Non-technical summary
In the oesophagus the ion channel TRPV4 senses multiple stimuli, including heat and mechanical stimulation. TRPV4 activation causes ATP release from oesophageal cells, which could be important in oesophageal disease mechanisms.
Abstract
Abstract
Gastro-oesophageal reflux disease (GERD) is a multi-factorial disease that may involve oesophageal hypersensitivity to mechanical or heat stimulus as well as acids. Intraganglionic laminar endings (IGLEs) are the most prominent terminal structures of oesophageal vagal mechanosensitive afferents and may modulate mechanotransduction via purinergic receptors. Transient receptor potential channel vanilloid 4 (TRPV4) can detect various stimuli such as warm temperature, stretch and some chemicals, including 4α-phorbol 12,13-didecanoate (4α-PDD) and GSK1016790A. TRPV4 is expressed in many tissues, including renal epithelium, skin keratinocytes and urinary bladder epithelium, but its expression and function in the oesophagus is poorly understood. Here, we show anatomical and functional TRPV4 expression in mouse oesophagus and its involvement in ATP release. TRPV4 mRNA and protein were detected in oesophageal keratinocytes. Several known TRPV4 activators (chemicals, heat and stretch stimulus) increased cytosolic Ca2+ concentrations in cultured WT keratinocytes but not in TRPV4 knockout (KO) cells. Moreover, the TRPV4 agonist GSK1016790A and heat stimulus evoked TRPV4-like current responses in isolated WT keratinocytes, but not in TRPV4KO cells. GSK1016790A and heat stimulus also significantly increased ATP release from WT oesophageal keratinocytes compared to TRPV4KO cells. The vesicle-trafficking inhibitor brefeldin A (BFA) inhibited the ATP release. This ATP release could be mediated by the newly identified vesicle ATP transporter, VNUT, which is expressed by oesophageal keratinocytes at the mRNA and protein levels. In conclusion, in response to heat, chemical and possibly mechanical stimuli, TRPV4 contributes to ATP release in the oesophagus. Thus, TRPV4 could be involved in oesophageal mechano- and heat hypersensitivity.
Introduction
Gastro-oesophageal reflux disease (GERD) is a multi-factorial disease that often impairs health-related quality of life (Flook & Wiklund, 2007). The cardinal symptoms of GERD are heartburn and regurgitation and affect 10–20% of adults in western countries and about 5% of adults in Asia (Dent et al. 2005; Wong & Kinoshita, 2006). Oesophageal acid exposure in GERD patients may be sufficiently severe to cause endoscopically visible mucosal damage (erosive oesophagitis), but many patients have no endoscopically visible oesophageal mucosal damage despite having GERD symptoms and oesophageal acid exposure. This latter condition is termed endoscopy-negative reflux disease (ENRD) and is quite common worldwide (Dent, 2007). Although symptoms for most GERD patients resolve with proton pump inhibitor (PPI) therapy, 19–44% of GERD patients only exhibit partial or no response (van Pinxteren et al. 2001; Donnellan et al. 2005). Oesophageal hypersensitivity and functional heartburn are the most prevalent causes of such PPI-resistant GERD patients (Mainie et al. 2006; Sharma et al. 2008). Many studies demonstrated that this patient population also has increased oesophageal sensitivity to mechanical or heat stimuli (Trimble et al. 1995; Drewes et al. 2006; Reddy et al. 2007).
Intraganglionic laminar endings (IGLEs) (Rodrigo et al. 1975) are derived from nodose ganglion neurons (Neuhuber, 1987; Berthoud & Powley, 1992) and represent the most prominent terminal structures of vagal mechanosensitive afferent fibres throughout the gastrointestinal tract (Berthoud et al. 1997). Immunohistochemical studies identified P2X2 and P2X3 purinergic receptors in the IGLEs (Raab & Neuhuber, 2005), and electrophysiological experiments demonstrated that adenosine triphosphate (ATP) may play a modulatory role in IGLE mechanotransduction processes (Zagorodnyuk et al. 2003). However, the underlying molecular mechanisms of mechano-ATP transduction are poorly understood.
TRPV4 was originally reported as an osmo- or mechano-sensor (Liedtke et al. 2000; Strotmann et al. 2000) that can be activated by diverse chemical stimuli, including the synthetic phorbol ester 4α-phorbol 12,13-didecanoate (4α-PDD), GSK1016790A (Watanabe et al. 2002a; Willette et al. 2008), as well as moderate warmth (>27°C) (Guler et al. 2002; Watanabe et al. 2002b). TRPV4 is widely expressed throughout the body, including renal epithelia, skin keratinocytes, hippocampal neurons, endothelial cells and urinary bladder epithelia, thereby contributing to numerous physiological processes (Shibasaki et al. 2007; Sokabe et al. 2010). We recently showed that TRPV4 was highly expressed in the complex urothelium lining the luminal side of the bladder and that isolated urothelial cells release ATP upon mechanical stimulus that induced TRPV4 activation (Mochizuki et al. 2009). Loss of TRPV4 leads to an increased micturition threshold, a larger bladder capacity and disrupted voiding behaviour, suggesting that normal sensitivity to bladder distension is lost in TRPV4-deficient mice (Gevaert et al. 2007). In contrast, intravesical instillation of GSK1016790A resulted in a decreased micturition threshold and increased voiding frequency, suggesting that excessive TRPV4 activation can induce bladder overactivity (Thorneloe et al. 2008). Furthermore, the TRPV4 antagonist HC-067047 was shown to be a potential treatment for cystitis-induced bladder dysfunction (Everaertsa et al. 2010b). In the last three decades, ATP has become widely recognized as a neurotransmitter in the central nervous system and also in peripheral tissues, including the gastrointestinal tract (Burnstock et al. 1978; Edwards et al. 1992; Galligan & Bertrand, 1994; McConalogue et al. 1996). SLC17A9 (vesicular nucleotide transporter (VNUT)) has been recently identified as a novel vesicular nucleotide transporter, and VNUT is reportedly involved in vesicular storage and ATP exocytosis in taste cells and during T-cell activation (Sawada et al. 2008; Iwatsuki et al. 2009; Tokunaga et al. 2010).
