Abstract
Introduction
To date, there is no evidence that conventional remineralization techniques using calcium and phosphate ion- containing media will completely remineralize carious lesions in regions where remnant apatite seed crystallites are absent. Conversely, guided tissue remineralization using biomimetic analogs of dentin matrix proteins is successful in remineralizing thin layers of completely demineralized dentin.
The hypothesis
Conventional remineralization strategy depends on epitaxial growth over existing apatite crystallites. If there are no or few crystallites, there will be no remineralization. Guided tissue remineralization uses biomimetic analogs of dentin matrix proteins to introduce sequestered amorphous calcium phosphate nanoprecursors into the internal water compartments of collagen fibrils. Attachment of templating analogs of matrix phosphoproteins to the collagen fibrils further guided the nucleation and growth of apatite crystallites within the fibril. Such a strategy is independent of apatite seed crystallites. Our hypothesis is that 250–300 microns thick artificial carious lesions can be completely remineralized in vitro by guide tissue remineralization but not by conventional remineralization techniques.
Evaluation of the hypothesis
Validation of the hypothesis will address the critical barrier to progress in remineralization of caries- affected dentin and shift existing paradigms by providing a novel method of remineralization based on a nanotechnology-based bottom-up approach. This will also generate important information to support the translation of the proof-of-concept biomimetic strategy into a clinically-relevant delivery system for remineralizing caries-affected dentin created by micro-organisms in the oral cavity.
Keywords: Biomimetic, Caries-affected dentin, Guided tissue remineralization, Intrafibrillar remineralization
Introduction
The hallmark of minimally invasive dentistry is conservative treatment of carious teeth to preserve their potential for remineralization. After caries excavation, the clinical bonding substrate is likely to be a combination of normal dentin in the periphery and caries-affected dentin in the center of the lesion. Mineral distribution of caries-affected dentin is highly variable and the lesion depth can extend hundreds of microns below the excavated surface [1]. Unlike caries-infected dentin that is denatured, the collagen matrix of caries-affected dentin demonstrates cross-banding when examined using transmission electron microscopy (TEM) [2] and is physiologically remineralizable [3, 4].
Conventional remineralization of artificial carious dentin often involves the use of calcium and phosphate ion-containing solutions in presence of various concentrations of fluoride [5, 6]. It is well established that conventional remineralization does not occur by spontaneous nucleation of mineral on the organic matrix but rather by growth of residual apatite seed crystallites in the partially demineralized carious dentin [7–9]. The mineral content of the lesion surface layer influences the characteristics of subsequent remineralization, including the location and density of mineral deposition [5]. Although fluoride enhances mineral uptake, it causes hypermineralization of the lesion surface [5, 10] and prevents effective remineralization of the deeper parts of the carious lesion [6]. Thus, slightly elevated fluoride levels is considered less effective in preventing lesion progression in dentin than in enamel [11].
The mineral phase in mineralized dentin is classified as intrafibrillar and extra-fibrillar. Intrafibrillar apatites are deposited within the gap zones of collagen fibril and extend along the microfibrillar spaces within the fibril. Extrafibrillar apatites are deposited within the interstitial spaces separating the collagen fibrils [12–14]. Intrafibrillar mineralization contributes significantly to the mechanical properties of dentin [15]. The end-point for assessing success or failure of remineralization by mineral density alone [16, 17] has been challenged as heterogeneous precipitation of extrafibrillar apatite contributes minimally to the mechanical properties of remineralized dentin [18, 19]. Transmission electron microscopy is required to provide the resolution for differentiation between intrafibrillar and extrafibrillar minerals within the collagen matrix [12, 13].
