Abstract
Dorsoventral (D/V) patterning in Drosophila oogenesis is initiated by the transmission of a TGF-α-like ligand, Gurken (Grk), from the oocyte to the anterodorsal follicle cells, activating the EGF receptor (Egfr) signaling pathway. The zinc-finger transcription factor CF2 is a negative regulator of the rhomboid (rho) gene that encodes an essential membrane-bound component of the dorsalizing pathway. Expression of CF2 itself is negatively regulated by the activated Egfr. In this report, we demonstrate that CF2 is the target of down-regulation by the MAPK kinase cascade, and that this down-regulation is independent of the Rho function. These results suggest that D/V patterning involves a two-step signaling process: the initial Egfr signal, which represses CF2 and induces rho expression; and the subsequent Egfr + Rho signal, which determines the dorsal cell fates. Furthermore, we show that CF2 down-regulation occurs at the post-translational level through a mechanism involving coupled cytoplasmic retention and degradation.
Keywords: Post-translational regulation, Egfr signaling, dorsoventral patterning, MAP kinase
The Drosophila epidermal growth factor receptor (Egfr) is involved in many developmental processes, including establishment of ventral ectodermal cell fates in early embryo, germ band retraction, wing development, eye development, determination of posterior follicle cell fates during oogenesis, and dorsoventral patterning in the egg chambers, among others (for review, see Perrimon and Perkins 1997; Schweitzer and Shilo 1997). A conserved signaling cassette consisting of at least 10 components is utilized by most, if not all, of these developmentally diverse Egfr functions. The signaling cassette includes the mammalian homologs of Ras, Raf, mitogen-activated protein kinase (MAPK) kinase (MEK) and MAPK, which are encoded by Ras1, Draf, Dsor1 (Dmek), and rolled (rl), respectively. Because the same intracellular signaling components can function in different tissues, developmental specificity is achieved at least at two other levels in the signaling pathways: by developmentally regulated production of extracellular ligands and by downstream gene regulation events that respond to these extracellular signals. Importantly, these signals and responses are coordinated by positive and negative feedback loops.
The complex regulatory web of Egfr signaling can be illustrated by the molecular events that determine the follicle cell fates during oogenesis (for review, see Ray and Schüpbach 1996). Early in oogenesis (up to stage 6), the oocyte nucleus is located at the posterior. The RNA and protein products of gurken (grk), which encodes a TGF-α-like ligand, are synthesized in the oocyte and restricted to the posterior cortex. Grk then acts across the oocyte membrane and helps specify the posterior follicle cell fates. The D/V patterning process is initiated later at stage 8 of oogenesis, after the oocyte nucleus migrates to an anterodorsal position. At this stage, both grk RNA and Grk protein become similarly localized in the anterodorsal region of the oocyte near the nucleus (Neuman-Silberberg and Schüpbach 1993; González-Reyes et al. 1995; Roth et al. 1995). The Grk protein acts as a spatially localized ligand to activate the ubiquitous Egfr located on the surrounding follicle cells (Schüpbach 1987; Price et al. 1989; Schejter and Shilo 1989). Grk binding initiates the Egfr signal transduction pathway in the anterodorsal follicle cells, which involves homologs of Ras (Schnorr and Berg 1996), Raf (Brand and Perrimon 1994), MEK (Hsu and Perrimon 1994; Lu et al. 1994) and, by inference, MAPK. Activation of Egfr signaling in the anterodorsal follicle cells leads to down-regulation of the Cis2–His2 zinc-finger transcription factor CF2 (Shea et al. 1990; Hsu et al. 1992, 1996). Absence of CF2 in turn results in induction of transcription of rho (Hsu et al. 1996), which encodes a seven-transmembrane-domain protein (Bier et al. 1990; Ruohola-Baker et al. 1993; Sturtevant et al. 1993). Subsequently, the combined functions of Egfr and Rho specify the dorsal follicle cell fates, including elaboration of the dorsal appendages (Schüpbach et al. 1991; Ruohola-Baker et al. 1993; Hsu et al. 1996). (Note that the Rho protein described here is the product of Drosophila gene rhomboid, which bears no relationship with the mammalian membrane-bound GTPase Rho.) It is not clear whether or not CF2 protein is also down-regulated in the posterior follicle cells in early oogenesis, which are also specified by the Grk function. The expression level of CF2 at these early stages is barely detectable (Hsu et al. 1996).
Genetic studies showed that without the rho function, activated Egfr signaling could not induce dorsal cell fates. Also, the rho function could not by itself manifest the dorsal cell fates without the function of Egfr (Ruohola-Baker et al. 1993). It has been suggested that Rho may be involved in the production of a second Egfr ligand (Golembo et al. 1996; Perrimon and Perkins 1997) but the exact mechanism is not known at this time. Because Egfr alone cannot induce dorsal cell fates, the signaling pathway can be viewed as a two-step process with CF2 being a central coordinator. That is, Egfr alone activated by Grk leads to CF2 down-regulation and Rho expression; and subsequently, determination of the dorsal follicle cell fates is achived by the combined functions of Rho and Egfr.
The D/V signaling pathway is also regulated by a negative feedback loop. It has been shown that the expression of the ETS-domain transcription factor Pointed (PntP1) is induced by Egfr and that ectopically expressed PntP1 can repress the Egfr singaling function (Morimoto et al. 1996). In the embryonic ventral ectoderm, PntP1 has been shown to induce the expression of Argos (Gabay et al. 1996), a putative extracellular inhibitor of Egfr (Schweitzer et al. 1995). It is not known whether Argos is also expressed in the follicle cells.