In this study, we investigated the mechanism of ATP release from oesophageal keratinocytes in response to mechanical, heat or chemical stimuli. Our results indicate that ATP release from oesophageal keratinocytes is regulated by TRPV4 channel-dependent Ca2+ influx and mediated by exocytosis with vesicles expressing VNUT.
Methods
Animals
Male C57BL/6NCr mice (6–8 weeks old; SLC) were used for a control. TRPV4-deficient (TRPV4KO) mice (Mizuno et al. 2003) and TRPV3-deficient (TRPV3KO) mice (Moqrich et al. 2005) were both backcrossed on a C57BL/6NCr background. Mice were housed in a controlled environment (12 h light–12 h dark cycle; room temperature, 22–24°C; 50–60% relative humidity) with free access to food and water. All procedures involving the care and use of animals were approved by The Institutional Animal Care and Use Committee of the National Institutes of Natural Sciences and carried out in accordance with the NIH Guide for the care and use of laboratory animals (NIH publication no. 85–23, revised 1985).
Reverse transcription PCR analysis
To examine TRP channel mRNA expression in the mouse oesophagus, total RNA (1 μg) isolated from whole oesophagus and cultured oesophageal keratinocytes was used for reverse transcription using the Superscript III first-strand synthesis system for RT-PCR (Invitrogen). PCR was performed using rTaq DNA polymerase (TaKaRa) in an iCycler (Bio-Rad) with specific primer sets (Supplemental Table S1) for several TRP channels including TRPV2, TRPV3 and TRPV4, the keratinocyte marker cytokeratin 14 (CK14), the skeletal muscle marker myosin heavy chain 2 (MHC2) and vesicular nucleotide transporter (VNUT). PCR conditions used were: 1 cycle at 94°C for 2 min; 40 cycles at 94°C for 10 s; 55°C for 10 s; and 72°C for 30 s; and 1 cycle at 72°C for 2 min.
Immunochemistry
All experiments were repeated on specimens from at least three mice. Antibody information is summarized in Table 1. For section preparation, mice were anaesthetized with diethyl ether and perfused through the heart with 4% paraformaldehyde in PBS. The oesophagi were removed and further fixed at 4°C for 6 h. Tissues were washed in PBS (3 times, 15 min each), placed in PBS–sucrose (PBS containing 20% sucrose) and stored at 4°C overnight. Next, they were embedded in OCT compound (Tissue Tek, Elkhart, IN, USA), and 14-μm-thick sections were collected onto slides and dried at room temperature for 1 h. Preparations were cleared with PBS plus 0.3% Triton X-100 (PBS–T 0.3%) 3 times for 5 min each. Non-specific antibody binding was reduced by incubation in BlockAce (Yukijirushi, Sapporo, Japan) in PBS–T 0.3% for 1 h at room temperature prior to antibody exposure. Preparations were analysed using a fluorescent microscope and a confocal laser scanning microscope (LSM 510, Carl Zeiss).
Table 1.
Characteristics of primary and secondary antisera used for immunochemistry
| Tissue antigen | Host | Dilution | Source (Reference) |
|---|---|---|---|
| CK14 | Rabbit | 1:500 | COVANCE |
| TRPV4 | Rabbit | 1:500 | B. Nilius (Mochizuki et al. 2009) |
| VNUT | Rabbit | 1:500 | (Tokunaga et al. 2010) |
| DAPI | 1:1000 | Dojin Chemical Corp. | |
| Secondary antibodies used for immunochemistry. | |||
| Antibody label | Dilution | Source | |
| Goat anti-rabbit IgG-Alexa488 | 1:1500 | Invitrogen, Inc. | |
| Goat anti-rabbit IgG-Alexa635 | 1:1500 | Invitrogen, Inc. | |
For immunocytochemistry, wild-type (WT) keratinocytes cultured on glass coverslips for 3 days were used. The cells were fixed at 4°C for 20 min with the same fixative. Samples were cleared with PBS–T 0.1% 3 times for 5 min each. PBS–T 0.1% with 3% bovine serum albumin (BSA; Sigma) was used as a blocking solution. Preparations were analysed similarly to the oesophagus sections.
Primary culture of oesophageal keratinocytes
Male C57BL/6NCr mice (6–8 weeks old; SLC), TRPV4KO and TRPV3KO mice were killed by cervical dislocation following light diethyl ether anaesthesia. The entire lengths of oesophagi were placed in cold (4°C) PBS without Ca2+ and Mg2+. The muscle layer was removed using forceps and the remaining inner tissue containing the keratinous layer was incubated at 4°C in 1 ml trypsin solution (Invitrogen) for 5–8 h. The gelatinized submucosal layer and lamina propria mucosae were peeled away and the keratinocytes harvested with a cell scraper (Greiner Bio-one). The resulting cells were plated on glass coverslips, dishes or fibronectin-coated silicone chambers after filtering with a cell strainer (BD Falcon). The cells were incubated in MCDB 153 medium (modified from that described in Sokabe et al. 2010) containing 5 μg ml−1 insulin, 0.4 μg ml−1 hydrocortisone, 14.1 μg ml−1 phosphorylethanolamine, 10 ng ml−1 epidermal growth factor (all from Sigma), 10 μg ml−1 transferrin (Funakoshi, Japan), 40 μg ml−1 bovine pituitary gland extract (Kyokuto, Japan), 25 μg ml−1 gentamicin, 50 U ml−1 penicillin, 50 μg ml−1 streptomycin and 10% fetal bovine serum (FBS). After incubating at 37°C for 24 h, the medium was changed to a medium lacking FBS. Cells were then maintained at 37°C in a humidified atmosphere of 5% CO2 in air. Half of the medium was changed every 2 days.