Guide tissue remineralization (GTR) represents a novel strategy in collagen biomineralization. This strategy utilizes nanotechnology and biomimetic principles to achieve intrafibrillar and extrafibrillar remineralization of a collagen matrix in the absence of apatite seed crystallites [20, 21]. In this strategy, two polyanionic analogs are involved to mimic the sequestration and templating functions of matrix proteins in biomineralization. This particle-mediated [22], bottom-up [23] mineralization strategy is different from conventional remineralization techniques currently employed in dentistry in two aspects. Firstly, it is a biomimetic process that recapitulates the progressive dehydration mechanism of natural biomineralization [24] by replacing free and loosely-bound water within a collagen matrix by apatite crystallites via the use of polyanion-stabilized amorphous calcium phosphate nanoprecursors [25]. Secondly, this particle-based assembly approach proceeds in the absence of apatite seed crystallites in a collagen matrix [21]. Whereas epitaxial growth over existing seed crystallites is a thermodynamically more favorable process [26], mineralization in the absence of seed crystallites requires alternative kinetically-driven protein/polymer-modulated pathways for lowering the activation energy barrier for crystal nucleation via sequential steps of phase transformations, as depicted by non-classical crystallization theory [27].
In guided tissue remineralization, a polycarboxylic acid-based biomimetic analog is employed as a sequestration agent to stabilize amorphous calcium phosphate derived from set Portland cement and a simulated body fluid in the form of nanoparticles that are moldable enough to infiltrate the internal water compartments of a collagen fibril (Figure 1). A phosphorus-based analog of matrix phosphoproteins is also attached to the collagen via electrostatic binding or chemical phosphorylation mechanisms to attract these nanoprecursors to the gap zones between the collagen molecules (Figure 2). Self assembly of the amorphous calcium phosphate nanoprecursors and their subsequent transformation into apatite nano-crystals result in intrafibrillar mineralization. This biomimetic remineralization process represents a bottom-up approach to create nanocrystals that are small enough to fit into the gap zones between adjacent collagen molecules, and to establish hierarchical order within the mineralized collagen. It is different from top-down remineralization strategies in that the latter invariably require the presence of a pre-established conformational order that is achieved by dentin noncollagenous proteins such as phosphophoryn and dentin matrix protein 1 during dentinogenesis. Partial demineralization of a mineralized collagen fibril by bacterial acids represents a top-down approach in generating apatite seed crystallites, with the latter encompassing the nanoscale details (e.g. crystalline lattice) for subsequent epitaxial growth. Expressed in conventional crystallization terminology, current remineralization strategies lack the mechanisms for inducing apatite nucleation and hierarchical assembly of apatites within a collagen matrix [28].
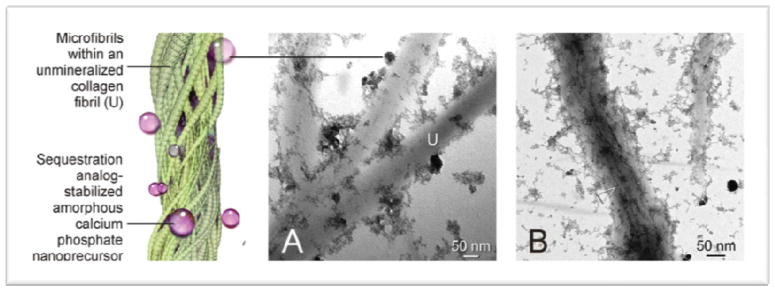
Figure 1.
Function of a sequestration biomimetic analog. A. Attachment of sequestration analog-stabilized amorphous calcium phosphate to unmineralized, reconstituted type I collagen fibrils (U). B. Infiltration of these nanoprecursors into the collagen fibrils results in the formation of electron-dense mineral strands (open arrowhead) along the microfibrils within the collagen fibrils.
Figure 2.
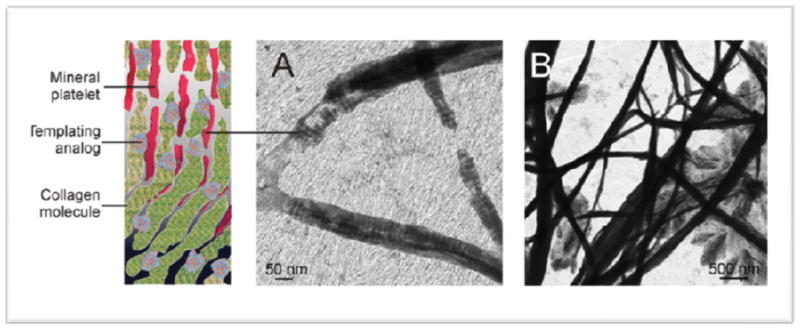
Function of a templating biomimetic analog of matrix phosphoprotein. A. Mineral deposition is guided by the attachment of templating analogs to the collagen molecules. B. Subsequent intrafibrillar mineralization of the collagen fibrils.