Together, these experimental data suggest a complex network of coordinated signals and responses. This model may very well provide a paradigm for the regulation of signal transduction pathways in general. However, a complete description of the molecular events in this signaling process is not yet clear. For example, direct regulatory targets of MAPK have not been identified. This is important to establish because although the Ras/MAPK signaling cassette is necessary for specifying dorsal follicle cell fates, it is not known whether it functions upstream of Rho, downstream of Rho, or both.
In this report we present evidence that CF2 is the direct target of the Ras–Raf—MEK–MAPK signaling cascade and that ectopically expressed Rho cannot induce CF2 down-regulation. Most interestingly, we demonstrate that, contrary to the generally accepted mechanism, MAPK does not simply modify the activity of CF2. Instead, down-regulation of CF2 is specifically targeted by the Ras/MAPK signaling and is achieved by a coupled mechanism involving cytoplasmic retention and degradation.
Results
CF2 is negatively regulated by the Ras–Raf–MEK signaling cascade but not by Rho
During mid-oogenesis, CF2 protein level is down-regulated in the anterodorsal population of the oocyte-associated follicle cells, where Egfr signaling occurs (Fig. 1A). For brevity, the oocyte-associated follicle cells will henceforth be referred to simply as follicle cells. The other cell type, nurse cell-associated follicle cells, will be specifically identified when necessary.
Figure 1.
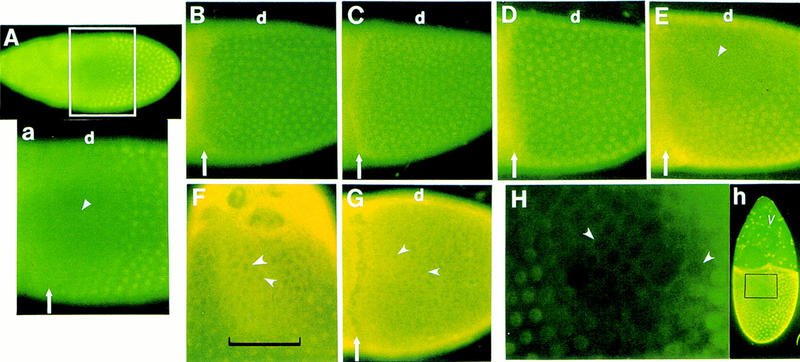
Control of down-regulation of CF2 protein level in the anterodorsal follicle cells. All eggs were stained with anti-CF2 antibody. (A) The wild-type CF2 expression pattern in a stage 10 egg from a y, w female (dorsal–lateral view). The anterodorsal region of the follicular epithelium is devoid of CF2 protein (▴). A close-up view of the anterior population of the follicle cells is shown in a. Note that in posterior–ventral cells, CF2 is localized in the nuclei (well-defined round spots). (Arrow) The anterior margin of the follicle cell layer, which also delineates the border between the oocyte (posterior to the arrow) and the nurse cells. (d) Dorsal side of the egg. (B–E) Lateral close-up views of stage 10 eggs of the similar region as in A. Other designations are also the same as in A. (B) Ras1D38N/Ras15703. (C) Homozygous DrafHM7. (D) Heat-treated temperature-sensitive Dmek mutant, DmekLH10; P[w+, Dmekts]. In these loss-of-function mutants there is no down-regulation of CF2. (E) Heat-treated hsp70–rho transgenic. The egg is oriented more ventrally than the one in A, so more ventral nuclei are visible here. CF2 expression pattern is essentially the same as that in the wild type with down-regulation in the anterodorsal follicle cells (Δ, about nine cells wide laterally) and no suppression in the posterior–ventral cells. (F) Close-up view of the dorsal surface of a y, w stage 12 egg. Notice the increased cytoplasmic level of CF2 in the anterodorsal region (delineated by the bracket). Examples of empty nuclei are marked by arrowheads. Anterior is up. (G) Heat-treated Draf gain-of-function mutant hsp70–ΔDrafF22 (lateral view). There is increased cytoplasmic accumulation of CF2 throughout the follicle cell layer. Examples of empty nuclei are marked by arrowheads. Other markings are the same as in A. (H) Detailed view of the dorsal follicular surface of a stage 10 egg from the hsp70–CF2 transgenic fly (h), induced at 37°C for 10 min and dissected 0.5 hr later. Ectopically expressed CF2 is not present in the nuclei of the anterodorsal follicle cells (arrowheads). Anterior is up. Ectopic induction of the CF2 transgene is confirmed by the expression in the nurse-cell-associated follicle cells (v), which do not express the endogenous CF2 (cf. A).
We hypothesized that CF2 is the direct target of regulation by the Egfr-activated kinase cascade. In support of this hypothesis, Ras1 and Draf hypomorphic as well as temperature-sensitive Dmek mutants no longer suppress CF2 protein level in the anterodorsal follicle cells (Fig. 1B–D). The penetrant levels, judged by the effect on CF2 down-regulation, are >95% for Ras1 and Draf (241/246 and 151/158 stage 10 eggs examined, respectively); and ∼70% (140/198 stage 10 eggs examined) for Dmek. Such pattern is reminiscent of that observed in maternal alleles of Egfr (Egfrtop) and grk mutants, as reported previously (Hsu et al. 1996). These results support the notion that the anterodorsal follicular expression of CF2 is down-regulated by the activated Ras–Raf–MEK signaling cascade.
