Non-technical summary
During a sustained maximal contraction, motoneurones rapidly become less responsive to input. This decrease is ameliorated by a high level of voluntary drive from the cortex. Here, we tested whether the excitability of motor cortical neurones or spinal motoneurones is suppressed during fatigue produced by a sustained submaximal contraction in which only some of the motoneurones are active. We found that motoneurone responses were gradually suppressed as fatigue developed. The results suggest that only the active motoneurones were affected and the decreased responsiveness was less when voluntary drive was present. In contrast, the excitability of the motor cortex appeared unaffected.
Abstract
Abstract
During fatigue caused by a sustained maximal voluntary contraction (MVC), motoneurones become markedly less responsive when tested during the silent period following transcranial magnetic stimulation (TMS). To determine whether this reduction depends on the repetitive activation of the motoneurones, responses to TMS (motor evoked potentials, MEPs) and to cervicomedullary stimulation (cervicomedullary motor evoked potentials, CMEPs) were tested during a sustained submaximal contraction at a constant level of electromyographic activity (EMG). In such a contraction, some motoneurones are repetitively activated whereas others are not active. On four visits, eight subjects performed a 10 min maintained-EMG elbow flexor contraction of 25% maximum. Test stimuli were delivered with and without conditioning by TMS given 100 ms prior. Test responses were MEPs or CMEPs (two visits each, small responses evoked by weak stimuli on one visit and large responses on the other). During the sustained contraction, unconditioned CMEPs decreased ∼20% whereas conditioned CMEPs decreased ∼75 and 30% with weak and strong stimuli, respectively. Conditioned MEPs were reduced to the same extent as CMEPs of the same size. The data reveal a novel decrease in motoneurone excitability during a submaximal contraction if EMG is maintained. Further, the much greater reduction of conditioned than unconditioned CMEPs shows the critical influence of voluntary drive on motoneurone responsiveness. Strong test stimuli attenuate the reduction of conditioned CMEPs which indicates that low-threshold motoneurones active in the contraction are most affected. The equivalent reduction of conditioned MEPs and CMEPs suggests that, similar to findings with a sustained MVC, impaired motoneurone responsiveness rather than intracortical inhibition is responsible for the fatigue-related impairment of the MEP during a sustained submaximal contraction.
Introduction
A significant portion of force loss during fatiguing exercise is due to a failure within the central nervous system (Gandevia 2001). Fatigue-related changes occur at both motor cortical and motoneuronal levels. Changes at a cortical level can be demonstrated with transcranial magnetic stimulation (TMS) of the motor cortex. The electrical response to TMS recorded at the muscle is termed the motor evoked potential (MEP) and the size of this response increases during sustained maximal or submaximal contractions (e.g. Gandevia et al. 1996; Taylor et al. 1996). If TMS is delivered during a voluntary contraction, the MEP is followed by a period of silence in surface electromyographic activity (EMG) recorded at the muscle. The duration of this silent period increases with the development of fatigue (e.g. Gandevia et al. 1996; McKay et al. 1996; Taylor et al. 1996; Benwell et al. 2007). Fatigue-related changes at the spinal motoneurones can be demonstrated with subcortical stimulation of the corticospinal tract at the level of the cervicomedullary junction (e.g. Gandevia et al. 1999). This stimulus evokes a single descending corticospinal volley (Berardelli et al. 1991; Ugawa et al. 1991) and an EMG response termed the cervicomedullary motor evoked potential (CMEP). Collision experiments have shown that MEPs and CMEPs travel largely in the same corticospinal axons (Ugawa et al. 1991; Gandevia et al. 1999; Taylor et al. 2002) and so comparison of the responses can delineate cortical versus spinal contributions to central fatigue. Changes at a motoneuronal level during fatigue include a decrease of CMEP size during the last quarter of a sustained 2 min maximal voluntary contraction (MVC; Butler et al. 2003; Martin et al. 2006b) and a gradual increase of CMEP size during a sustained submaximal contraction (Levenez et al. 2008; Hoffman et al. 2009). If torque is maintained during a sustained submaximal effort, voluntary EMG increases progressively, which suggests descending drive increases to counteract fatigue of the exercising muscle fibres (e.g. Lippold et al. 1960; Bigland-Ritchie et al. 1986; Fuglevand et al. 1993). It is likely that the increased excitatory drive to the motoneurones accounts for the increase in the CMEP in such contractions (Levenez et al. 2008).
We recently used single and paired motor cortical stimuli (a test stimulus in isolation versus 100 ms after a conditioning stimulus) to investigate the effect of fatigue on a MEP recorded in the silent period (conditioned) relative to an MEP recorded with voluntary drive intact (unconditioned; McNeil et al. 2009). In unfatigued muscle, the conditioned MEP is typically smaller than the unconditioned MEP and this phenomenon is referred to as long-interval intracortical inhibition (Valls-Sole et al. 1992). During a sustained 2 min MVC, the conditioned MEP in the silent period is rapidly and completely suppressed, whereas the unconditioned MEP in the presence of voluntary drive increases in size (McNeil et al. 2009). We first interpreted these results as evidence of a profound fatigue-related increase in long-interval intracortical inhibition. However, substitution of the second motor cortical stimulus with a cervicomedullary stimulus led to an equally rapid and profound suppression of the conditioned CMEP. This equivalence indicates that the marked decrease of the conditioned MEP was largely mediated at the spinal cord. The rapidity and completeness of the CMEP suppression during sustained maximal effort precluded the observation of the hypothesised increase in intracortical inhibition during fatigue (McNeil et al. 2009).
Thus, one aim of the present study was to assess the effect of fatigue on conditioned MEPs and CMEPs during a sustained submaximal contraction designed to produce a gradual increase in long-interval inhibition. The moderate intensity contraction also represented physiological conditions which were closer than our previous work to the fatigue produced by repeated submaximal contractions during everyday activities. We hypothesised that while spinal mechanisms would contribute to a progressive reduction of the conditioned CMEP, inhibitory mechanisms within the motor cortex would cause a faster and greater suppression of the conditioned MEP compared to CMEP. The other aim was to determine the effect of fatigue on unconditioned CMEPs (i.e. to assess motoneurone excitability) when EMG, rather than torque, was held constant during a sustained submaximal contraction. Under these conditions, in which motoneurone output remains consistent, we hypothesised that unconditioned CMEP size would gradually decrease rather than increase.
Methods
Subjects
Eight healthy subjects (36 ± 11 years, mean ± SD; 2 female) participated in four protocols performed on separate days in a pseudo-random order. All studies were approved by the institutional ethics committee and conformed to the Declaration of Helsinki. Written consent was obtained from each of the participants.
