Abstract
Abstract
The adaptive-filter model of the cerebellar microcircuit is in widespread use, combining as it does an explanation of key microcircuit features with well-specified computational power. Here we consider two methods for its evaluation. One is to test its predictions concerning relations between cerebellar inputs and outputs. Where the relevant experimental data are available, e.g. for the floccular role in image stabilization, the predictions appear to be upheld. However, for the majority of cerebellar microzones these data have yet to be obtained. The second method is to test model predictions about details of the microcircuit. We focus on features apparently incompatible with the model, in particular non-linear patterns in Purkinje cell simple-spike firing. Analysis of these patterns suggests the following three conclusions. (i) It is important to establish whether they can be observed during task-related behaviour. (ii) Highly non-linear models based on these patterns are unlikely to be universal, because they would be incompatible with the (approximately) linear nature of floccular function. (iii) The control tasks for which these models are computationally suited need to be identified. At present, therefore, the adaptive filter remains a candidate model of at least some cerebellar microzones, and its evaluation suggests promising lines for future enquiry.
Paul Dean (left) received the MA degree in physiology with psychology from the University of Cambridge, Cambridge, UK, and the DPhil degree from the University of Oxford, Oxford, UK. He is currently an Emeritus Professor with the Department of Psychology and a Member of the Centre for Signal Processing in Neuroimaging and Systems Neuroscience, University of Sheffield, Sheffield, UK. His research interests include producing computational models of neural systems that are based on both biological data and developments in control engineering, signal processing and robotics, which serve as a vehicle for two-way communication between biological and physical sciences, allowing roboticists to use new discoveries in biology and biologists to interpret their findings in light of current developments in signal processing. John Porrill (right) received the MA degree in mathematics and the PhD degree from the University of Cambridge, Cambridge, UK, where he worked with J. Stewart on topics in classical general relativity. He is currently a Reader in Psychology and a member of the Centre for Signal Processing in Neuroimaging and Systems Neuroscience in the Department of Psychology, University of Sheffield, Sheffield, UK. His research centres around the computational modelling of the neural processes controlling sensory and motor systems, the role of the cerebellum in their adaptive calibration, and the application of these biological principles to the control of biomimetic robot devices.
 |
The adaptive-filter model of the cerebellar cortical microcircuit was introduced by Fujita (1982), based on the original ideas of Marr (1969) and Albus (1971). Versions of the adaptive filter are now widely used for modelling how the cerebellum learns to make accurate movements, particularly of the eyes [e.g. smooth pursuit, vestibulo-ocular reflex (VOR)], eyelids (eyeblink conditioning) and arms (reaching; see references in Dean et al. 2010). The adaptive filter has the following three key features (Fig. 1): ‘analysis’ of input signals into a large number of component signals; ‘synthesis’ of these components by weighting them individually and then summing to produce the filter output; and adjustment of the weights by a teaching signal. Its general structure has similarities to a simplified version of the cerebellar cortical microcircuit (Fig. 2) and offers explanations for two of the most striking features of the microcircuit.
Figure 1. Adaptive filter.
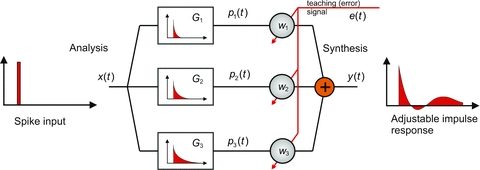
A commonly used adaptive filter architecture is the analysis–synthesis filter. A bank of fixed filters 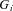 analyses the input signal
analyses the input signal 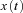 into component signals
into component signals 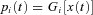 . This figure shows an example of a bank of three leaky-integrator filters with different time constants. The component signals are recombined to form the output signal
. This figure shows an example of a bank of three leaky-integrator filters with different time constants. The component signals are recombined to form the output signal 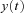 , with the amount of a given component in the output controlled by an adjustable weight
, with the amount of a given component in the output controlled by an adjustable weight 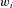 so that
so that 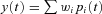 . Weights are adjusted automatically by the learning rule,
. Weights are adjusted automatically by the learning rule,  , where
, where 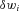 is the change in weight,
is the change in weight, 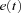 is a teaching signal carrying information about errors in filter output,
is a teaching signal carrying information about errors in filter output, 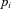 is the input signal to the weight
is the input signal to the weight 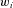 and
and 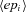 denotes the covariance of
denotes the covariance of  with
with 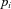 . This learning rule is called the covariance learning rule (Sejnowski, 1977) or the least mean square rule, because when
. This learning rule is called the covariance learning rule (Sejnowski, 1977) or the least mean square rule, because when  is the error in the output
is the error in the output 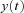 , it can be shown to minimize the mean square performance error, or the decorrelation learning rule because learning stops when errors
, it can be shown to minimize the mean square performance error, or the decorrelation learning rule because learning stops when errors  are uncorrelated with all filter inputs
are uncorrelated with all filter inputs 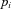 . Although the filter output is linear in the weights
. Although the filter output is linear in the weights 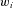 , by including appropriate non-linear component filters
, by including appropriate non-linear component filters 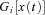 in the analysis layer, it can be used to model non-linear filters. Adapted from Fig. 1A of Dean & Porrill (2010).
in the analysis layer, it can be used to model non-linear filters. Adapted from Fig. 1A of Dean & Porrill (2010).
Figure 2. Simplified diagram of cerebellar cortical microcircuit.
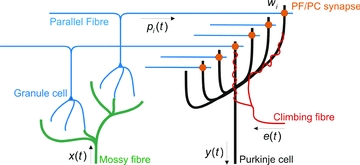
The input signals to the cerebellum are carried by mossy fibres, which synapse on granule cells. Granule cells axons bifurcate and form parallel fibres (PFs), which extend over the surface of the cerebellum in parallel fibre beams, synapsing extensively on Purkinje cells, which are the output cells of the cerebellum, and causing them to produce simple spikes. In addition to its many PF inputs, a Purkinje cell takes input from a single climbing fibre, which winds around the dendrites of the Purkinje cell and produces complex spikes on a one-to-one basis. Note that this simplified figure omits many details of the microcircuit, such as the ascending granule-cell axon inputs, inhibitory projections from granule cells to Purkinje cells via stellate and basket cells, and the recurrent connection of granule cells via Golgi cells. The Marr–Albus interpretation of the microcircuit maps elegantly onto the adaptive filter architecture shown in Fig. 1. Processing of the mossy-fibre input signal in the granule cell layer is interpreted as analysis by a bank of filters, to produce component signals carried on the parallel fibres. Combination of these PF inputs weighted by the efficacies of the PF–Purkinje cell (PC) synapses to produce Purkinje-cell output is interpreted as the synthesis stage. The climbing-fibre input is interpreted as a teaching signal, which adjusts synaptic weights according to a spike-timing dependent plasticity rule in which weights are decreased (LTD) when the parallel fibre and climbing-fibre input to a synapse are positively correlated and increased (LTP) when they are negatively correlated; this learning rule is equivalent to the covariance learning rule described in Fig. 1. Adapted from Fig. 1A of Porrill et al. (2004).
One feature is the enormous proliferation of granule cells, which are estimated to constitute ∼80% of all neurons in the human brain (Herculano-Houzel, 2009). In the adaptive-filter model, they are needed to provide a set of (possibly non-linear) component signals that is large enough to allow synthesis of all desired output signals. The second feature is the unusual behaviour of climbing fibres. These fire on average at ∼1 Hz, apparently too low a frequency to have significant impact on Purkinje-cell output (∼40 Hz). However, a single climbing-fibre action potential produces a large, widespread calcium transient throughout the Purkinje-cell dendritic tree in a manner thought to be related to plasticity at the estimated 150 000 parallel-fibre synapses on the tree (e.g. Ohtsuki et al. 2009). This combination of properties is exactly that required by an adaptive-filter teaching signal, which must alter all the weights appropriately without contaminating the filter output.