Because rho is involved in dorsal follicle cell fate determination, it will be interesting to see whether or not the Rho function is also required for maintaining CF2 down-regulation. In the rho gain-of-function (rhogof) background induced by overexpression of the hsp70–rho transgene (Ruohola-Baker et al. 1993), CF2 expression in the ventral follicle cells is not down-regulated (Fig. 1E). This result suggests that the Rho function is not involved in CF2 down-regulation, despite that the same rhogof flies generate ∼80% dorsalized egg chambers (Ruohola-Baker et al. 1993; E. Yu. Mantrova and T. Hsu, unpubl.). We also note that in the rho hypomorphic background induced by overexpression of the hsp70–antisense-rho transgenes (Ruohola-Baker et al. 1993), CF2 down-regulation pattern remains unchanged (data not shown).
Down-regulation of CF2 is at the post-transcriptional level
To establish whether or not down-regulation of CF2 gene expression occurs at the transcriptional level, RNA in situ hybridization was performed. Endogenous CF2 RNA is uniformly expressed in all follicle cells and shows no spatial restriction either in the wild-type, or in the loss-of-function and gain-of-function D/V patterning mutants (Fig. 2A–D). Thus, the Egfr-mediated down-regulation is neither at the level of transcription nor at the level of mRNA metabolism.
Figure 2.
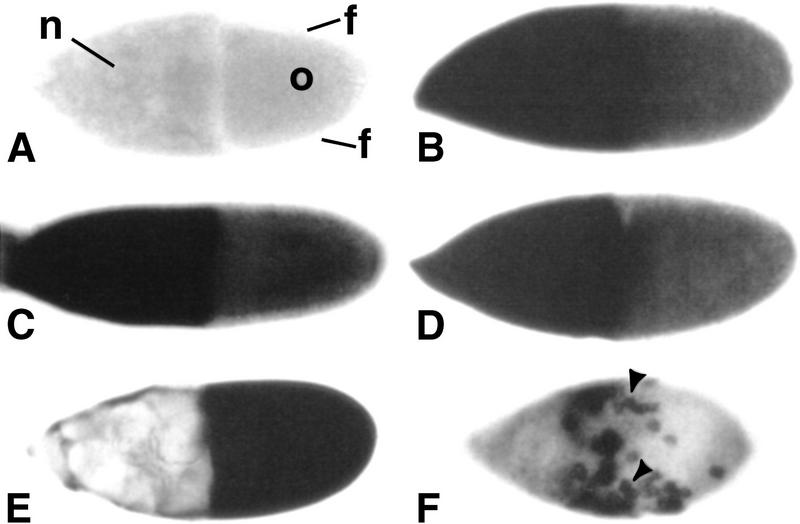
CF2 RNA expression patterns show no spatial restriction. CF2 RNA was detected by whole-mount in situ hybridization with digoxigenin-labeled RNA probes. All eggs are shown in lateral view. Dorsal sides are up, and anterior is to the left. (A) Stage 10 egg from a y, w female hybridized with a CF2 sense-strand RNA probe. This hybridization serves as a negative control. The sample is shown in optical sagittal section to show different cell types: nurse cells (n), follicle cells (f), and the oocyte (o). (B–F) Hybridizations with CF2 antisense RNA probes. For B–F, the images were focused on the follicular surface; the oocyte is not visible at this focal point. (B) A stage 10 egg from a y, w female. (C) A stage 10 egg from a homozygous DrafHM7 female. (D) Stage 10 egg from a heat-treated Draf gain-of-function mutant hsp70–ΔDrafF22. (E) Stage 10 egg from a hsp70–CF2 transgenic fly, induced at 37°C for 10 min and dissected 0.5 hr later. (F) Stage 9 egg from a y, w; P[w+, GAL4]55B/P[w+, UAS–CF2wt] female. Arrowheads point to examples of specific CF2-expressing cells. Because of the low level of endogenous CF2 RNA, color reactions were developed for 6 hr at 37°C for A–D. In contrast, ectopically overexpressed CF2 RNA in E and F could be detected at very high levels in the follicle cells within 0.5 hr of color reaction, whereas nurse cells were barely stained (cf. C–D).
Interestingly, among stage 10–12 wild-type egg chambers examined for CF2 protein expression, about 10% (64/652 examined) showed elevated cytoplasmic level of CF2 in the anterodorsal region. This pattern is particularly striking when the elevated cytoplasmic level of CF2 is contrasted by the empty anterodorsal nuclei (Fig. 1F). Such a pattern indicates that the Egfr signaling cascade may negatively regulate CF2 function by controlling its subcellular localization. In support of this hypothesis, cytoplasmic accumulation of CF2 can be enhanced in the presence of ectopically expressed constitutively active Raf (Fig. 1G). In addition, CF2 must be subjected to rapid degradation as most of the wild-type egg chambers show a complete elimination of CF2 protein in the anterodorsal follicle cells (Fig. 1A). This is a mechanism opposite to that of Dorsal protein regulation (Roth et al. 1989), in which the Dorsal protein function is activated by translocating into the nucleus and the protein is stable throughout its steady state in the cytoplasm.