Experimental set-up
Subjects were seated with their right arm positioned in an isometric myograph and an angle of ∼90 deg flexion at both the shoulder and elbow joints. The forearm was supinated and a strap at the wrist firmly secured the arm to the myograph. Elbow flexor torque was measured with a linear strain gauge (Xtran, Melbourne, Australia). EMGs of biceps brachii and brachioradialis were recorded via adhesive Ag–AgCl electrodes (10 mm diameter) arranged in a monopolar fashion. The recording electrodes were positioned on the belly of each muscle and the reference electrode over the distal tendon for biceps and ∼5 cm distal to the recording electrode for brachioradialis.
In all experiments, torque and EMG data were recorded to computer using a 12-bit A/D converter (CED 1401 Plus; Cambridge Electronic Design Ltd, Cambridge, UK) in conjunction with Spike2 software (v. 6.06; Cambridge Electronic Design). The torque and EMG data were sampled at 1000 and 2000 Hz, respectively. EMG data were amplified (×100) and bandpass filtered (16–1000 Hz) using CED 1902 amplifiers (Cambridge Electronic Design). EMG of the biceps was also rectified and integrated using a 100 ms time constant (Neurolog System NL124A module; Digitimer, Welwyn Garden City, UK) and displayed on an oscilloscope for subject feedback.
Brachial plexus stimulation
A constant current stimulator (DS7AH, Digitimer) was used to deliver single electrical stimuli (100 μs pulse width; 400 V) to the brachial plexus at Erb's point to record the maximal compound muscle action potential (Mmax) in biceps. The cathode and anode were placed in the supraclavicular fossa and over the acromion, respectively. Stimulus intensity was set to 150% of the current required to produce a maximal response (50–210 mA).
Transcranial magnetic stimulation
Stimulation of the motor cortex was delivered over the vertex using a circular coil (13.5 cm outside diameter) attached via a BiStim unit to two Magstim 200 stimulators (Magstim, Dyfed, UK). One stimulator delivered the conditioning stimulus and the other delivered the test stimulus. Three different patterns of stimulation were used: a single conditioning stimulus, a single test stimulus (producing what is referred to as the unconditioned response), and paired-stimuli in which the conditioning stimulus preceded the test stimulus by 100 ms (producing what is referred to as the conditioned response). This interstimulus interval was chosen because motoneurone excitability is fully recovered 100 ms after conditioning TMS, i.e. the effect of conditioning TMS at this time is a withdrawal of voluntary drive rather than a spinal inhibition (Fuhr et al. 1991; Roick et al. 1993; Uncini et al. 1993; Ziemann et al. 1993). The intensity of the conditioning cortical stimulus (85–100% stimulator output) was set to produce a silent period of ∼200 ms during an effort to a target EMG of 25% MVC. The test stimulus intensity (55–100% stimulator output) was set to evoke a conditioned MEP of either ∼15 or 50%Mmax during brief control contractions at 25% MVC.
Cervicomedullary stimulation
In two of the four protocols, the TMS test stimulus was replaced by stimulation of the corticospinal tract at the cervicomedullary level. A high-voltage electrical current (100 μs duration, Digitimer DS7AH) was passed between adhesive Ag–AgCl electrodes fixed to the skin over the mastoid processes (Ugawa et al. 1991; Gandevia et al. 1999). The stimulation intensity (195–325 mA) was set to evoke a conditioned CMEP of either ∼15 or 50%Mmax during brief control contractions at 25% MVC.
Experimental procedures
Protocol A
MEPs were evoked by paired stimuli or a single test stimulus during a sustained 10 min contraction in which the level of EMG was maintained at that produced by the fresh muscle at 25% MVC. Data collection began with determination of Mmax in the relaxed biceps. Stimulus current was increased incrementally for successive stimuli until the amplitude of the compound muscle action potential reached a plateau. Intensity was then increased to 150% of the current required to evoke Mmax and a further three stimuli were delivered. Next, resting motor threshold of biceps was determined using single magnetic stimuli. Resting motor threshold was taken as the minimum stimulator output required to yield MEPs of 50 μV in 5 of 10 stimuli delivered at 5 s intervals. Two or three brief MVCs of the elbow flexors were performed to establish peak torque. Strong verbal encouragement and visual feedback of elbow flexion torque were provided during each effort. Peak torque was used to set a target torque of 25% MVC. Using visual feedback, subjects performed a contraction of the elbow flexors to this target torque for 5–10 s. During this contraction, an investigator monitored the biceps integrated EMG signal on an oscilloscope and used a cursor to mark the level of EMG produced during the 25% MVC effort. Subjects used this EMG-based target on the oscilloscope for the remainder of the experiment. This EMG target is subsequently referred to as 25% MVC. Intensity of the conditioning stimulus was established as described above.
Two Mmax responses were recorded during brief 25% MVC contractions and the mean amplitude was used to estimate the target size of the conditioned MEP, i.e. 15%Mmax. To evoke this size of conditioned MEP, paired stimuli were delivered during brief 25% MVC efforts with varying test stimulus intensities. When the desired conditioned MEP size was obtained (actual amplitude of 17.8 ± 3.7%Mmax), this test stimulus intensity (124.2 ± 24.5% resting motor threshold) was used for the remainder of the experiment. Pre-fatigue (control) values for unconditioned MEP, conditioned MEP and Mmax responses were obtained during 25% MVC efforts of ∼12 s. Subjects slowly increased biceps EMG output to the target and a stimulation sequence was initiated ∼1 s after the target was attained. This sequence involved a single test stimulus to the cortex (unconditioned MEP), paired cortical stimuli (conditioned MEP) and an Erb's point stimulus (Mmax) delivered at 5 s intervals (Fig. 1). Three of these ‘control’ contractions were performed and at least 1 min of rest separated each contraction.
Figure 1. Schematic diagram of the experimental protocol.
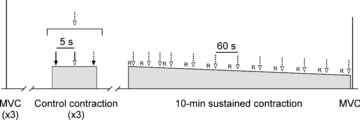
Three brief control contractions were performed at the level of EMG produced at 25% MVC. During each control contraction, a stimulation sequence was delivered (dotted arrow, open arrowhead) which included a single test stimulus (continuous arrow, filled arrowhead), paired conditioning–test stimuli (continuous arrow, open arrowhead), and a brachial plexus stimulus (dotted arrow, filled arrowhead). This stimulation sequence was also delivered during the sustained 10 min contraction. Ratings of perceived effort and muscle pain (R) were collected during the during the sustained contraction. To assess the fatigue induced by the sustained contraction, a brief MVC was performed ∼5 s afterward.