The hypothesis
As conventional remineralization strategies depend on epitaxial growth, a carious lesion with a high surface mineral content will differentially remineralize along the lesion surface. Restriction of ion diffusion creates a “self-strangulation” effect that prevents optimal remineralization of the basal part of the lesion. Conversely, a carious lesion with a low surface mineral content will result in remineralization of the basal part of the lesion only [5]. Thus, it is impossible to completely remineralize carious lesions using conventional remineralization strategies. Conversely, the particle-mediated guide tissue remineralization strategy is independent of seed crystallites and ion transport. In presence of appropriate biomimetic analogs, the initially formed metastable amorphous calcium phosphate is sequestrated into nanophases. In the presence of dentin matrix phosphoprotein analogs, these amorphous nanophases are directly towards the gap zones of collagen matrix, and subsequently transformed into crystalline apatite [20]. Both totally and partially demineralized dentin as well as reconstituted type I collagen fibrils that are completely devoid of matrix phosphoproteins have been successfully remineralized via this strategy [20, 21, 29]. For partially demineralized dentin, remineralization is achieved by a combination of epitaxial growth over remnant seed crystallites at the basal part of the collagen matrix and infiltration of fluid amorphous nanophases in top part of the matrix that is devoid of seed crystallites [29]. Therefore, it is reasonable to hypothesize that an artificial carious lesion that is 250–300 micron thick can be coletely remineralized by guide tissue remineralization, but not by conventional remineralization strategy in vitro.
Evaluation of the hypothesis
To date, no conventional remineralization studies have demonstrated complete remineralization of artificial carious lesions from lesion surface to the base. Moreover, studies based on transverse microradiography alone provides information only on mineral density and do not provide ultrastructural evidence of intrafibrillar remineralization that is critical for restoring the mechanical properties of the remineralized dentin matrix. Thus, we propose to test our hypothesis using a combination of mineral density assessment, transmission electron microscopy and evaluation of dynamic mechanical properties of the remineralized dentin.
We will create a dentin substrate that resembles caries-affected intertubular dentin via pH cycling for 14 days. This produces a 250–300 μm thick artificial carious lesion with a gradient of demineralization from the surface to the base of the lesion [30]. The artificial carious lesions will be randomly divided into two groups and remineralized for four months using a solution-based conventional remineralization technique and the guided tissue remineralization strategy, respectively. In previous studies on remineralization of carious dentin, transverse microradiography (TMR) has been used as a standardized method to assess changes in mineral density within the lesion. However, this method examines sections as thin as 100 pm [16, 17]. Recently, micro-computed tomography (micro-CT) has been used to examine mineralized tissues non-destructively in three dimensions and show similar changes as TMR in mineral density and lesion depth of the artificial carious lesions after remineralization [31]. Thus, we propose to examine 3-mm thick sections of remineralized dentin using a micro-CT to enable us to collect more information to validate our hypothesis. Transverse microradiography or micro-CT are valuable for longitudinal tracking of the changes in mineral density and lesion depth but cannot delineate between intrafibrillar and extrafibrillar apatite deposition. To date, such information is unavailable from remineralization studies as none of these studies incorporated an ultra-structural component in their evaluation protocols. The combination of hardness measurement and synchrotron micro-CT, for example, generates minimal information on how hardness is related to the mode of remineralization within the collagen matrix [32]. As intrafibrillar remineralization is critical for restoring the mechanical properties of remineralized dentin, we will use transmission electron microscopy to complement our micro-CT results. The latter has sufficient resolution to examine the dimension and hierarchy of apatite deposition within the remineralized collagen matrix [12, 13]. The adjunctive use of selected area electron diffracttion during transmission electron microscopy [20, 21] will identify whether the mineral phase produced after remineralization is apatite or other