Ca2+ imaging
Fura-2 fluorescence was measured in primary oesophageal keratinocytes cultured for 2–3 days with a standard bath solution containing 140 mm NaCl, 5 mm KCl, 2 mm MgCl2, 2 mm CaCl2, 10 mm Hepes and 10 mm glucose at pH 7.4 (adjusted with NaOH). The ratios of fluorescence intensities obtained with fura-2 emission at 340 nm and 380 nm are shown. 4α-PDD and GSK1016790A were used as TRPV4 agonists, and a broad TRP channel blocker, ruthenium red (RR), was used as an antagonist (both from Sigma). Ionomycin (Sigma) was used to confirm cell viability. Temperature changes were achieved using an inline-heater (SH-27B and TC-344B, Warner Instruments) with a thermocouple (TA-30, Warner Instruments) used to measure bath temperature (Mandadi et al. 2009). Stretch stimulus was applied with an elastic silicone chamber (STB-CH-04, STREX, Osaka, Japan) and an extension device (modified version of STB150, STREX), which were established for use with primary urothelial cells (Mochizuki et al. 2009). All experiments were performed at room temperature (25°C). F340/F380 was calculated and acquired with an imaging processing system (IP-Lab, Scanalytics Inc., Rockville, MD, USA) and ImageJ software (http://rsb.info.nih.gov/ij/). Changes in ratio (Δ) were calculated by subtracting mean basal from peak values.
Electrophysiology
The standard bath solution for the patch-clamp experiments was the same as that used for the Ca2+ imaging experiments. Pipette solutions for whole-cell recordings contained 140 mm KCl, 5 mm EGTA and 10 mm Hepes, pH 7.4. Primary oesophageal keratinocytes were used within 3 h of isolation. Heat stimulus was applied in the same system used for Ca2+ imaging experiments. Whole-cell recording data were sampled at 10 kHz and filtered at 5 kHz for analysis (Axon 200B amplifier with pClamp software, Molecular Devices, Foster City, CA, USA). Voltage ramp pulses from –100 mV to +100 mV (500 ms) were applied every 6–10 s to generate an I–V curve.
Measurement of ATP release (luciferin–luciferase assay)
The concentration of ATP released from oesophageal keratinocytes cultured in 24-well plates was measured by a luciferin–luciferase assay using an ATP analyser (AF-100, DKK-TOA Co., Tokyo, Japan) and a method slightly modified from that previously described (Dutta et al. 2004). Primary oesophageal keratinocytes cultured in 24-well plates for 4–5 days to full confluence were incubated in Tyrode solution for 1 h at 37°C to measure basal ATP release. The superfusate was collected for measurements of released ATP and replaced with Tyrode solution containing the TRPV4 agonist GSK or an exocytic stimulator, 5-nitro-2-(3-phenylpropylamino)-benzoate (NPPB; Sigma) (Dolovcak et al. 2009). The superfusate was collected after 15 min and the ratio of released ATP (15 min stimulation/control condition) was calculated. For measurement of heat-induced ATP release, the solution was first maintained at room temperature (25°C) for 1 h in Tyrode solution, after which the solution was heated to 38.5°C by addition of preheated solution and incubated for an additional 15 min at 38.5°C. To block ATP vesicle trafficking, cells were pretreated with brefeldin A (10 μm, BFA; Wako, Japan) for 1 h (Tokunaga et al. 2010). An aliquot (500 μl) of superfusate was mixed with 50 μl luciferin–luciferase assay mixture for luminometric ATP measurements.
Data analysis and statistics
Values in Ca2+ imaging, patch-clamp experiments and ATP measurements are presented as means ± SEM from three or more independent experiments. Student's t test or non-parametric Bonferroni-type multiple comparison were used. P < 0.05 was considered statistically significant.
Results
TRPV4 and VNUT mRNAs are expressed in oesophageal keratinocytes
Given the previous findings of TRPV3 and TRPV4 expression in mouse keratinocytes (Mandadi et al. 2009; Sokabe et al. 2010), the mechanosensitivity of TRPV2 (Muraki et al. 2003; Mihara et al. 2010; Shibasaki et al. 2010) and the contribution of VNUT to ATP exocytosis (Sawada et al. 2008), we wanted to first confirm mRNA expression of TRPV2, TRPV3, TRPV4 and VNUT in the oesophagus and cultured oesophageal keratinocytes. mRNA for TRP channels and VNUT together with the control CK14 (a keratinocyte marker) was detected in whole oesophagus and in cultured oesophageal keratinocytes (Fig. 1).
Figure 1. Expression of TRPV4 and VNUT mRNAs in whole oesophagus and cultured oesophageal keratinocytes.
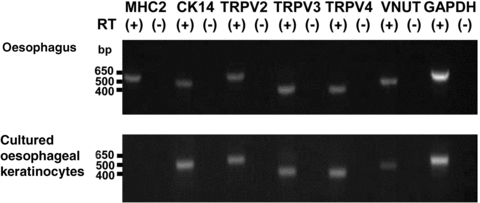
Expression of mRNA for MHC2, CK14, TRPV2, TRPV3, TRPV4, VNUT (vesicular nucleotide transporter) and GAPDH was examined with (+) and without (–) RT reaction. Expected sizes of the amplified fragments were 562, 523, 552, 421, 404, 466 and 545 bp, respectively. TRPV2, TRPV3, TRPV4 and VNUT mRNAs were also detected in cultured oesophageal keratinocytes. MHC2. skeletal muscle marker; CK14, keratinocyte marker.
Expression of TRPV4 protein in oesophageal keratinocytes
To examine TRPV4 expression at a protein level, immunochemistry of oesophageal tissue from mice was performed. TRPV4-like immunoreactive (IR) cells were observed in WT oesophageal keratinous layers with the CK14 keratinocyte marker, while TRPV4-like IR cells were not observed in TRPV4KO oesophagus (Fig. 2). TRPV4-like immunoreactivity appeared homogeneous in keratinous layers.
Figure 2. TRPV4 immunoreactivity in WT and TRPV4KO mouse oesophagus.
