Non-technical summary
Shivering is an involuntary somatic motor response that occurs in skeletal muscles to produce heat during exposure to cold environments or during the development of fever. This study describes the brain circuitry mechanism that produces shivering. The reception of either cutaneous cool-sensory signals or pyrogenic signals by neurons in the preoptic area, a thermoregulatory and febrile centre, leads to activation of descending excitatory signalling through hypothalamic and medullary sites to drive shivering. Intriguingly, this central command pathway for shivering parallels that for sympathetically regulated non-shivering thermogenesis in brown adipose tissue. The present results promote our understanding of the brain mechanisms for thermal homeostasis that orchestrate the regulation of the somatic and autonomic motor systems to meet the critical demand for regulation of the body and brain temperatures.
Abstract
Abstract
Shivering is a remarkable somatomotor thermogenic response that is controlled by brain mechanisms. We recorded EMGs in anaesthetized rats to elucidate the central neural circuitry for shivering and identified several brain regions whose thermoregulatory neurons comprise the efferent pathway driving shivering responses to skin cooling and pyrogenic stimulation. We simultaneously monitored parameters from sympathetic effectors: brown adipose tissue (BAT) temperature for non-shivering thermogenesis and arterial pressure and heart rate for cardiovascular responses. Acute skin cooling consistently increased EMG, BAT temperature and heart rate and these responses were eliminated by inhibition of neurons in the median preoptic nucleus (MnPO) with nanoinjection of muscimol. Stimulation of the MnPO evoked shivering, BAT thermogenesis and tachycardia, which were all reversed by antagonizing GABAA receptors in the medial preoptic area (MPO). Inhibition of neurons in the dorsomedial hypothalamus (DMH) or rostral raphe pallidus nucleus (rRPa) with muscimol or activation of 5-HT1A receptors in the rRPa with 8-OH-DPAT eliminated the shivering, BAT thermogenic, tachycardic and pressor responses evoked by skin cooling or by nanoinjection of prostaglandin (PG) E2, a pyrogenic mediator, into the MPO. These data are summarized with a schematic model in which the shivering as well as the sympathetic responses for cold defence and fever are driven by descending excitatory signalling through the DMH and the rRPa, which is under a tonic inhibitory control from a local circuit in the preoptic area. These results provide the interesting notion that, under the demand for increasing levels of heat production, parallel central efferent pathways control the somatic and sympathetic motor systems to drive thermogenesis.
Introduction
Shivering is a remarkable thermogenic response in homeothermic animals, including humans, that involves rapid, repeated skeletal muscle contractions leading to heat production through the inefficiency of ATP utilization (Jubrias et al. 2008). This involuntary thermoregulatory response is driven by a central neuronal mechanism that is triggered by physiological stimuli to increase thermogenesis, such as exposure to a cold environment or reception of pyrogenic immune signals by the brain during infection. Only limited information is available on the central circuitry through which shivering is initiated and controlled.
Within the framework of central thermoregulation, neurons in the preoptic area (POA) play pivotal roles by receiving and integrating information on peripheral (e.g. cutaneous and visceral) and local brain temperatures and by providing appropriate command signals to peripheral thermoregulatory effectors (for reviews, see Romanovsky, 2007; Morrison et al. 2008; Morrison & Nakamura, 2011). Recently, we have identified thermosensory pathways from skin thermoreceptors to the POA that mediate feedforward signalling required to elicit rapid thermoregulatory responses, including shivering, to changes in environmental temperature (Nakamura & Morrison, 2008a, 2010). Cool and warm sensory signals from the skin are transmitted by separate pathways through the spinal dorsal horn and the lateral parabrachial nucleus to the midline portion of the POA including the median preoptic nucleus (MnPO) (Nakamura & Morrison, 2008a, 2010).
Recent studies on the efferent pathways from the POA mediating skin cooling- and pyrogen-evoked non-shivering thermogenesis in brown adipose tissue (BAT) support the hypothesis that descending, GABAergic projection neurons in the medial POA (MPO) provide an inhibitory regulation of the activity of BAT sympathoexcitatory neurons in the dorsomedial hypothalamus (DMH) (Chen et al. 1998; Osaka, 2004; Nakamura et al. 2005b; Nakamura & Morrison, 2007). In a cold environment, the descending inhibition from the POA is attenuated by MnPO neurons that are activated by cutaneous cool signals (Nakamura & Morrison, 2008b). In the case of infection, immune signalling results in the brain vasculature production (Matsumura et al. 1998) of prostaglandin (PG) E2, a pyrogenic mediator, which can act through the EP3 subtype of PGE receptors in the POA (Nakamura et al. 1999, 2000, 2002; Lazarus et al. 2007) to potentially attenuate the activity of descending GABAergic projection neurons. The cooling- or pyrogen-triggered attenuation of the tonic descending inhibition from the POA is hypothesized to disinhibit the BAT sympathoexcitatory neurons in the DMH and sympathetic premotor neurons in the rostral medullary raphe region, including the rostral raphe pallidus nucleus (rRPa), which provide the excitatory drive for the sympathetic outflow determining BAT thermogenesis (Nakamura et al. 2002, 2004, 2005b; Morrison, 2003; Madden & Morrison, 2003, 2004; Zaretskaia et al. 2003; Nakamura & Morrison, 2007).
The present study was undertaken to determine if a neural circuit model similar to that for the control of BAT thermogenesis is also applicable to the central efferent mechanism for shivering thermogenic responses to cooling and pyrogenic stimuli. In this investigation, we recorded skin cooling-evoked and pyrogen-evoked shivering EMG responses in anaesthetized rats and examined the effects of drug applications into candidate brain regions on the amplitude of these evoked increases in EMG activity. Our results suggest that, despite the differences in their respective output systems (sympathetic vs somatomotor), thermoregulatory and febrile activations of BAT thermogenesis and of shivering thermogenesis are mediated by altered neuronal discharge in the same regions of the hypothalamus and medulla oblongata.
Methods
Animal preparation
Sixty male Wistar rats (250–450 g) contributed to the present study. The animals were housed with ad libitum access to food and water in a room air-conditioned at 22–23°C with a standard 12 h light–dark cycle. All procedures conform to the guidelines for animal care of the Institute of Laboratory Animals, Faculty of Medicine, Kyoto University and to the regulations detailed in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Research Committee, Graduate School of Medicine, Kyoto University and by the Animal Care and Use Committee of the Oregon Health and Science University.
Rats, breathing spontaneously, were anaesthetized with 2.0% isoflurane in 100% O2 through a tracheal cannula. Adequacy of anaesthesia was verified by the absence of a hindlimb withdrawal to foot pinch and/or by the absence of eye blink response to gentle touch of the cornea. Arterial pressure and heart rate (HR) were recorded from a cannulated femoral artery and rectal temperature was monitored with a copper–constantan thermocouple (Physitemp, Clifton, NJ, USA) as an indication of body core temperature. The trunk was shaved, a thermocouple to monitor skin temperature was taped onto the abdominal skin, and the trunk was wrapped with a plastic water jacket to cool and re-warm the skin. The animal was positioned in a stereotaxic apparatus and a needle-type thermocouple (0.33 mm diameter; Physitemp) was inserted perpendicularly into the basal forebrain (2.2 mm anterior to bregma, 1.3 mm left to the midline, 7.0–7.5 mm ventral to the brain surface) to monitor brain temperature. BAT temperature (TBAT) was monitored with a thermocouple inserted into the left interscapular BAT pad. Rectal temperature was maintained at 35.5–37.0°C by perfusing the water jacket with warm or cold water. All the thermocouples were connected to a thermocouple meter (TC-2000, Sable Systems, Las Vegas, NV, USA) for computer acquisition of the analog signals.
Bipolar needle electrodes for EMG recording were inserted into nuchal muscles and the signal was filtered (10–1000 Hz) and amplified (×2000) with a CyberAmp 380 (Axon Instruments, Union City, CA, USA). During EMG measurement, the isoflurane concentration was reduced to 0.6–0.8% and under this anaesthetic condition, the animals never showed any movement except for shivering. Alternatively, in the ventromedial medullary mapping experiments, the rats were anaesthetized with Inactin (120 mg kg−1 initial dose, 12 mg kg−1 h−1 supplements after 4 h; Sigma). Inactin-anaesthetized rats exhibited skin cooling-evoked shivering responses that were indistinguishable from those of isoflurane-anaesthetized rats. EMG amplitude was quantified (Spike2, CED, Cambridge, UK) in sequential 4 s bins as the square root of the total power (root mean square) in the 0–500 Hz band of the autospectra of each 4 s segment of EMG. Physiological variables were digitized and recorded to a computer hard disk using Spike2. To determine the mean frequency of the shivering-related bursts in EMG activity, the EMG signal was demeaned, rectified, smoothed and lowpass-filtered below 100 Hz. An averaged autospectrum was calculated from 5–10, 0.8 s segments of the EMG signal and the mean frequency of the EMG spike activity was then obtained as the weighted mean of the four sequential averaged autospectrum frequency bins (1.2 Hz bin−1) with the greatest power values.