The fatigue protocol was a sustained 10 min contraction in which biceps EMG was held at the level produced at a torque of 25% MVC. Visual feedback of biceps integrated EMG was provided throughout the contraction and subjects were routinely reminded to maintain their feedback signal as near as possible to the target cursor. The same stimulation sequence used during the control contractions was delivered throughout the fatiguing contraction. It was delivered twice in each of the first two minutes and then once a minute thereafter (Fig. 1). About 10 s prior to each stimulation sequence (except the first sequence of the second minute), subjects were asked to provide a rating of their perceived effort and their perceived pain in the elbow flexors using an 11-point Borg scale (Borg 1990). A brief MVC was performed 5 s after the fatiguing contraction to quantify the impairment to maximal torque. Raw traces showing the conditioned MEPs before and during the 10 min contraction are displayed for a single subject in Fig. 2A (left panel).
Figure 2. Individual traces of biceps EMG recorded from single subjects using weak (A) or strong (B) test stimuli.
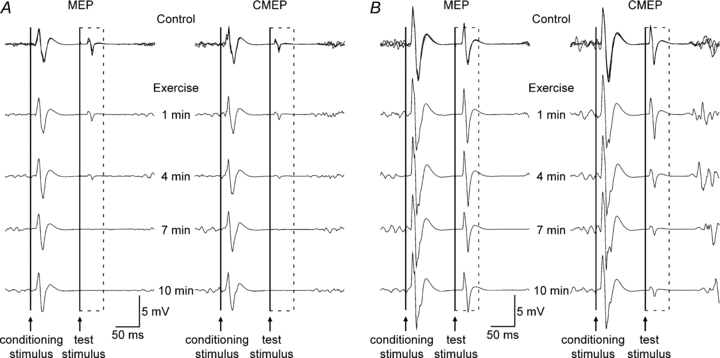
Data in panels A and B were collected from different subjects. Responses obtained during the three brief control contractions with paired conditioning–test stimulation are overlaid. The time course of stimulation during the 10 min contraction is indicated between the two sets of traces. The dashed box surrounds the conditioned test MEPs (left) and CMEPs (right) evoked in the silent period following the conditioning TMS stimulus. The continuous vertical lines indicate the timing of the conditioning and test stimuli. A, using weak test stimuli, conditioned MEPs and CMEPs decreased in parallel. B, using strong test stimuli, the conditioned CMEPs decreased more than conditioned MEPs but this did not occur in all subjects (see Fig. 5). The suppression of both conditioned MEPs and CMEPs was less with strong than weak test stimuli.
Protocol B
To differentiate cortical and spinal contributions to the changes in MEPs observed in protocol A, in protocol B electrical cervicomedullary stimulation replaced TMS as the test stimulus. Subjects completed control and fatigue procedures as in protocol A with the exception that resting motor threshold for TMS was not determined. Subjects were familiarised to cervicomedullary stimulation with a small number of stimuli in a relaxed muscle just prior to the attempt to match conditioned CMEP amplitude to ∼15%Mmax (actual amplitude of 17.1 ± 4.2%Mmax). The right panel of Fig. 2A shows data from a single subject for the conditioned CMEPs before and during the 10 min contraction.
Protocols C and D
These experiments were conducted to assess if the fatigue-related changes to the MEP and CMEP observed in protocols A and B were affected by the size of the conditioned potentials in the control state. Procedures were identical to protocols A and B except that the test stimulus intensities were increased to evoke conditioned MEP and CMEPs with amplitudes of ∼50%Mmax during control contractions. The test stimulus intensity for the MEP was 155.8 ± 43.0% resting motor threshold and actual amplitudes were 46.2 ± 4.7 and 46.8 ± 6.4%Mmax for the conditioned MEP and CMEP, respectively. Raw traces showing the conditioned MEPs (left panel) and CMEPs (right panel) before and during the 10 min contraction are displayed for a single subject in Fig. 2B. A conditioned CMEP of ∼50%Mmax could not be obtained in one subject and thus protocol D has only seven subjects.
Data analysis and statistics
During off-line analysis, Signal software (v. 3.05; Cambridge Electronic Design) was used to determine all measures. Mean torque and voluntary EMG were calculated over 100 ms (in the interval 250 to 150 ms prior to the test stimulus). Non-fatigued MVC torque was calculated as the peak value of the brief contractions. Duration of the silent period was taken as the time from conditioning stimulus to the return of voluntary EMG (as determined by visual inspection). The areas of Mmax, MEPs and CMEPs were measured between cursors marking the initial deflection from the baseline to the second crossing of the horizontal axis (Martin et al. 2006a). Long-interval inhibition was calculated as the ratio (expressed as a percentage) between the area of a conditioned response and the area of the preceding unconditioned response (conditioned/unconditioned × 100). The control level of inhibition was obtained using the mean values of the conditioned and unconditioned responses. To aid comparison with other studies, we also report control values of Mmax, MEPs, CMEPs and long-interval inhibition as amplitudes. However, because the area of Mmax is preferred to amplitude for studies of fatigue, all measures during fatigue are based on area.
Repeated measures ANOVAs were used to compare non-fatigued MVC, post-fatigue MVC, Mmax and silent period data across protocols A–D (SPSS version 17; SPSS Inc., Chicago, IL, USA). Separately for weak and strong stimulus intensities, Student's t test for paired-samples was used to compare MEP and CMEP size and long-interval inhibition during control contractions. Two-way repeated measures ANOVAs, with time as one factor and protocol as the other, were used to compare unconditioned and conditioned responses and the inhibition ratio (each normalised to the control value) across protocols of the same sized conditioned potentials. When no differences existed between protocols, data were pooled and results of paired-samples t tests were compared to a two-tailed Dunnett's table to control for multiple comparisons and determine time points which differed from the control vaue. If the main effect for protocol or the protocol × time interaction was significant, a one-way repeated measures ANOVA was used for each protocol to assess a main effect for time. If this main effect was significant, paired-samples t tests and a Dunnett's table were used as above to determine which time points were different from the control value. Perceived effort and muscle pain were compared across all protocols using two-way repeated measures ANOVAs. All data are reported in the text as the mean ± SD. The significance level was P < 0.05.
Results
During a 10 min sustained submaximal contraction of maintained EMG (25% MVC), perceived effort increases progressively despite a decline in torque. CMEP size decreases moderately with fatigue and markedly so when tested in the transient absence of voluntary drive (silent period). There is an equivalent reduction in the size of the CMEP and MEP during the silent period throughout the sustained effort, which argues against a fatigue-related increase in intracortical inhibition. High-intensity stimulation greatly attenuates the reduction of the CMEP (and MEP) in the silent period, which suggests differential changes across the motoneurone pool.