Post-transcriptional regulation is further demonstrated by ectopic expression of CF2 from a heterologous promoter. We showed earlier that strong heat-shock treatment could induce the hsp70–CF2 transgene to overcome repression of CF2 function in the anterodorsal follicle cells and lead to ventralization (Hsu et al. 1996). We also noticed, however, that when hsp70–CF2 was induced at a lower level, for example, 37°C for 10 min, very few phenocopies were generated (E. Yu. Mantrova and T. Hsu, unpubl.) and repression fo CF2 persisted in the anterodorsal follicle cells (Fig. 1H, h; see also Fig. 5, below). Note that the ectopic overexpression of CF2 RNA from the hsp70 promoter showed no spatial restriction (Fig. 2E). This observation supports a post-transcriptional mechanism for repression of CF2 as the ectopically expressed CF2 is under control of a heterologous promoter. Interestingly, the newly synthesized CF2 (within 0.5 hr of heat induction) is never detected in the nuclei of the anterodorsal follicle cells (Fig. 1H). Although it is possible that the ectopic CF2 can enter the nuclei and then be rapidly exported out, it would seem more plausible that the CF2 protein is prevented from nuclear entry in the anterodorsal follicle cells.
Figure 5.

Down-regulation of CF2 in the anterodorsal follicle cells is independent of nuclear localization. y, w females carrying two copies of hsp70–CF2wt, hsp70–CF2A40, hsp70–CF2Δwt or hsp70–CF2ΔA40 were conditioned in the presence of live yeast at room temperature for 3 days. To induce ectopic CF2 expression, females were heat treated at 37°C for 10 min and allowed to recover at 25°C for 3 hr. Egg chambers were then dissected and processed for immunostaining. The fly strains are indicated. (A) CF2wt, (B) CF2A40, and (D) CF2ΔA40 are shown in lateral views. (C) CF2Δwt is shown in a dorsal view. The ectopic expression of CF2 and CF2Δ proteins are observed in the nurse-cell-associated follicle cells (▴), which do not express the endogenous CF2 protein. Difference between nuclear staining (bright round dots in A and B) and cytoplasmic staining (generalized smear of staining in C and D) is easily discernible. Both CF2wt and CF2Δwt are depleted in the anterodorsal follicle cells (arrows), whereas both CF2A40 and CF2ΔA40 remain stable in these cells (arrowheads). Note that the CF2 antibody titer is reduced by 50% in this experiment as compared to those shown in Figure 1. This enabled us to highlight the overexpressed CF2 protein.
Predicted MAPK phosphorylation site in CF2 is required for cytoplasmic accumulation
Maternal alleles of mapk that affect dorsoventral patterning in egg chambers are currently not available, but MAPK is likely involved in the signaling event as the same Ras–MEK–MAPK signaling cassette has been shown to be involved in many other Egfr signaling pathways analyzed so far (Perrimon and Perkins 1997; Schweitzer and Shilo 1997). Examination of the CF2 amino acid sequence revealed one optimal consensus MAPK phosphorylation site at residue 40 (PAT40P; see Materials and Methods), where the threonine residue is presumed to be the phosphorylation target. We tested the importance of the MAPK site by site-specific mutagenesis, quantifying the effect in cultured cells. Because the cultured S2 cells have been shown to be less efficient in protein degradation than the in vivo systems (Rebay and Rubin 1995), they may also provide a better assay condition for examining cytoplasmic accumulation of CF2.
Cultured Drosophila S2 cells were cotransfected with CF2-coding sequences (either wild-type, CF2wt, or the MAPK-site mutant form, CF2A40; see Materials and Methods) and coding sequences for either constitutively active Ras (Ras1V12; Fortini et al. 1992; Rebay and Rubin 1995) or constitutively active MAPK (MAPKSem; Brunner et al. 1994). Note that the A40 mutation does not by itself alter the stability of CF2 in the absence of activated MAPK, at least when its half-life was measured in the S2 cells (E. Yu. Mantrova and T. Hsu, unpubl.). As shown in Figure 3A, three types of subcellular localization of CF2 were observed; nuclear (N), cytoplasmic (C), and both (N + C). Quantitation of the results is shown in Figure 3B. When expressed alone from an actin 5c gene promoter, CF2wt protein is localized to the nucleus in over 92% of all CF2-expressing cells. In the presence of either Ras1V12 or MAPKSem, there is a significant increase in cytoplasmic accumulation of CF2wt: The percentages of cytoplasmic CF2-containing cells (N + C and C) increased to 44% with Ras1V12 and 52% with MAPKSem. In contrast, the percentages of cells expressing cytoplasmic CF2A40 mutant protein remains largely unchanged at the background level in the presence of Ras1V12 or MAPKSem.
Figure 3.
Constitutively active MAPK and Ras induce cytoplasmic accumulation of CF2. Cultured S2 cells were cotransfected with CF2-coding sequences (CF2wt or CF2A40) together with one of the following: vector, pPac; MAPKSem plasmid encoding the constitutively active MAPK; or Ras1V12 plasmid encoding the constitutively active Ras. Cells were collected and immunostained with anti-CF2 antibody. (A) Three types of CF2 localization patterns are shown from cells cotransfected with CF2wt and MAPKSem: (N) exclusively nuclear; (N + C) nuclear and cytoplasmic; (C) exclusively cytoplasmic. (B) In repeated experiments, the percentages of the three types described in A were determined for different combinations of expression plasmids as indicated. Three independent experiments were performed. The numbers are the average from these repeat experiments and represent the percentages of all CF2-expressing cells. Cell numbers counted for each experiment ranged from 199 to 510; however, the total cell numbers counted for each transfection data set are between 767 and 1390.
Note that the Ras- and MAPK-induced responses are not observed in all cells in the transfection assay. This may be attributable to the high level of overexpression of CF2, a lack of efficient Egfr-regulated protein metabolism in the S2 cells, and/or inefficient activities of the Ras1V12 and MAPKSem mutant proteins on CF2.
