Abstract
Over the past twenty years, Tallal and colleagues have directed their research toward defining the neuropathological mechanisms responsible for developmental dysphasia. We have hypothesized that higher level auditory processing dysfunction, which has previously been associated with developmental dysphasia, may result from more basic temporal processing deficits which interfere with the resolution of rapidly presented, brief duration stimuli. This temporal processing deficit interferes with adequate perception of specific verbal stimuli which require resolution of brief duration formant transitions, resulting in disordered language development. The temporal processing deficit occurs across multiple sensory modalities, and also affects rapid and sequential motor production skills. Despite relatively normal clinical neuroradiological examinations, in vivo morphological analysis, utilizing magnetic resonance imaging techniques for quantitative volumetric measurements of specific brain structures, has identified abnormalities in superior parietal, prefrontal, and temporal cortices, as well as diencephalic and caudate nuclei. Abnormalities in structures which are involved in multimodal processing and sensory motor integration is consistent with the behavioral profile of developmental dysphasia. Two alternative hypotheses regarding the neurophysiological basis of the multimodal temporal processing disorder include: dysfunction in specifc cellular systems which subserve rapid, transient processing; and abnormal gating of sensory relay by intralaminar and reticular thalamic nuclei.
Keywords: developmental dysphasia, dyslexia, temporal processing, MRI, thalamic nuclei, caudate nuclei
INTRODUCTION
Developmental dysphasia is defined as a specific dysfunction in the development of speech and language expression and/or reception, in the absence of other causal disabilities such as defects of hearing, peripheral speech structures, mental subnormality, personality disorder, brain trauma, or psychoaffective or psychotic disorders (Benton, 1964). The physiological etiology of this disorder is unknown, however recent research has focused on elucidating the neuropsychological, physiological, and possible genetic dysfunction underlying this developmental language deficit. The diagnosis is made based on gross behavioral findings, and exclusion of other disorders. Although often termed developmental aphasia, this disorder may differ significantly both in terms of functional deficit and physiological mechanism from the disorder for which the term “aphasia” was coined, traumatic acquired aphasia. Therefore, the disorder will be termed “developmental dysphasia” in this paper, referring to a specific developmental disturbance in language functions, of unknown origin.
This paper will review a series of psychophysical, neuropsychological and anatomical studies which have been directed toward defining the dysfunction of, and elucidating the physiological mechanisms responsible for developmental dysphasia. The research, conducted over the past 20 years by Tallal and colleagues has focused on 4 major areas: analysis of the nonverbal auditory processing deficit association with developmental dysphasia, assessment of the modality specificity of this perceptual deficit and examination of the relationship between the nonverbal processing deficit and verbal dysfunction. Recent work has focused on elucidating the physiological deficit in developmental dysphasia through in vivo morphological analysis of brain structures via magnetic resonance imaging (MRI) techniques (Jernigan, Tallal, and Hesselink, 1987; Jernigan, Hesselink, Sowell, and Tallal, in press).
Language processing depends on intact basic sensory reception and processing functions, which culminate in comprehension. These broad steps are necessarily hierarchical, requiring intact signal reception and processing before adequate language comprehension can occur. The basic premise of our research has been the hypothesis that important aspects of acoustic processing are critical for normal speech perception. If acoustic processing is disrupted during development, then this nonverbal processing disorder will interfere with language development.
Behavioral, as well as evoked response audiometry has demonstrated that the peripheral hearing status of children with developmental dysphasia cannot account for their language deficit (Grillon, Couchesne, and Akshoonoff, 1989). In other words, brainstem evoked responses have been found to be relatively normal or, if abnormalities occur, they are insufficient to account for the severity of the language disorder. Despite normal hearing, previous studies have suggested that children with developmental dysphasia have significant “higher level” auditory processing deficits and have focused on two major parameters of auditory perception: auditory sequencing and auditory memory (see Tallal, 1978 for a review). Hirsh (1959) has described these more complex auditory skills as dependent on two more basic abilities: auditory temporal resolution, the perception of two sounds as distinct, and auditory discrimination, the ability to perceive two different sounds as different. Tallal’s first set of experiments focused on determining the integrity of these two basic functions in developmentally dysphasic children.
ACOUSTIC PERCEPTION
In order to avoid verbal response requirements, Tallal and Peircy (1973a) developed an operant conditioning paradigm in which subjects were trained to respond to two complex steady state tones of different frequencies presented in rapid succession by pressing one of two response panels. Three basic paradigms were utilized: a same/different paradigm to determine auditory discrimination, a two alternative forced choice paradigm, in which the subjects were required to indicate the order of presentation of the two stimuli by pushing the response panels in the order of stimulus presentation, and a serial memory paradigm, in which the same tones were presented in random order, but in increasingly longer strings of 2,3,4, and 5 elements.