Experimental procedure and data analysis
Skin cooling experiments
All the animals exhibited consistent cold-defensive physiological responses to repeated cooling of the trunk skin by perfusing the water jacket with cold water (Nakamura & Morrison, 2007, 2008a). Each cooling episode lasted for 150–200 s until the skin temperature was lowered to a level consistent with preceding cooling episodes and then the skin was re-warmed by switching to perfusion of the jacket with warm water. After control cooling episodes, we examined the effects of nanoinjections of saline vehicle and, subsequently, a drug into the MnPO, DMH, posterior hypothalamus (PH) or rRPa on cold-defensive responses evoked by subsequent cooling episodes. Drugs were obtained from Sigma (St Louis, MO, USA) dissolved in 0.9% saline and nanoinjections were made by pressure-ejection through stereotaxically positioned glass micropipettes (tip inner diameter, 20–30 μm) using a Picospritzer II (General Valve, Fairfield, NJ, USA). The MnPO was injected with muscimol (2 mm, 100–200 nl), the DMH and PH were bilaterally injected with muscimol (2 mm, 60 nl site−1), and the rRPa was injected with muscimol (2 mm, 60 nl) or the 5-HT1A receptor agonist, (±)-8-hydroxy-2-(di-n-propylamino)tetralin hydrobromide (8-OH-DPAT, 10 mm, 60 nl).
Baseline values of all physiological variables were the averages during the 30 s period immediately before skin cooling. Skin cooling-evoked response values for TBAT and HR were obtained at the end of the skin cooling episode and those for EMG and mean arterial pressure (MAP) were the averages during the 30 s period immediately before the end of skin cooling. Skin cooling-evoked changes in these variables from their baseline values were compared between skin cooling episodes after saline and after drug injections (Figs 4D and 6E and F). Statistical significance was evaluated with Student's two-tailed paired t test. In Fig. 2D, skin cooling-evoked changes were compared among cooling episodes before injections, after saline injection and after muscimol injection by using a repeated measures one-way ANOVA followed by a Bonferroni post hoc test.
Figure 4. Muscimol injections into the DMH block shivering, non-shivering thermogenic and cardiac responses to skin cooling.
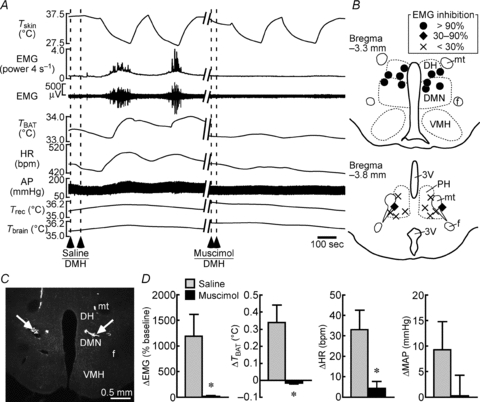
A, changes in physiological variables that are evoked by repeated skin cooling. Saline or muscimol was bilaterally nanoinjected into the DMH at the dashed lines. B, composite drawing of sites of muscimol injections into the DMH or PH with their inhibitory effects on skin cooling-evoked increase in EMG. Inhibition of EMG activity was expressed as a percentage of EMG response evoked by skin cooling before injections. The DMH consists of the dorsomedial hypothalamic nucleus (DMN) and dorsal hypothalamic area (DH). f, fornix; mt, mammillothalamic tract; VMH, ventromedial hypothalamic nucleus. C, representative view of sites of bilateral injections into the DMN. D, group data showing skin cooling-evoked changes in physiological variables following saline or muscimol injections into the DMH (n = 5 per group). *P < 0.05, compared with the cooling after saline injection (two-tailed paired t test).
Figure 6. Muscimol or 8-OH-DPAT injection into the rRPa blocks shivering, non-shivering thermogenic and cardiac responses to skin cooling.
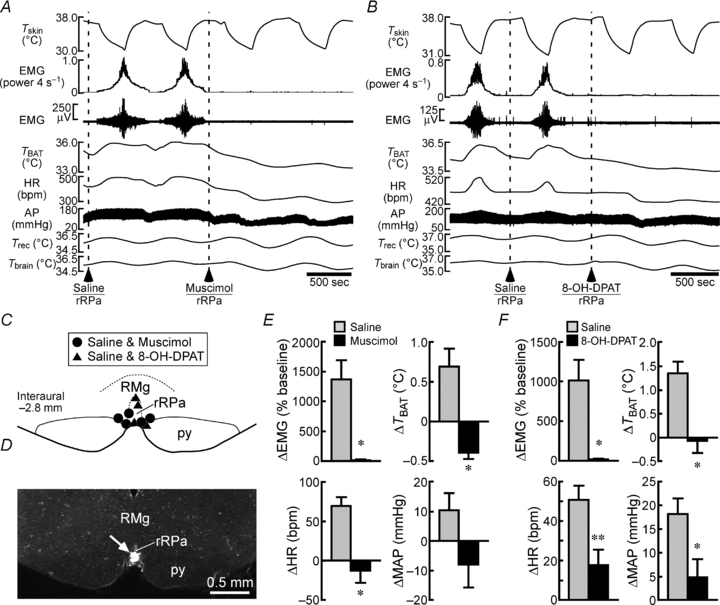
A and B, changes in physiological variables that are evoked by repeated skin cooling. Saline, muscimol (A) or 8-OH-DPAT (B) was nanoinjected into the rRPa at the dashed lines. C, location of the sites of saline, muscimol and 8-OH-DPAT injections. py, pyramidal tract. D, representative view of a site of an injection into the rRPa (arrow). E and F, group data showing skin cooling-evoked changes in physiological variables following saline or muscimol injection into the rRPa (E, n = 5 per group) or following saline or 8-OH-DPAT injection into the rRPa (F, n = 4 per group). *P < 0.05; **P < 0.01, compared with the cooling after saline injection (two-tailed paired t test).
Figure 2. Muscimol injection into the MnPO blocks shivering, non-shivering thermogenic and cardiac responses to skin cooling.
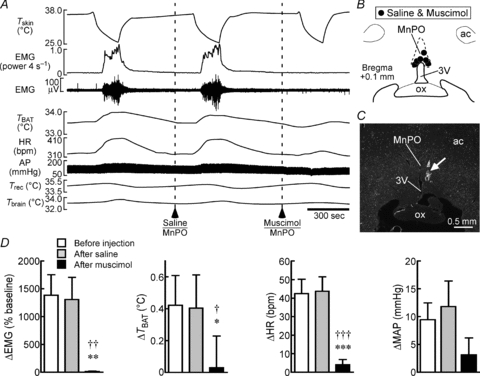
A, changes in Tskin, EMG, TBAT, HR, arterial pressure (AP), rectal temperature (Trec) and brain temperature (Tbrain) that are evoked by repeated skin cooling. Saline or muscimol was nanoinjected into the MnPO at the dashed lines. B, location of the sites of saline and muscimol injections. 3V, third ventricle; ac, anterior commissure; ox, optic chiasm. C, representative view of a site of an injection into the MnPO. The injection site is clearly identified as a cluster of fluorescent beads (arrow). D, group data showing skin cooling-evoked changes in physiological variables before injection, after saline injection or after muscimol injection into the MnPO (n = 6 per group). †P < 0.05; ††P < 0.01; †††P < 0.001, compared with the cooling before injection. *P < 0.05; **P < 0.01; ***P < 0.001, compared with the cooling after saline injection (Bonferroni post hoc test following a repeated measures one-way ANOVA).
PGE2 injection experiments
Animals received a unilateral nanoinjection of PGE2 (1 mg ml−1, 60 nl) into the MPO. At least 5 min after PGE2 injection, saline (60 nl site−1) was nanoinjected into the DMH (bilaterally) or the rRPa. Five to ten minutes after the saline injections, 2 mm muscimol (DMH, rRPa) or 10 mm 8-OH-DPAT (rRPa) was injected at the same stereotaxic coordinates. Baseline values of all physiological variables were the averages during the 30 s period immediately before PGE2 injection. PGE2-evoked response values for TBAT and HR were obtained immediately before the first injection of saline, muscimol or 8-OH-DPAT and those for EMG and MAP were the averages during the 30 s period immediately before the first injection of saline, muscimol or 8-OH-DPAT. Effect values after injections into the DMH or the rRPa for TBAT and HR were taken at 5 min (TBAT) or 3 min (HR) after the completion of the saline or drug injections into the DMH or the rRPa and those for EMG and MAP were the averages during the 30 s period beginning at 3 min after the completion of the injections into the DMH or the rRPa. Changes in these variables that were evoked by the injections into the DMH or the rRPa were compared between variable values following saline and drug injections (Figs 5C and 7D and E). Statistical significance was evaluated with a two-tailed paired t test.
Figure 5. Muscimol injections into the DMH block shivering, non-shivering thermogenic, cardiac and pressor responses to PGE2 injection into the MPO.