As well as offering explanations for important structural features of the microcircuit, the adaptive-filter model has very desirable functional properties. It uses the covariance learning rule (Fig. 1), which is both biologically plausible and equivalent to the least mean square rule in artificial systems (Widrow & Stearns, 1985). This rule can be shown to be optimum in the sense of minimizing the mean square difference between desired and actual output, and its effect for appropriately connected filters is to decorrelate all the component signals from the teaching signal, a procedure exactly suited to basic tasks such as noise cancellation (Fig. 3) and learning accurate movements (Fig. 4) that are associated with the cerebellum. Moreover, because the basic function of the adaptive filter is to deal with time-varying signals, the adaptive-filter model of the cerebellum copes naturally with the issues of timing that are associated with cerebellar function. Finally, the adaptive filter is a natural candidate for the essential adaptive element in the wide variety of signal-processing and control schemes that have been recently proposed to explain cerebellar function, such as internal models, state estimation, Kalman filters and Smith predictors (further details in Dean et al. 2010). Such schemes have been suggested to play roles not only in sensory and motor processing, but also in cognition (e.g. Ito, 2008).
Figure 3. Architecture for adaptive noise cancellation.
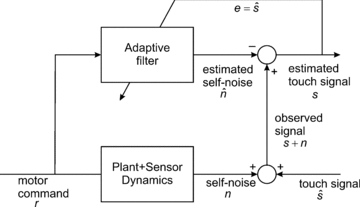
In many applications, a signal of interest s is contaminated by additive noise  so that the observable signal is
so that the observable signal is  . Although the noise component
. Although the noise component  is not itself observable, it may be known to be generated by a noise channel whose input
is not itself observable, it may be known to be generated by a noise channel whose input  , called the reference noise, is observable. If an adaptive filter can be trained to mimic the properties of the noise channel, it can be applied to the reference noise to obtain an estimate
, called the reference noise, is observable. If an adaptive filter can be trained to mimic the properties of the noise channel, it can be applied to the reference noise to obtain an estimate  of
of  . Subtracting this estimated noise from the observed signal
. Subtracting this estimated noise from the observed signal  then gives an estimate
then gives an estimate 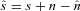 of the signal of interest. When the noise is successfully removed, the signal estimate
of the signal of interest. When the noise is successfully removed, the signal estimate 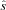 will be decorrelated from the reference noise
will be decorrelated from the reference noise  ; hence, it can be used as the teaching signal e in the covariance learning rule. The decorrelation algorithm is thus beautifully suited to the task of noise cancellation. The noise cancellation architecture has been applied in a biomimetic whisking robotic to removing the component of whisker sensor signals generated by whisker self-motion (Anderson et al. 2010). Whisker sensory signals are a combination of ‘touch’ signals generated by deflections on contact with objects and the ‘self-noise’ signals generated by free whisking. These self-noise signals are generated via motor commands to whiskers, and these commands can be regarded as a reference noise signal. This example demonstrates that the adaptive filter is an essential component of theories which propose that the cerebellum implements forward and inverse models; here the adaptive filter learns a forward model of the plant dynamics which transforms motor commands into sensory self-noise signals. This architecture is related to that proposed for sensory noise cancellation in ‘cerebellum-like’ structures, such as the electrosensory lateral line lobe in mormyrid electric fish (Dean et al. 2002), but differs crucially in providing a role for the inferior olive (Dean & Porrill, 2010). Diagram adapted from Fig. 1B of Anderson et al. (2010).
; hence, it can be used as the teaching signal e in the covariance learning rule. The decorrelation algorithm is thus beautifully suited to the task of noise cancellation. The noise cancellation architecture has been applied in a biomimetic whisking robotic to removing the component of whisker sensor signals generated by whisker self-motion (Anderson et al. 2010). Whisker sensory signals are a combination of ‘touch’ signals generated by deflections on contact with objects and the ‘self-noise’ signals generated by free whisking. These self-noise signals are generated via motor commands to whiskers, and these commands can be regarded as a reference noise signal. This example demonstrates that the adaptive filter is an essential component of theories which propose that the cerebellum implements forward and inverse models; here the adaptive filter learns a forward model of the plant dynamics which transforms motor commands into sensory self-noise signals. This architecture is related to that proposed for sensory noise cancellation in ‘cerebellum-like’ structures, such as the electrosensory lateral line lobe in mormyrid electric fish (Dean et al. 2002), but differs crucially in providing a role for the inferior olive (Dean & Porrill, 2010). Diagram adapted from Fig. 1B of Anderson et al. (2010).
Figure 4. Architecture for learning accurate tracking movements.
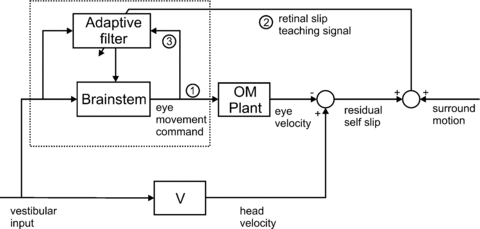
The architecture shown in Fig. 3 removes the effects of self-movement from an internal estimate of a sensory input. An alternative strategy, adopted in the vestibulo-ocular reflex, is to counteract the self-movement (in this case of the head) by moving the sensor itself (in this case the retina). This strategy requires three major modifications to the architecture shown in Fig. 3 so that it can be used to learn the required accurate tracking eye movements. (i) The output of the cancellation module is now a motor command to the oculomotor (OM) plant rather than an estimate of the self-induced slip; when learning is complete, retinal slip will be decorrelated from the motor command, i.e. it will have no self-generated component. (ii) As the cancellation of head velocity by eye velocity is now physical rather than internal, the signal required for the teaching signal is no longer an internal signal but is the measured retinal slip itself. This sensory signal must be made available on the climbing fibre. (iii) A copy of the motor command signals must be made available to the adaptive filter. Also, since the direct path via the brainstem contributes to the vestibulo-ocular reflex, the cerebellum only learns a ‘partial’ forward model. In this example, the required connections of the adaptive filter can be compared in detail with the biological evidence. The relevant area of the cerebellum is the flocculus; its involvement in image stabilization is well established, both by classical inactivation and lesion studies in a variety of species and by more recent studies in mutant mice (e.g.De Zeeuw & Yeo, 2005). Its two main types of mossy-fibre input convey vestibular information and an efference copy of eye-movement commands (e.g. Miles et al. 1980) exactly as required by the architecture shown (Dean et al. 2002; Porrill et al. 2004). Its climbing-fibre input signals retinal slip (e.g. Simpson et al. 1996), and its Purkinje cells project to ocular motoneurons, with the most direct projections having either two or three synapses. Their simple-spike firing rates are related to eye movements (>30 in vivo studies in primates alone), with a number of studies providing detailed quantitative analyses of firing rates in relation to eye position, velocity and acceleration (e.g. Gomi et al. 1998). Diagram adapted from Fig. 3B of Dean & Porrill (2010).
Signal processing by cerebellar microcomplexes
The power and plausibility of the adaptive-filter model suggests its suitability for detailed evaluation, and in this and these next sections we consider two different evaluative procedures. The first is to assess how far the signal processing by a particular functional subregion of the cerebellum is consistent with the operations of an adaptive filter. The flocculus is probably the subregion whose signal processing has been most extensively investigated and, as argued elsewhere (Dean & Porrill, 2010), its connectivity with respect to retinal image stabilization appears to conform well to that expected from an adaptive filter (Fig. 4). Moreover, the changes in Purkinje-cell firing that accompany changes in VOR gain induced by appropriate training appear to be those expected from an adaptive filter. This issue has in fact turned out to be much less straightforward than originally envisioned, partly because the primate flocculus is also involved in smooth pursuit as well as in image stabilization (see references in Medina & Lisberger, 2008), and partly because there is an additional site of synaptic plasticity relevant to VOR adaptation in the vestibular nuclei (see references in Boyden et al. 2004; Ke et al. 2009). However, simulation studies that attempt to take these complexities into account suggest the adaptive-filter model is able to explain the pattern of experimental results observed for VOR adaptation (Porrill & Dean, 2007; Menzies et al. 2010); though see McElvain et al. (2010).