In the first set of experiments, twelve 6–9 year old dysphasic children and nine age matched controls were tested. Two 75 msec tones varying in frequency were used. The interstimulus interval (ISI) was varied between 8 and 4062 msec. A criterion of 20/24 correct responses was used. All subjects reached criterion at 428 msec, but the dysphasic’s performance rapidly deteriorated with shorter ISI’s, with no dysphasic subjects reaching criterion at 305 msec ISI’s or shorter. All controls were able to reach criterion at ISI’s of 8 msec. A similar pattern of results was demonstrated in the same/different and sequential ordering paradigms. This clearly demonstrated that the sequencing deficit identified in dysphasic children is a secondary sequelea due to the more primary deficit in tone discrimination of rapidly presented stimuli. That is, at rapid rates of presentation, tone stimuli can not be discriminated and therefore, also cannot be sequenced. (i.e., if a subject cannot determine if two stimuli are different, they certainly could not determine the temporal order of those stimuli.)
In the next set of experiments, the role of stimulus duration on auditory perception was examined (Tallal and Piercy, 1973b). The most significant findings were on the serial memory task. Whereas all controls reached criterion on the three element serial memory task at 75 msec stimulus tone durations, only two out of twelve dysphasics reached criterion. However, when stimulus duration was increased to 250 msec, ten out of twelve dysphasics reached criterion on three element patterns. It is significant to note that control performance did not deteriorate significantly as a function of increasing number of elements per sequence, up to five items. However, severe deterioration in the dysphasic subjects’ performance was demonstrated at sequence lengths above three elements, even with longer duration stimuli. Thus, it is clear from these results that increasing the duration of the stimulus improves the serial memory performance of dysphasic children, but that serial memory remains impaired in comparison to controls for longer length sequences. Therefore, the time available for acoustic processing is clearly important for sequential memory performance. However, the impairment in serial memory performance may not be entirely attributable to temporal processing deficits, since increasing stimulus duration did not prevent further deterioration in serial memory for tone sequences of greater than three elements. On the other hand, it seems likely that because of the developmental nature of this disorder, the primary temporal processing deficit may cause a form of auditory deprivation. That is, faulty acoustic information processing may deprive the affected children of developmental learning experiences which involve practice with processing clearly differentiated auditory stimuli. The effect of this deprivation may result in, among other things, retarded development of auditory memory skills.
MODALITY SPECIFICITY
The previous experiments clearly indicate an auditory temporal processing deficit in developmentally dysphasic children. The next set of experiments were designed to determine the modality specificity of the temporal processing deficit in developmental dysphasics. In the next series of experiments, the same paradigms described above were presented again; however, in these studies, visual presentation of 2 light stimuli (2 shades of green) replaced the auditory tones (Tallal and Peircy, 1973b). The same subjects were tested again with visual stimuli. No significant group differences were found, which indicated that the temporal processing deficit was specific to the auditory modality. However, these results do not rule out the possibility of a general cross modality temporal processing disorder, in which the specific stimulus quality and temporal constraints vary according to modality. The use of shading differences was chosen because a subtle difference in light frequency appeared at least physically analogous to sound frequency differences. However, the neural mechanisms responsible for the discrimination of sound frequency differences may be more analogous to other types of visual discrimination such as orientation or form discrimination. Therefore this study did provide evidence against cross modality effects of the temporal processing deficit, but in itself was inconclusive.
In a later set of experiments (Tallal, Stark, Kallman, and Mellits, 198 l), the paradigm was modified to examine modality specificity of temporal processing across a larger age span. In these experiments, three types of visual stimuli were used: two shades of green, two nonsense letter-like characters (ε and φ), and two letters (E and K). In these experiments, shorter ISI’s resulted in equally impaired performance on both auditory and visual tasks for dysphasics ranging in age from 5 to 6 yrs. However, performance on auditory tasks among the 7 and 8 year olds was two times worse than performance on visual tasks. Therefore, the temporal processing deficit appeared to be general, across modalities in the younger age group, but was selective to the auditory modality in the older group. One explanation for this discrepancy may be that the cross sectional sample may have been skewed according to age group, with greater diversity in the degree and scope of impairment in the younger group. Only the most seriously impaired children may continue to be specifically language impaired at the older ages and thus may represent a more homogeneous disorder. On the other hand, differential temporal parameters may govern the processing of sequentially presented visual or auditory stimuli during the course of normal development. In other words, at the younger ages the auditory and visual systems may be functioning under similar temporal constraints, while the visual system may function under different time constraints than the auditory system in the older group. The presentation parameters used for the perception paradigm may not be sensitive enough to pick up significant errors on visual tasks in the older group.