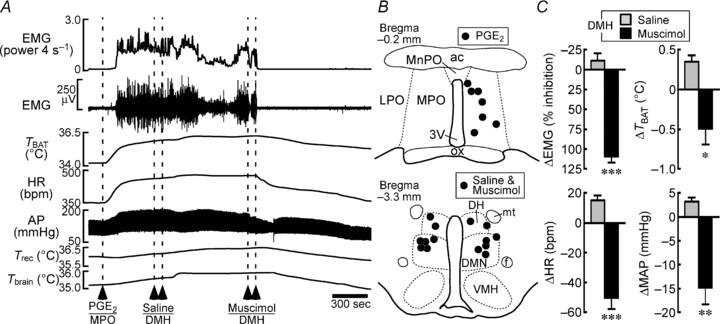
A, changes in physiological variables following PGE2 nanoinjection into the MPO. Saline and muscimol were bilaterally nanoinjected into the DMH during the febrile response to a PGE2 injection into the MPO. B, location of the sites of PGE2, saline and muscimol injections. C, group data showing the saline- or muscimol-induced changes in the physiological variables that were elevated by PGE2 injection (n = 7 per group). *P < 0.05; **P < 0.01; ***P < 0.001, compared with the saline-induced changes (two-tailed paired t test).
Figure 7. Muscimol or 8-OH-DPAT injection into the rRPa blocks shivering, non-shivering thermogenic, cardiac and pressor responses to PGE2 injection into the MPO.
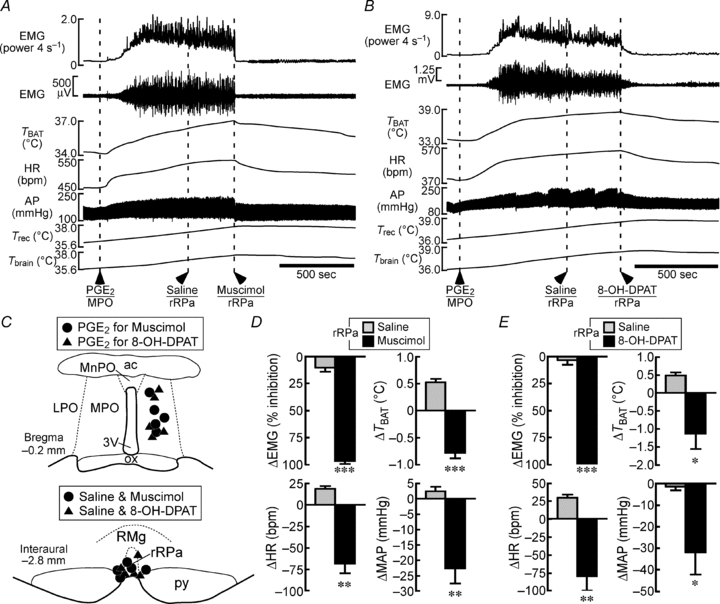
A and B, changes in physiological variables following PGE2 nanoinjection into the MPO. Saline, muscimol (A) or 8-OH-DPAT (B) was nanoinjected into the rRPa during the febrile response to a PGE2 injection into the MPO. C, location of the sites of PGE2, saline, muscimol and 8-OH-DPAT injections. D and E, group data showing the saline-, muscimol- (D) or 8-OH-DPAT-induced (E) changes in the physiological variables that were elevated by PGE2 injection (n = 5 per group). *P < 0.05; **P < 0.01; ***P < 0.001, compared with the saline-induced changes (two-tailed paired t test).
Experiments injecting bicuculline into the MnPO
The GABAA receptor antagonist, (–)-bicuculline methiodide (2 mm, 60 nl) was nanoinjected into the MnPO. During the response evoked by this injection, bilateral nanoinjections of saline or bicuculline (2 mm, 60 nl site−1) were made into the MPO. Baseline values of all physiological variables were the averages during the 30 s period immediately before the injection into the MnPO. The values of the increases in TBAT and HR evoked by bicuculline into the MnPO were taken immediately before the first of the subsequent bilateral saline or bicuculline injections into the MPO and those for EMG and MAP were the averages during the 30 s period immediately before the first saline or bicuculline injection into the MPO. To determine the effect of the bilateral saline or bicuculline injections into the MPO, values for TBAT and HR were taken at 5 min (TBAT) or 3 min (HR) after the completion of the bilateral injections and those for EMG and MAP were the averages during the 30 s period beginning at 3 min after the completion of the bilateral injections. For each variable, the statistical significance of the change due to the bicuculline injections into the MPO was detected with a two-tailed paired t-test comparing the response value following bicuculline into the MnPO and the effect value after bilateral bicuculline injections into the MPO (Fig. 3D).
Figure 3. Bicuculline injections into the MPO reverse shivering, non-shivering thermogenic and cardiac responses to MnPO stimulation.
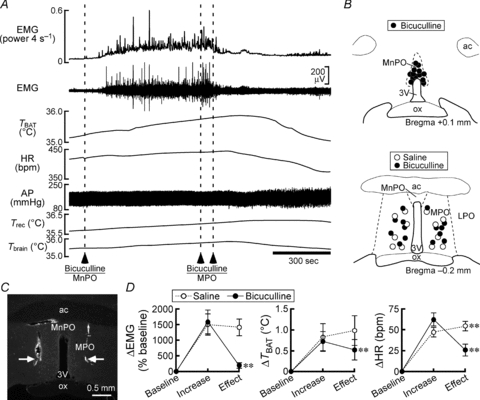
A, changes in physiological variables after a bicuculline nanoinjection into the MnPO followed by bilateral nanoinjections of bicuculline into the MPO. B, location of the sites of saline and bicuculline injections. C, representative view of sites of bilateral injections into the MPO (arrows). D, group data showing the effect of saline (n = 6) or bicuculline (n = 7) injections into the MPO on increases in the physiological variables that were evoked by bicuculline injection into the MnPO. *P < 0.05; **P < 0.01, compared with the increase value (two-tailed paired t test).
Experiments injecting NMDA into the ventromedial medulla
NMDA (0.2 mm, 45 nl) was nanoinjected into sites in the ventromedial medulla, with a minimum 10 min recovery period between injections. At the completion of the mapping in each rat, a minimum of four representative injection sites were marked with small deposits (∼5 nl) of 0.2% fluorescent microspheres (diameter, 0.1 μm; Invitrogen) in saline. The NMDA-evoked change (% of baseline) in EMG activity was determined by dividing the average EMG amplitude during the 30 s of maximum response by that during the 30 s just prior to the NMDA injection. The NMDA-evoked increases in TBAT, HR and MAP were determined by subtracting the baseline values just prior to NMDA injection from the peak values following the NMDA injection. In cases where there was no obvious response to an NMDA injection, changes in variable values were determined from values at the times following such NMDA injections at which responses occurred to NMDA injections at responsive sites in the same experiment. To assess and plot the relative responsiveness of the ventromedial medullary sites at which NMDA injections were made, the EMG responses to NMDA injections within each experiment were grouped into three categories: 0–10%, 30–75% and 75–100% of the maximum EMG response in that experiment and the sites in these categories were plotted with three symbols on atlas drawings (Paxinos & Watson, 2007) of the ventromedial medulla (Fig. 8B).
Figure 8. Sites in the rostral medullary raphe region at which nanoinjection of NMDA elicits shivering.
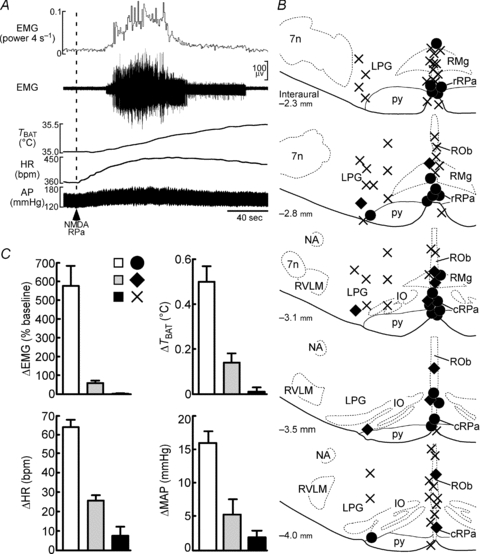
A, typical, ‘maximal’ activation of nuchal EMG following nanoinjection of NMDA (dashed line) into a site in the rRPa, which also resulted in increases in TBAT, HR and MAP. B, drawings of coronal sections through the rostral medullary raphe region, corresponding to atlas drawings (modified from Paxinos & Watson, 2007) at indicated distances caudal to the interaural line. The relative amplitudes of the increases in nuchal EMGs elicited by a 45 nl injection of 0.2 mm NMDA are indicated by a filled circle (75–100% of maximum), a filled diamond (25–50% of maximum) or a cross (0–10% of maximum) for 87 injection sites in 5 rats. 7n, facial nucleus; cRPa, caudal raphe pallidus nucleus; IO, inferior olivary complex; LPG, lateral paragigantocellular nucleus; NA, ambiguus nucleus; ROb, raphe obscurus nucleus; RVLM, rostral ventrolateral medulla. C, bar graphs indicating the relative increases in EMG, TBAT, HR and MAP evoked by NMDA injections at a sampling of the sites indicated by filled circles (n = 12), filled diamonds (n = 6) and crosses (n = 12).