Control measures prior to fatigue
MVC torque (P = 0.539), Mmax area (P = 0.118), and silent period duration (P = 0.667) were similar across the four protocols. Group means were 70.4 ± 17.2 N m, 0.127 ± 0.027 mV s, and 199.3 ± 24.2 ms, respectively. The amplitudes and areas of Mmax, MEPs and CMEPs, as well as the control levels of long-interval inhibition for MEPs and CMEPs are reported for each protocol in Table 1. In brief, as intended, conditioned MEP and CMEP amplitude was well matched at low (P = 0.643) and high test stimulus intensities (P = 0.843). The area of the potentials was less well matched than amplitude but MEPs and CMEPs were still equivalent at both low (P = 0.227) and high intensities (P = 0.061). Unconditioned MEP area was significantly greater than CMEP area at low (P = 0.001) and high test stimulus intensity (P = 0.014). The control level of long-interval inhibition was similar across protocols at high intensity (P = 0.586) but nearly greater for the MEP than CMEP at low intensity (P = 0.051). The large difference between the MEP and CMEP ratios at low intensity (22.4 ± 6.8 and 67.5 ± 57.1, respectively) failed to achieve statistical significance because of a large standard deviation in the CMEP ratio caused by two subjects who had conditioned potentials that were facilitated, rather than inhibited, compared to unconditioned responses (192 and 113%).
Table 1.
Control potentials and long-interval inhibition during submaximal maintained-EMG contractions prior to fatigue
| Mmax | Conditioned potentials | Unconditioned potentials | Cond/Uncond ratio | |||||
|---|---|---|---|---|---|---|---|---|
| Amplitude (mV) | Area (mV s) | Amplitude (mV) | Area (mV s) | Amplitude (mV) | Area (mV s) | Amplitude (%) | Area (%) | |
| Weak | ||||||||
| MEP | 23.6 ± 5.5 | 0.123 ± 0.026 | 4.3 ± 1.4 | 0.019 ± 0.007 | 13.9 ± 5.0* | 0.086 ± 0.029* | 32.1 ± 11.3 | 22.4 ± 6.8 |
| CMEP | 24.3 ± 5.2 | 0.129 ± 0.027 | 4.2 ± 1.3 | 0.017 ± 0.006 | 7.8 ± 4.0 | 0.035 ± 0.020 | 68.4 ± 48.4 | 67.5 ± 57.1 |
| P = 0.379 | P = 0.093 | P = 0.643 | P = 0.227 | P = 0.007 | P = 0.001 | P = 0.071 | P = 0.051 | |
| Strong | ||||||||
| MEP | 24.3 ± 6.3 | 0.129 ± 0.030 | 11.2 ± 2.8 | 0.063 ± 0.020 | 15.8 ± 4.8 | 0.101 ± 0.029* | 71.9 ± 8.5 | 62.8 ± 14.8 |
| CMEP | 24.6 ± 5.6 | 0.128 ± 0.031 | 11.4 ± 2.7 | 0.049 ± 0.015 | 15.2 ± 6.4 | 0.080 ± 0.040 | 81.1 ± 22.0 | 69.9 ± 26.2 |
| P = 0.465 | P = 0.182 | P = 0.843 | P = 0.061 | P = 0.309 | P = 0.014 | P = 0.252 | P = 0.586 | |
Data are mean values (± SD) of the four protocols. Long-interval inhibition was calculated for each subject as the ratio (expressed as a percentage) between the mean area of conditioned responses and the mean area of unconditioned responses. Asterisks (*) denote a difference between the weak MEP and CMEP or the strong MEP and CMEP (P values < 0.05 are indicated by bold text).
Measures of fatigue across all protocols
Subjects successfully maintained the integrated EMG target as biceps RMS EMG was unchanged by exercise (P = 0.744) for all protocols (P = 0.825; Fig. 3A). Brachioradialis RMS EMG was also unchanged by exercise (P = 0.492) for all protocols (P = 0.711). Although EMG output was maintained, torque decreased progressively during the 10 min contraction (P < 0.001; Fig. 3A). The decline in torque was similar across all protocols (P = 0.503). Just prior to the end of exercise, mean torque had declined to 60.4 ± 4.1% of the value at the beginning of the contraction. MVC torque was impaired by the fatigue protocol (P = 0.016) to a similar extent (81.2 ± 13.0% of the pre-fatigue value) for all protocols (P = 0.321). During exercise, ratings of effort and muscle pain increased progressively (P < 0.001 for both) and to a similar extent in each protocol (P = 0.119, P = 0.477, respectively; Fig. 3B). Mmax area increased slightly during exercise (P < 0.001) with no difference between protocols (P = 0.740) and just prior to the end of exercise was 105.8 ± 1.9% of the control value.
Figure 3. Elbow flexor torque and biceps RMS EMG (A) and ratings of perceived effort and muscle pain (B) during a submaximal maintained-EMG contraction.
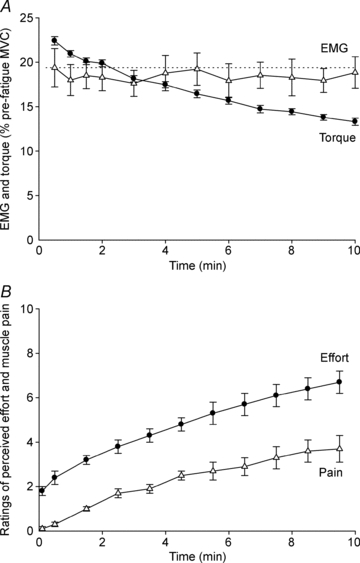
Data are mean values (± SEM) of the four protocols. A, elbow flexor torque (•) and biceps RMS EMG (▵) obtained over 100 ms just prior to each unconditioned test stimulus. Both variables are expressed as a percentage of their value during the pre-fatigue MVC with the greatest peak torque. B, ratings of perceived effort (•) and muscle pain (▵) collected using an 11-point Borg scale.