MAPK target site mediates down-regulation of CF2 in vivo
To confirm the importance of the MAPK site in vivo, transgenic flies were constructed that carried wild-type (CF2wt) or MAPK site mutant (CF2A40) versions of the CF2-coding sequences under control of the yeast GAL4-dependent enhancer element UAS (Brand and Perrimon 1993). Flies bearing the UAS–CF2 transgene were crossed with another transgenic fly strain w; P[w+, GAL4]55B that expresses the yeast transfection factor GAL4 in the anterior population of the oocyte-associated follicle cells (Brand and Perrimon 1994; Fig. 4A, top panels), including the critical anterodorsal region, during mid-oogenesis. In female progeny bearing both GAL4 and UAS–CF2 transgenes, CF2 protein is expected to be overexpressed in a specific GAL4-dependent pattern, that is, throughout the anterior population of the oocyte-associated follicle cells. However, wild-type CF2 expressed from the UAS–CF2 transgene is still absent from the anterodorsal region (Fig. 4A, middle panels), despite that the ectopically expressed CF2 RNA shows no spatial restriction (Fig. 2F). This result again indicates that the down-regulation is post-transcriptional. Note that CF2 protein is indeed ectopically expressed in the anteroventral cells. By contrast, the MAPK-site mutant CF2 protein (CF2A40) expressed from the UAS–CF2 transgene can be detected at a high level in both the anterodorsal and anteroventral regions. (Fig. 4A, bottom panels). Furthermore, overexpression of CF2A40 results in a loss of dorsal appendage material (Fig. 4B), as expected. The phenotype is similar to that observed in the weak Ras1 hypomorphs (Schnorr and Berg 1996). The percentage of phenocopies in the transgenic line shown in Figure 4B is 16% (51/319 stage 14 eggs examined). Three other independent lines were also examined, and they showed similar results (data not shown).
Figure 4.
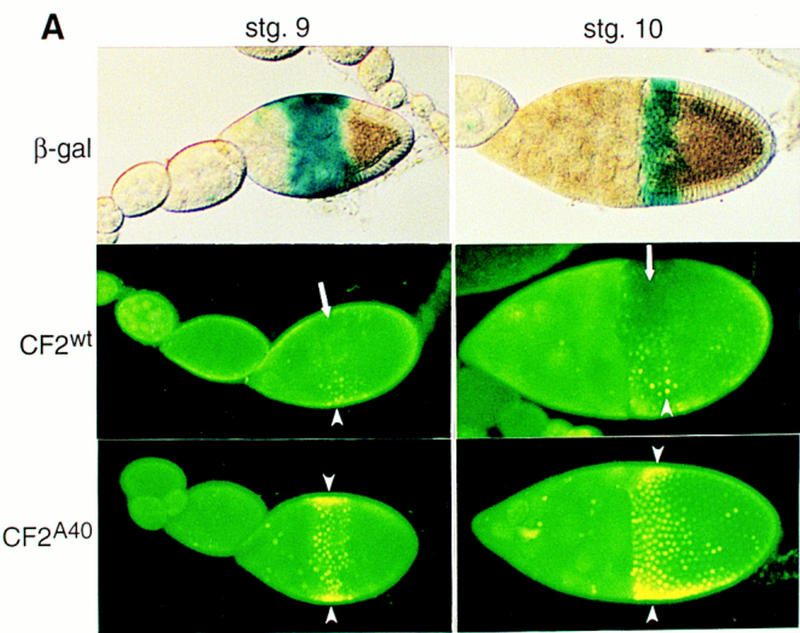
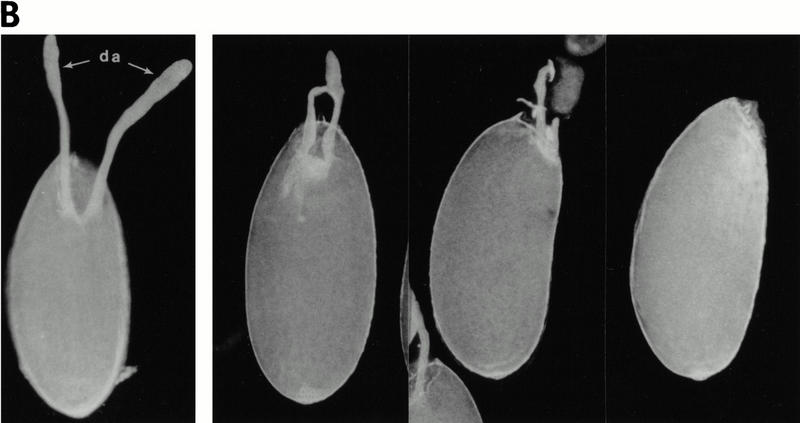
Down-regulation of CF2 protein in vivo is mediated by the MAPK target site. (A) Transgenic fly strain w; P[w+, GAL4]55B was crossed with transgenic lines carrying P[w+, UAS–lacZ], P[w+, UAS–CF2wt], or P[w+, UAS–CF2A40]. The resulting flies were trans-heterozygous for the third chromosome. Newly eclosed females were conditioned at 25°C in the presence of live yeast for 3 days, and egg chambers were dissected and stained either with X-gal (for the β-gal line) or with anti-CF2 antibody (for the CF2wt and CF2A40 lines). Staining for β-galactosidase activity establishes the spatial expression pattern of GAL4 (top panels), which appears at stage 9 in a band of follicle cells just anterior to the oocyte. This band of expression narrows and encircles the anterior margin of the oocyte at stage 10. Ectopic expression of CF2 follows this pattern, except that CF2wt is eliminated from the anterodorsal region in the same manner as the endogenous CF2 expression (arrows, middle panels). The UAS–CF2wt transgene was induced because overexpressed CF2 protein is detected in the anteroventral cells (arrowheads). In contrast, CF2A40 is ectopically expressed throughout the anterior cells. Arrowheads indicate the overexpressed CF2 protein. Note that the CF2 antibody titer was reduced by 50% in this experiment as compared to those shown in Fig. 1, A–G. This enabled us to highlight the overexpressed CF2 protein. (B) Constitutively stable CF2 mutant protein results in reduction of dorsal appendages. Matured eggs were dissected from either y, w (wild type; left panel) or y, w; P[w+, GAL4]55B/P[w+, UAS–CF2A40] flies (with various degrees of expressivity; three right panels). The mutant eggs show weak Ras1-like phenotypes: reduced dorsal appendages (da). Three other independent lines were also examined and showed similar results.