To mark other drug injection sites, 5–10 nl injections of fluorescent microspheres were made at the same coordinates as the drug injections through the same micropipette. After the physiological recordings, the animals were transcardially perfused with a 4% formaldehyde solution and the brain tissue was sectioned. The locations of the injections were identified by detecting the fluorescent microspheres under an epifluorescence microscope. Most anatomical definitions of brain structures followed the brain atlas of Paxinos & Watson (2007); however, the raphe pallidus nucleus was divided into two parts: rostral (rRPa) and caudal (caudal raphe pallidus nucleus) to the rostral end of the inferior olivary complex (Nakamura et al. 2002, 2004).
All data are presented as the means ± SEM and statistical results with a P value of <0.05 were considered significant.
Results
Frequency analysis of EMG activities evoked by skin cooling, PGE2 injection into the POA and NMDA stimulation of rRPa neurons
At core temperatures below 36°C, trunk skin cooling consistently evoked a shivering response during which the EMG exhibited large amplitude oscillations (Fig. 1Aa and b) that were accompanied by clearly visible, rapid muscle contractions. The large amplitude oscillations in EMG activity during cooling could take the form of individual ‘spikes’ (Fig. 1Ab) or of bursts of spikes as in the examples of PGE2 injection into the POA (Fig. 1Bb) and of NMDA injection into the rRPa (Fig. 1Cb). From the averaged autospectrum of the EMG (Fig. 1Ac), the predominant frequency of the bursts in EMG activity in Fig. 1A was 34 Hz. The average frequency of the spike bursting in the cold-evoked shivering EMG in the anaesthetized rat nuchal muscle was 33 ± 0.9 Hz (n = 9). PGE2 injection into the POA was followed by a large and prolonged increase in EMG spike bursting activity (Fig. 1Ba and b) that was accompanied by rapid muscle contractions. In this example, the mean frequency (Fig. 1Bc) of the bursts in EMG activity was 38 Hz. The average frequency of the nuchal EMG spike activity producing PGE2-evoked shivering was 33 ± 0.9 Hz (n = 8). NMDA injection into the rRPa elicited a large increase in EMG spike activity (Fig. 1Ca and b) and rapid muscle contractions resembling those of cold-evoked and PGE2-evoked shivering. In this example, the mean frequency (Fig. 1Cc) of the NMDA-evoked bursts in EMG activity was 37 Hz. The average predominant frequency of the nuchal EMG spike activity following NMDA stimulation of neurons in the rRPa was 33 ± 0.6 Hz (n = 12). There was no significant difference among the frequencies of the bursts of spikes in nuchal EMG elicited by cooling, injection of PGE2 into the POA or excitation of neurons in the rRPa with NMDA injections.
Figure 1. Frequency analysis of shivering EMGs.
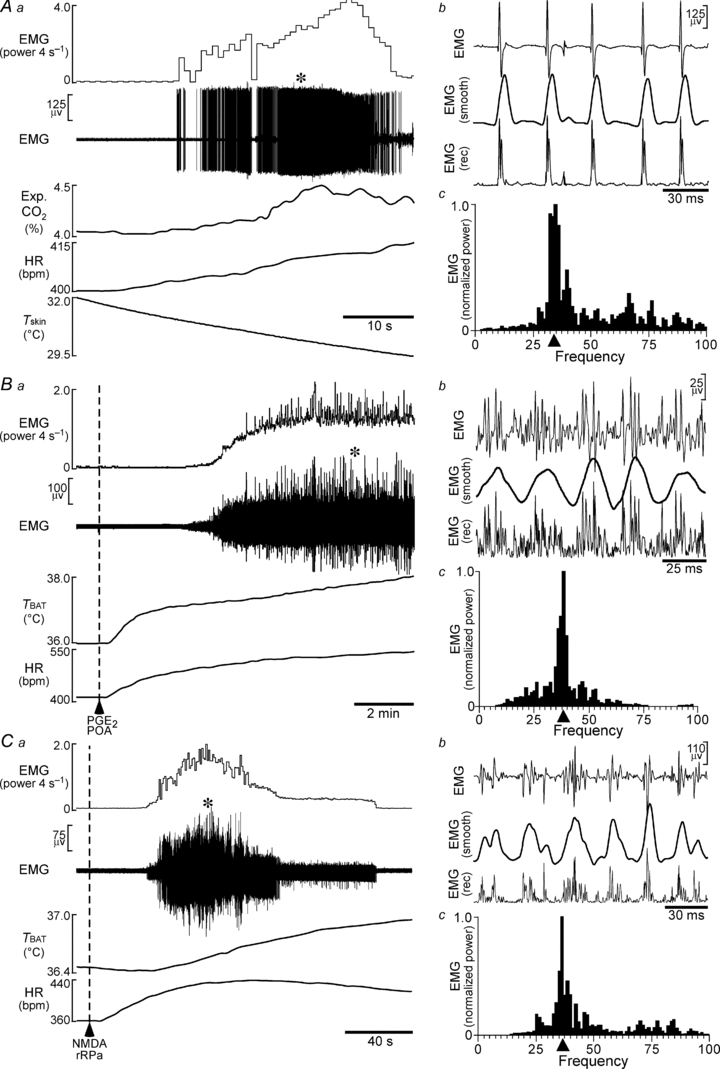
Aa, increases in nuchal EMG, expired CO2 (Exp. CO2) and HR elicited by reducing skin temperature (Tskin). Ab, expanded traces of EMG (at the time in Aa indicated by *), of the rectified EMG signal (EMG (rec)) and of the rectified EMG signal after smoothing (EMG (smooth)). Ac, averaged autospectrum of four 0.8 s segments of smoothed EMG (at * in Aa). Filled triangle indicates the mean of the 4 EMG spike burst frequencies with the greatest power values. Ba, increases in nuchal EMG, TBAT and HR elicited by nanoinjection of PGE2 into the POA. Bb, expanded traces of EMG (at * in Ba), of EMG (rec) and of EMG (smooth). Bc, averaged autospectrum of four 0.8 s segments of EMG (smooth) (at * in Ba). Filled triangle indicates the mean of the EMG spike burst frequencies containing the most power. Ca, increases in nuchal EMG, TBAT and HR elicited by nanoinjection of NMDA into the rRPa. Cb, expanded traces of EMG (at * in Ca), of EMG (rec) and of EMG (smooth). Cc, averaged autospectrum of four 0.8 s segments of EMG (smooth) (at * in Ca). Filled triangle indicates the mean of the EMG spike burst frequencies with the greatest power values.
Shivering responses to skin cooling require activation of MnPO neurons
To determine whether induction of the shivering response to reduced environmental temperature involves activation of MnPO neurons, we examined the effect of inhibition of MnPO neurons with nanoinjection of muscimol, a GABAA receptor agonist that inhibits local neurons, on the shivering EMG responses to skin cooling. Repeated episodes of trunk skin cooling evoked consistent increases in EMG and skeletal muscle shivering; in TBAT, an indicator of non-shivering thermogenic activity; and in HR, a contributor to cardiac output (Fig. 2A and D). As reported previously (Nakamura & Morrison, 2007), skin cooling-evoked changes in MAP were not consistent among animals. Skin cooling following a saline injection into the MnPO (Fig. 2B and C) increased EMG, TBAT and HR to levels comparable to those evoked by pre-injection skin cooling challenges (Fig. 2A and D). In contrast, skin cooling following a muscimol injection into the MnPO failed to evoke any of the thermogenic or cardiac responses (Fig. 2A and D). The blockade of cooling-evoked BAT thermogenesis and tachycardia by inhibition of MnPO neurons is consistent with our previous study (Nakamura & Morrison, 2008b). These results indicate that activation of neurons in the MnPO is an essential central process for the shivering response to environmental cooling as well as for the cold-defensive responses of non-shivering thermogenesis in BAT and of tachycardia.
The increase in EMG evoked by activation of MnPO neurons requires GABA transmission in the MPO
Blockade of skin cooling-evoked shivering by inhibition of MnPO neuronal activity led us to examine the effect of stimulation of MnPO neurons on EMG activity. Nanoinjection into the MnPO of bicuculline, a GABAA receptor antagonist that disinhibits local neurons, elicited a robust increase in EMG (Fig. 3A). The same injection also elevated TBAT and HR, but not MAP (Fig. 3A) (Nakamura & Morrison, 2008b). Since skin cooling-evoked BAT thermogenesis and tachycardia require cold afferent signal-triggered activation of GABAergic transmission from the MnPO to the MPO (Nakamura & Morrison, 2008b), we examined the effect of antagonizing GABA neurotransmission in the MPO on the EMG response evoked by disinhibitory activation of MnPO neurons. Bilateral nanoinjection of bicuculline, but not saline, into the MPO completely reversed the EMG response to bicuculline injection into the MnPO (Fig. 3). The blockade of GABAA receptors in the MPO also reversed the MnPO-triggered BAT thermogenesis and tachycardia (Fig. 3) (Nakamura & Morrison, 2008b). These results suggest that cutaneous cold afferent-triggered activation of GABAergic transmission from the MnPO to MPO mediates the shivering response as well as the BAT non-shivering thermogenic and the tachycardic responses to environmental cooling.
