] [Google Scholar]
- Van Der Want JJ, Wiklund L, Guegan M, Ruigrok T, Voogd J. Anterograde tracing of the rat olivocerebellar system with phaseolus vulgaris leucoagglutinin (PHA-L). Demonstration of climbing fiber collateral innervation of the cerebellar nuclei. J Comp Neurol. 1989;288:1–18. doi: 10.1002/cne.902880102. [DOI] [PubMed] [Google Scholar]
- Voogd J, Barmack NH. Oculomotor cerebellum. In: Büttner-Ennever JA, editor. Neuroanatomy of the Oculomotor System. Elsevier; 2006. pp. 231–268. [Google Scholar]
- Voogd J, Glickstein M. The anatomy of the cerebellum. Trends Neurosci. 1998;21:370–375. doi: 10.1016/s0166-2236(98)01318-6. [DOI] [PubMed] [Google Scholar]
- Voogd J, Pardoe J, Ruigrok TJH, Apps R. The distribution of climbing and mossy fiber collateral branches from the copula pyramidis and the paramedian lobule: congruence of climbing fiber cortical zones and the pattern of zebrin banding within the rat cerebellum. J Neurosci. 2003;23:4645–4656. doi: 10.1523/JNEUROSCI.23-11-04645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JP. Functional significance of climbing-fiber synchrony. A population coding and behavioral analysis. Annals N Y Acad Sci. 2002;978:188–204. doi: 10.1111/j.1749-6632.2002.tb07567.x. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Lang EJ, Suglhara I, Llinás R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374:453–457. doi: 10.1038/374453a0. [DOI] [PubMed] [Google Scholar]
- Wise AK, Cerminara NL, Marple-Horvat DE, Apps R. Mechanisms of synchronous activity in cerebellar Purkinje cells. J Physiol. 2010;588:2373–2390. doi: 10.1113/jphysiol.2010.189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HS, Sugihara I, Shinoda Y. Projection patterns of single mossy fibers originating from the lateral reticular nucleus in the rat cerebellar cortex and nuclei. J Comp Neurol. 1999;411:97–118. doi: 10.1002/(sici)1096-9861(19990816)411:1<97::aid-cne8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Zanjani HS, Vogel MW, Delhaye-Bouchaud N, Martinou JC, Mariani J. Increased cerebellar Purkinje cell numbers in mice overexpressing a human bcl-2 transgene. J Comp Neurol. 1996;374:332–341. doi: 10.1002/(SICI)1096-9861(19961021)374:3<332::AID-CNE2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. Long-term depression at the mossy fiber-deep cerebellar nucleus synapse. J Neurosci. 2006;26:6935–6944. doi: 10.1523/JNEUROSCI.0784-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N, Raman IM. Ca currents activated by spontaneous firing and synaptic disinhibition in neurons of the cerebellar nuclei. J Neurosci. 2009;29:9826–9838. doi: 10.1523/JNEUROSCI.2069-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng N, Raman I. Synaptic inhibition, excitation, and plasticity in neurons of the cerebellar nuclei. Cerebellum. 2010;9:56–66. doi: 10.1007/s12311-009-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