In summary, it is clear that the younger age group demonstrated a lack of modality specificity in their temporal processing disorder. However, the ability of the paradigm to resolve visual temporal processing deficits in older children may be poor due to age dependent changes in the visual system, or due to group variance differences across ages.
In an additional set of experiments, a battery of clinical neurological and neuropsychological tests were given to dysphasic children revealing significant deficits in discrimination of simultaneously presented tactile stimuli, and deficits in producing rapid alternating and sequential movements (Katz, Tallal and Curtiss, submitted; Tallal, Stark, and Mellits, 1985a; Johnson, Stark, Mellits, and Tallal, 1981). Based on these findings, it appears that the perceptual deficit associated with developmental dysphasia may affect multiple modalities. It is unclear if the dysfunction in producing rapid motor behaviors is due to primary efferent system deficits, or whether these effects are secondary to deficits in somatosensory processing. Although the majority of data indicates a temporal processing deficit, dysphasic children demonstrated poor tactile discrimination to simultaneously presented stimuli. This may be representative of a dysfunction in temporal resolution (i.e., IS1 = 0), or spatial resolution. Whether the deficit involves primarily temporal processing or spatial processing may vary depending on the modality. Indeed, spatial resolution is a more salient feature of tactile perception than auditory perception. More specific studies of the somatosensory involvement in developmental dysphasia is required to answer this question. It is however, physiologically consistent for a temporal and spatial resolution deficit to exist concurrently, since both spatial and temporal gating mechanisms probably involve similar lateral and recurrent inhibition mechanisms.
One mechanim which may be hypothesized to account for the temporal processing deficits seen in developmental dysphasics is a temporal gating mechanism involving “edge sharpening” of stimuli. Edge sharpening, as used here, refers to the clarification of receptive field edges by lateral inhibition mechanisms, thereby providing a perceptual “window” for stimulus resolution. Such lateral inhibition mechanisms have been described for visual, tactile, and auditory systems (Berne and Levy, 1988). Temporal edge sharpening may involve recurrent feedback mechanisms which function to gate the frequency of incoming sensory impulses, and therefore provide, perceptually, a temporal discrimination window. Clearly a common dysfunction in “edge sharpening” functions may be occurring across modalities in developmental dysphasics.
A second hypothesis has been raised by Livingstone, who suggested that the parallel magnocellular and parvocellular systems identified in the visual system may have counterparts in other modalities (Livingstone and Galaburda, 1990). These two pathways remain relatively segregated throughout the visual system. At the level of the retinal ganglion cells through the lateral geniculate, the response properties of the two systems vary significantly (Perry, Oehler, and Cowey, 1984). The magnocellular system receives input mostly from peripheral retinal photoreceptors, has low spatial resolution, is not color coded, has little contrast sensitivity, and responds best to high frequency stimuli. On the other hand, the parvocellular system receives mostly from central retina, has high spatial resolution, is color coded, is highly contrast sensitive, and responds best to low frequency stimuli (Perry and Shapely, 1986; Kaplan and Shapley, 1982). The magnocellular system appears designed to respond best to rapid, transient or moving stimuli presented in peripheral fields, while the parvocellular system responds best to detailed, static stimuli presented foveally. Livingstone proposed that similar functionally segregated parallel subsystems could exist in a variety of sensory systems. Selective impairment in the subsystems subserving fast transient responses may underlie the temporal processing deficit in developmental dysphasia. In opposition to this hypothesis, the errors in visual discrimination presented above, while temporally dependent, occurred in response to color differences, as well as detailed form differences in black and white stimuli, which were regarded foveally. The role of the magnocellular system in processing these stimuli would be expected to be minimal. However, it is possible that activation of the magnocellular system during rapid transient stimulation is necessary for adequate parvocellular processing. A more detailed analysis of the visual processing deficits which occur across stimuli selected specifically to represent each of these two visual subsystems would provide a better understanding of the possible neural dysfunction underlying developmental dysphasia.
VERBAL PERCEPTION
The previously described psychoacoustic work supported the hypothesis that a temporal processing deficit is primary to developmental dysphasia, and may be prerequisite to the speech and language dysfunction. Based on an understanding of how phonemes transmit information about speech, various predictions were made. Specific elements of the acoustic signal within a phoneme are essential for perceptual discrimination. For example, steady-state vowels transmit the same acoustic information throughout their spectra. However, stop consonant-vowel syllables have a transitional period between the release of the consonant and the initiation of the vowel during which the frequencies change very rapidly in time. Information carried within these brief formant transitions is critical for syllable discrimination. We predicted that dysphasic children would be unimpaired in discriminating between speech sounds which are characterized by steady-state acoustic spectra, such as vowels. On the other hand, they would be significantly impaired in discriminating speech sounds such as stop consonant-vowel syllables, which incorporate very rapidly changing formant transitions. Experimental results were consistent with these predictions (Tallal and Peircy, 1974, 1975). The critical stimulus parameter interfering with successful performance on discrimination tasks proved to be the rate of temporal change, not whether the stimulus was verbal or nonverbal.