Activation of DMH neurons is required for cold-defensive and febrile shivering
We next examined the role of neurons in the DMH in the central mechanism that drives skin cooling-evoked shivering responses by inhibiting DMH neurons with muscimol nanoinjections. Following a few skin cooling episodes, we first injected saline bilaterally into the DMH, an area consisting of the dorsomedial hypothalamic nucleus and the dorsal hypothalamic area (Fig. 4). Skin cooling episodes following the saline injections into the DMH consistently evoked increases in EMG, TBAT and HR (Fig. 4). Following subsequent injections of muscimol into the same bilateral sites in the DMH, skin cooling failed to evoke any of the shivering, non-shivering BAT thermogenic or cardiac responses (Fig. 4). The blockade of skin cooling-evoked BAT thermogenesis and tachycardia by inhibition of DMH neurons is consistent with our previous study (Nakamura & Morrison, 2007).
A previous study reported that cooling-evoked shivering can be inhibited by injecting muscimol not only into the DMH, but also into the PH (Tanaka et al. 2001). Since their injection volume was large (0.3 μl; Tanaka et al. 2001), the muscimol targeting the PH is likely to have also diffused into the DMH. To resolve this question, we tested the effect of smaller injections (60 nl site−1) of muscimol into the PH on skin cooling-evoked shivering. Bilateral muscimol injections into the PH attenuated skin cooling-evoked increases in EMG by 26.4 ± 9.9% (n = 5) of the EMG responses evoked by pre-injection cooling episodes (Fig. 4B). This inhibition of cooling-evoked shivering was significantly lower than that by muscimol injections of the same volume made into the DMH (98.8 ± 1.0%, n = 5, P < 0.0001, two-tailed unpaired t test; Fig. 4B). These results indicate that skin cooling-evoked shivering requires activation of DMH neurons and such shivering-mediating neurons are localized mostly in the DMH rather than in the PH.
We next determined whether inhibition of DMH neurons also blocks shivering evoked by a pyrogenic stimulation. Nanoinjection of the pyrogenic mediator PGE2 into the MPO evoked a remarkable increase in EMG activity (at the first saline injection into the DMH, +1233 ± 568% of the baseline level, n = 7), accompanied by strong shivering responses, by activation of non-shivering thermogenesis in BAT detected as an elevation of TBAT (+0.8 ± 0.2°C), by tachycardia (+55 ± 11 bpm) and by an increase in MAP (+15 ± 3 mmHg) (Fig. 5A). Bilateral injections of saline into the DMH during the PGE2-evoked febrile responses had no significant effect, whereas subsequent bilateral injections of muscimol into the DMH immediately reversed all these elevated physiological parameters (Fig. 5). These results indicate that DMH neurons mediate febrile signalling, as well as skin cooling signalling, from the POA leading to shivering and to non-shivering thermogenic and cardiovascular responses.
Cold-defensive and febrile shivering responses require activation of rostral medullary raphe neurons and are modulated by activation of local 5-HT1A receptors
We then examined the involvement of neurons in the rostral medullary raphe region in skin cooling-evoked shivering. Skin cooling episodes following nanoinjection of saline into the rostral medullary raphe region centred on the rRPa consistently evoked increases in EMG, TBAT and HR (Fig. 6A and C–E). In contrast, these cold-defensive physiological responses were never evoked by subsequent skin cooling episodes after nanoinjection of muscimol into the same site (Fig. 6A and C–E).
Skin cooling-evoked BAT thermogenesis and tachycardia are inhibited by activation of 5-HT1A receptors in the rostral medullary raphe region (Nakamura & Morrison, 2007). In the present study, therefore, we examined whether 5-HT1A receptors in this region might also modulate the central shivering mechanism. Whereas skin cooling episodes after saline injection into the rRPa increased EMG, TBAT and HR, those after injection into the rRPa of 8-OH-DPAT, a 5-HT1A receptor agonist, failed to evoke such responses (Fig. 6B, C and F).
We also examined the involvement of rostral medullary raphe neurons in febrile shivering responses. Increases in EMG, TBAT, HR and MAP evoked by injection of PGE2 into the MPO were not affected by saline injection into the rRPa, whereas subsequent injection of muscimol into the same site immediately reversed these increased physiological parameters (Fig. 7A, C and D). Nanoinjection of 8-OH-DPAT during the febrile responses to PGE2 into the MPO also reversed the elevated EMG, TBAT, HR and MAP to their baseline levels (Fig. 7B, C and E). These results indicate that both cold-defensive and febrile shivering responses require activation of rostral medullary raphe neurons and can be modulated by serotonergic inputs to this raphe region through 5-HT1A receptors.
Consistent with this notion, stimulation of rostral medullary raphe neurons with local nanoinjection of NMDA elicited increases in EMG, TBAT, HR and MAP (Fig. 8A and C). We used the amplitude of the EMG responses evoked by NMDA injections made in ventromedial medullary sites to identify the rostral medullary raphe sites where shivering-mediating neurons are localized (Fig. 8B). The most responsive sites were located near the midline in the region of the raphe pallidus nucleus between 2.3 and 3.5 mm caudal to the interaural line, corresponding to an antero-posterior level between 400 μm caudal to 800 μm rostral to the caudal border of the facial nucleus. Strong shivering responses could also be occasionally elicited from sites at the lateral border of the pyramid. Shivering was never elicited at midline sites within 2 mm of the dorsal surface of the medulla, nor at most sites in the lateral paragigantocellular nucleus.
Discussion
The present investigation used an in vivo electrophysiological technique to provide a model (Fig. 9) of the efferent neuronal pathway from the POA that leads to shivering in skeletal muscles. Our present findings highlight the role of neurons in the POA, DMH and rRPa in the efferent mechanisms for eliciting shivering responses to environmental cooling and to infection or inflammation. These brain regions also mediate skin cooling-evoked and pyrogen-evoked non-shivering BAT thermogenic responses and cardiovascular responses (Nakamura et al. 2002, 2004, 2005b; Morrison, 2003; Madden & Morrison, 2003, 2004; Zaretskaia et al. 2003; Nakamura & Morrison, 2007, 2008b). In the present study, we recorded the nuchal EMG activity simultaneously with TBAT, HR and arterial pressure to compare the effects of chemical modulations in localized brain sites on the shivering responses and the non-shivering thermogenic and cardiovascular responses evoked by cooling or pyrogenic stimulation. We observed that the drug injections made in these brain regions exerted comparable effects on these physiological parameters. Because shivering is driven through the somatomotor system and BAT thermogenesis and cardiovascular responses are controlled through the sympathetic system, this observation raises the interesting notion that these two different motor systems are governed by parallel and co-localized central efferent thermoregulatory mechanisms when strong heat production is demanded.
Figure 9. Schematic model of the mechanism for shivering responses to skin cooling and infection.
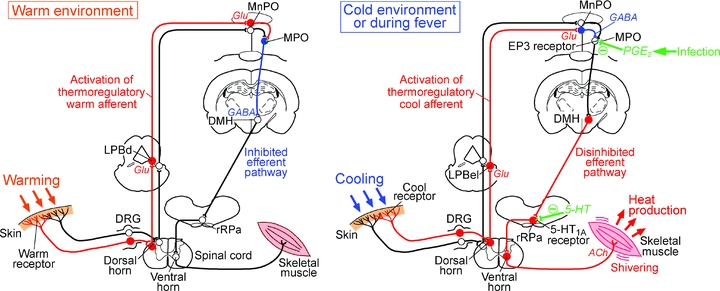
In a warm environment (left), cutaneous warm receptors are activated and the warm sensory signals ascend to the POA through the dorsal horn and the dorsal part of the lateral parabrachial nucleus (LPBd) and activate GABAergic projection neurons in the MPO, which then halt the efferent output by inhibiting DMH neurons. In a cold environment (right), cutaneous cool receptors are activated and the cool sensory signals ascend to the POA through the dorsal horn and the external lateral part of the lateral parabrachial nucleus (LPBel) and activate GABA neurons in the MnPO, which then inhibit the GABAergic projection neurons in the MPO. Resultant disinhibition of DMH neurons leads to activation of neurons in the rRPa, which finally activate the somatomotor output from ventral horn neurons leading to shivering. In the case of infection, PGE2, which is produced in brain vasculature, attenuates the tonic activity of the GABAergic projection neurons in the MPO through EP3 receptors and thereby disinhibits DMH neurons, which drive the febrile shivering output through the rRPa and ventral horn. Serotonin input to the rRPa can inhibit the activity of the premotor neurons through 5-HT1A receptors. Filled red, filled blue and open black circles denote cell bodies of activated excitatory neurons, activated inhibitory neurons and suppressed or resting neurons, respectively. DRG, dorsal root ganglion.