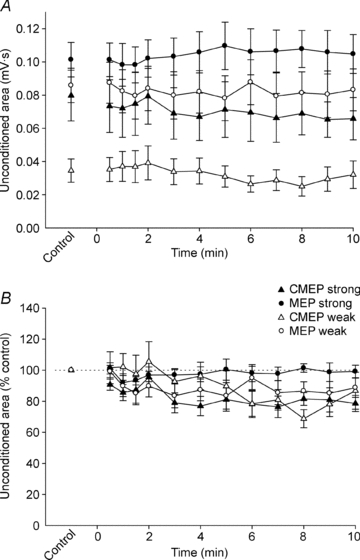
Absolute areas of unconditioned and conditioned potentials are displayed in Fig. 4A and 5A, respectively. To enable comparison across protocols, values during exercise were normalised to the control values (Figs. 4B and 5B). Using these normalised values, the effect of exercise on the area of the unconditioned potentials was similar for weak and strong test stimuli (P = 0.908; Fig. 4B). In contrast, the area of normalised conditioned potentials decreased to a greater extent with weak compared to strong test stimuli (P = 0.001; Fig. 5B). Consequently, the decrease of the normalised conditioned to unconditioned ratio was greater with weak than strong test stimuli (P = 0.001; Fig. 6). The effects of stimulus type (MEP vs. CMEP) and time are discussed below separately for weak and strong test stimuli.
Figure 4. Unconditioned test potentials during a submaximal maintained-EMG contraction.
Data are mean values (± SEM) for unconditioned MEPs (○) and CMEPs (▵). Open and filled symbols show data from protocols with weak and strong test stimuli, respectively. A, absolute area of unconditioned MEPs and CMEPs. B, areas of unconditioned MEPs and CMEPs are normalised to Mmax recorded 10 s later and then expressed as a percentage of the mean normalised value obtained during the control contractions.
Figure 5. Conditioned test potentials during a submaximal maintained-EMG contraction.
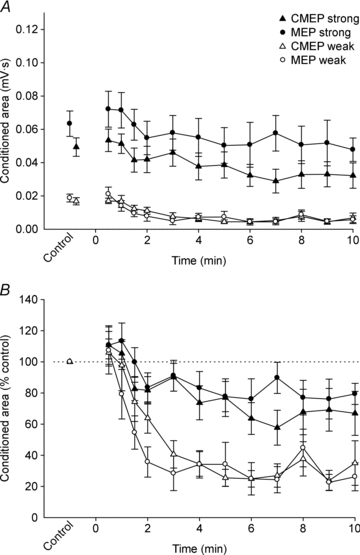
Data are mean values (± SEM) for conditioned MEPs (○) and CMEPs (▵). Open and filled symbols show data from protocols with weak and strong test stimuli, respectively. A, absolute area of conditioned MEPs and CMEPs. B, area of conditioned MEPs and CMEPs are expressed as a percentage of the mean value obtained during the control contractions.
Figure 6. Ratio of conditioned to unconditioned test potentials during a submaximal maintained-EMG contraction.
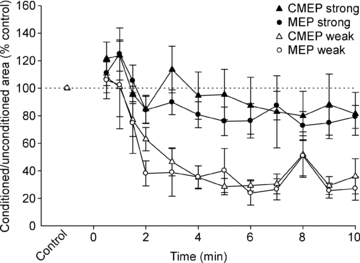
Data are mean values (± SEM) for MEP (○) and CMEP (▵) inhibition ratios. Open and filled symbols show data from protocols with weak and strong test stimuli, respectively. The ratios of conditioned to unconditioned potentials are expressed as a percentage of the mean value obtained during the control contractions.
Weak test stimuli
Using weak test stimuli, normalised MEPs and CMEPs behaved similarly. Specifically, unconditioned and conditioned potentials as well as the inhibition ratios were all equivalent during the fatiguing exercise (P = 0.424, P = 0.470, P = 0.677, respectively; Figs. 4–6). Thus, the statistical analyses for main effects of time (exercise) are based on the combined MEP and CMEP data. Unconditioned area decreased significantly during exercise (P = 0.004) and was significantly smaller than control from 7–9 min (Fig. 4B). As shown for a single subject, both the conditioned MEP and the conditioned CMEP decreased gradually during the fatiguing contraction (Fig. 2A). The group data show that the area of the conditioned responses decreased rapidly during the first 3 min of exercise and only modestly thereafter (P < 0.001; Fig. 5B). After correction for multiple comparisons, the conditioned responses first became statistically less than control values at 1.5 min of exercise. Because conditioned potentials were much more affected by fatigue than unconditioned potentials, the pattern of decrease for the ratios of the conditioned to unconditioned responses (P < 0.001; Fig. 6) closely resembled the decline of the conditioned potentials (Fig. 5B). The ratios were first significantly less than control at 2 min of exercise.
Strong test stimuli
Some differences existed between normalised MEPs and CMEPs in the comparison across the strong test stimulus protocols. Unconditioned MEPs were larger than CMEPs during exercise (P = 0.038; Fig. 4B). MEP area did not change with exercise (P = 0.381), whereas CMEP area decreased with exercise (P < 0.001) and was statistically smaller than control at 8 and 10 min of exercise. In contrast, conditioned MEPs and CMEPs responded similarly during exercise (P = 0.377) and were smaller than control at 6 min of exercise (P < 0.001; Fig. 5B). MEP and CMEP ratios were similar during exercise (P = 0.596) and although there was a main effect of time (P < 0.001), no time points were statistically different from control after correction for multiple comparisons (Fig. 6). Compared to the ratios with weaker test stimuli, the decrease from control was relatively small (minimum values of ∼80% control) when test stimuli were strong (Fig. 6).
Discussion
In this study, subjects performed fatiguing submaximal maintained-EMG contractions. Multiple measures were tracked during the development of fatigue. Measures included torque, perceived effort and muscle pain, MEPs and CMEPs of two sizes, as well as MEPs and CMEPs of two sizes recorded during a TMS-induced silent period. In addition to the answer to our first aim, comparisons among these measures provide several novel insights into motor cortical and motoneuronal behaviour during muscle fatigue. As anticipated, a sustained submaximal contraction slowed the fatigue-related decrease of motoneuronal responsiveness during the silent period compared to a sustained maximal effort (McNeil et al. 2009). However, contrary to the hypothesis of our first aim, there was still no evidence of additional inhibition within the motor cortex. Stronger test stimuli reduced the magnitude of the fatigue-related suppression of the conditioned MEP but did not alter the conclusion that a spinal, rather than cortical, mechanism appears responsible for the phenomenon. As indicated above, there were novel insights beyond the site of long-interval inhibition in a fatiguing submaximal contraction. First, in support of the hypothesis to our second aim, unconditioned CMEPs decreased with fatigue when EMG, rather than torque, was maintained in a sustained submaximal effort. Second, comparison of large and small evoked responses, particularly CMEPs, suggests that large (high-threshold) and small (low-threshold) motoneurones are affected differentially in this form of exercise. Third, comparison of the MEPs and CMEPs evoked during ongoing voluntary EMG with those evoked during the silent period emphasises the role of descending drive in counterbalancing the profound reductions in excitability of the motoneurones. Finally, comparison of EMG, torque and perceived effort confirms the large mismatch between effort and voluntary output that has been reported previously for fatiguing submaximal contractions in which torque was held constant (e.g. Sogaard et al. 2006; Smith et al. 2007).