A key feature allowing this assessment to be made is that the links between floccular output and its climbing-fibre input are reasonably well understood. The relationship between floccular Purkinje-cell simple-spike firing and eye-movement parameters has been the subject of systematic investigation for more than 30 years (Boyden et al. 2004); the effect of eye movements on retinal image slip can be easily calculated; and floccular climbing fibre input is known to be dominated by retinal slip signals (Simpson et al. 1996; Highstein et al. 2005). Thus, the loop between output and input via the external world (Fig. 4) can be characterized. This is not the case for most regions of the cerebellum, however, and even where there are exceptions the evidence is still incomplete.
For example, one extensively studied task that is dependent upon the cerebellum is delay eyeblink conditioning. This task involves part of the hemisphere of lobule VI, possibly zone D0 (Mostofi et al. 2010), and it has been suggested that the role of the cerebellum can be explained at a general level by the ideas of Marr and Albus (e.g. Yeo & Hesslow, 1998), and more specifically by an adaptive-filter-type model (Medina et al. 2000). However, the problem here is not that the relationship between cerebellar output and climbing-fibre input is unknown; rather, the classical conditioning model ensures that there can be no such relationship in the external world, because by definition the unconditioned stimulus is delivered regardless of the animal's response. One solution to this problem has been to postulate an internal comparator that subtracts a version of the conditioned response command from the unconditioned stimulus signal (e.g. Medina et al. 2002). Although this addition permits an adaptive-filter model of the cerebellum to learn which stimuli predict the unconditioned stimulus, it does not specify values for conditioned response amplitude and duration (Lepora et al. 2010). It is not currently understood how these parameters are in fact specified, and it is possible that the C2 zone of lobule VI is also involved (Chen & Evinger, 2006; Lepora et al. 2010). This problem is highlighted in the well-defined adaptive-filter model, but applies to all models that lack criteria to allow the system to assess whether a conditioned response is the ‘correct’ shape.
A second example where connectivity appears to be relatively well understood is the oculomotor vermis (zone A) located in lobules VIb, VIc and VII (for recent review see Iwamoto & Kaku, 2010). This region is concerned with calibrating saccadic accuracy, and its operations have been modelled by versions of an adaptive filter (Schweighofer et al. 1996; Ebadzadeh & Darlot, 2003; Gad & Anastasio, 2010). However, whereas some experiments indicate that the signal conveyed by climbing fibres to the oculomotor vermis contains the information about saccadic inaccuracy required for the adaptive-filter learning rule (Soetedjo & Fuchs, 2006; Soetedjo et al. 2008, 2009), other studies suggest the climbing-fibre signal increases rather than decreases in magnitude as saccadic adaptation proceeds (Catz et al. 2005; see also Dash et al. 2010). Possible reasons for this discrepancy have been discussed (e.g. Highstein et al. 2005; Iwamoto & Kaku, 2010), but general agreement has yet to be reached.
These examples illustrate the difficulties of identifying the critical input–output signals for a functional cerebellar subregion and help to explain why, at present, so few have been properly characterized. Cerebellar cortex can be divided into microzones, each with a distinctive input from a particular region of the inferior olive (reviewed by, e.g. Apps & Garwicz, 2005; Apps & Hawkes, 2009). An individual microzone projects in turn to a particular region of the deep cerebellar nuclei, in which some cells project back to exactly the part of the inferior olive that innervates both the parent microzone and the nuclear region. This loop (termed a microcomplex) suggests a functional subunit, and it has been estimated that the cat cerebellum contains about 5000 of them (Dean et al. 2010). Thus, even if it turns out that the cerebellar roles in both eyeblink conditioning and saccadic adaptation can be explained by the adaptive-filter model, that conclusion would have been established for well under 1% of cerebellar microcomplexes.
Microcircuit features
The second evaluative procedure is to assess how far the model's theoretical description is consistent with particular features of the cerebellar microcircuit. Although the simplified circuitry shown in Fig. 2 is compatible with the basic structure of an analysis–synthesis filter, much more is now known about details of the microcircuit than when the main features were originally described. These new discoveries provide the ground for a more rigorous assessment of the adaptive-filter model (Dean et al. 2010), and highlight a general issue concerning model evaluation. The model shown in Fig. 1 is formulated at an abstract, signal-processing level. To explain more detailed microcircuit features, it needs to be reformulated at the appropriate level, in accord with the general framework outlined by the International Union of Physiological Sciences for modelling physiological entities at a range of different levels from molecules to systems (Hunter & Nielsen, 2005).
If the appropriate level of modelling can be achieved, three main evaluative outcomes are possible. The first outcome is that a new feature proves to be strikingly congruent with the predictions of the model. Examples of such congruency have been reviewed elsewhere (Dean et al. 2010); here a brief summary is given to indicate how a model originally proposed in 1982 is able to account for recent experimental discoveries.
Congruent features
While early studies of plasticity at the synapses between parallel fibres and Purkinje cells focused on the long-term depression (LTD) produced by pairing parallel-fibre and climbing-fibre stimulation, more recent investigations have indicated that long-term potentiation (LTP) can also be obtained (Jörntell & Hansel, 2006). This LTP is produced by parallel-fibre stimulation in the absence of climbing-fibre activation, and is able to reverse the effects of LTD. Such bidirectional plasticity is exactly that required by the covariance rule used in the adaptive filter.
A second recent discovery is that many (85–98%) synapses between parallel fibres and Purkinje cells appear to be silent (Isope & Barbour, 2002; Ekerot & Jorntell, 2003). The very large number (∼150,000) of parallel-fibre synapses suggests that many will be carrying signals irrelevant to a particular learning task; moreover, even some of those that do convey relevant signals are likely to be corrupted by internal or external noise. In the adaptive-filter model, irrelevant or noisy signals that drive Purkinje-cell output produce errors that appear in the climbing-fibre signal. For example, in the VOR architecture illustrated in Fig. 4, noisy Purkinje-cell output will produce eye movements that generate rather than remove retinal slip. The resultant correlation between parallel-fibre and climbing-fibre inputs will cause the synapses involved to be driven to zero by the covariance learning rule (Porrill & Dean, 2008; Dean et al. 2010). Thus, the adaptive-filter model accounts naturally for the preponderance of silent parallel-fibre Purkinje-cell synapses.
A third microcircuit feature congruent with the model concerns plasticity in the pathway from granule cells to Purkinje cells via stellate and basket cells. In the basic adaptive filter model (Fig. 1), weights can take either positive or negative values (Fig. 1), whereas actual synapses are of course constrained to either excitatory or inhibitory. When this constraint is introduced into the model, it becomes apparent that a second, inhibitory pathway from the analysis stage of the filter to the synthesis stage is required, corresponding to an inhibitory pathway between granule cells and Purkinje cells (Porrill & Dean, 2008). Exactly such a pathway is provided via the molecular layer interneurons, and its properties regarding synaptic plasticity (Jörntell & Ekerot, 2002; Ekerot & Jorntell, 2003) appear to be those predicted by the adaptive-filter model (Dean et al. 2010).
Finally, a very recent study of mice with Purkinje-cell specific knockout of the protein phosphatase PP2B points to the importance of cerebellar LTP in VOR gain adaptation (Schoneville et al. 2010). This importance is consistent with the predictions from the model that most parallel-fibre synapses will be silent, and that therefore for some tasks the initial acquisition cannot take place via LTD but must instead use LTP (Dean & Porrill, 2008; Porrill & Dean, 2008; Dean et al. 2010).
Unrelated features?