calcium phosphate phases such as octacalcium phosphate [32]. The use of a combined micro-CT and transmission electron microscopy approach will also delineate factors that may jeopardize the success of the guided tissue remineralization strategy, such as denaturation of the collagen matrix by endogenous matrix metalloproteinases [34, 35] before complete remineralization of the thick, partially demineralized collagen matrix is accomplished. This will generate valuable information on whether the use of matrix metalloproteinase inhibitors such as chlorhexidine [36, 37] is necessary to prevent collagen degradation during guided tissue remineralization. A modification of the protocol using intentionally denatured collagen matrices created in artificial carious lesions may be used to evaluate whether caries-infected dentin [2], which is not amendable to remineralization with conventional approaches [3], can be remineralized, at least non-hierarchically, with the application of guided tissue remineralization on a gelatinous matrix (degraded collagen). Understanding these important issues will have strong potential impact in extending the dimensions of minimal invasive dentistry to include the preservation of caries-infected dentin [38] in classic restorative dentistry as well as in the atraumatic restorative techniques.
As type I collagen mineralizes, their modulus of elasticity increases up to 400-fold with increasing amount of intrafibrillar minerals deposited within the collagen fibrils [39]. Thus, we further propose to validate our hypothesis by examining the dynamic nanomechanical properties [40] of dentin remineralized using conventional vs guided remineralization approaches. As we anticipate variations in the viscoplastic properties across the remineralized caries-affected dentin, we will accomplish this goal using a triboindentor in the scanning mode [41]. The latter will be performed simultaneously with scanning probe microscopy to produce images of the remineralized caries-affected dentin that correspond to 256 × 256 values of complex modulus, storage modulus and loss modulus of the remineralized substrate over a 50 × 50 μm area. The storage modulus provides information on the ability of a material to store elastic energy and to fully recover from elastic stress. The loss modulus indicates how easily stored energy is dissipated. The complex modulus is the sum of the storage and loss moduli and is analogous to the modulus of elasticity derived from quasistatic nanoindentation [42] or ultrasonic acoustic techniques [43].
Unfortunately, studies remineralization of caries-affected dentin using conventional remineralization approaches rarely take into consideration the improvement of nanomechanical properties of the remineralized substrate. Understanding these critically important attributes using state-of-the-art evaluation of dynamic mechanical properties with a scanning nanoindentation approach will challenge existing paradigms on dentin remineralization and develop new methodologies for quantifying the efficacy of guided tissue remineralization of caries-affected dentin. In artificial carious lesions, diffusion of nanoprecursors derived from guided tissue remineralization is anticipated to occur via the dentinal tubules and the anastomosis of their lateral branches.
In the evaluation of our hypothesis, we must also consider the limitations of the artificial caries model that has been universally employed for the evaluation of dentin remineralization.
Unlike artificial carious lesions, naturally occurring caries-affected dentin produced by bacterial acid challenge in the oral cavity is highly heterogeneous and with the dentinal tubules blocked by occluding minerals that restrict diffusion of large molecular species into the intertubular collagen matrix [44, 45].
Thus, the ultimate test of our hypothesis will be accomplished using extracted carious teeth derived from the oral cavity [1] to enable us to fully access how biomimetic analogs of dentin matrix proteins and amorphous calcium phosphate nanoprecursors may be most effectively delivered to the intertubular carious dentin matrix when dentinal tubules are occluded by minerals. Information gathered from such exploratory studies will pave the way for more detailed investigations on the development of clinically-relevant delivery systems to remineralize “real” caries-affected dentin in the future, using a guided tissue remineralization mechanism to recapitulate the process of biomineralization during dentinogenesis.
It is at the nanoscale dimensions that one anticipates the greatest expansion in horizons in the translation of this exciting proof-of-concept mechanism into a clinically applicable technique.