A subsequent experiment was carried out to determine if the poor performance found on tests with stop consonant-vowel syllables was due to impaired ability to process transitional elements of auditory information or simply due to the short duration of the stimulus period (Tallal and Peircy, 1975). In this experiment the previous paradigms were carried out with computer generated speech stimuli that were modified to change their temporal components. Vowel-vowel syllables were constructed to include approximately 50 msec steady state durations, while stop consonant-vowel syllables were constructed with synthetically extended formant transitions. Results demonstrated that the dysphasics’ performance on tests using steady-state vowel sounds, but of brief duration, was significantly impaired. Conversely, extending the formant transition within stop consonant-vowel syllables resulted in significantly improved performance. Thus, the clear conclusion of these studies was that a highly specific auditory temporal deficit is sufficient to interfere with the perception of brief duration acoustic information essential for normal speech discrimination, regardless of whether or not stimuli are transitional or steady-state. Subsequent studies demonstrated a highly significant relation between the degree of temporal processing deficit and the extent of receptive language impairment in dysphasic children (Tallal, Stark, and Mellits, 1985b).
There may also be a striking convergence of data between dysphasic and dyslexic children. Longitudinal studies have demonstrated that the vast majority of developmental dysphasic children have inordinate difficulty learning to read (see Tallal, Curtiss, and Kaplan, 1988 for a review). A broad body of research now suggests that phonological awareness and coding deficits may be at the heart of developmental reading disorders (Liberman, 1988). But, what is the physiological basis of disorders in phonological awareness and coding? Struck by the considerable overlap between developmental dysphasia and dyslexia, Tallal and colleagues carried out experiments with two groups of dyslexic children. One group had depressed scores not only on standardized reading tests, but on measures of oral language as well. The other group was equally impaired in reading, but was within normal limits on tests of oral language. The phonological coding, as well as temporal processing abilities of these two groups of dyslexics were assessed and compared to that of dysphasic children. The results were clear. The dyslexic children with concomitant oral language disabilities had significant deficits in both phonological coding (reading nonsense words) and non-verbal temporal processing. Furthermore, these deficits were highly correlated with each other in these dyslexics (r = 0.81). Interestingly, the dyslexics with normal oral language scores had neither phonological coding or temporal processing deficits. Thus, deficits in basic temporal processing may interfere with phoneme analysis leading to initial speech perception and/or language deficits, and subsequent deficits in phonological awareness and reading development (Tallal, 1980; Tallal and Stark, 1982; Stark and Tallal et al., 1988). It is particularly relevant to note that genetic studies have found that decoding deficits in reading nonsense words may be among the best phenotype markers for developmental dyslexia (see Olson, Gillis, Rack, and DeFries, in press; Stevenson, in press).
Our studies suggest that the physiological basis of phoneme awareness deficits in dyslexia may be basic temporal integration and serial memory deficits, and that it may be these deficits which are transmitted genetically. A recent study completed in Tallal’s laboratory lends direct support to this hypothesis. Language/reading impaired (L/RI)1 children with other affected first degree relatives were compared to matched L/RI children without other affected family members. Few behavioral differences in phenotype emerged. However, a significant group difference was found on a battery of nonverbal auditory attention and temporal processsing tests, with L/RI children with a positive family history for language and/or learning disability performing significantly more poorly than those without a positive family history (Tallal, Townsend, Curtiss, and Wulfeck, in press). Taken together, these data suggest that there may be a continuum between developmental language disorders and the types of reading disorders which are characterized by deficits in phonological awareness. The genetic basis for this continua may be in a physiological deficit which slows the rate of basic sensory information processing in the nervous system. The search for an anatomical/morphological substrate for such a deficit was investigated in the following series of studies.
CEREBRAL MORPHOLOGY
A series of studies of cerebral morphology, utilizing magnetic resonance imaging (MRI) to calculate volumetric data, was undertaken recently by Jemigan and Tallal (Jernigan, Tallal, and Hesselink, 1987; Jemigan, Hesselink, Sowell, and Tallal, in press). Volumetric measurements of cerebral grey matter, both cortical and subcortical was obtained for twenty language impaired and twelve control subjects in the age range of 8 to 10 years. The sample was drawn from a larger sample of ninety five specifically language impaired children and sixty age, nonverbal I.Q., race, and SES matched controls who were identified at 4 years old and studied longitudinally until age 8. The method of utilizing magnetic resonance imaging techniques for in vivo quantitative volumetric analysis of brain morphology presents a powerful, and non-invasive technique for exploring the anatomical and physiological basis of chronic, non life-threatening nervous system disorders such as developmental dysphasia. Therefore, the methods will be explained here in some depth (see Jernigan et al., in press, for a more detailed discussion).