A second outcome of evaluating microcircuit features is that the feature in question turns out to be unrelated to the computational properties of the model, but is relevant instead to implementation-level issues concerning the properties of neural tissue. For example, Purkinje cells are very vulnerable to glutamate-mediated excitotoxicity (see references in Piochon et al. 2010), so it is possible that certain forms of plasticity, for example at synapses between parallel fibres and Purkinje cells, act to reduce this vulnerability by keeping Purkinje-cell firing rates within bounds (Dean et al. 2010). This suggestion has been considered in the general context of homeostatic plasticity (e.g. Turrigiano & Nelson, 2004), and more specifically as a functional explanation of LTD at synapses between parallel fibres and Purkinje cells (De Schutter, 1995). Thus, not all forms of plasticity identified in the cerebellar microcircuit need be accounted for by the computational adaptive-filter model.
Other examples of features that may not be related directly to computational competence have emerged from recent studies of granular-layer processing. While some studies have emphasized the apparently almost trivial nature of the transformation between mossy-fibre input and granule-cell output (see references in Dean et al. 2010), others have described complex signal-processing features, such as delayed responses (‘time windowing’), oscillations and plasticity (D'Angelo & De Zeeuw, 2009; D'Angelo et al. 2009; Mapelli et al. 2010). At present, it is unclear whether these differences in emphasis represent differences in granular-layer processing between microzones (Dean & Porrill, 2010) or in currently unidentified differences in experimental approach. Three points emerge from the adaptive-filter perspective. Firstly, although the filter requires an extensive range of ‘analysed’ responses to a given input (Fig. 1), and the obvious location for generating those responses is the granular layer (Fig. 2), a substantial contribution from the already-existing diversity of mossy-fibre responses is not in the least ruled out (Dean et al. 2010). Secondly, theoretical analyses of adaptive-filter learning indicate that it can be greatly facilitated by plasticity at the analysis stage that can produce an efficient code specifically tailored to an individual sensorimotor problem. A key task for current research is to assess whether the experimentally demonstrated forms of granular-layer plasticity are in fact suited to this computational role. Finally, it is at present unclear whether granular-layer oscillations are needed for a central computational function or are primarily a byproduct of neural implementation (see previous paragraph). To return to the point made above, multiscale modelling is needed to help resolve this problem, in particular a new detailed implementation of the adaptive-filter model that embodies the relevant complexities of cerebellar neurons.
Incompatible features: patterns and pauses in simple-spike firing
The third outcome is that a particular feature of the microcircuit appears definitely incompatible with the adaptive-filter model. The implications of this outcome are considered here for a particular example, namely the presence of ‘patterns and pauses’ in Purkinje-cell simple-spike firing (De Schutter & Steuber, 2009).
It is first important to distinguish ‘patterns and pauses’ from an apparently similar phenomenon described for Purkinje-cell simple-spike firing, namely bistability (De Schutter & Steuber, 2009). Some aspects of the relation between bistability and the adaptive filter have been briefly considered elsewhere (Dean & Porrill, 2010; Dean et al. 2010); here the major points are summarized to clarify the differences between bistability on the one hand and patterns and pauses on the other.
Bistability
Purkinje cells can alternate between ‘up’ states, with depolarized membrane potential and simple-spike firing, and ‘down’ states, with hyperpolarized membrane potential and no simple spikes (Fig. 5A; Llinás & Sugimori, 1980; Loewenstein et al. 2005). However, these states are relatively long lasting (>1 s) and appear to have been described primarily in vitro or in anaesthetized animals, rather than in awake animals performing a relevant task (Schonewille et al. 2006). In particular, they have not been described in the large number of studies of floccular firing in awake animals referred to above, which have found relationships between simple-spike firing parameters and behavioural features, such as eye position and velocity, that indicate little if any significant interference from the intrusion of long-duration down-states.
Figure 5. Bistability, patterns and pauses in Purkinje cell firing.
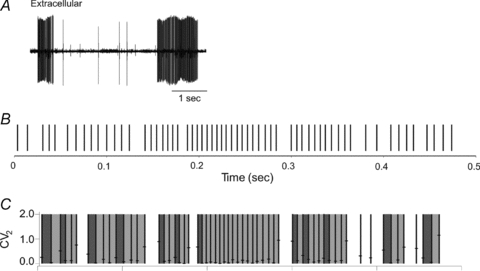
A shows an in vivo extracellular recording from a guinea-pig Purkinje cell. High-frequency bursts of simple spikes are separated by quiescent periods of the order of ∼1 s associated with membrane potential down-states. Reproduced with permission from Loewenstein et al. (2005). B shows a segment of a Purkinje cell simple-spike train recorded in vivo. Spikes are classified as initiating pauses if followed by an interspike interval of >12 ms, or as part of a single pattern if the coefficient of short-range variation (CV2) of the interspike intervals on either side of the spike is <0.2 (further details in main text). The results of the spike classification are shown in C, with pauses unshaded, patterns shaded, and the first interspike interval in each pattern shaded more darkly. The analysis reveals sequences of patterns and pauses which are incompatible with a renewal process. Reproduced with permission from Elsevier from De Schutter & Steuber (2009).
It remains possible that bistability is functionally significant in cerebellar microzones other than the flocculus. Urbano et al. (2006) compared the properties of neurons in two regions of the inferior olive, one projecting to the flocculus and the other to the lateral D zone in the hemispheres (Apps & Hawkes, 2009). Only the latter showed subthreshold oscillations and robust rhythmicity at 6–12 Hz, features that could contribute to the generation of bistability by allowing complex spikes to toggle between states (Loewenstein et al. 2005). In this case, Purkinje-cell bistability might reflect a new mode of motor control or planning, made necessary by the huge complexity of the multijoint somatomotor system (e.g. Urbano et al. 2006), and requiring olivary input to play a novel role by the direct driving of motor output by generating temporal patterns (e.g. Jacobson et al. 2008).
Evaluation of this new version of the cerebellar algorithm reveals two difficulties. Firstly, many recordings of Purkinje-cell firing in the lateral and intermediate regions of the cerebellum concerned with arm movements do not report bistability, but instead suggest that firing rates are related to task variables (see references in e.g. Norris et al. 2004; Roitman et al. 2009), just as in the flocculus. Indeed, one recent study of the lateral D zone in cats concluded that its ‘Purkinje-cell activity reflects the operation of an internal model’ (Cerminara et al. 2009, p. 429) of a moving visual target. Such a role would be entirely consistent with the adaptive-filter algorithm. It appears that experimental demonstration of task-related bistability in awake animals remains a critical goal in evaluating the new algorithm.
Secondly, the adaptive-filter algorithm is known to be effective in a very wide range of signal-processing and control-engineering contexts, and it is understood how in principle it could contribute to putative cerebellar roles, such as learning internal models or state estimation (Dean et al. 2010). In contrast, the computational properties of the new algorithm are not well understood at either a theoretical or a practical level (for example, how they could be used to control a multijoint robot arm). Addressing this issue will be important for evaluating all models that require the inferior olivary input to help drive cerebellar output rather than act as a teaching signal (Llinás et al. 2004; Bandyopadhyay et al. 2009).
Patterns and pauses: data
Returning to the main topic of patterns and pauses, we first briefly describe the relevant data. Shin & De Schutter (2006) investigated synchronous pauses in simple-spike firing patterns obtained from Purkinje cells in crus II of rats anaesthetized with ketamine and xylazine. Simple-spike pauses were defined as those interspike intervals (ISIs) equal to or larger than a defined threshold of 12 ms (corresponding to a mean firing rate of 83 spikes s−1). The main findings were that ∼13% of all pauses were synchronized in nearby Purkinje cells (<100 μm apart), and that these pauses had a median ISI of 20 ms. In fact almost no ISIs, synchronized or otherwise, were greater than 50 ms, a finding that emphasizes the distinction between ‘patterns and pauses’ and bistability. ‘It is unlikely that the …. pauses we observed correspond to quiescent down states because the latter last on average for several seconds’ (Shin & De Schutter, 2006, p. 3490). A very similar ISI distribution is shown in Fig. 2 of Holtzman et al. (2006), also from anaesthetized rats (usually urethane). It is, however, unclear why Shin & De Schutter did not observe down-states, given that Loewenstein et al. (2005) found that, in Sprague–Dawley rats under ketamine and xylazine anaesthesia, 24 of 24 Pukinje cells showed bistability with 52% of time spent in the down-state (Loewenstein et al. 2006).