Acknowledgments
This study has been financially supported by National Institute of Dental and Craniofacial Research, NIH (grant number: R21 DE019213-02).
List of abbreviations
- Micro-CT
Micro-computed tomography
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflicts of interests
The authors declare that they have no competing interest.
Authors’ contributions
YL wrote the paper.
ZS, LD and JM contributed to discussions on how the paper could be improved.
SK performed the literature search.
DHP revised the paper critically for important intellectual content.
FRT initiated the hypothesis and performed transmission electron microscopy of the remineralized collagen.
References
- 1.Neves ADA, Coutinho E, Cardoso MV, Jaecques SV, Van Meerbeek B. Micro-CT based quantitative evaluation of caries excavation. Dent Mater. 2010;26:597–88. doi: 10.1016/j.dental.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Yoshiyama M, Tay FR, Torii Y, Nishitani Y, Doi J, Itou K, Ciucchi B, Pashley DH. Resin adhesion to carious dentin. Am J Dent. 2003;16:47–52. [PubMed] [Google Scholar]
- 3.Kuboki Y, Ohgushi K, Fusayama T. Collagen biochemistry of the two layers of carious dentin. J Dent Res. 1977;56:1233–7. doi: 10.1177/00220345770560102301. [DOI] [PubMed] [Google Scholar]
- 4.Nakornchai S, Atsawasuwan P, Kitamura E, Surarit R, Yamauchi M. Partial biochemical characterisation of collagen in carious dentin of human primary teeth. Arch Oral Biol. 2004;49:267–73. doi: 10.1016/j.archoralbio.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Kawasaki K, Ruben J, Tsuda H, Huysmans MCDNJM, Takagi O. Relationship between mineral distributions in dentin lesions and subsequent remineralization in vitro. Caries Res. 2000;34:395–403. doi: 10.1159/000016614. [DOI] [PubMed] [Google Scholar]
- 6.Preston KP, Smith PW, Higham SM. The influence of varying fluoride concentrations on in vitro remineralisation of artificial dentinal lesions with differing lesion morphologies. Arch Oral Biol. 2008;53:20–6. doi: 10.1016/j.archoralbio.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Klont B, ten Cate JM. Remineralization of bovine incisor root lesions in vitro: the role of the collagenous matrix. Caries Res. 1991;25:3945. doi: 10.1159/000261340. [DOI] [PubMed] [Google Scholar]
- 8.Klont B, ten Cate JM. Susceptibility of the collagenous matrix from bovine incisor roots to proteolysis after in vitro lesion formation. Caries Res. 1991;25:46–50. doi: 10.1159/000261341. [DOI] [PubMed] [Google Scholar]
- 9.Wefel JS. Root caries histopathology and chemistry. Am Dent J. 1994;7:261–5. [PubMed] [Google Scholar]
- 10.Baysan A, Lynch E, Ellwood R, Davies R, Petersson L, Borsboom P. Reversal of primary root caries using dentifrices containing 5,000 and 1100 ppm fluoride. Caries Res. 2001;35:41–6. doi: 10.1159/000047429. [DOI] [PubMed] [Google Scholar]
- 11.Ten Cate JM, Buijs MJ, Damen JJ. pH-cycling of enamel and dentin lesions in the presence of low concentrations of fluoride. Eur J Oral Sci. 1995;103:362–7. doi: 10.1111/j.1600-0722.1995.tb01858.x. [DOI] [PubMed] [Google Scholar]
- 12.Arsenault AL. Crystal-collagen relationships in calcified turkey leg tendons visualized by selected-area dark field electron microscopy. Calcif Tissue Int. 1988;43:202–12. doi: 10.1007/BF02555136. [DOI] [PubMed] [Google Scholar]