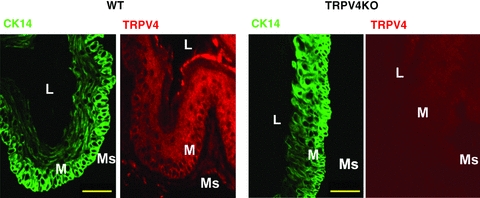
TRPV4 immunoreactivity was observed in WT but not TRPV4KO oesophageal mucosal layers. Scale bars indicate 50 μm. CK14, keratinocyte marker; L, lumen; M, mucosa; Ms, muscle.
TRPV4-mediated cytosolic Ca2+ increase in primary oesophageal keratinocytes
To confirm functional TRPV4 expression in primary oesophageal keratinocytes, we used a fluorescent Ca2+ imaging system (10 μm fura-2 AM) to examine the response to TRPV4 agonists (4α-PDD and GSK1016790A) and physiological stimuli (heat and mechanical stimuli) in primary oesophageal keratinocytes. We first confirmed the cellular purity of cultured oesophageal keratinocytes using CK14 immunoreactivity (Supplemental Fig. S1), which indicated that almost all cells were CK14-IR keratinocytes. Response traces for 4α-PDD with WT and TRPV4KO keratinocytes are shown in Fig. 3A. 4α-PDD and the TRPV4-specific agonist, GSK1016790A, significantly increased intracellular Ca2+ concentrations ([Ca2+]i) in WT keratinocytes, but not in TRPV4KO cells. These increases were also abolished by pretreatment with a broad TRP channel blocker, ruthenium red (RR), or in the absence of extracellular Ca2+ (Ca2+(–)) (Fig. 3A and B). Heat stimulus (25 to 40°C) also increased [Ca2+]i in oesophageal keratinocytes from WT, TRPV4KO and TRPV3KO mice, although the [Ca2+]i increases were significantly smaller for TRPV4KO keratinocytes (Fig. 3C and D). Omitting Ca2+ from the bath solution or pretreating with RR significantly reduced the [Ca2+]i increases, suggesting that heat-induced [Ca2+]i increases were mediated by Ca2+ influx mainly through plasma membrane TRPV4 channels (Fig. 3C and D). On the other hand, heat-induced [Ca2+]i increases were not significantly affected by the loss of TRPV3, another heat-sensitive channel (Fig. 3D) while camphor, a TRPV3 agonist, induced [Ca2+]i increases that were significantly larger in WT oesophageal keratinocytes compared to TRPV3KO cells (Supplemental Fig. S2), suggesting that the contribution of TRPV3 to heat-evoked [Ca2+]i increases could be modest. Another physiological stimulus, mechanical stretch, increased [Ca2+]i, in WT keratinocytes, but not in TRPV4KO cells but only in the presence of extracellular Ca2+ (Fig. 3E and F). These data indicate that TRPV4 is functionally expressed in oesophageal keratinocytes and senses physiological stimuli such as heat and mechanical stretch. In addition, TRPV4 seems to be more predominantly involved in heat-evoked responses than is TRPV3.
Figure 3. TRPV4-mediated cytosolic Ca2+ increase in primary oesophageal keratinocytes.
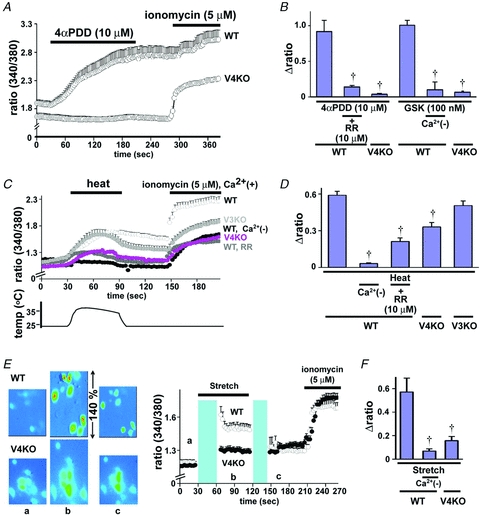
A, traces for [Ca2+]i changes (340/380 nm ratio) in the response to the TRPV4-specific agonist 4α-PDD, in WT and TRPV4KO (V4KO) keratinocytes (mean ± SEM). Bars indicate the duration of chemical application. B, 4α-PDD and another TRPV4-specific agonist GSK1016790A (GSK), increased [Ca2+]i in WT oesophageal keratinocytes, but not in TRPV4KO cells. The increases were significantly inhibited by a broad TRP channel blocker, ruthenium red (RR), or in the absence of extracellular Ca2+ (Ca2+(–)) (†, *P < 0.05 vs. WT). Changes in ratio (Δ) were calculated by subtracting mean basal from peak values. C, traces for [Ca2+]i changes in response to heat stimuli (25 to 40°C) in WT (in the presence or absence of extracellular Ca2+) or TRPV4KO keratinocytes (mean ± SEM). Bars indicate the duration of heat stimuli or a chemical application. D, [Ca2+]i increase was larger in WT than in TRPV4KO keratinocytes and inhibited by RR or in the Ca2+(–) condition (†P < 0.05 vs. WT). [Ca2+]i increase in TRPV3KO keratinocytes was similar to that for WT. E, representative pseudocolour images and traces for [Ca2+]i changes in response to mechanical stimuli (140%) in WT and TRPV4KO keratinocytes cultured on silicon chambers (mean ± SEM). Images were obtained at points a, b and c indicated on the traces. A mechanical stimulus was applied between the blue boxes (black bar). F, [Ca2+]i increase was significantly smaller in TRPV4KO than in WT keratinocytes and was inhibited significantly in the absence of Ca2+ (Ca2+(–), †P < 0.05 vs. WT).