Control efforts
During submaximal contractions of unfatigued muscle, comparison of the conditioned to unconditioned MEP ratio and the conditioned to unconditioned CMEP ratio for the weak stimuli indicates the presence of intracortical inhibition. Conditioned MEPs were ∼75% smaller than unconditioned MEPs whereas conditioned CMEPs averaged 30% smaller than unconditioned CMEPs. Thus, the suppression of the conditioned MEP was greater than that of the CMEP, which suggests that intracortical inhibition causes suppression beyond the disfacilitation of the motoneurones. In contrast, when strong stimuli were used, the conditioned MEPs and CMEPs had an equivalent suppression of ∼35% relative to their unconditioned counterparts. This suggests that MEPs elicited by high intensity TMS, unlike those elicited by weaker stimuli, are not subject to long-interval intracortical inhibition (see also McNeil et al. 2011a).
Fatiguing contraction
When compared with the reduction of the conditioned MEP during a 2 min MVC (McNeil et al. 2009), a sustained 10 min 25% MVC contraction caused a slower and smaller reduction for MEPs that were initially of equivalent size in the two studies. However, similar to the previous finding, the conditioned CMEP evoked by stimulation of the corticospinal tract was suppressed to the same degree as the MEP, which suggests an absence of a measurable motor cortical contribution to the fatigue-related reduction of the MEP. Stronger test stimuli attenuated the reduction of the conditioned MEP and CMEP but the two potentials were still similarly affected by fatigue. Hence, a reduction in motoneurone excitability rather than a cortical effect seems likely to be responsible for the decrease in the size of conditioned MEPs (and CMEPs) during a fatiguing sustained contraction of 25–100% MVC.
Cervicomedullary stimulation and TMS evoke single and multiple descending volleys, respectively, and so an alternative, albeit more complex, interpretation of the comparable effect of fatigue on MEPs and CMEPs could allow for an intracortical component to the inhibition of the MEP. It is possible that fatigue-related intracortical inhibition reduces the size or number of the multiple volleys contributing to the MEP but temporal summation and facilitation of the volleys at the motoneurones mitigate the reduction of the conditioned MEP (Lemon & Mantel 1989). In this argument, suppression of the conditioned CMEP would reflect a reduction in motoneurone excitability which affects only a single descending volley and hence would not strongly influence the MEP. However, in our opinion, the similarity of the suppression of MEPs and CMEPs with both weak and strong stimuli (and in both maximal (McNeil et al. 2009) and submaximal contractions) argues against a fortuitous confluence of events at cortical and spinal levels that matches the suppression of the two responses. The proposal that there are precisely balanced effects at cortical and spinal sites when multiple corticospinal volleys are induced by cortical stimulation highlights that surprisingly little is known about the transformation by motoneurones of the complex high-frequency burst of corticospinal input that constitutes the cortical output produced by TMS (cf. Lemon & Mantel 1989).
The lack of a likely increase in the intracortical component of the long-interval inhibition of the MEP contrasts with the increase in silent period duration which is often seen during sustained fatiguing contractions and is taken as evidence of increased intracortical inhibition (McKay et al. 1996; Taylor et al. 1996; Sacco et al. 1997). The absence of fatigue-related inhibition within the cortex during sustained maximal (McNeil et al. 2009) and now submaximal contractions combined with the decrease in long-interval intracortical inhibition observed during relaxation between intermittent brief MVCs (Benwell et al. 2007) suggests that the duration of the silent period may not be a good marker of intracortical inhibition.
Comparison of the changes in the smaller and larger conditioned responses reveals the possibility of a differential effect of fatigue produced by a submaximal contraction on the excitability of small and large motoneurones (see also Carpentier et al. 2001). Motor unit recruitment features prominently in the increase of force from biceps brachii motoneurone pool (Kukulka & Clamann 1981) and the consequences for motoneuronal output have been determined using CMEPs (Martin et al., 2006a). In the current study, the responses to weak stimuli (both CMEPs and MEPs) are more inhibited than responses to strong stimuli. One interpretation of the greater inhibition of responses to weak stimuli is that motoneurones active during the submaximal task get progressively more difficult to excite but that larger motoneurones (i.e. those not used during the moderate sustained voluntary contraction) are generally unaffected by fatigue in the present task. As muscle afferent inputs are widely distributed, albeit not homogeneously, across the motoneurone pool (see Binder et al. 1996; Powers & Binder 2001 for reviews), the differential response to weak and strong stimuli does not support our earlier suggestion that reduced spindle discharge contributes strongly to the decreased motoneurone excitability in the silent period (McNeil et al. 2009). Hence, an additional suggested mechanism, changes to intrinsic motoneuronal properties, may have a more influential role than first proposed (McNeil et al. 2009). Evidence for such intrinsic changes due to repetitive motoneurone discharge include spike-frequency adaptation (e.g. Kernell & Monster 1982; Spielmann et al. 1993; Sawczuk et al. 1997) and the increased excitatory drive needed to maintain motoneurone output in isolated preparations (see Powers & Binder 2001 for review) and to maintain the firing rate of low-threshold single motor units (Johnson et al. 2004).
The comparatively modest decrease of unconditioned relative to conditioned potentials (Fig. 4Bvs. 5B) demonstrates the efficiency with which descending drive is able to overcome fatigue-related reductions in motoneurone excitability (Rothwell 2009). This facilitatory effect is more clearly seen with weak than strong stimuli because the weak stimulus tests the responsiveness of the small motoneurones which are active in the fatiguing contraction. However, the most convincing evidence for the potency of descending drive comes from our previous study in which motoneurone responsiveness during the silent period was absent ∼30 s into a sustained maximal effort yet the unconditioned CMEP was increased (relative to the unfatigued size) at that time (McNeil et al. 2009). Given the importance of voluntary drive, one might question the physiological relevance of analysing fatigue mechanisms by monitoring changes to CMEPs (or MEPs) during the silent period. While important information on fatigue mechanisms can be obtained from stimuli delivered to relaxed muscle after the contraction, many fatigue processes recover rapidly after the exercise is stopped. Further, interrupting sustained contractions to perform such tests will interfere with the development of fatigue. Hence, tests of spinal and cortical excitability in the silent period enable one to monitor the development of fatigue mechanisms during ongoing contractions (McNeil et al. 2011b).