The following techniques were utilized to define and quantify brain structure volumes from MRI data. To facilitate and standardize the determination of structure edges, the method involved a semi-automated classification of each voxel within a brain section on the basis of its signal characteristics on two spatially registered MR images of that section. These classifications corresponded to major tissue types: grey matter, white matter, cerebral spinal fluid (CSF), and tissue abnormalities. The full series of axial brain sections was analyzed, beginning at the bottom of the cerebellar hemispheres and extending through the vertex.
Specific brain structures were then defined. Subcortical structures were identified by visually identifying groups of pixels classified as grey matter which were located within caudate nuclei, lenticular nuclei, and dience-phalic grey matter structures (including mammillary bodies, hypothalamic grey, septal nuclei, and thalamus). Cerebral regions were defined relative to the centromedial structural midline and two consistently identifiable points: the most anterior point on the genu, and the most posterior point on the splenium of the corpus callosum. By calculating rotation angles using these landmarks, it was possible to perform a three dimensional rotation of the images, thus correcting each individual’s image data for rotation out of the optimal imaging plane. Regions could then be constructed which resulted in highly consistent placement of regional boundaries relative to gross anatomical landmarks.
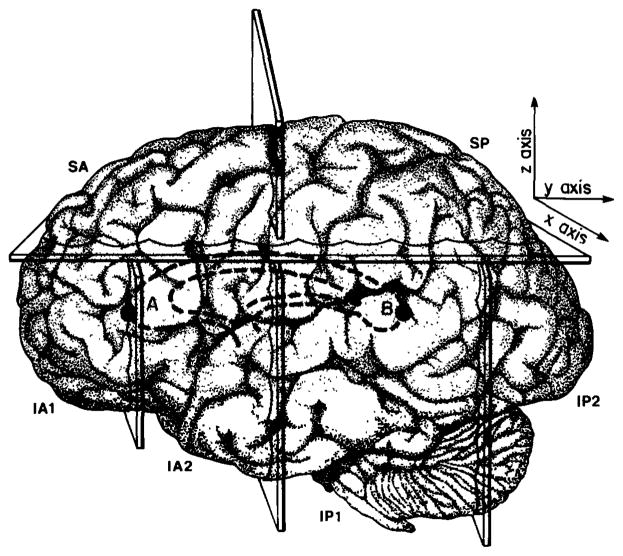
Six cerebral zones were identified relative to these planes: IA1, IA2, IP1, IP2, SA, SP. The first letter of the region describes its position relative to the axial dividing plane (inferior or superior), and the second to the coronal plane (anterior or posterior). Thus, IA is inferior to the axial and anterior to the coronal plane. This division resulted in the inclusion of all posterior perisylvian cortical structures in the IP zones, however the occipital lobe was also included in this zone. In addition, the IA zone included both anterior temporal and frontal cortical structures. In order to separate IA and IP into more anatomically distinct regions, two additional coronal dividing planes were added which subdivided the region inferior to the axial plane. The resulting six cerebral regions are defined in the table below and shown in figure 1.
Fig. 1.
Figure 1 shows the six brain regions used for morphometric analysis to compare MR images from language impaired and control children. SA = Superior Anterior; SP = Superior Posterior; IA1 = Inferior Anterior 1; IA2 = Inferior Anterior 2; IP1 = Inferior Posterior 1; IP2 = Inferior Posterior 2
Group comparisons were made on: cerebral asymmetry of the volumes of each of the six regions, and absolute hemispheric volumes of cerebral and subcortical structures. Asymmetry was expressed as a ratio of the total voxel quantity of the left hemisphere region to the total voxel quantity of the homologous right hemisphere region.
Results revealed generally unremarkable findings on routine clinical neuroradiological examination. However, quantitative volumetric analysis revealed significant morphological differences in dysphasic children vs. controls. Results indicated significantly different asymmetries in dysphasics vs. controls for both IA1 and SP regions.
In IA1, or prefrontal cortex, controls demonstrated symmetry between the two hemispheres, while dysphasics demonstrated a smaller volume in the left than in the right hemisphere. In SP, the superior parietal and parieto-occipital regions, controls showed larger right than left volumes, while dysphasics showed the opposite pattern (left larger than right). In terms of comparisons of absolute volumes, the IP1 or posterior perisylvian region (the region which has been most clearly associated with receptive language function), was significantly reduced bilaterally in dysphasics. In addition, subcortical volumes in the right diencephalon and caudate were also significantly reduced in dysphasics compared to controls. Ratios of tissue type: grey matter, white matter, and CSF were similar for dysphasics and controls.