TRPV4-mediated current responses in primary oesophageal keratinocytes
We next performed patch-clamp experiments using acute isolated oesophageal keratinocytes. GSK1016790A (300 nm) evoked current responses with a strongly outwardly rectifying current–voltage (I–V) relationship (Fig. 4A–C) in WT keratinocytes, but not in TRPV4KO cells. This I–V relationship is consistent with a previous report (Willette et al. 2008). In addition, GSK1016790A-induced currents could be blocked by RR (10 μm). Currents at –100 mV were significantly larger in WT keratinocytes than in TRPV4KO cells (Fig. 4C). Heat stimulus (25 to 45°C) also evoked inward currents at –60 mV with an outwardly rectifying I–V relationship (Fig. 4D and E), but the currents were significantly smaller in TRPV4KO keratinocytes compared to WT and TRPV3KO cells (Fig. 4F), which is consistent with the Ca2+ imaging data (Fig. 3C and D).
Figure 4. TRPV4 agonist- and heat-induced current responses in primary oesophageal keratinocytes.
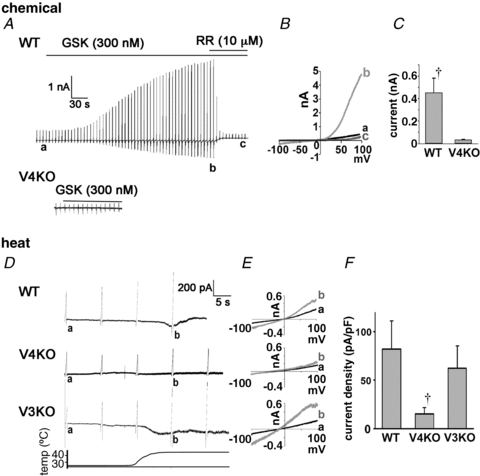
A, GSK1016790A (GSK, 300 nm) evoked current responses in WT, but not in TRPV4KO cells. The currents were blocked by ruthenium red (RR; 10 μm). B, currents in response to ramp pulses at points a, b and c shown in A show a strongly outwardly rectifying I–V relationship. C, significantly larger inward currents at – 100 mV were obtained from WT keratinocytes compared with TRPV4KO cells (†P < 0.05). D, heat stimuli (25 to 45°C) evoked inward currents at –60 mV in WT and TRPV3KO (V3KO) keratinocytes, but not in TRPV4KO cells. E, currents in response to ramp pulses at points a and b shown in D show outwardly rectifying I–V relationship in WT and TRPV3KO cells. F, significantly larger inward currents at −60mV were obtained from WT keratinocytes compared with TRPV4KO (†P < 0.05 vs. WT or TRPV3KO).
VNUT immunoreactivity in oesophageal keratinocytes
We previously showed that urinary bladder epithelium senses mechanical stretch via TRPV4 and releases ATP (Mochizuki et al. 2009). On the other hand, it was reported that T-cells can translate the Ca2+ signals into ATP release via VNUT (Tokunaga et al. 2010) and that ATP acts as a neuromodulator for mechanotransduction in IGLEs via a (P2X2)–P2X3 receptor-mediated pathway, especially in the abdominal portion of the mouse oesophagus (Raab & Neuhuber, 2005; Kestler et al. 2009). These results led us to hypothesize that mechanical stimulus activates TRPV4 expressed in oesophageal keratinocytes, leading to VNUT-dependent exocytotic ATP release, followed by transmission of signals to the CNS via activation of purinergic receptors expressed in IGLEs. To verify this hypothesis, we examined oesophageal VNUT expression. VNUT-like immunoreactivity was observed in both the WT oesophageal keratinous layer (Fig. 5A) and cultured keratinocytes (Fig. 5B). The punctate expression pattern observed in cultured keratinocytes might indicate vesicle clusters.
Figure 5. VNUT immunoreactivity in the oesophagus and cultured keratinocytes.
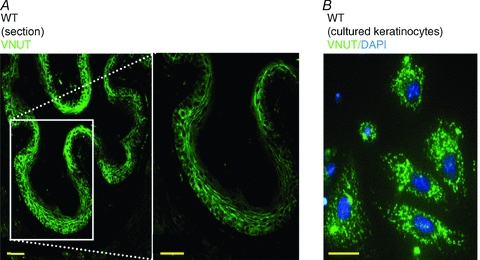
A, VNUT immunoreactivity in WT oesophagus mucosal layer. B, a punctate pattern of VNUT immunoreactivity was detected in cultured keratinocytes. Scale bars indicate 50 μm.
TRPV4 activators increased ATP release from oesophageal keratinocytes via exocytosis
To examine whether TRPV4-mediated [Ca2+]i increases are involved in ATP release from keratinocytes, we measured ATP release in chemical- or heat-stimulated cells from WT, TRPV4KO and TRPV3KO mice using a luciferin–luciferase assay. As the amount of released ATP varied among the agonists/antagonist used, we compared the ratio of stimulated responses to control values (data from WT or TRPV4KO cells kept at 37°C for 15 min). The TRPV4 agonist GSK1016790A robustly increased ATP release from cultured WT oesophageal keratinocytes, but not from TRPV4KO cells (Fig. 6A). The [Ca2+]i increases were abolished in the absence of extracellular Ca2+ or by RR treatment (Fig. 6A), indicating that TRPV4 is essential for GSK1016790A-evoked ATP release. We next examined the effects of heat on ATP release in oesophageal keratinocytes. For this assay, we expressed the results as the actual released ATP amounts because control values were obtained at 25°C instead of 37°C (control). Heat stimulus (from 25 to 38.5°C and kept at 38.5°C for 15 min) also induced ATP release, although the responses were significantly larger in WT keratinocytes than in TRPV4KO cells (Fig. 6B). The heat-evoked ATP releases were indistinguishable between WT and TRPV3KO cells, further confirming the specific involvement of TRPV4 in the heat-evoked response. To confirm that the ATP-releasing ability is not affected by the loss of TRPV4, ATP release was examined in the presence of 5-nitro-2-(3-phenylpropylamino)-benzoate (NPPB), a specific exocytosis activator (Dolovcak et al. 2009). The presence of NPPB caused similar ATP release in both WT and TRPV4KO keratinocytes (Fig. 6C), indicating that TRPV4 keratinocytes have a similar ability to release ATP through an exocytotic mechanism. To demonstrate that ATP release involving TRPV4 is mediated by exocytosis, we examined the effect of a vesicle-trafficking inhibitor, brefeldin A (BFA). Pretreatment with BFA (10 μm) significantly reduced GSK1016790A-induced ATP release from WT keratinocytes (Fig. 6D), supporting the concept that TRPV4 activation induces ATP exocytosis.