Although skin cooling evoked parallel changes in EMG, TBAT and HR, the onset of the increase in EMG activity was, in most cases, slightly delayed in comparison to those in TBAT and HR (see Fig. 2A). This observation is consistent with the possibility that the threshold skin temperature to trigger a shivering response is lower than those required to trigger BAT thermogenesis and tachycardia. In this case, the different neuronal populations in the POA, DMH and rRPa that mediate shivering, non-shivering BAT thermogenesis and cardiovascular responses would exhibit different skin temperature thresholds for the alteration of their discharge, consistent with similar conclusions drawn from experiments with centrally positioned thermodes (Banet et al. 1978). In this regard, we cannot eliminate the possibility that the magnitude of the differences in thermal thresholds for the activation of different thermal effectors may be influenced by anaesthesia. Given the role of somatic muscles in movement and posture, as well as in shivering thermogenesis, somatic shivering responses may also be selectively influenced by the activity of neurons in brain regions other than those studied here.
Based on the present findings, the local circuitry in the POA could be considered as a command centre for the regulation of cold-defensive shivering. In the present study, inhibition of MnPO neurons blocked skin cooling-evoked shivering EMG responses and stimulation of MnPO neurons elicited shivering EMG responses. These results are consistent with our previous finding that skin cooling afferent signalling elicits shivering responses mediated through the lateral parabrachial–MnPO pathway (Nakamura & Morrison, 2008a; see also Fig. 9). These lines of evidence indicate an essential role of MnPO neurons in the central reception and integration of cutaneous cool afferent signals for triggering cold-defensive shivering.
The present result that the shivering response to stimulation of MnPO neurons was eliminated by antagonizing GABAA receptors in the MPO indicates that the shivering efferent mechanism that is triggered by the cool afferent-mediated activation of MnPO neurons requires GABAergic inhibition of MPO neurons. This conclusion is supported by the findings that antagonizing GABAA receptors in the MPO blocks cooling-evoked shivering (Osaka, 2004) and that inhibition of MPO neurons elicits a shivering response, whereas stimulation of these neurons inhibits cooling-evoked shivering (Zhang et al. 1995; Osaka, 2004). Many neurons whose firing rates increase in response to local warming (warm-sensitive neurons) can be electrophysiologically identified in the POA (Nakayama et al. 1961; Boulant & Hardy, 1974) and are predominantly GABAergic (Lundius et al. 2010). Local cooling and warming in the POA elicit and inhibit shivering, respectively (Hammel et al. 1960; Kanosue et al. 1991; Zhang et al. 1995). Furthermore, the MPO contains GABAergic neurons that innervate the DMH neurons projecting to the rRPa (Nakamura et al. 2005b). These lines of evidence support a model (Fig. 9) in which cutaneous cool signals disinhibit shivering-promoting DMH neurons by attenuating the tonic descending inhibition that they receive from GABAergic projection neurons, potentially warm-sensitive neurons, in the MPO. Based on the present results, we propose that such cooling-induced attenuation of the tonic activity of inhibitory MPO neurons is mediated by local GABAergic inhibition from the MnPO neurons that are activated by cutaneous cool afferent signals (Fig. 9). The idea that MnPO neurons provide a local GABAergic connection to the MPO is also supported by previous anatomical observations that the MnPO contains many GABAergic neurons (Nakamura et al. 2002; Gong et al. 2004) and some MnPO neurons innervate the MPO (Uschakov et al. 2007). Furthermore, many neurons in the MnPO, rather than the MPO or lateral POA, are activated (express Fos protein) in response to reduced environmental temperature (Bratincsák & Palkovits, 2004) and the extracellular level of GABA in the POA is elevated during cold exposure and reduced during heat exposure in free-moving rats (Ishiwata et al. 2005).
Shivering (i.e. chills) thermogenesis is a well-recognized component of the febrile response to infection and inflammation and the central application of PGEs evokes shivering as one of the physiological responses to fever (Cooper et al. 1976). We observed a robust shivering response evoked by an injection of PGE2 into the MPO. The MPO and MnPO contain many neurons that express the EP3 subtype of PGE receptor (Nakamura et al. 1999, 2000), which mediates the febrile action of PGE2 (Lazarus et al. 2007). This metabotropic receptor is mostly coupled to Gi, an inhibitory GTP-binding protein (Narumiya et al. 1999), and most of the neurons that express this receptor are GABAergic (Nakamura et al. 2002). Furthermore, there is a projection from EP3 receptor-expressing POA neurons to the DMH (Nakamura et al. 2005b, 2009). Together, these observations are consistent with skin cooling or administration of PGE2 into the MPO leading to a disinhibition of the DMH neurons whose activation then leads to an excitatory drive for shivering (Fig. 9), although the source(s) of excitatory inputs to shivering-promoting neurons in the DMH is unknown. Shivering-mediating neurons seem to be focally distributed within the DMH, since nanoinjections of muscimol into the DMH, but not the nearby PH, eliminated shivering responses. Supporting this localization, neural tracing studies have shown that rRPa-projecting neurons cluster in the region consisting of the dorsal hypothalamic area and the dorsal tip of the dorsomedial hypothalamic nucleus, but such neurons are few in the PH (Hosoya et al. 1987; Samuels et al. 2004; Nakamura et al. 2005b).
Such rRPa-projecting neurons in the DMH potentially provide an excitatory input to premotor neurons in the rostral medullary raphe region that mediate shivering (Fig. 9), although our data do not rule out the possibility that neurons in an intermediate region mediate the signalling from the DMH to the raphe region. The present results indicate that skin cooling-evoked and pyrogen-evoked shivering responses are dependent on activation of neurons in the rostral medullary raphe region including the rRPa. The neurons whose activation leads to shivering are distributed primarily in the rRPa with a rostrocaudal distribution from the middle of the facial nucleus to the rostral pole of the inferior olivary complex, as well as in the parapyramidal region immediately dorsolateral to the pyramidal tract. This region overlaps with those reported in earlier studies to play a role in shivering (Nason & Mason, 2004; Brown et al. 2008) and with that identified with muscimol injection mapping to mediate PGE2-evoked BAT thermogenesis and rise in core temperature (Nakamura et al. 2002). There is a monosynaptic innervation of spinal somatomotor neurons from this medullary region (Zagon & Bacon, 1991) and both serotonergic and non-serotonergic neurons in this medullary region project to the ventral horn (Allen & Cechetto, 1994). The spinally projecting neurons in this ventromedial medullary location of putative shivering premotor neurons include many of vesicular glutamate transporter 3-expressing sympathetic premotor neurons that control BAT and skin blood vessels and are activated in response to cold exposure or central administration of PGE2 (Nakamura et al. 2004, 2005a). This medullary region also contains serotonergic neurons that are multi-synaptically connected to BAT and are activated during cold exposure (Cano et al. 2003). The neurochemical phenotypes of the medullospinal somatic premotor neurons for shivering remain to be determined.
There are several observations suggesting the existence of medullary raphe neurons that are involved in the control of both the sympathetic and somatomotor systems. A retrograde tracing study reported that some rostral medullary raphe neurons send their axon collaterals both to the ventral horn and to the thoracic intermediolateral cell column, where sympathetic preganglionic neurons are located (Allen & Cechetto, 1994). Furthermore, a trans-synaptic tracing study using two different pseudorabies virus strains showed that some neurons in the rostral medullary raphe region multi-synaptically innervate both the gastrocnemius muscle and adrenal gland, although such neurons tend to be distributed slightly dorsal to the raphe region that we found to mediate shivering (Kerman et al. 2003). Such medullary raphe neurons could contribute to the parallel changes in shivering activity and the sympathetic parameters that we observed in the present study. On the other hand, the different thermal thresholds and the different latencies to activation for shivering and for sympathetic thermoregulatory responses support the existence of target-specific components within efferent pathways mediating cold-defence responses.
Activation of 5-HT1A receptors in the rostral medullary raphe region eliminated shivering responses to skin cooling and to PGE2 injection into the MPO. The 5-HT1A receptor is negatively coupled to adenylate cyclase via Gi proteins probably leading to inhibition of neuronal functions (Barnes & Sharp, 1999) and these receptors are located on spinal-projecting neurons in the medullary raphe region (Helke et al. 1997), consistent with the potential localization of 5-HT1A receptors on the cell bodies of putative shivering-mediating premotor neurons in the rRPa. Application of 8-OH-DPAT in the piglet medullary raphe or in the lateral paragigantocellular nucleus attenuated cooling-evoked shivering (Hoffman et al. 2007; Brown et al. 2008), although the time course of the microdialysis application allowed for the possibility that the 8-OH-DPAT applied in the lateral paragigantocellular nucleus diffused into the rostral medullary raphe region to inhibit shivering. The present immediate and complete elimination of the shivering responses by volume-controlled nanoinjection of 8-OH-DPAT into the midline site of the rRPa suggests that the major site of action of this agonist was in the rostral medullary raphe region.
Systemic or central administration of 5-HT1A receptor agonists induces hypothermia in humans and rodents (Goodwin et al. 1985; Hjorth, 1985; Blier et al. 2002). We have reported that 8-OH-DPAT injection into the rRPa eliminates BAT thermogenic, metabolic and tachycardic responses to intravenous administration of leptin or skin cooling (Morrison, 2004; Nakamura & Morrison, 2007). Here, we showed that the same injection reverses febrile BAT thermogenic and tachycardic responses to PGE2 into the POA. Application of 8-OH-DPAT into the rRPa also disrupts sleep (Brown et al. 2008). Together with the present blockade of shivering responses with 8-OH-DPAT, these observations suggest that serotonergic neurotransmission through 5-HT1A receptors in the rostral medullary raphe region has an important modulatory role in many homeostatic functions. The source(s) of serotonergic input to these receptors remains to be identified.