It is clear from this work that although routine clinical neuroradiological examinations revealed no significant structural defects in developmentally dysphasic children, abnormalities in brain morphology were significant when measured quantitatively. There is evidence of bilateral reduction in the perisylvian volume, the brain region which has been most clearly related to functional receptive language deficits. Significant morphological abnormalities were also demonstrated in posterior parietal and prefrontal cortices, as well as diencephalic and caudate nuclei. These structures represent areas of multimodality sensory processing and sensory motor integration. Thus, the behavioral results from Tallal’s laboratory, revealing a significant multimodal temporal processing deficit, are consistent with morphological abnormalities in these structures.
Preliminary analysis aimed at directly linking the verbal and temporal perceptual deficits of L/RI children with the morphological differences found on MRI have proved promising. Significant correlations are found between degree of volumetric reduction in the left IP1 region and a reduction in verbal IQ score. Similarly, significant correlations have emerged between auditory sequential memory performance and hemispheric asymmetry ratio differences in the IA1 and SP regions. More detailed analysis are currently in progress to reveal the precise pattern of relationship between functional and structural patterns in L/RI children.
SUMMARY
Major results from this line of research demonstrate the involvement of a significant deficit in temporal analysis of auditory information, which predicts both the pattern of speech perception and production deficits, as well as the degree of receptive language deficit in developmental dysphasia. In addition, the evidence implicates cross modality gating mechanisms, possibly involving a deficit in temporal and spatial “edge sharpening” functions. The morphological findings from MRI studies implicate cortical as well as subcortical structures. It is possible to speculate that during neurogenesis, a primary subcortical structure fails to develop normally, resulting in the spectrum of morphological deficits seen in L/RI children. Given the subcortical involvement demonstrated on volumetric analysis together with the behavioral deficits in temporal processing and motor control which have been shown to characterize these children, we hypothesize that thalamic gating mechanisms may be involved, The results of evoked potential studies suggest that the deficit may involve normal input to brainstem and thalamus, but abnormal thalamic or post-thalamic processing. The widespread cortical morphological involvement, including the temporal lobes bilaterally, as well as parietal and frontal association cortices, supports the involvement of subcortical structures which provide widespread input to cortex, as well as reciprocal innervation of nonprimary cortices. In addition, based on the MRI data, the primary affected structure would also be expected to provide heavy innervation of caudate nucleus, since this structure is also significantly reduced in volume in the brains of L/RI children.
Nuclei which receive input from all modalities as well as from wide-spread cortical regions include the nonspecific nuclei of the thalamus. The intralaminar nuclei have reciprocal innervations with caudate nucleus, widespread nonprimary cortical sites, and thalamic reticular nuclei (Macchi and Bentivoglio, 1986). The reticular nuclei have been shown to provide powerful inhibitory input to specific thalamic nuclei (Macchi and Bentivoglio, 1986). Since reticular nuclei receive input from the same specific thalamic sites to which they project, these nuclei provide a possible mechanism for recurrent feedback mechanisms.
A possible thalamic mechanism involved in temporal gating of sensory information in the temporal range shown to be disordered in dysphasic children (20 hz to 5 hz) may involve, in part, intrathalamic circuits which involve input from specific “relay” nuclei, and which provide feedback to the same nuclei. The intralaminar and reticular nuclei provide a possible anatomical substrate for such an interaction. In addition, the mechanism would be expected to be modulated by association cortical areas, where judgements regarding priority and relevance of information would be expected to be made. Again, the anatomical substrate is relevant. The nonspecific nuclei input to specific “relay” cells, may provide a filtering function by modulating the excitability of these cells following phasic activation. This could be achieved through modulation of specific potassium conductances, such as IA or calcium dependent conductances which would result in altered after-hyperpolarization durations (see Strong and Kaczmarek, 1987, for a detailed discussion of these potassium conductantes). Based on behavioral work, implicating a multiple modality temporal gating mechanism, and morphological work implicating nonprimary cortical, diencephalic and striatal involvement, it may be hypothesized that nonspecific thalamic nuclei are involved in the primary pathology of developmental dysphasia. In support of such a hypothesis, there is a growing body of evidence that damage to thalamic structures results in acquired aphasic disorders in some adult patients (Jones, 1982; Luria, 1977; Moher, Watter, and Duncan, 1975). Similarly, Ojemann described language production and verbal memory deficits as a result of nongeniculate thalamic lesions and in response to thalamic electrical stimulation (Ojemann, 1984).