As noted in the Introduction, the increase in EMG (drive) when torque is maintained is likely to explain the increase in CMEP size reported during submaximal fatigue (Levenez et al. 2008; Hoffman et al. 2009). Hence, in an attempt to clamp motoneuronal output, we deliberately used a sustained submaximal contraction in which EMG, rather than torque, was held constant. Under these conditions, any fatigue-related loss of torque is largely the result of changes within the muscle fibres. It should be noted that maintenance of biceps EMG does not guarantee that this surface EMG will be made up of firing of the same motor units at the same frequencies throughout the contraction. Furthermore, maintenance of EMG does not necessarily indicate maintenance of voluntary descending drive as this should vary to compensate for any changes in afferent input or intrinsic motoneurone properties. As it is impossible to quantify descending drive in intact humans, there is no direct way to ascertain whether voluntary drive increased, decreased or remained unchanged during the present fatigue task. Nevertheless, it is well established that drive increases when a submaximal torque is maintained and more motor units are recruited progressively and so a submaximal contraction with maintained EMG was better suited to our purpose. This was to attempt to hold motoneurone output steady and thereby define two levels of motoneurone activity (active throughout the contraction or not active at all).
During the maintained EMG contraction, using weak test stimuli, both the unconditioned MEPs and CMEPs decreased ∼15–20%. This suggests that motoneurone excitability decreased but the cortical elements activated by weak TMS did not undergo a net change in excitability even though voluntary cortical output presumably had to increase to maintain output from the less excitable motoneurones. The progressive increase in perceived effort supports this latter suggestion of increased voluntary output from the cortex. With strong test stimuli, the unconditioned CMEP decreased ∼20% whereas the unconditioned MEP was similar throughout exercise. This suggests that strong cervicomedullary stimulation could not overcome the reduction in motoneurone excitability but that cortical elements activated by the strong TMS may be facilitated. Alternatively, the MEP induced by strong TMS could be limited in size during control contractions because it recruits all of the motoneurone pool and it may thus be insensitive to changes in input to the motoneurones with exercise. That is, the similarity of MEP size throughout exercise could reflect an initially supramaximal input to the motoneurones which simply becomes less supramaximal when the motoneurones are harder to drive.
Similar to results from submaximal contractions of constant torque (Sogaard et al. 2006; Smith et al. 2007), the rating of perceived effort increased steadily during exercise which indicates a mismatch between sense of effort and motor output, even when motoneurone output is maintained. However, unlike contractions of constant torque in which voluntary EMG increases, albeit to a lesser extent than perceived effort (Sogaard et al. 2006; Smith et al. 2007), torque actually decreased ∼40% in the current exercise protocol while EMG was maintained and perceived effort increased. Perhaps the most effective demonstration of the mismatch between perceived effort and actual capacity to generate torque is the comparison between perceived effort at the end of the submaximal contraction and the torque produced seconds later during a brief MVC. Subjects reported that a ‘very large’ effort (6.7 ± 1.4) was required to maintain the EMG target and produce a torque of ∼15% of the initial MVC during the last minute of the sustained contraction, yet the MVC performed immediately afterward was 81.2 ± 13.0% of the pre-fatigue value. Like perceived effort, the rating of pain in the elbow flexors increased steadily during exercise. Fatigue-sensitive small-diameter muscle afferents which are responsible for the sensation of pain are unlikely to contribute to the decrease in excitability in the elbow flexor motoneurone pool (Taylor et al. 2000; Butler et al. 2003). However, these afferents may influence the generation of motor cortical output and thus contribute to the increase in perceived effort (Smith et al. 2007).
In conclusion, our data from a sustained submaximal maintained-EMG contraction reveal several novel insights into motoneurone behaviour during muscle fatigue. When descending voluntary drive is present, CMEP size decreases modestly with fatigue, which suggests a reduction in motoneurone excitability. The reduction in CMEP size is much greater in the silent period. This contrast shows the potency of voluntary drive in maintaining motoneurone excitability during muscle fatigue. The equivalent reduction of MEPs and CMEPs in the silent period indicates that, similar to a maximal effort, cortical mechanisms do not contribute appreciably to the fatigue-related increase in long-interval inhibition. Stronger test stimuli attenuate much of the reduction of the CMEP in the silent period, which suggests that large (high-threshold) motoneurones are less affected by this exercise.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia and the Natural Sciences and Engineering Research Council of Canada.
Glossary
Abbreviations
- CMEP
cervicomedullary motor evoked potential
- EMG
electromyographic activity
- MEP
motor evoked potential
- Mmax
maximal compound muscle action potential
- MVC
maximal voluntary contraction
- TMS
transcranial magnetic stimulation
Author contributions
Each author contributed to all aspects of the study. All experiments were performed at Neuroscience Research Australia (formerly Prince of Wales Medical Research Institute) in Sydney, Australia.
References
- Benwell NM, Mastaglia FL, Thickbroom GW. Differential changes in long-interval intracortical inhibition and silent period duration during fatiguing hand exercise. Exp Brain Res. 2007;179:255–262. doi: 10.1007/s00221-006-0790-2. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Inghilleri M, Rothwell JC, Cruccu G, Manfredi M. Multiple firing of motoneurones is produced by cortical stimulation but not by direct activation of descending motor tracts. Electroencephalogr Clin Neurophysiol. 1991;81:240–242. doi: 10.1016/0168-5597(91)90078-c. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie BR, Dawson NJ, Johansson RS, Lippold OC. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol. 1986;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder MD, Heckman CJ, Powers RK. The physiological control of motoneuron activity. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. Bethesda, SD: American Physiological Society; 1996. pp. 3–53. [Google Scholar]
- Borg G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health. 1990;16(Suppl 1):55–58. doi: 10.5271/sjweh.1815. [DOI] [PubMed] [Google Scholar]