- 13.Traub W, Arad T, Weiner S. Three-dimensional ordered distribution of crystals in turkey tendon collagen fibers. Proc Natl Acad Sci USA. 1989;86:9822–6. doi: 10.1073/pnas.86.24.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landis WJ, Hodgens KJ, Arena J, Song MJ, McEwen BF. Structural relations between collagen and mineral in bone as determined by high voltage electron microscopic tomography. Microsc Res Tech. 1996;33:192–202. doi: 10.1002/(SICI)1097-0029(19960201)33:2<192::AID-JEMT9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 15.Jäger I, Fratzl P. Mineralized collagen fibrils: a mechanical model with a staggered arrangement of mineral particles. Biophys J. 2000;79:1737–46. doi: 10.1016/S0006-3495(00)76426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawasaki K, Ruben J, Stokroos I, Takagi O, Arends J. The remineralization of EDTA-treated human dentine. Caries Res. 1999;33:275–80. doi: 10.1159/000016529. [DOI] [PubMed] [Google Scholar]
- 17.Ten Cate JM. Remineralization of caries lesions extending into dentin. J Dent Res. 2001;80:1407–11. doi: 10.1177/00220345010800050401. [DOI] [PubMed] [Google Scholar]
- 18.Kinney JH, Habelitz S, Marshall SJ, Marshall GW. The importance of intrafibrillar mineralization of collagen on the mechanical properties of dentin. J Dent Res. 2003;82:957–61. doi: 10.1177/154405910308201204. [DOI] [PubMed] [Google Scholar]
- 19.Bertassoni LE, Habelitz S, Kinney JH, Marshall SJ, Marshall GW., Jr Biomechanical perspective on the remineralization of dentin. Caries Res. 2009;43:70–7. doi: 10.1159/000201593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tay FR, Pashley DH. Guided tissue remineralisation of partially demineralised human dentine. Biomaterials. 2008;29:1127–37. doi: 10.1016/j.biomaterials.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Kim YK, Gu L-S, Bryan TE, Kim JR, Chen L, Liu Y, Yoon JC, Breschi L, Pashley DH, Tay FR. Mineralisation of reconstituted collagen using polyvinylphosphonic acid/polyacrylic acid templating matrix protein analogues in the presence of calcium, phosphate and hydroxyl ions. Biomaterials. doi: 10.1016/j.biomaterials.2010.04.060. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gower LB. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem Rev. 2008;108:4551–627. doi: 10.1021/cr800443h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong T-S, Brough B, Ho C-M. Creation of functional micro/nano systems through top- down and bottom-up approaches. MCB. 2009;6:1–55. [PMC free article] [PubMed] [Google Scholar]
- 24.Chesnick IE, Mason JT, Giuseppetti AA, Eidelman N, Potter K. Magnetic resonance microscopy of collagen mineralization. Biophys J. 2008;95:2017–26. doi: 10.1529/biophysj.107.120923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim YK, Mai S, Mazzoni A, Liu Y, Tezvergil-Mutluay A, Takahashi K, Zhang K, Pashley DH, Tay FR. Biomimetic remineralization as a progressive dehydration mechanism of collagen matrices - Implications in the aging of resin-dentin bonds. Acta Biomater. 2010 doi: 10.1016/j.actbio.2010.03.021. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Nancollas GH. Pathways to biomineralization and biodemineralization of calcium phosphates: the thermodynamic and kinetic controls. Dalton Trans. 2009;15:2665–72. doi: 10.1039/b815887h. [DOI] [PubMed] [Google Scholar]
- 27.Niederberger M, Cölfen H. Oriented attachment and mesocrystals: non-classical crystallization mechanisms based on nanoparticle assembly. Phys Chem Chem Phys. 2006;8:327187. doi: 10.1039/b604589h. [DOI] [PubMed] [Google Scholar]