The proposed mechanism is a speculative integration of these behavioral and morphological findings into a single primary structural pathology. An alternative hypothesis is that single developmental events, such as the presence of abnormal cell adhesion proteins, may result in a wide spectrum of structural abnormalities which are functionally and anatomically unrelated, or which represent a dysfunction in similar cellular systems across multiple modalities, such as the magnocellular components of the visual system. However, in support of a mechanism which functionally integrates the morphological data, preliminary results have indicated a significant correlation between extent of morphological involvement and the severity of the language deficit. As such, the studies described in this paper provide a solid foundation upon which to base further research into the specific neurophysiological basis of the temporal modulation mechanism which appears to be primary in the neuropathology of specific developmental language and reading disorders.
Table 1.
Summary of cortical structures within each cerebral region
| Inferior Anterior 1 (IA1): |
| Prefrontal cortex inferior to a plane above the frontal operculum, including orbitofrontal, dorsolateral and mesial frontal lobe. |
| Inferior Anterior 2 (IA2) |
| The temporal poles, uncus, and some amygdala. |
| Anterior perforated substance and adjacent orbitofrontal cortex. |
| Anterior insular cortex and frontal operculum. |
| Inferior Posterior 1 (IP1): |
| Most of temporal cortex, including all mesial temporal lobe structures posterior to the amygdala (only the temporal pole is included in IA2). |
| Perisylvian parietal cortex and parietal operculum. |
| Inferior Posterior 2 (IP2) |
| Most of the occipital lobe (only a small portion of the superior occipital cortex was included in SP). |
| A small part of the most posterior gyri of the temporal lobe on the lateral cortical surface. |
| Superior Anterior (SA): |
| Superior parts of the dorsolateral and mesial frontal lobes (above the frontal operculum). |
| Superior Posterior (SP): |
| Superior parietal lobe above the parietal operculum. |
| A small portion of the most superior occupital lobe. |
Note: From Jemigan et al., in press
Footnotes
L/RI refers to children selected at age 4 as developmental dysphasics and followed longitudinally to age 8, at which time they were both language and reading impaired.
References
- Benton AL. Developmental aphasia and brain damage. Cortex. 1964;1:40–52. [Google Scholar]
- Berne R, Levy M. Physiology. 2. Washington, D.C.: The C. V. Mosby Co.; 1988. pp. 106–111.pp. 140pp. 176 [Google Scholar]
- Grillon C, Courchesne E, Akshoonoff N. Brainstem and middle latency auditory evoked potentials in autism and developmental language disorder. Journal of Autism and Developmental Disorders. 1989;19(2):255–69. doi: 10.1007/BF02211845. [DOI] [PubMed] [Google Scholar]
- Hirsh IJ. Auditory perception of temporal order. Journal of the Acoustic Society of America. 1959;3:157–78. [Google Scholar]
- Jernigan T, Hesselink J, Sowell E, Tallal P. Cerebral morphology on MRI in language-learning children. Archives of Neurology. doi: 10.1001/archneur.1991.00530170103028. (in press) [DOI] [PubMed] [Google Scholar]
- Jemigan T, Tallal P, Hesselink J. Cerebral morphology on magnetic resonance imaging in developmental dysphasia. Society of Neuroscience Abstracts. 1987;13(1):651. [Google Scholar]
- Johnson R, Stark R, Mellits E, Tallal P. Neurological status of language impaired and normal children. Annals of Neurology. 1981;10:159–163. doi: 10.1002/ana.410100206. [DOI] [PubMed] [Google Scholar]
- Jones S. The thalamus and aphasia, including transcortical aphasia: a review. Journal of Communication Disorders. 1982;15(1):3l–44. doi: 10.1016/0021-9924(82)90042-9. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Shapely R. X and Y cells in the lateral geniculate of macaque monkeys. Journalof Phsiology (London) 1982;330:125–146. doi: 10.1113/jphysiol.1982.sp014333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz WF, Tallal P, Curtiss DS. Rapid autmomatized naming and gestures by normal and language impaired children. (Submitted) [DOI] [PubMed] [Google Scholar]
- Liberman IY. Phonology and beginning reading revisisted. In: Von Euler Lundberg, Lennerstrand, editors. Bruin and Reading. London: Mac Millan Press; 1988. pp. 112–143. [Google Scholar]
- Livingstone M, Galaburda A. Physiological evidence for a magnocellular defect in development dyslexia. Paper presented at the National Dyslexia Research Foundation Conference: The exceptional Brain; Barcelona, Spain. 1990. [Google Scholar]