- Butler JE, Taylor JL, Gandevia SC. Responses of human motoneurons to corticospinal stimulation during maximal voluntary contractions and ischemia. J Neurosci. 2003;23:10224–10230. doi: 10.1523/JNEUROSCI.23-32-10224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier A, Duchateau J, Hainaut K. Motor unit behaviour and contractile changes during fatigue in the human first dorsal interosseus. J Physiol. 2001;534:903–912. doi: 10.1111/j.1469-7793.2001.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglevand AJ, Zackowski KM, Huey KA, Enoka RM. Impairment of neuromuscular propagation during human fatiguing contractions at submaximal forces. J Physiol. 1993;460:549–572. doi: 10.1113/jphysiol.1993.sp019486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol. 1996;490:529–536. doi: 10.1113/jphysiol.1996.sp021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Petersen N, Butler JE, Taylor JL. Impaired response of human motoneurones to corticospinal stimulation after voluntary exercise. J Physiol. 1999;521:749–759. doi: 10.1111/j.1469-7793.1999.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman BW, Oya T, Carroll TJ, Cresswell AG. Increases in corticospinal responsiveness during a sustained submaximal plantar flexion. J Appl Physiol. 2009;107:112–120. doi: 10.1152/japplphysiol.91541.2008. [DOI] [PubMed] [Google Scholar]
- Johnson KV, Edwards SC, Van Tongeren C, Bawa P. Properties of human motor units after prolonged activity at a constant firing rate. Exp Brain Res. 2004;154:479–487. doi: 10.1007/s00221-003-1678-z. [DOI] [PubMed] [Google Scholar]
- Kernell D, Monster AW. Time course and properties of late adaptation in spinal motoneurones of the cat. Exp Brain Res. 1982;46:191–196. doi: 10.1007/BF00237176. [DOI] [PubMed] [Google Scholar]
- Kukulka CG, Clamann HP. Comparison of the recruitment and discharge properties of motor units in human brachial biceps and adductor pollicis during isometric contractions. Brain Res. 1981;219:45–55. doi: 10.1016/0006-8993(81)90266-3. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Mantel GW. The influence of changes in discharge frequency of corticospinal neurones on hand muscles in the monkey. J Physiol. 1989;413:351–378. doi: 10.1113/jphysiol.1989.sp017658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenez M, Garland SJ, Klass M, Duchateau J. Cortical and spinal modulation of antagonist coactivation during a submaximal fatiguing contraction in humans. J Neurophysiol. 2008;99:554–563. doi: 10.1152/jn.00963.2007. [DOI] [PubMed] [Google Scholar]
- Lippold OCJ, Redfearn JWT, Vuco J. The electromyography of fatigue. Ergonomics. 1960;3:121–131. [Google Scholar]
- Martin PG, Gandevia SC, Taylor JL. Output of human motoneuron pools to corticospinal inputs during voluntary contractions. J Neurophysiol. 2006a;95:3512–3518. doi: 10.1152/jn.01230.2005. [DOI] [PubMed] [Google Scholar]
- Martin PG, Smith JL, Butler JE, Gandevia SC, Taylor JL. Fatigue-sensitive afferents inhibit extensor but not flexor motoneurons in humans. J Neurosci. 2006b;26:4796–4802. doi: 10.1523/JNEUROSCI.5487-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay WB, Stokic DS, Sherwood AM, Vrbova G, Dimitrijevic MR. Effect of fatiguing maximal voluntary contraction on excitatory and inhibitory responses elicited by transcranial magnetic motor cortex stimulation. Muscle Nerve. 1996;19:1017–1024. doi: 10.1002/mus.880190803. [DOI] [PubMed] [Google Scholar]
- McNeil CJ, Martin PG, Gandevia SC, Taylor JL. The response to paired motor cortical stimuli is abolished at a spinal level during human muscle fatigue. J Physiol. 2009;587:5601–5612. doi: 10.1113/jphysiol.2009.180968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Martin PG, Gandevia SC, Taylor JL. Long-interval intracortical inhibition in a human hand muscle. Exp Brain Res. 2011a;209:287–297. doi: 10.1007/s00221-011-2552-z. [DOI] [PubMed] [Google Scholar]
- McNeil CJ, Martin PG, Gandevia SC, Taylor JL. A novel way to test human motoneurone behaviour during muscle fatigue. Physiology News. 2011b;82:29–31. [Google Scholar]
- Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol. 2001;143:137–263. doi: 10.1007/BFb0115594. [DOI] [PubMed] [Google Scholar]
- Roick H, von Giesen HJ, Benecke R. On the origin of the postexcitatory inhibition seen after transcranial magnetic brain stimulation in awake human subjects. Exp Brain Res. 1993;94:489–498. doi: 10.1007/BF00230207. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. The fatigued spinal cord. J Physiol. 2009;587:5517–5518. doi: 10.1113/jphysiol.2009.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco P, Newberry R, McFadden L, Brown T, McComas AJ. Depression of human electromyographic activity by fatigue of a synergistic muscle. Muscle Nerve. 1997;20:710–717. doi: 10.1002/(sici)1097-4598(199706)20:6<710::aid-mus8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Sawczuk A, Powers RK, Binder MD. Contribution of outward currents to spike-frequency adaptation in hypoglossal motoneurons of the rat. J Neurophysiol. 1997;78:2246–2253. doi: 10.1152/jn.1997.78.5.2246. [DOI] [PubMed] [Google Scholar]
- Smith JL, Martin PG, Gandevia SC, Taylor JL. Sustained contraction at very low forces produces prominent supraspinal fatigue in human elbow flexor muscles. J Appl Physiol. 2007;103:560–568. doi: 10.1152/japplphysiol.00220.2007. [DOI] [PubMed] [Google Scholar]
- Sogaard K, Gandevia SC, Todd G, Petersen NT, Taylor JL. The effect of sustained low-intensity contractions on supraspinal fatigue in human elbow flexor muscles. J Physiol. 2006;573:511–523. doi: 10.1113/jphysiol.2005.103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmann JM, Laouris Y, Nordstrom MA, Robinson GA, Reinking RM, Stuart DG. Adaptation of cat motoneurons to sustained and intermittent extracellular activation. J Physiol. 1993;464:75–120. doi: 10.1113/jphysiol.1993.sp019625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Allen GM, Gandevia SC. Changes in motor cortical excitability during human muscle fatigue. J Physiol. 1996;490:519–528. doi: 10.1113/jphysiol.1996.sp021163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Petersen N, Butler JE, Gandevia SC. Ischaemia after exercise does not reduce responses of human motoneurones to cortical or corticospinal tract stimulation. J Physiol. 2000;525:793–801. doi: 10.1111/j.1469-7793.2000.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Petersen NT, Butler JE, Gandevia SC. Interaction of transcranial magnetic stimulation and electrical transmastoid stimulation in human subjects. J Physiol. 2002;541:949–958. doi: 10.1113/jphysiol.2002.016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD. Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Ann Neurol. 1991;29:418–427. doi: 10.1002/ana.410290413. [DOI] [PubMed] [Google Scholar]
- Uncini A, Treviso M, Di Muzio A, Simone P, Pullman S. Physiological basis of voluntary activity inhibition induced by transcranial cortical stimulation. Electroencephalogr Clin Neurophysiol. 1993;89:211–220. doi: 10.1016/0168-5597(93)90098-a. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Netz J, Szelenyi A, Homberg V. Spinal and supraspinal mechanisms contribute to the silent period in the contracting soleus muscle after transcranial magnetic stimulation of human motor cortex. Neurosci Lett. 1993;156:167–171. doi: 10.1016/0304-3940(93)90464-v. [DOI] [PubMed] [Google Scholar]