Cytoplasmic localization is not sufficient for degradation
Two processes appear to be involved in CF2 down-regulation: cytoplasmic accumulation and degradation. The question was raised concerning whether or not degradation of CF2 is a default outcome of cytoplasmic localization. To address this question, deletion mutant CF2 proteins defective in nuclear localization were engineered by removal of the last 18 codons of the CF2 open reading frame, which encode a part of the last zinc-finger and the nuclear localization signal (Hsu et al. 1992; Vesque and Charnay 1992; Matheny et al. 1994). The deleted form of either wild-type (CF2Δwt) or the MAPK-site mutant (CFSΔA40) was ectopically expressed in the ovaries directed by the heat-shock promoter, which was induced by a mild heat treatment (37°C for 10 min). The intact CF2wt and CF2A40 coding sequences were also examined for comparison. The mild heat-induction allows the post-translational down-regulation of CF2wt to occur (Fig. 5A; also see Fig. 1H). Also in support of the earlier result using the UAS transgenic system (Fig. 4), CF2A40 becomes stable in the anterodorsal cell (Fig. 5B). Strikingly, the cytoplasmic CF2Δwt is still down-regulated in the anterodorsal follicle cells although the ectopically expressed truncated protein can be detected in the cytoplasm of all other follicle cells (Fig. 5C). By contrast, the cytoplasmic CF2ΔA40 is expressed throughout the follicle cell layer (Fig. 5D). These observations demonstrate that cytoplasmic localization does not necessarily lead to degradation and that degradation is also a specific mechanism for targeted proteins in the anterodorsal follicle cells, not simply a default outcome of cytoplasmic localization.
Discussion
CF2 is the target of Ras–Raf–MEK–MAPK signaling cassette
Previous studies have indicated that CF2 is down-regulated in the anterodorsal follicle cells in response to the activated Egfr (Hsu et al. 1996). It has also been shown that Ras, Raf, MEK, and, by extension, MAPK are downstream of the Egfr function. In this report, we show that at least one of the targets of the kinase cascade is the transcription factor CF2. CF2 is not down-regulated in the anterodorsal follicle cells in Ras1, Draf, and Dmek mutants (Fig. 1). The down-regulation is at the protein level because: First, down-regulation occurs when CF2 transgene is transcribed from heterologous promoter elements (Figs. 1H, 3, 4, and 5); second, there is no spatial restriction in CF2 mRNA expression (Fig. 2); and third, down-regulation can be abolished by alteration of the presumptive MAPK site in CF2 protein (Figs. 3–5). We have not yet been able to demonstrate that MAPKSem specifically phosphorylates CF2 proteins at the predicted phosphorylation site. In cultured cells as well as in cell-free phosphorylation reactions, both CF2wt and CF2A40 were phosphorylated at a low level by MAPKSem (E. Yu. Mantrova and T. Hsu, unpubl.). We did note that although PAT40P is the only optimal MAPK target in CF2, six other suboptimal sites also exist, in the form of SP or TP, and these suboptimal sites may be phosphorylated in vitro. In any case, these other sites at best play only auxiliary roles in D/V patterning as only the optimal site is required for MAPK-mediated protein processing in vivo (Figs. 4 and 5).
Although the mapk gain-of-function allele rlSem could induce cytoplasmic accumulation of CF2 in cultured cells (Fig. 3), it did not show D/V patterning defects in the egg chamber (Brunner et al. 1994). This may be because the expressivity level of this allele is not high enough to afford D/V patterning phenotypes. This hypothesis is supported by the observation that the mutant rlSem gene product is only partially effective in inducing cytoplasmic accumulation of CF2 in the transfection assay (Fig. 3). Alternatively, the rl gene product may not be the MAPK activity expressed (or utilized) in the dorsal follicle cells. At least two other mapk-related genes have been identified recently (see FlyBase and GenBank entries).