Figure 6. TRPV4 activators increase ATP release from oesophageal keratinocytes via exocytosis.
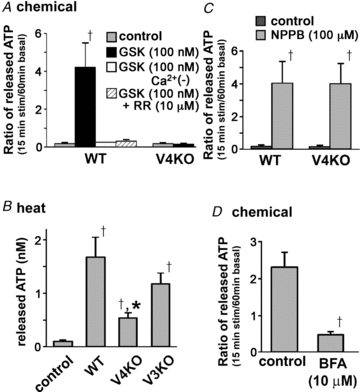
A, the TRPV4 agonist GSK1016790A (GSK) increased ATP release from WT cultured oesophageal keratinocytes, but not from TRPV4KO cells. The increase was abolished in the absence of extracellular Ca2+ or by ruthenium red (RR) treatment in WT cells (†P < 0.05 vs. control). B, heat stimulus (from 25 to 38.5°C and kept at 38.5°C for 15 min) significantly increased ATP release from WT cultured keratinocytes compared with WT cells incubated at 37°C for 15 min (control) (†P < 0.05 vs. control). The increase was significantly smaller in TRPV4KO cells compared with WT or TRPV3KO cells (*P < 0.05). C, an exocytic stimulator, 5-nitro-2-(3-phenylpropylamino)-benzoate (NPPB), induced ATP release from TRPV4KO keratinocytes similar to that from WT cells (†, *P < 0.05 vs. control). D, the vesicle-trafficking inhibitor brefeldin A (BFA, 10 μm) significantly inhibited GSK-induced ATP release (†P < 0.05).
Discussion
We identified anatomical and functional TRPV4 expression in mouse oesophageal keratinocytes (Figs 1–4) and showed that TRPV4 activation by GSK1016790A and heat induced ATP release (Figs 5 and 6). ATP released from keratinocytes may be readily metabolized by ectonucleotidases, which could prevent the released ATP from reaching the IGLEs located in tunica muscularis. However, because even a keratinocyte monolayer released nanomolar levels of ATP (Fig. 6B) and because micromolar ATP release upon heating was estimated for the cultured skin keratinocytes (Mandadi et al. 2009), it follows that the oesophageal keratinous layers could release up to micromolar amounts of ATP, which could reach and activate P2X receptors expressed in IGLEs as they are in intestine (Bertrand & Bornstein, 2002). Alternatively, vagal and spinal mucosal afferents could detect released ATP because they are reported to abut on and even penetrate the epithelium with small branches (Dutsch et al. 1998; Wank & Neuhuber, 2001). Thus, ATP released from oesophageal keratinocytes could act on nerve endings that innervate the oesophagus in different ways. Indeed, P2X3-like immunoreactivity was observed within nerve fibre arborization in rat pharynx mucosa (Wang & Neuhuber, 2003). Other P2X receptors could also be expressed in mucosal afferents and act as receptors for released ATP. Therefore, upon detecting various stimuli, oesophageal keratinocyte TRPV4 could transmit the information to different kinds of neurons via released ATP.
In a human study, slow heat stimulation of the oesophagus evoked vague perceptions of mild hot and burning sensations, which is indicative of C-fibre activation, even below the threshold temperatures for TRPV1 activation (∼43°C) (Olesen et al. 2009), suggesting the existence of thermosensors besides TRPV1. Nodose ‘nociceptive-like’ fibres are exclusively C-fibres that are sensitive to P2X receptor analogues, whereas jugular ‘nociceptive-like’ fibres contain both C- and Aδ-fibres that are insensitive to P2X receptor analogues in guinea-pigs (Yu et al. 2005). These reports suggest that ATP released from keratinocytes might activate P2X receptors expressed in C-fibres that innervate from the nodose ganglion (or dorsal root ganglion). Taken together, TRPV4-mediated ATP release might be involved in vague slow heat-evoked sensations.
Endogenous candidate stimuli for TRPV4 activation are mechanical stimulus, temperature and chemicals. Since TRPV4 is thought to be constitutively active at body temperature, basal TPV4 activation by body temperature could enhance TRPV4 activity induced by mechanical and chemical stimuli (Everaerts et al. 2010a). This concept could be supported by the result showing that GSK1016790A increased ATP release from keratinocytes kept at 37°C (Fig. 6A). TRPV4 can be activated by an endogenously produced 5,6-epoxyeicosatrieonic (5,6-EET) acid, an arachidonic acid (AA) metabolite in endothelial cells (Watanabe et al. 2003). Concentrations of 5,6-EET could be elevated in some conditions in the oesophagus. Moreover, ATP-mediated activation of phospholipase C (PLC) is known to modulate TRPV4 activity by sensitizing TRPV4 to EETs (Fernandes et al. 2008). Thus, ATP released from damaged tissues could enhance TRPV4 activity, which in turn further enhances ATP release. Such positive feedback mechanisms might explain the mechano- and heat hypersensitivity seen in GERD patients that do not have histological changes.
In conclusion, TRPV4 is functionally expressed in mouse oesophageal keratinocytes and functions as a transducer of heat, chemicals and possibly mechanical stimuli, leading to ATP release via exocytosis. Our results suggest that TRPV4 could be a promising novel therapeutic target for oesophageal hypersensitivity in PPI-resistant GERD patients.
Acknowledgments
This work was supported by grants to M.T from the Ministry of Education, Culture, Sports, Science and Technology in Japan. We thank R. Z. Sabirov NIPS (National Institute for Physiological Sciences) for his suggestions for ATP detection, B. Nilius (Katholieke Universiteit, Belgium) for his generous gift of antibodies and T. Sokabe (NIPS) for his technical assistance. The authors declare that no conflict of interest exists.