A subsequent analysis of simple-spike trains in anaesthetized rats (Shin et al. 2007b) focused on their high coefficients of variation (CVs), which suggested that they might be generated by a renewal process (in which case the ISIs would be independent and identically distributed; more details below). A measure of short-range variability (CV2) was used to separate segments of firing that were regular (termed ‘patterns’) from the remainder (termed ‘singles’). The latter did not correspond simply to pauses, though the data (Figs 9C and 10A of Shin et al. 2007b) suggest that almost all the ISIs greater than 50 ms are to be found in singles. Statistical fitting of γ-process parameters to these two classes indicated that they had separate properties (though it should be noted that at least 20 consecutive ISIs were needed to obtain a correct γ-order estimate, so that only 1.3% of all regular patterns could be used). This work was extended by Shin et al. (2007a), who also analysed recordings from the flocculus or paramedian lobule of both anaesthetized and awake mice. While CVs in these cases were 1.74 and 1.39 (3.93 for anaesthetized rats), values of CV2 were 0.30 and 0.39 (0.51), ‘suggestive of much more regular firing at short time scales’ (Shin et al. 2007a, p. 3). When a CV2 value of 0.2 used to identify regular patterns, 72% of patterns were only two or three ISIs long, with only 4% greater than 10 ISIs in anaesthetized rodents and only 0.4% in awake animals.
Finally, Steuber et al. (2007) re-analysed simple-spike data from floccular Purkinje cells in both wild-type and mutant (L7-PKCi) mice during performance of the optokinetic reflex (Goossens et al. 2004) and found that the proportion of longer ISIs in the range 20–60 ms was higher in the mutant animals.
Patterns and pauses: theoretical interpretation
These observations have been interpreted in a framework that retains important features of the adaptive-filter model (De Schutter & Steuber, 2009). For example, it is argued that regular patterns of simple-spike firings might plausibly be produced by Purkinje cells passively integrating ‘strong excitatory inputs composed of numerous small inputs, which are not balanced by inhibitory inputs’ (Shin et al. 2007b, p. 792), perhaps with a contribution from the intrinsic firing properties of Purkinje cells, whereas singles could be produced by inputs from ascending axons, or by feedforward inhibition from granule cells via molecular-layer interneurons. As the climbing-fibre input does not directly induce pauses (there was no evidence of complex spikes triggering transitions between high and low firing rate regimes), it retains its interpretation as a teaching signal, modulating synaptic weights via complex spikes. Just as in the adaptive filter, it is the simple-spike train which carries the ‘informative’ output of the Purkinje cell. In this regard, the computational framework proposed for patterns and pauses differs much less from that of the adaptive filter than frameworks associated with bistability.
Nonetheless, two important theoretical issues emerge. The first concerns the role of Purkinje-cell outputs, because the new theory assigns specific roles to two different components of the Purkinje-cell simple-spike train. Regular spiking patterns function as a rate code and set the ‘amplitude’ (which can be zero) of subsequent rebound bursts in deep cerebellar nuclei (DCN) neurons, while pauses provide a temporal coding signal which, when sufficiently synchronized across afferent Purkinje cells, will evoke a rebound burst at the preset amplitude. This complex non-linear output coding is quite different from the simple linear code assumed by the adaptive-filter model.
The second theoretical issue concerns the mechanism by which pauses and patterns are generated. It has been suggested that pauses be understood in the context of a particular perceptron-like model (Steuber et al. 2007), in which learning is dominated by LTD of synapses with initially ‘high’ weights. The finding by Steuber et al. (2007) that such burst-pause inputs to the DCN are likely to be stronger before training Purkinje neurons with LTD than afterwards is opposite to the original prediction by the Marr–Albus theory that LTD learning leads to an increase in pause responses (Albus, 1971).
Possible artefact
As in the case of Purkinje-cell bistability, it is first necessary to establish whether the findings apparently incompatible with the adaptive-filter model are artefactual. Specifically, evidence is needed that pauses and patterns can be observed in task-related Purkinje-cell firing, because the data from awake animals in the study of Shin et al. (2007a) came from restrained mice that were not performing any behavioural task. Although many studies of task-related firing (particularly in the flocculus) report only relations with task variables, it should be noted that ‘pauses and patterns’ require particular statistical analyses for their detection; pauses are only ∼50 ms long (consistent with a 20 Hz firing rate) and so are much shorter than the ∼1 s pauses required by bistability. Patterns are identified by reference to the CV2 statistic, not normally used in the analysis of floccular discharge. Thus, whether the patterns and pauses observed in apparently idling cerebellar cortex can also be seen in working cortex is still to be determined. It should be noted that the analyses reported by Steuber et al. (2007) are not directly relevant to the issue, because their focus was on differences between normal and mutant behaviour, not on normal behaviour as such. In any case, the differences found in these analyses were very subtle and of obscure functional significance, given that the original study (Goossens et al. 2004) concluded that both simple-spike and complex-spike ‘discharge dynamics appeared to be very similar in wild-type and transgenic P[urkinje]-cells at all stimulus frequencies’ (p. 687) and in any case ‘L7-PKC1 mutants exhibit no deficits in their default oculomotor performance’ (p. 696).
However, even if patterns and pauses were demonstrated in ‘working’ Purkinje cells, that in itself need not invalidate the adaptive-filter model. Although it has been argued that the high CV values for simple-spike firing are consistent with Poisson-like irregularity (Shin et al. 2007a,b;), on shorter time scales the predicted simple-spike output under the rate-coded adaptive-filter hypothesis would almost certainly be band-limited coloured noise. For example, in adaptive-filter models of the VOR, the Purkinje-cell output codes head velocity, transformed via the partial forward model encoded in the adaptive filter (Dean et al. 2002). As head velocity is usually modelled as coloured noise for free head movements, filter output will also be coloured noise. It is likely that the relatively weak CV2 <20% criterion (Shin et al. 2007a) used for similarity of adjacent ISIs would also identify patterns in coloured noise signals, while pauses would be generated whenever negative signals were coded as very low firing rates. Hence, it is vital that the statistical analysis is repeated in a well-understood system where the adaptive filter model provides a realistic null hypothesis for simple-spike statistics.
New algorithm: decoding
As outlined above in the section on theoretical interpretation, it has been suggested that the patterns and pauses observed in simple-spike firing denote a complex non-linear output coding which is quite different from the simple linear code assumed by the adaptive-filter model, and which points to the operation of a new algorithm for motor control. However, it is unclear whether this new algorithm is used in the VOR, given the linear properties of vestibular neurons and the infrequency of long pauses in their response to typical head movements (see e.g. Bagnall et al. 2008). Thus, even though the subset of vestibular nucleus neurons that receive input from the flocculus does show rebound firing (Sekirnjak & du Lac, 2006), it appears unlikely that such firing is triggered by typical head movements. It would therefore appear that if the proposed non-linear coding mechanism is used at all, it is by some regions of the cerebellum and not others (cf. ‘Bistability’ above).
However, even for DCN neurons themselves the functionality of rebound firing is far from established (e.g. Alvina et al. 2008). Sangrey & Jaeger (2010) distinguish between two modes of input processing by DCN neurons. One mode, which they term the continuous mode, appears compatible with the linear processing required by the adaptive filter, and also with some studies of the task-related firing of DCN neurons (see references in e.g. Casabona et al. 2010). The second mode is evoked by strong bursts of inhibitory inputs, which in vitro produce strong rebound firing when the burst terminates. However, the ‘role of rebound properties in DCN neurons in the control of cerebellar output in behaving animals remains unclear at this point … rebounds at present represent a specialized response property of DCN neurons in specific situations that yet need to be clarified with physiological recordings in behaving animals’ (Sangrey & Jaeger, 2010, p. 1655). Likewise, a recent review has commented, ‘even the potential to record rebound bursts in DCN cells in the live animal remains a source of debate, with the functional significance of this activity relatively unknown’ (Tadayonnejad et al. 2010, p. 370).