The frequency of the bursts of spikes in the shivering nuchal EMG discharge elicited by a physiological stimulus (i.e. skin and core cooling) was the same as that evoked by central injection of PGE2 into the POA or by excitation of neurons in the rRPa with NMDA. This frequency (∼33 Hz) is in close agreement with that (∼31 Hz) determined by Günther et al. (1983) from an EMG analysis during cold tremor in rat leg muscles. This frequency is markedly higher than the burst frequency (∼4 Hz) of the sympathetic nerve discharge recorded from the input to interscapular BAT (Morrison, 1999). Our results suggest that the neurochemically evoked activations of EMG discharge via injections of NMDA into the rRPa engaged the same central neural network responsible for the rhythmic, high-frequency bursting in the EMG as that mediating physiologically evoked shivering. Since NMDA stimulation of rRPa neurons produces a rhythmic EMG bursting indistinguishable from that during physiological shivering and activation of neurons in the rRPa is required for physiological shivering, it is likely that the neural network ‘oscillator’ for shivering is located within the medulla and/or the spinal cord. The early demonstration that cold-evoked shivering has the same frequency in intact and spinally transected animals (Simon, 1974) suggests a cold-sensing mechanism within the spinal cord in addition to cutaneous thermoreception and is consistent with a spinal localization of those components of the shivering efferent circuit that determine the frequency of the shivering EMG bursting pattern.
Shivering has a sustained, low-intensity component, i.e. ‘thermal muscular tone’ preceding overt shivering (Burton & Bronk, 1937), mediated by low-threshold, type I, lipid fuel-preferring muscle fibres as well as phasic, high-intensity bursts due to high-threshold, type II, carbohydrate-utilizing muscle fibres (Haman, 2006). The balance between bursts of high-intensity shivering and continuous, low-intensity shivering is critical for determining the fuel used by muscle to sustain shivering thermogenesis (Haman, 2006) and this balance and the frequency of high-intensity shivering bursts are strongly influenced by the size of the available glycogen stores (Haman et al. 2005). The complexity of such coordination of the differential activation of different pools of α-motoneurons during shivering, and of the influence of the available fuel sources on this coordination suggests that these critical aspects of the control of shivering thermogenesis (1) are mediated within the central neural circuits providing the excitatory drive to α-motoneurons during shivering, (2) involve metabolic afferent signalling that impinges on these pathways and (3) could be influenced by a sympathetic efferent regulation of muscle metabolism (Braun & Marks, 2011). The present identification of the principal synaptic integration sites in the generation of shivering and their overlap with central sites regulating sympathetic outflow to thermoregulatory effectors provides insight into potential sites at which metabolic signals may influence the pattern of muscle recruitment during shivering.
In summary, our demonstration of the essential roles of neurons in the MPO, MnPO, DMH and rostral medullary raphe region in the efferent neuronal circuits driving cold-defensive and febrile shivering responses supports the model of the central efferent pathways for shivering illustrated in Fig. 9. The present results not only validate the basic framework (hypothalamic control of supraspinal premotor neurons) of an earlier model (Nagashima et al. 2000; Romanovsky, 2007), but the model proposed here also significantly enhances the anatomical, neurotransmitter and functional specificity of the synaptic integration sites within both the central efferent pathway for shivering and the central afferent pathway conveying cutaneous thermal information to the preoptic area (Nakamura & Morrison, 2008a,b;). We look for future models to include mechanisms underlying the coordination of the shivering and non-shivering thermogenic and cardiovascular responses to cold and febrile stimuli comprising the integrated physiological repertoire that maintains homeostatic brain and body temperatures.
Acknowledgments
The authors thank Chika Tanizawa and Jane Igoe for anatomical assistance. This study was supported by a Special Coordination Fund for Promoting Science and Technology (to K.N.) and Grants-in-Aid for Scientific Research (21890114 and 22689007 to K.N.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, by the Funding Program for Next Generation World-Leading Researchers from the Japan Society for the Promotion of Science (LS070 to K.N.), by the US National Institutes of Health grant (NS40987 to S.F.M.) and by grants from the Nakajima Foundation, Takeda Science Foundation and Kowa Life Science Foundation (to K.N.).
Glossary
Abbreviations
- BAT
brown adipose tissue
- DMH
dorsomedial hypothalamus
- HR
heart rate
- MAP
mean arterial pressure
- MnPO
median preoptic nucleus
- MPO
medial preoptic area
- PG
prostaglandin
- PH
posterior hypothalamus
- POA
preoptic area
- rRPa
rostral raphe pallidus nucleus
- TBAT
brown adipose tissue temperature
Author contributions
The experiments were performed both in Kyoto University and Oregon Health and Science University. Both authors designed the study, carried out the experiments, analysed the data, wrote the paper and approved the final version for publication.
References
- Allen GV, Cechetto DF. Serotoninergic and nonserotoninergic neurons in the medullary raphe system have axon collateral projections to autonomic and somatic cell groups in the medulla and spinal cord. J Comp Neurol. 1994;350:357–366. doi: 10.1002/cne.903500303. [DOI] [PubMed] [Google Scholar]
- Banet M, Hensel H, Liebermann H. The central control of shivering and non-shivering thermogenesis in the rat. J Physiol. 1978;283:569–584. doi: 10.1113/jphysiol.1978.sp012520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Blier P, Seletti B, Gilbert F, Young SN, Benkelfat C. Serotonin 1A receptor activation and hypothermia in humans: lack of evidence for a presynaptic mediation. Neuropsychopharmacology. 2002;27:301–308. doi: 10.1016/S0893-133X(02)00318-4. [DOI] [PubMed] [Google Scholar]
- Boulant JA, Hardy JD. The effect of spinal and skin temperatures on the firing rate and thermosensitivity of preoptic neurones. J Physiol. 1974;240:639–660. doi: 10.1113/jphysiol.1974.sp010627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratincsák A, Palkovits M. Activation of brain areas in rat following warm and cold ambient exposure. Neuroscience. 2004;127:385–397. doi: 10.1016/j.neuroscience.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Braun TP, Marks DL. Hypothalamic regulation of muscle metabolism. Curr Opin Clin Nutr Metab Care. 2011;14:237–242. doi: 10.1097/MCO.0b013e328345bbcd. [DOI] [PubMed] [Google Scholar]
- Brown JW, Sirlin EA, Benoit AM, Hoffman JM, Darnall RA. Activation of 5-HT1A receptors in medullary raphé disrupts sleep and decreases shivering during cooling in the conscious piglet. Am J Physiol Regul Integr Comp Physiol. 2008;294:R884–R894. doi: 10.1152/ajpregu.00655.2007. [DOI] [PubMed] [Google Scholar]
- Burton AC, Bronk DW. The motor mechanism of shivering and of thermal muscular tone. Am J Physiol. 1937;119:284. [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- Chen XM, Hosono T, Yoda T, Fukuda Y, Kanosue K. Efferent projection from the preoptic area for the control of non-shivering thermogenesis in rats. J Physiol. 1998;512:883–892. doi: 10.1111/j.1469-7793.1998.883bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper KE, Preston E, Veale WL. Effects of atropine, injected into a lateral cerebral ventricle of the rabbit, on fevers due to intravenous leucocyte pyrogen and hypothalamic and intraventricular injections of prostaglandin E1. J Physiol. 1976;254:729–741. doi: 10.1113/jphysiol.1976.sp011255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong H, McGinty D, Guzman-Marin R, Chew KT, Stewart D, Szymusiak R. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J Physiol. 2004;556:935–946. doi: 10.1113/jphysiol.2003.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin GM, De Souza RJ, Green AR. The pharmacology of the hypothermic response in mice to 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT): a model of presynaptic 5-HT1 function. Neuropharmacology. 1985;24:1187–1194. doi: 10.1016/0028-3908(85)90153-4. [DOI] [PubMed] [Google Scholar]
- Günther H, Brunner R, Klussmann FW. Spectral analysis of tremorine and cold tremor electromyograms in animal species of different size. Pflügers Arch. 1983;399:180–185. doi: 10.1007/BF00656712. [DOI] [PubMed] [Google Scholar]
- Haman F. Shivering in the cold: from mechanisms of fuel selection to survival. J Appl Physiol. 2006;100:1702–1708. doi: 10.1152/japplphysiol.01088.2005. [DOI] [PubMed] [Google Scholar]
- Haman F, Péronnet F, Kenny GP, Massicotte D, Lavoie C, Weber JM. Partitioning oxidative fuels during cold exposure in humans: muscle glycogen becomes dominant as shivering intensifies. J Physiol. 2005;566:247–256. doi: 10.1113/jphysiol.2005.086272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel HT, Hardy JD, Fusco MM. Thermoregulatory responses to hypothalamic cooling in unanesthetized dogs. Am J Physiol. 1960;198:481–486. doi: 10.1152/ajplegacy.1960.198.3.481. [DOI] [PubMed] [Google Scholar]