Glossary
Abbreviations
- 4α-PDD
4α-phorbol 12,13-didecanoate
- BFA
brefeldin A
- CK14
cytokeratin 14
- EET
epoxyeicosatrienoic acids
- ENRD
endoscopy-negative reflux disease
- GERD
gastro-oesophageal reflux disease
- GSK
GSK1016790A, a TRP4 agonist
- IGLEs
intraganglionic laminar endings
- MHC2
myosin heavy chain 2
- NPPB
5-nitro-2-(3-phenylpropylamino)-benzoate
- PPI
proton pump inhibitor
- RR
ruthenium red
- TRPV4
transient receptor potential vanilloid 4
- VNUT
vesicular nucleotide transporter
Author contributions
Conception and design of the experiments: H.M., A.B. and M.T.; data collection: H.M and A.B.; data analysis and interpretation: all authors; drafting manuscript: H.M. and M.T. All authors approved the final version.
Supplementary material
Supplemental Table 1
Supplemental Figure 1
Supplemental Figure 2
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Berthoud HR, Patterson LM, Neumann F, Neuhuber WL. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol (Berl) 1997;195:183–191. doi: 10.1007/s004290050037. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Powley TL. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol. 1992;319:261–276. doi: 10.1002/cne.903190206. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Bornstein JC. ATP as a putative sensory mediator: activation of intrinsic sensory neurons of the myenteric plexus via P2X receptors. J Neurosci. 2002;22:4767–4775. doi: 10.1523/JNEUROSCI.22-12-04767.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Cocks T, Kasakov L, Wong HK. Direct evidence for ATP release from non-adrenergic, non-cholinergic (‘purinergic’) nerves in the guinea-pig taenia coli and bladder. Eur J Pharmacol. 1978;49:145–149. doi: 10.1016/0014-2999(78)90070-5. [DOI] [PubMed] [Google Scholar]
- Dent J. Microscopic esophageal mucosal injury in nonerosive reflux disease. Clin Gastroenterol Hepatol. 2007;5:4–16. doi: 10.1016/j.cgh.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710–717. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolovcak S, Waldrop SL, Fitz JG, Kilic G. 5-Nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) stimulates cellular ATP release through exocytosis of ATP-enriched vesicles. J Biol Chem. 2009;284:33894–33903. doi: 10.1074/jbc.M109.046193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan C, Sharma N, Preston C, Moayyedi P. Medical treatments for the maintenance therapy of reflux oesophagitis and endoscopic negative reflux disease. Cochrane Database Syst. 2005:CD003245. doi: 10.1002/14651858.CD003245.pub2. [DOI] [PubMed] [Google Scholar]
- Drewes AM, Reddy H, Pedersen J, Funch-Jensen P, Gregersen H, Arendt-Nielsen L. Multimodal pain stimulations in patients with grade B oesophagitis. Gut. 2006;55:926–932. doi: 10.1136/gut.2005.067769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutsch M, Eichhorn U, Worl J, Wank M, Berthoud HR, Neuhuber WL. Vagal and spinal afferent innervation of the rat esophagus: a combined retrograde tracing and immunocytochemical study with special emphasis on calcium-binding proteins. J Comp Neurol. 1998;398:289–307. [PubMed] [Google Scholar]
- Dutta AK, Sabirov RZ, Uramoto H, Okada Y. Role of ATP-conductive anion channel in ATP release from neonatal rat cardiomyocytes in ischaemic or hypoxic conditions. J Physiol. 2004;559:799–812. doi: 10.1113/jphysiol.2004.069245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- Everaerts W, Nilius B, Owsianik G. The vanilloid transient receptor potential channel TRPV4: From structure to disease. Prog Biophys Mol Biol. 2010a;103:2–17. doi: 10.1016/j.pbiomolbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, et al. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci U S A. 2010b;107:19084–19089. doi: 10.1073/pnas.1005333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes J, Lorenzo IM, Andrade YN, Garcia-Elias A, Serra SA, Fernandez-Fernandez JM, Valverde MA. IP3 sensitizes TRPV4 channel to the mechano- and osmotransducing messenger 5′-6′-epoxyeicosatrienoic acid. J Cell Biol. 2008;181:143–155. doi: 10.1083/jcb.200712058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flook NW, Wiklund I. Accounting for the effect of GERD symptoms on patients' health-related quality of life: supporting optimal disease management by primary care physicians. Int J Clin Pract. 2007;61:2071–2078. doi: 10.1111/j.1742-1241.2007.01586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galligan JJ, Bertrand PP. ATP mediates fast synaptic potentials in enteric neurons. J Neurosci. 1994;14:7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, et al. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest. 2007;117:3453–3462. doi: 10.1172/JCI31766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–6414. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki K, Ichikawa R, Hiasa M, Moriyama Y, Torii K, Uneyama H. Identification of the vesicular nucleotide transporter (VNUT) in taste cells. Biochem Biophys Res Commun. 2009;388:1–5. doi: 10.1016/j.bbrc.2009.07.069. [DOI] [PubMed] [Google Scholar]
- Kestler C, Neuhuber WL, Raab M. Distribution of P2X3 receptor immunoreactivity in myenteric ganglia of the mouse esophagus. Histochem Cell Biol. 2009;131:13–27. doi: 10.1007/s00418-008-0498-4. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainie I, Tutuian R, Shay S, Vela M, Zhang X, Sifrim D, Castell DO. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut. 2006;55:1398–1402. doi: 10.1136/gut.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConalogue K, Todorov L, Furness JB, Westfall DP. Direct measurement of the release of ATP and its major metabolites from the nerve fibres of the guinea-pig taenia coli. Clin Exp Pharmacol Physiol. 1996;23:807–812. doi: 10.1111/j.1440-1681.1996.tb01184.x. [DOI] [PubMed] [Google Scholar]