Finally, the computational features of the proposed coding scheme have yet to be determined. A model of cerebellar memory recall incorporating rebound firing in the DCN has been described by Wetmore et al. (2008), but its compatibility with the patterns-and-pauses scheme of De Schutter & Steuber (2009) has yet to be explored, as are its capacities for learning the kind of signal-processing tasks successfully managed by the adaptive-filter model. In fact, the tasks or circumstances in which the suggested non-linear coding scheme would be useful have yet to be identified.
New algorithm: learning patterns and pauses
As outlined above in the section on theoretical interpretation, it has been suggested that pauses and patterns in Purkinje-cell firing could be generated by a perceptron-like learning scheme implemented by cerebellar cortex (Steuber et al. 2007). It is not in general clear how the pattern-classifying properties of perceptrons could be adapted for motor-control problems that use continuous variables (Dean et al. 2010), and this difficulty is accentuated in the particular scheme of Steuber et al. (2007), which appears to require strong, synchronized granule-cell burst inputs (Jaeger, 2007). As some granule-cell inputs appear not to use bursts but instead (for example) encode head velocity in continuous mode (Arenz et al. 2008), it appears that the proposed model cannot be a general one. Once again, it would be extremely helpful to identify those particular motor-control problems for which the proposed scheme is especially suited, so that its computational properties can be compared with those of the adaptive-filter model. Finally, it is essential to demonstrate that the learning rules for these control problems, when combined with the non-linear coding schemes and output phenomena suggested, are as computationally powerful as the simple covariance learning rule used in the adaptive filter.
Conclusions
The attempt here to evaluate the adaptive-filter model of the cerebellar microcircuit has illuminated the following general problems relevant to any model of cerebellar function.
There are remarkably few microzones where the information about inputs and outputs required for evaluation is available. In particular, it is not generally known how the output of a microzone affects its climbing-fibre input. This is particularly relevant to the adaptive-filter model, because when wired appropriately adaptive filters are theoretically capable of many proposed cerebellar functions (Dean et al. 2010), such as learning internal models (Wolpert et al. 1998; Kawato, 1999), state estimation (Miall et al. 2007) and Smith prediction (Miall et al. 1993). These functions are of great current interest, but in no case has the detailed circuitry required been mapped onto neural substrates (cf. Lisberger, 2009).
The behaviour of the cerebellar microcircuit is being described at an increasingly detailed level. An important role for functional models is to suggest which of these details are of computational significance. One aspect of this role is to raise concerns about microcircuit behaviours that are only observed in in vitro or anaesthetized preparations.
The competence of functional models needs to be demonstrated. Complex non-linear schemes have been proposed, but at present their computational virtues remain unclear, especially in the basic contexts of sensory analysis or motor control. It seems reasonable to suppose, given the mounting evidence of (approximate) linearity for floccular processing in gaze stabilization, that highly non-linear models cannot be applied generally. Hence, it is particularly important to identify the particular computational difficulties that their complexity is needed to address.
A far as the adaptive-filter model itself is concerned, the evidence reviewed here suggests that it provides a reasonable account of processing in at least some regions of the cerebellum, and that the extent to which other cerebellar regions employ a different algorithm is an important issue for future enquiry. In general terms, the attempt at evaluation appears to raise types of questions that point to fruitful avenues for further research. In particular, multilevel modelling will be needed to relate the adaptive-filter model to the relevant complexities of cerebellar neurons.
Acknowledgments
Preparation of this paper was supported by grants from the European Union (BIOTACT, ICT-215910; REALNET, 270434 FP7).
Glossary
Abbreviations
- CV
coefficient of variation
- CV2
coefficient of short-range variation (see text)
- DCN
deep cerebellar nuclei
- ISI
interspike interval
- LTD
long-term depression
- LTP
long-term potentiation
- VOR
vestibulo-ocular reflex
References
- Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61. [Google Scholar]
- Alvina K, Walter JT, Kohn A, Ellis-Davies G, Khodakhah K. Questioning the role of rebound firing in the cerebellum. Nat Neurosci. 2008;11:1256–1258. doi: 10.1038/nn.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SR, Pearson MJ, Pipe A, Prescott T, Dean P, Porrill J. Adaptive cancelation of self-generated sensory signals in a whisking robot. IEEE Trans Robot. 2010;26:1065–1076. [Google Scholar]
- Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci. 2005;6:297–311. doi: 10.1038/nrn1646. [DOI] [PubMed] [Google Scholar]
- Apps R, Hawkes R. Cerebellar cortical organization: a one-map hypothesis. Nat Rev Neurosci. 2009;10:670–681. doi: 10.1038/nrn2698. [DOI] [PubMed] [Google Scholar]
- Arenz A, Silver RA, Schaefer AT, Margrie TW. The contribution of single synapses to sensory representation in vivo. Science. 2008;321:977–980. doi: 10.1126/science.1158391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnall MW, McElvain LE, Faulstich M, du Lac S. Frequency-independent synaptic transmission supports a linear vestibular behavior. Neuron. 2008;60:343–352. doi: 10.1016/j.neuron.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay PR, Singh SN, Thivierge DP, Annaswamy AM, Leinhos HA, Fredette AR, Beal DN. Synchronization of animal-inspired multiple high-lift fins in an underwater vehicle using olivo-cerebellar dynamics. IEEE J Ocean Eng. 2009;33:563–578. [Google Scholar]
- Boyden ES, Katoh A, Raymond JL. Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Ann Rev Neurosci. 2004;27:581–609. doi: 10.1146/annurev.neuro.27.070203.144238. [DOI] [PubMed] [Google Scholar]
- Casabona A, Bosco G, Perciavalle V, Valle MS. Processing of limb kinematics in the interpositus nucleus. Cerebellum. 2010;9:103–110. doi: 10.1007/s12311-009-0149-x. [DOI] [PubMed] [Google Scholar]
- Catz N, Dicke PW, Thier P. Cerebellar complex spike firing is suitable to induce as well as to stabilize motor learning. Curr Biol. 2005;15:2179–2189. doi: 10.1016/j.cub.2005.11.037. [DOI] [PubMed] [Google Scholar]
- Cerminara NL, Apps R, Marple-Horvat DE. An internal model of a moving visual target in the lateral cerebellum. J Physiol. 2009;587:429–442. doi: 10.1113/jphysiol.2008.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FP, Evinger C. Cerebellar modulation of trigeminal reflex blinks: interpositus neurons. J Neurosci. 2006;26:10,569–10,576. doi: 10.1523/JNEUROSCI.0079-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo E, De Zeeuw CI. Timing and plasticity in the cerebellum: focus on the granular layer. Trends Neurosci. 2009;32:30–40. doi: 10.1016/j.tins.2008.09.007. [DOI] [PubMed] [Google Scholar]
- D'Angelo E, Koekkoek SK, Lombardo P, Solinas S, Ros E, Garrido J, Schonewille M, De Zeeuw CI. Timing in the cerebellum: oscillations and resonance in the granular layer. Neuroscience. 2009;162:805–815. doi: 10.1016/j.neuroscience.2009.01.048. [DOI] [PubMed] [Google Scholar]