CF2 is down-regulated by a cytoplasmic retention and degradation system
Cytoplasmic accumulation is observed in only about 10% of the stage 10 to stage 12 wild-type eggs, probably because of rapid degradation of the cytoplasmic CF2. However, increased levels of cytoplasmic accumulation can be induced in all follicle cells when constitutively active Raf is ectopically expressed (Fig. 1G); and in cultured cells by co-expression of constitutively active Ras1 or MAPK (Fig. 3). The simplest model for CF2 down-regulation would involve phosphorylation of CF2 in the cytoplasm, thus blocking nuclear import and inducing protein degradation. This model is in contrast to a nuclear export mechanism, in which CF2 will need to enter the nucleus, modified by MAPK, and transported out again. The cytoplasmic retention model is supported by the expression patterns of a truncated form of CF2 protein (CF2Δ) defective in nuclear localization (Fig. 5). The otherwise wild-type version of the cytoplasmic CF2Δ still shows specific down-regulation pattern in the anterodorsal follicle cells, whereas the A40 version again becomes constitutively stable. This indicates that CF2Δ is targeted by MAPK in the cytoplasm. Also, cytoplasmic localization of CF2Δ is not sufficient for the subsequent degradation since it remains stable in the ventral follicle cells and the cytoplasmic CF2ΔA40 remains stable in both dorsal and ventral follicle cells. Therefore, we suggest that down-regulation of CF2 is through a coupled cytoplasmic retention and degradation system, and the existing nuclear CF2 may simply diffuse out into the cytoplasm when there is no more incoming CF2 protein (Fig. 6). It should be noted that at this time we cannot vigorously exclude the possibility that MAPK can also target the existing nuclearly localized CF2 and induce its nuclear export.
Figure 6.
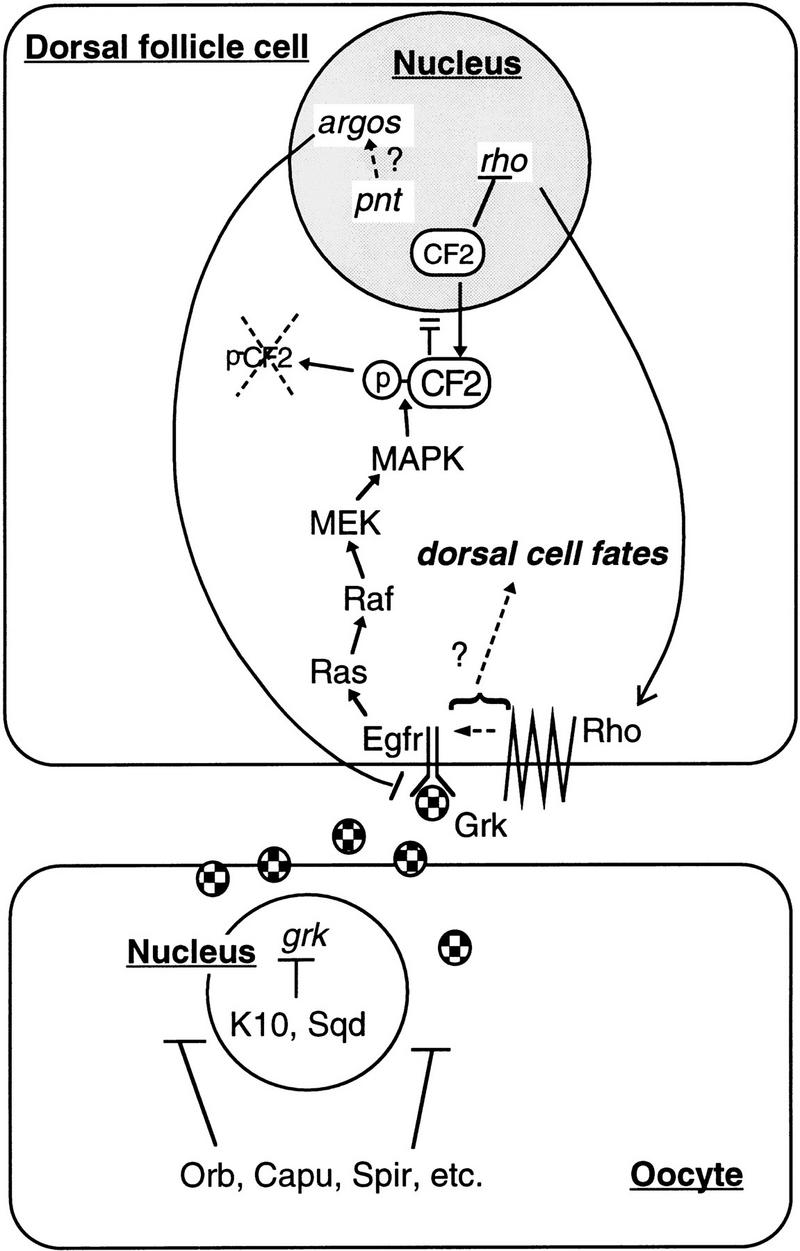
A model for the Egfr signaling pathway in dorsal follicle cell fate determination. In mid-oogenesis, grk gene product is sequestered to the anterodorsal region of the oocyte, aided by gene functions such as fs(1)K10, sqd, spir, capu, orb, etc. Grk acts across the oocyte membrane and activates the Egfr on the neighboring follicle cells. See text for other details on the resulting signaling events.
Proteins designated for degradation very often contain PEST sequence motifs (Rogers et al. 1986). Interestingly, CF2 has one PEST motif at the amino terminus overlapping the presumptive MAPK site: 15RPEDQSPAPPPPPPSSATTSTAAPATPTH43, the underlined residues being the putative MAPK site. It is possible that phosphorylation at the presumptive MAPK site induces conformational changes so as to expose the PEST motif. The motif is then recognized by either ubiquitin or other cellular proteins and the tagged protein is eventually processed by the degradation machinery.