- Mandadi S, Sokabe T, Shibasaki K, Katanosaka K, Mizuno A, Moqrich A, et al. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch. 2009;458:1093–1102. doi: 10.1007/s00424-009-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara H, Boudaka A, Shibasaki K, Yamanaka A, Sugiyama T, Tominaga M. Involvement of TRPV2 activation in intestinal movement through NO production in mice. J Neurosci. 2010;30:16536–16544. doi: 10.1523/JNEUROSCI.4426-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno A, Matsumoto N, Imai M, Suzuki M. Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol. 2003;285:C96–C101. doi: 10.1152/ajpcell.00559.2002. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Sokabe T, Araki I, Fujishita K, Shibasaki K, Uchida K, et al. The TRPV4 cation channel mediates stretch-evoked Ca2 +influx and ATP release in primary urothelial cell cultures. J Biol Chem. 2009;284:21 257–21 264. doi: 10.1074/jbc.M109.020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ Res. 2003;93:829–838. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- Neuhuber WL. Sensory vagal innervation of the rat esophagus and cardia: a light and electron microscopic anterograde tracing study. J Auton Nerv Syst. 1987;20:243–255. doi: 10.1016/0165-1838(87)90153-6. [DOI] [PubMed] [Google Scholar]
- Olesen SS, Olesen AE, Gravesen F, Poulsen JL, Funch-Jensen P, Gregersen H, Drewes AM. An endoscopic method for thermal and chemical stimulation of the human oesophagus. Neurogastroenterol Motil. 2009;21:1250–e116. doi: 10.1111/j.1365-2982.2009.01362.x. [DOI] [PubMed] [Google Scholar]
- Raab M, Neuhuber WL. Number and distribution of intraganglionic laminar endings in the mouse esophagus as demonstrated with two different immunohistochemical markers. J Histochem Cytochem. 2005;53:1023–1031. doi: 10.1369/jhc.4A6582.2005. [DOI] [PubMed] [Google Scholar]
- Reddy H, Staahl C, Arendt-Nielsen L, Gregersen H, Drewes AM, Funch-Jensen P. Sensory and biomechanical properties of the esophagus in non-erosive reflux disease. Scand J Gastroenterol. 2007;42:432–440. doi: 10.1080/00365520600973099. [DOI] [PubMed] [Google Scholar]
- Rodrigo J, Hernandez J, Vidal MA, Pedrosa JA. Vegetative innervation of the esophagus. II. Intraganglionic laminar endings. Acta Anat (Basel) 1975;92:79–100. doi: 10.1159/000144431. [DOI] [PubMed] [Google Scholar]
- Sawada K, Echigo N, Juge N, Miyaji T, Otsuka M, Omote H, et al. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci U S A. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Agrawal A, Freeman J, Vela MF, Castell D. An analysis of persistent symptoms in acid-suppressed patients undergoing impedance-pH monitoring. Clin Gastroenterol Hepatol. 2008;6:521–524. doi: 10.1016/j.cgh.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Shibasaki K, Murayama N, Ono K, Ishizaki Y, Tominaga M. TRPV2 enhances axon outgrowth through its activation by membrane stretch in developing sensory and motor neurons. J Neurosci. 2010;30:4601–4612. doi: 10.1523/JNEUROSCI.5830-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki K, Suzuki M, Mizuno A, Tominaga M. Effects of body temperature on neural activity in the hippocampus: regulation of resting membrane potentials by transient receptor potential vanilloid 4. J Neurosci. 2007;27:1566–1575. doi: 10.1523/JNEUROSCI.4284-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokabe T, Fukumi-Tominaga T, Yonemura S, Mizuno A, Tominaga M. The TRPV4 channel contributes to intercellular junction formation in keratinocytes. J Biol Chem. 2010;285:18749–18758. doi: 10.1074/jbc.M110.103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, et al. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J Pharmacol Exp Ther. 2008;326:432–442. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- Tokunaga A, Tsukimoto M, Harada H, Moriyama Y, Kojima S. Involvement of SLC17A9-dependent vesicular exocytosis in the mechanism of ATP release during T cell activation. J Biol Chem. 2010;285:17406–17416. doi: 10.1074/jbc.M110.112417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble KC, Pryde A, Heading RC. Lowered oesophageal sensory thresholds in patients with symptomatic but not excess gastro-oesophageal reflux: evidence for a spectrum of visceral sensitivity in GORD. Gut. 1995;37:7–12. doi: 10.1136/gut.37.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pinxteren B, Numans M, Bonis PA, Lau J. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2001;4:CD002095. doi: 10.1002/14651858.CD002095. [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Neuhuber WL. Intraganglionic laminar endings in the rat esophagus contain purinergic P2X2 and P2X3 receptor immunoreactivity. Anat Embryol (Berl) 2003;207:363–371. doi: 10.1007/s00429-003-0351-4. [DOI] [PubMed] [Google Scholar]
- Wank M, Neuhuber WL. Local differences in vagal afferent innervation of the rat esophagus are reflected by neurochemical differences at the level of the sensory ganglia and by different brainstem projections. J Comp Neurol. 2001;435:41–59. doi: 10.1002/cne.1192. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002a;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002b;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, et al. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse. Part 2. J Pharmacol Exp Ther. 2008;326:443–452. doi: 10.1124/jpet.107.134551. [DOI] [PubMed] [Google Scholar]
- Wong BC, Kinoshita Y. Systematic review on epidemiology of gastroesophageal reflux disease in Asia. Clin Gastroenterol Hepatol. 2006;4:398–407. doi: 10.1016/j.cgh.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Yu S, Undem BJ, Kollarik M. Vagal afferent nerves with nociceptive properties in guinea-pig oesophagus. J Physiol. 2005;563:831–842. doi: 10.1113/jphysiol.2004.079574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Costa M, Brookes SJ. Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea-pig oesophagus. J Physiol. 2003;553:575–587. doi: 10.1113/jphysiol.2003.051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