- Dash S, Catz N, Dicke PW, Thier P. Specific vermal complex spike responses build up during the course of smooth-pursuit adaptation, paralleling the decrease of performance error. Exp Brain Res. 2010;205:41–55. doi: 10.1007/s00221-010-2331-2. [DOI] [PubMed] [Google Scholar]
- Dean P, Porrill J. Adaptive filter models of the cerebellum: computational analysis. Cerebellum. 2008;7:567–571. doi: 10.1007/s12311-008-0067-3. [DOI] [PubMed] [Google Scholar]
- Dean P, Porrill J. The cerebellum as an adaptive filter: a general model? Funct Neurol. 2010;25:173–180. [PubMed] [Google Scholar]
- Dean P, Porrill J, Ekerot CF, Jorntell H. The cerebellar microcircuit as an adaptive filter: experimental and computational evidence. Nat Rev Neurosci. 2010;11:30–43. doi: 10.1038/nrn2756. [DOI] [PubMed] [Google Scholar]
- Dean P, Porrill J, Stone JV. Decorrelation control by the cerebellum achieves oculomotor plant compensation in simulated vestibulo-ocular reflex. Proc R Soc Lond B Biol Sci. 2002;269:1895–1904. doi: 10.1098/rspb.2002.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Schutter E. Cerebellar long-term depression might normalize excitation of Purkinje cells: a hypothesis. Trends Neurosci. 1995;18:291–295. doi: 10.1016/0166-2236(95)93916-l. [DOI] [PubMed] [Google Scholar]
- De Schutter E, Steuber V. Patterns and pauses in Purkinje cell simple spike trains: experiments, modeling and theory. Neuroscience. 2009;162:816–826. doi: 10.1016/j.neuroscience.2009.02.040. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Yeo CH. Time and tide in cerebellar memory formation. Curr Opin Neurobiol. 2005;15:667–674. doi: 10.1016/j.conb.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Ebadzadeh M, Darlot C. Cerebellar learning of bio-mechanical functions of extra-ocular muscles: modeling by artificial neural networks. Neuroscience. 2003;122:941–966. doi: 10.1016/s0306-4522(03)00569-4. [DOI] [PubMed] [Google Scholar]
- Ekerot CF, Jorntell H. Parallel fiber receptive fields: a key to understanding cerebellar operation and learning. Cerebellum. 2003;2:101–109. doi: 10.1080/14734220309411. [DOI] [PubMed] [Google Scholar]
- Fujita M. Adaptive filter model of the cerebellum. Biol Cybern. 1982;45:195–206. doi: 10.1007/BF00336192. [DOI] [PubMed] [Google Scholar]
- Gad YP, Anastasio TJ. Simulating the shaping of the fastigial deep nuclear saccade command by cerebellar Purkinje cells. Neural Netw. 2010;23:789–804. doi: 10.1016/j.neunet.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Gomi H, Shidara M, Takemura A, Inoue Y, Kawano K, Kawato M. Temporal firing patterns of Purkinje cells in the cerebellar ventral paraflocculus during ocular following responses in monkeys I. Simple spikes. J Neurophysiol. 1998;80:818–831. doi: 10.1152/jn.1998.80.2.818. [DOI] [PubMed] [Google Scholar]
- Goossens HHLM, Hoebeek FE, van Alphen AM, Van Der Steen J, Stahl JS, De Zeeuw CI, Frens MA. Simple spike and complex spike activity of floccular Purkinje cells during the optokinetic reflex in mice lacking cerebellar long-term depression. Eur J Neurosci. 2004;19:687–697. doi: 10.1111/j.0953-816x.2003.03173.x. [DOI] [PubMed] [Google Scholar]
- Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Front Hum Neurosci. 2009;3:31. doi: 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highstein SM, Porrill J, Dean P. Report on a workshop concerning the cerebellum and motor learning, held in St Louis October 2004. Cerebellum. 2005;4:140–150. doi: 10.1080/14734220510007987. [DOI] [PubMed] [Google Scholar]
- Holtzman T, Rajapaksa T, Mostofi A, Edgley SA. Different responses of rat cerebellar Purkinje cells and Golgi cells evoked by widespread convergent sensory inputs. J Physiol. 2006;574:491–507. doi: 10.1113/jphysiol.2006.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter P, Nielsen P. A strategy for integrative computational physiology. Physiology. 2005;20:316–325. doi: 10.1152/physiol.00022.2005. [DOI] [PubMed] [Google Scholar]
- Isope P, Barbour B. Properties of unitary granule cell→Purkinje cell synapses in adult rat cerebellar slices. J Neurosci. 2002;22:9668–9678. doi: 10.1523/JNEUROSCI.22-22-09668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- Iwamoto Y, Kaku Y. Saccade adaptation as a model of learning in voluntary movements. Exp Brain Res. 2010;204:145–162. doi: 10.1007/s00221-010-2314-3. [DOI] [PubMed] [Google Scholar]
- Jacobson GA, Rokni D, Yarom Y. A model of the olivo-cerebellar system as a temporal pattern generator. Trends Neurosci. 2008;31:617–625. doi: 10.1016/j.tins.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Jaeger D. Pauses as neural code in the cerebellum. Neuron. 2007;54:9–10. doi: 10.1016/j.neuron.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Jörntell H, Ekerot CF. Reciprocal bidirectional plasticity of parallel fiber receptive fields in cerebellar Purkinje cells and their afferent interneurons. Neuron. 2002;34:797–806. doi: 10.1016/s0896-6273(02)00713-4. [DOI] [PubMed] [Google Scholar]
- Jörntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron. 2006;52:227–238. doi: 10.1016/j.neuron.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Kawato M. Internal models for motor control and trajectory planning. Curr Opin Neurobiol. 1999;9:718–727. doi: 10.1016/s0959-4388(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Ke MC, Guo CC, Raymond JL. Elimination of climbing fiber instructive signals during motor learning. Nat Neurosci. 2009;12:1171–1179. doi: 10.1038/nn.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepora N, Porrill J, Yeo CH, Dean P. Sensory prediction or motor control? Application of Marr–Albus models of cerebellar function to classical conditioning. Front Comput Neurosci. 2010;4:140. doi: 10.3389/fncom.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger SG. Internal models of eye movement in the floccular complex of the monkey cerebellum. Neuroscience. 2009;162:763–776. doi: 10.1016/j.neuroscience.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Leznik E, Makarenko VI. The olivo-cerebellar circuit as a universal motor control system. IEEE J Ocean Eng. 2004;29:631–639. [Google Scholar]
- Loewenstein Y, Mahon S, Chadderton P, Kitamura K, Sompolinsky H, Yarom Y, Hausser MH. Bistability of cerebellar Purkinje cells modulated by sensory stimulation. Nat Neurosci. 2005;8:202–211. doi: 10.1038/nn1393. [DOI] [PubMed] [Google Scholar]
- Loewenstein Y, Mahon S, Chadderton P, Kitamura K, Sompolinsky H, Yarom Y, Hausser MH. Purkinje cells in awake behaving animals operate at the upstate membrane potential – author reply. Nat Neurosci. 2006;9:461. doi: 10.1038/nn0406-459. [DOI] [PubMed] [Google Scholar]
- McElvain LE, Bagnall MW, Sakatos A, du Lac S. Bidirectional plasticity gated by hyperpolarization controls the gain of postsynaptic firing responses at central vestibular nerve synapses. Neuron. 2010;68:763–775. doi: 10.1016/j.neuron.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapelli J, Gandolfi D, D'Angelo E. High-pass filtering and dynamic gain regulation enhance vertical bursts transmission along the mossy fiber pathway of cerebellum. Front Cell Neurosci. 2010;4:14. doi: 10.3389/fncel.2010.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Garcia KS, Nores WL, Taylor NM, Mauk MD. Timing mechanisms in the cerebellum: testing predictions of a large-scale computer simulation. J Neurosci. 2000;20:5516–5525. doi: 10.1523/JNEUROSCI.20-14-05516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Lisberger SG. Links from complex spikes to local plasticity and motor learning in the cerebellum of awake-behaving monkeys. Nat Neurosci. 2008;11:1185–1192. doi: 10.1038/nn.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JF, Nores WL, Mauk MD. Inhibition of climbing fibres is a signal for the extinction of conditioned eyelid responses. Nature. 2002;416:330–333. doi: 10.1038/416330a. [DOI] [PubMed] [Google Scholar]