- 28.Gajjeraman S, Narayanan K, Hao J, Qin C, George A. Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J Biol Chem. 2007;282:1193–204. doi: 10.1074/jbc.M604732200. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Arola DD, Gu L, Kim YK, Mai S, Liu Y, Pashley DH, Tay FR. Functional biomimetic analogs help remineralize apatite-depleted demineralized resin-infiltrated dentin via a bottom-up approach. Acta Biomater. 2010;6:2740–50. doi: 10.1016/j.actbio.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marquezan M, Corrêa FN, Sanabe ME, Rodrigues Filho LE, Hebling J, Guedes-Pinto AC, Mendes FM. Artificial methods of dentine caries induction: A hardness and morphological comparative study. Arch Oral Biol. 2009;54:1111–7. doi: 10.1016/j.archoralbio.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Lo EC, Zhi QH, Itthagarun A. Comparing two quantitative methods for studying remineralization of artificial caries. J Dent. 2010;38:3529. doi: 10.1016/j.jdent.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Delbem AC, Sassaki KT, Vieira AE, Rodrigues E, Bergamaschi M, Stock SR, Cannon ML, Xiao X, De Carlo F, Delbem AC. Comparison of methods for evaluating mineral loss: hardness versus synchrotron microcomputed tomography. Caries Res. 2009;43:359–65. doi: 10.1159/000231573. [DOI] [PubMed] [Google Scholar]
- 33.Dorozhkin SV. Calcium orthophosphates in nature, biology and medicine. Materials. 2009;2:399–498. [Google Scholar]
- 34.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. J Dent Res. 2004;83:216–21. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 35.Carrilho MR, Tay FR, Donnelly AM, Agee KA, Tjaderhane L, Mazzoni A, Breschi L, Foulger S, Pashley DH. Host-derived loss of dentin matrix stiffness associated with solubilization of collagen. J Biomed Mater Res B Appl Biomater. 2009;90:373–80. doi: 10.1002/jbm.b.31295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hebling J, Pashley DH, Tjäderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J Dent Res. 2005;84:741–6. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 37.Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjaderhane L, Ruggeri A, Jr, Tay FR, Dorigo Ede S, Pashley DH. Chlorhexidine stabilizes the adhesive interface: a 2-year in vitro study. Dent Mater. 2010;26:320–5. doi: 10.1016/j.dental.2009.11.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson V, Craig RG, Curro FA, Green WS, Ship JA. Treatment of deep carious lesions by complete excavation or partial removal: a critical review. J Am Dent Assoc. 2008;139:70512. doi: 10.14219/jada.archive.2008.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landis WJ, Librizzi JJ, Dunn MG, Silver FH. A study of the relationship between mineral content and mechanical properties of turkey gastrocnemius tendon. J Bone Mineral Res. 1995;10:859–67. doi: 10.1002/jbmr.5650100606. [DOI] [PubMed] [Google Scholar]
- 40.Donnelly E, Williams RM, Downs SA, Dickinson ME, Baker SP, van der Meulen MCH. Quasistatic and dynamic nanomechanical properties of cancellous bone tissue relate to collagen content and organization. J Mater Res. 2006;21:2106–17. [Google Scholar]
- 41.Chaudhry B, Ashton H, Muhamed A, Yost M, Bull S, Frankel D. Nanoscale viscoelastic properties of an aligned collagen scaffold. J Mater Sci Mater Med. 2009;20:257–63. doi: 10.1007/s10856-008-3574-3. [DOI] [PubMed] [Google Scholar]
- 42.Angker L, Swain MV. Nanoindentation: Application to dental hard tissue investigations. J Mater Res. 2006;21:1893–1905. [Google Scholar]
- 43.Rabe U, Amelio S, Kopycinska MS, Hireskorn S, Kempf M, Goeken M, Arnold W. Imaging and measurements of local mechanical material properties by atomic force acoustic microscopy. Surf Interface Anal. 2002;33:65–70. [Google Scholar]
- 44.Marshall GW, Habelitz S, Gallagher R, Balooch M, Balooch G, Marshall SJ. Nanomechanical properties of hydrated carious human dentin. J Dent Res. 2001;80:1768–71. doi: 10.1177/00220345010800081701. [DOI] [PubMed] [Google Scholar]
- 45.Zavgorodniy AV, Rohanizadeh R, Bulcock S, Swain MV. Ultrastructural observations and growth of occluding crystals in carious dentine. Acta Biomater. 2008;4:1427–39. doi: 10.1016/j.actbio.2008.04.010. [DOI] [PubMed] [Google Scholar]