- Luria AR. On quasi-aphasic disturbances in lesions of deep structures of the brain. Brain and Language. 1977;4(3):432–459. doi: 10.1016/0093-934x(77)90036-0. [DOI] [PubMed] [Google Scholar]
- Macchı G, Bentivoglio M. The thalamic intralaminar nuclei and the cerebral cortex. In: Jones E, Peters A, editors. Cerebral Cortex. New York: Plenum Press; 1986. pp. 335–401. [Google Scholar]
- Moher J, Watter W, Duncan G. Thalamic hemorrhage and aphasia. Bruin and Language. 1975;2:3–l7. doi: 10.1016/s0093-934x(75)80050-2. [DOI] [PubMed] [Google Scholar]
- Ojemann GA. Common cortical and thalamic mechanisms for language and motor functions. American Journal of Physiological. 1984;15:901–903. doi: 10.1152/ajpregu.1984.246.6.R901. [DOI] [PubMed] [Google Scholar]
- Olson RK, Gillis JJ, Rack JP, DeFries JC, Fulker DW. Confirmatory factor analysis of word recognition and process measures in the Colorado Reading Project. Reading and Writing: An Interdisciplinary Journal (in press) [Google Scholar]
- Perry V, Oehler R, Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984;12:1l0l–1122. doi: 10.1016/0306-4522(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Perry V, Shapley R. Cat and monkey retinal ganglion cells and their functional roles. Trends in Neurosciences. 1986;9:1–9. [Google Scholar]
- Stark RE, Tallal P. Language, Speech, and Reading Disorders in Children: Neurobiological Studies. San Diego: College Hall Press; 1988. [Google Scholar]
- Stevenson J. Which aspects of processing text mediate genetic effects? Reading and Writing: An Interdiciplinary Journal (in press) [Google Scholar]
- Strong J, Kaczmarek L. Potassium currents that regulate action potentials and repetitive firing. In: Kaczmarek L, Levitan I, editors. Neuromodulation. New York: Oxford University Press; 1987. pp. 119–l34. [Google Scholar]
- Tallal P. An experimental investigation of the role of auditory temporal processing in normal and disordered language development. In: Caramazza A, Zurif E, editors. Language Acquisition and Language Breakdown: Parallels and Divergences. Baltimore, MD: The John Hopkins University Press; 1978. pp. 25–61. [Google Scholar]
- Tallal P. Auditory temporal perception, phonics and reading disabilities in children. Brain and Language. 1980;9:182–198. doi: 10.1016/0093-934x(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Tallal P, Curtiss S, Kaplan R. The San Diego longitudinal study: Evaluating the outcomes of preschool impairment in language development. In: Gerber SE, Mencher GT, editors. Interntional perspectives on communication disorders. Washington, DC: Gallaudet University Press; 1988. pp. 86–l26. [Google Scholar]
- Tallal P, Peircy M. Defects of non-verbal auditory perception in children with developmental aphasia. Nature. 1973a;241:468–469. doi: 10.1038/241468a0. [DOI] [PubMed] [Google Scholar]
- Tallal P, Peircy M. Developmental aphasia: impaired rate of non-verbal processing as a function of sensory modality. Neuropsychologia. 1973b;11:389–398. doi: 10.1016/0028-3932(73)90025-0. [DOI] [PubMed] [Google Scholar]
- Tallal P, Peircy M. Developmental aphasia: Rate of auditory processing and selective impairment of consonant perception. Neuropsychologiu. 1974;12:83–93. doi: 10.1016/0028-3932(74)90030-x. [DOI] [PubMed] [Google Scholar]
- Tallal P, Peircy M. Developmental Aphasia: The perception of brief vowels and extended stop consonants. Neurosychologia. 1975;13:69–74. doi: 10.1016/0028-3932(75)90049-4. [DOI] [PubMed] [Google Scholar]
- Tallal P, Stark R. Perceptual/motor profiles of reading impaired children with or without concomitant oral language deficits. Annuals of Dyslexia. 1982;32:163–176. [Google Scholar]
- Tallal P, Stark R, Kallman C, Mellits E. A reexamination of some non-verbal perceptual abilities of language impaired and normal children as a function of age and sensory modality. Journal of Speech and Hearing Research. 1981;24:351–357. doi: 10.1044/jshr.2403.351. [DOI] [PubMed] [Google Scholar]
- Tallal P, Stark R, Mellits E. Identification of language impaired children on the basis of rapid perception and production skills. Brain and Language. 1985a;25:314–322. doi: 10.1016/0093-934x(85)90087-2. [DOI] [PubMed] [Google Scholar]
- Tallal P, Stark R, Mellits E. The relationship between auditory analysis and receptive language development: Evidence from studies of developmental language disorder. Neuropsychologia. 1985b;23:527–534. doi: 10.1016/0028-3932(85)90006-5. [DOI] [PubMed] [Google Scholar]
- Tallal P, Townsend I, Curtiss S, Wulfeck B. Phenotypic profiles of language impaired children based on genetic/family history. Brain and Language. doi: 10.1016/0093-934x(91)90112-e. (in press) [DOI] [PubMed] [Google Scholar]