- Menzies JRW, Porrill J, Dutia M, Dean P. Synaptic plasticity in medial vestibular nucleus neurons: comparison with computational requirements of VOR adaptation. PLoS One. 2010;5:e13182. doi: 10.1371/journal.pone.0013182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, Christensen LO, Cain O, Stanley J. Disruption of state estimation in the human lateral cerebellum. PLoS Biol. 2007;5:2733–2744. doi: 10.1371/journal.pbio.0050316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, Weir DJ, Wolpert DM, Stein JF. Is the cerebellum a Smith predictor? J Motor Behav. 1993;25:203–216. doi: 10.1080/00222895.1993.9942050. [DOI] [PubMed] [Google Scholar]
- Miles FA, Fuller JH, Braitman DJ, Dow BM. Long-term adaptive changes in primate vestibuloocular reflex. III. Electrophysiological observations in flocculus of normal monkeys. J Neurophysiol. 1980;43:1437–1476. doi: 10.1152/jn.1980.43.5.1437. [DOI] [PubMed] [Google Scholar]
- Mostofi A, Holtzman T, Grout AS, Yeo CH, Edgley SA. Electrophysiological localization of eyeblink-related microzones in rabbit cerebellar cortex. J Neurosci. 2010;30:8920–8934. doi: 10.1523/JNEUROSCI.6117-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris SA, Greger B, Hathaway EN, Thach WT. Purkinje cell spike firing in the posterolateral cerebellum: correlation with visual stimulus, oculomotor response, and error feedback. J Neurophysiol. 2004;92:1867–1879. doi: 10.1152/jn.01251.2003. [DOI] [PubMed] [Google Scholar]
- Ohtsuki G, Piochon C, Hansel C. Climbing fiber signaling and cerebellar gain control. Front Cell Neurosci. 2009;3:4. doi: 10.3389/neuro.03.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piochon C, Levenes C, Ohtsuki G, Hansel C. Purkinje cell NMDA receptors assume a key role in synaptic gain control in the mature cerebellum. J Neurosci. 2010;30:15,330–15,335. doi: 10.1523/JNEUROSCI.4344-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrill J, Dean P. Cerebellar motor learning: when is cortical plasticity not enough? PLoS Comput Biol. 2007;3:1935–1950. doi: 10.1371/journal.pcbi.0030197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrill J, Dean P. Silent synapses, LTP and the indirect parallel-fibre pathway: computational consequences of optimal noise processing. PLoS Comput Biol. 2008;4:e1000085. doi: 10.1371/journal.pcbi.1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrill J, Dean P, Stone JV. Recurrent cerebellar architecture solves the motor error problem. Proc R Soc Lond B Biol Sci. 2004;271:789–796. doi: 10.1098/rspb.2003.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman AV, Pasalar S, Ebner TJ. Single trial coupling of Purkinje cell activity to speed and error signals during circular manual tracking. Exp Brain Res. 2009;192:241–251. doi: 10.1007/s00221-008-1580-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangrey T, Jaeger D. Analysis of distinct short and prolonged components in rebound spiking of deep cerebellar nucleus neurons. Eur J Neurosci. 2010;32:1646–1657. doi: 10.1111/j.1460-9568.2010.07408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonewille M, Khosrovanl S, Winkelman BHJ, Hoebeek FE, De Jeu MTG, Larsen IM, Van Der Burg J, Schmolesky MT, Frens MA, De Zeeuw CI. Purkinje cells in awake behaving animals operate at the upstate membrane potential. Nat Neurosci. 2006;9:459–461. doi: 10.1038/nn0406-459. [DOI] [PubMed] [Google Scholar]
- Schonewille M, Belmeguenai A, Koekkoek SK, Houtman SH, Boele HJ, van Beugen BJ, Gao Z, Badura A, Ohtsuki G, Amerika WE, Hosy E, Hoebeek FE, Elgersma Y, Hansel C, De Zeeuw CI. Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning. Neuron. 2010;67:618–628. doi: 10.1016/j.neuron.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer N, Arbib MA, Dominey PF. A model of the cerebellum in adaptive control of saccadic gain. 1. The model and its biological substrate. Biol Cybern. 1996;75:19–28. doi: 10.1007/BF00238736. [DOI] [PubMed] [Google Scholar]
- Sejnowski TJ. Storing covariance with nonlinearly interacting neurons. J Math Biol. 1977;4:303–321. doi: 10.1007/BF00275079. [DOI] [PubMed] [Google Scholar]
- Sekirnjak C, du Lac S. Physiological and anatomical properties of mouse medial vestibular nucleus neurons projecting to the oculomotor nucleus. J Neurophysiol. 2006;95:3012–3023. doi: 10.1152/jn.00796.2005. [DOI] [PubMed] [Google Scholar]
- Shin SL, De Schutter E. Dynamic synchronization of Purkinje cell simple spikes. J Neurophysiol. 2006;96:3485–3491. doi: 10.1152/jn.00570.2006. [DOI] [PubMed] [Google Scholar]
- Shin SL, Hoebeek FE, Schonewille M, De Zeeuw CI, Aertsen A, De Schutter E. Regular patterns in cerebellar Purkinje cell simple spike trains. PLoS One. 2007a;2:e485. doi: 10.1371/journal.pone.0000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SL, Rotter S, Aertsen A, De Schutter E. Stochastic description of complex and simple spike firing in cerebellar Purkinje cells. Eur J Neurosci. 2007b;25:785–794. doi: 10.1111/j.1460-9568.2007.05308.x. [DOI] [PubMed] [Google Scholar]
- Simpson JI, Wylie DR, De Zeeuw CI. On climbing fiber signals and their consequence(s) Behav Brain Sci. 1996;19:384–398. [Google Scholar]
- Soetedjo R, Fuchs AF. Complex spike activity of Purkinje cells in the oculomotor vermis during behavioral adaptation of monkey saccades. J Neurosci. 2006;26:7741–7755. doi: 10.1523/JNEUROSCI.4658-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetedjo R, Fuchs AF, Kojima Y. Subthreshold activation of the superior colliculus drives saccade motor learning. J Neurosci. 2009;29:15,213–15,222. doi: 10.1523/JNEUROSCI.4296-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soetedjo R, Kojima Y, Fuchs AF. Complex spike activity in the oculomotor vermis of the cerebellum: a vectorial error signal for saccade motor learning? J Neurophysiol. 2008;100:1949–1966. doi: 10.1152/jn.90526.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuber V, Mittmann W, Hoebeek FE, Silver RA, De Zeeuw CI, Hausser M, De Schutter E. Cerebellar LTD and pattern recognition by Purkinje cells. Neuron. 2007;54:121–136. doi: 10.1016/j.neuron.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadayonnejad R, Anderson D, Molineux ML, Mehaffey WH, Jayasuriya K, Turner RW. Rebound discharge in deep cerebellar nuclear neurons in vitro. Cerebellum. 2010;9:3352–3374. doi: 10.1007/s12311-010-0168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Urbano FJ, Simpson JI, Llinas RR. Somatomotor and oculomotor inferior olivary neurons have distinct electrophysiological phenotypes. Proc Natl Acad Sci USA. 2006;103:16,550–16,555. doi: 10.1073/pnas.0607888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore DZ, Mukamel EA, Schnitzer MJ. Lock-and-key mechanisms of cerebellar memory recall based on rebound currents. J Neurophysiol. 2008;100:2328–2347. doi: 10.1152/jn.00344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widrow B, Stearns SD. Adaptive Signal Processing. Engelwood Cliffs, NJ: Prentice-Hall Inc.; 1985. [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338–347. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hesslow G. Cerebellum and conditioned reflexes. Trends Cogn Sci. 1998;2:322–330. doi: 10.1016/s1364-6613(98)01219-4. [DOI] [PubMed] [Google Scholar]