- Helke CJ, Capuano S, Tran N, Zhuo H. Immunocytochemical studies of the 5-HT1A receptor in ventral medullary neurons that project to the intermediolateral cell column and contain serotonin or tyrosine hydroxylase immunoreactivity. J Comp Neurol. 1997;379:261–270. [PubMed] [Google Scholar]
- Hjorth S. Hypothermia in the rat induced by the potent serotoninergic agent 8-OH-DPAT. J Neural Transm. 1985;61:131–135. doi: 10.1007/BF01253058. [DOI] [PubMed] [Google Scholar]
- Hoffman JM, Brown JW, Sirlin EA, Benoit AM, Gill WH, Harris MB, Darnall RA. Activation of 5-HT1A receptors in the paragigantocellularis lateralis decreases shivering during cooling in the conscious piglet. Am J Physiol Regul Integr Comp Physiol. 2007;293:R518–R527. doi: 10.1152/ajpregu.00816.2006. [DOI] [PubMed] [Google Scholar]
- Hosoya Y, Ito R, Kohno K. The topographical organization of neurons in the dorsal hypothalamic area that project to the spinal cord or to the nucleus raphé pallidus in the rat. Exp Brain Res. 1987;66:500–506. doi: 10.1007/BF00270682. [DOI] [PubMed] [Google Scholar]
- Ishiwata T, Saito T, Hasegawa H, Yazawa T, Kotani Y, Otokawa M, Aihara Y. Changes of body temperature and thermoregulatory responses of freely moving rats during GABAergic pharmacological stimulation to the preoptic area and anterior hypothalamus in several ambient temperatures. Brain Res. 2005;1048:32–40. doi: 10.1016/j.brainres.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Jubrias SA, Vollestad NK, Gronka RK, Kushmerick MJ. Contraction coupling efficiency of human first dorsal interosseous muscle. J Physiol. 2008;586:1993–2002. doi: 10.1113/jphysiol.2007.146829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanosue K, Niwa K, Andrew PD, Yasuda H, Yanase M, Tanaka H, Matsumura K. Lateral distribution of hypothalamic signals controlling thermoregulatory vasomotor activity and shivering in rats. Am J Physiol Regul Integr Comp Physiol. 1991;260:R486–R493. doi: 10.1152/ajpregu.1991.260.3.R486. [DOI] [PubMed] [Google Scholar]
- Kerman IA, Enquist LW, Watson SJ, Yates BJ. Brainstem substrates of sympatho-motor circuitry identified using trans-synaptic tracing with pseudorabies virus recombinants. J Neurosci. 2003;23:4657–4666. doi: 10.1523/JNEUROSCI.23-11-04657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10:1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- Lundius EG, Sanchez-Alavez M, Ghochani Y, Klaus J, Tabarean IV. Histamine influences body temperature by acting at H1 and H3 receptors on distinct populations of preoptic neurons. J Neurosci. 2010;30:4369–4381. doi: 10.1523/JNEUROSCI.0378-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Excitatory amino acid receptor activation in the raphe pallidus area mediates prostaglandin-evoked thermogenesis. Neuroscience. 2003;122:5–15. doi: 10.1016/s0306-4522(03)00527-x. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Excitatory amino acid receptors in the dorsomedial hypothalamus mediate prostaglandin-evoked thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2004;286:R320–R325. doi: 10.1152/ajpregu.00515.2003. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Cao C, Ozaki M, Morii H, Nakadate K, Watanabe Y. Brain endothelial cells express cyclooxygenase-2 during lipopolysaccharide-induced fever: light and electron microscopic immunocytochemical studies. J Neurosci. 1998;18:6279–6289. doi: 10.1523/JNEUROSCI.18-16-06279.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF. RVLM and raphe differentially regulate sympathetic outflows to splanchnic and brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 1999;276:R962–R973. doi: 10.1152/ajpregu.1999.276.4.R962. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Raphe pallidus neurons mediate prostaglandin E2-evoked increases in brown adipose tissue thermogenesis. Neuroscience. 2003;121:17–24. doi: 10.1016/s0306-4522(03)00363-4. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Activation of 5-HT1A receptors in raphe pallidus inhibits leptin-evoked increases in brown adipose tissue thermogenesis. Am J Physiol Regul Integr Comp Physiol. 2004;286:R832–R837. doi: 10.1152/ajpregu.00678.2003. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci. 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K, Nakai S, Tanaka M, Kanosue K. Neuronal circuitries involved in thermoregulation. Auton Neurosci. 2000;85:18–25. doi: 10.1016/S1566-0702(00)00216-2. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kaneko T, Yamashita Y, Hasegawa H, Katoh H, Ichikawa A, Negishi M. Immunocytochemical localization of prostaglandin EP3 receptor in the rat hypothalamus. Neurosci Lett. 1999;260:117–120. doi: 10.1016/s0304-3940(98)00962-8. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kaneko T, Yamashita Y, Hasegawa H, Katoh H, Negishi M. Immunohistochemical localization of prostaglandin EP3 receptor in the rat nervous system. J Comp Neurol. 2000;421:543–569. doi: 10.1002/(sici)1096-9861(20000612)421:4<543::aid-cne6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Hübschle T, Nakamura Y, Hioki H, Fujiyama F, Boldogköi Z, König M, Thiel HJ, Gerstberger R, Kobayashi S, Kaneko T. Identification of sympathetic premotor neurons in medullary raphe regions mediating fever and other thermoregulatory functions. J Neurosci. 2004;24:5370–5380. doi: 10.1523/JNEUROSCI.1219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J Neurosci. 2002;22:4600–4610. doi: 10.1523/JNEUROSCI.22-11-04600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Matsumura K, Kobayashi S, Kaneko T. Sympathetic premotor neurons mediating thermoregulatory functions. Neurosci Res. 2005a;51:1–8. doi: 10.1016/j.neures.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2007;292:R127–R136. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci. 2008a;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Preoptic mechanism for cold-defensive responses to skin cooling. J Physiol. 2008b;586:2611–2620. doi: 10.1113/jphysiol.2008.152686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci U S A. 2010;107:8848–8853. doi: 10.1073/pnas.0913358107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci. 2005b;22:3137–3146. doi: 10.1111/j.1460-9568.2005.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Morrison SF. Different populations of prostaglandin EP3 receptor-expressing preoptic neurons project to two fever-mediating sympathoexcitatory brain regions. Neuroscience. 2009;161:614–620. doi: 10.1016/j.neuroscience.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Eisenman JS, Hardy JD. Single unit activity of anterior hypothalamus during local heating. Science. 1961;134:560–561. doi: 10.1126/science.134.3478.560. [DOI] [PubMed] [Google Scholar]
- Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- Nason MW, Jr, Mason P. Modulation of sympathetic and somatomotor function by the ventromedial medulla. J Neurophysiol. 2004;92:510–522. doi: 10.1152/jn.00089.2004. [DOI] [PubMed] [Google Scholar]
- Osaka T. Cold-induced thermogenesis mediated by GABA in the preoptic area of anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R306–R313. doi: 10.1152/ajpregu.00003.2004. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th edn. London: Academic Press; 2007. [Google Scholar]
- Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol. 2007;292:R37–R46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- Samuels BC, Zaretsky DV, DiMicco JA. Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons. Am J Physiol Regul Integr Comp Physiol. 2004;287:R472–R478. doi: 10.1152/ajpregu.00667.2003. [DOI] [PubMed] [Google Scholar]
- Simon E. Temperature regulation: the spinal cord as a site of extrahypothalamic thermoregulatory functions. Rev Physiol Biochem Pharmacol. 1974;71:1–76. doi: 10.1007/BFb0027660. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Tonouchi M, Hosono T, Nagashima K, Yanase-Fujiwara M, Kanosue K. Hypothalamic region facilitating shivering in rats. Jpn J Physiol. 2001;51:625–629. doi: 10.2170/jjphysiol.51.625. [DOI] [PubMed] [Google Scholar]
- Uschakov A, Gong H, McGinty D, Szymusiak R. Efferent projections from the median preoptic nucleus to sleep- and arousal-regulatory nuclei in the rat brain. Neuroscience. 2007;150:104–120. doi: 10.1016/j.neuroscience.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Yanase-Fujiwara M, Hosono T, Kanosue K. Warm and cold signals from the preoptic area: which contribute more to the control of shivering in rats? J Physiol. 1995;485:195–202. doi: 10.1113/jphysiol.1995.sp020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagon A, Bacon SJ. Evidence of a monosynaptic pathway between cells of the ventromedial medulla and the motoneuron pool of the thoracic spinal cord in rat: electron microscopic analysis of synaptic contacts. Eur J Neurosci. 1991;3:55–65. doi: 10.1111/j.1460-9568.1991.tb00811.x. [DOI] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, DiMicco JA. Role of the dorsomedial hypothalamus in thermogenesis and tachycardia caused by microinjection of prostaglandin E2 into the preoptic area in anesthetized rats. Neurosci Lett. 2003;340:1–4. doi: 10.1016/s0304-3940(03)00047-8. [DOI] [PubMed] [Google Scholar]