Receptor tyrosine kinase signaling cascades have been shown to enhance the activity of transcription factors by phosphorylation (Karin 1994). In addition, protein stability and nuclear localization can be induced by kinase activation (Palombella et al. 1994; Hochstrasser 1995; Musti et al. 1997; for review, see Vandromme et al. 1996). To date, there have been only a handful of examples for negative regulation of transcription factors at the post-translational level. One example concerns the Drosophila ETS-domain transcription repressor Yan in the Sevenless signaling pathway during Drosophila eye development. In this case, an out-of-nucleus model was proposed, but the possibility of cytoplasmic retention was not ruled out (Rebay and Rubin 1995). More recently, it has been shown that proteasome-dependent degradation of the Tramtrack protein requires interaction with Phyllopod and Seven In Absentia proteins (Li et al. 1997; Tang et al. 1997). It is not yet clear, however, how this down-regulation is regulated by the Sevenless and the Egfr pathways.
In this report, we demonstrate that coupled functions of cytoplasmic retention and degradation are required for CF2 regulation; and that the target protein is designated by the Egfr/MAPK signal. We suggest that this novel regulatory mechanism can be considered a paradigm for the elimination of transcription factor functions as one of the immediate responses to extracellular signals.
CF2 is the coordinator in Egfr signaling
A model that integrates previous and current findings is presented in Figure 6. We propose that the Egfr-mediated D/V patterning is a two-step signaling process, demarcated by the appearance of Rho function. This model is based on three previous observations (Ruohola-Baker et al. 1993; Hsu et al. 1996): (1) down-regulation of CF2 precedes rho expression; (2) overexpression of CF2 can suppress rho expression; and (3) Egfr alone cannot induce dorsal cell fates without Rho. In this report, we have placed the Ras/MAPK signaling pathway upstream of CF2. But what are the changes in the signaling cascade, if any, brought on by Rho? It has been shown that Rho by itself cannot induce dorsal fates without Egfr, but it can dorsalize the egg chambers when ectopically expressed in the ventral follicle cells (Ruohola-Baker et al. 1993), despite that Egfr in the ventral follicle cells is not pre-activated by Grk. Interestingly, the level of CF2 in the ventral follicle cells is not affected by the ectopically expressed Rho (Fig. 1F). This indicates that the signal induced by Rho is at least different from that of Egfr alone with respect to CF2 regulation. It has been suggested that Rho is involved in processing a second ligand (Golembo et al. 1996; Perrimon and Perkins 1997). If this is correct, then Rho may induce a signaling cascade distinct from Ras–Raf–MEK–MAPK, or may modify the specificity of the existing cascade. Indirect evidence has emerged recently that other signaling pathway(s) parallel to that of Egfr may in fact exist (Goode et al. 1996; Schnorr and Berg 1996). Resolving the events downstream of Rho should be the next step in unraveling this complex developmental signaling process.
Materials and methods
Drosophila strains
The Ras1 alleles used in this study were kindly provided by C. Berg, University of Washington, Seattle, Washington: Ras1D38N/TM3, Sb; and Ras15703, ry506, cv-c, sbd/TM3, ryRK, Sb. These lines have high embryonic lethality but trans-heterozygotes are largely viable and show strong egg chamber patterning defects with an expressivity level of nearly 100% (Schnorr and Berg 1996).
The Dmek allele (kindly provided by N. Perrimon, Harvard Medical School, Boston, MA) has been described (Hsu and Perrimon 1994). It is a lethal allele DmekLH10 rescued by the temperature-sensitive hsp70–mekts transgene. To induce D/V patterning defects in the egg chamber, newly eclosed females were kept at 32°C for 3 days with two periods of 15-min heat shock at 37°C daily.
The Draf loss-of-function allele, y, DrafHM7, wa/FM7, and the gain-of-function allele, y, w; hsp70–ΔDrafF22, were also provided by N. Perrimon, and have been described (Brand and Perrimon 1994). To induce the gain-of-function phenotype, newly eclosed females were kept at 32°C for 3 days with two periods of 15-min heat-shock at 37°C daily.
The rho gain-of-function allele is the transgenic y, w flies carrying one copy of the hsp70–rho transgene, y, w; P[w+, hsp70–rho]/TM3, Sb (Ruohola-Baker et al. 1993). To induce ectopic expression of Rho, newly eclosed females were kept at 32°C for 3 days with two periods of 15-min heat shock at 37°C daily.
w; P[w+, GAL4]55B, and w; P[w+, UAS–lacZ]4-2-4B lines were obtained from the Bloomington Stock Center, Indiana University, with assistance from K. Matthews.
CF2 transgenes
The consensus MAPK phosphorylation site is located at around amino acid residue 40: PAT40P (P, proline; A, alanine; and T, threonine; Clark-Lewis et al. 1991). To generate the MAPK site mutant protein (CF2A40), the codon for threonine, ACG, was mutated to the alanine codon, GCG, using the Transformer Site-Directed Mutagenesis Kit (Clontech Laboratories). Nucleotide sequence of the primer introducing the mutation is 5′-CCAGCCGCGCCACGCAC-3′. In vitro mutagenesis was performed on the cDNA clone described previously (Hsu et al. 1992). The mutated DNA fragment was sequenced to confirm the presence of mutation and integrity of the rest of the coding sequence. The CF2-encoding sequences of either wild-type or the mutant form were released as a EagI–ClaI fragment as described before (Hsu et al. 1996). The fragments were subcloned into the pCaSpeR–hs vector under the control of the hsp70 gene promoter as described before (Hsu et al. 1996); into the pPac vector under the control of the actin5C gene promoter for expression in the cultured S2 cells; or into the pUAST vector (a gift from N. Perrimon) under the control of the GAL4-dependent UAS element.
To generate the cytoplasmic CF2 deletion protein, the cDNA clones encoding either CF2wt or CF2A40 were subjected to 3′-deletion up to the Eco47III restriction site located at 53 bp upstream of the termination codon. The deletion eliminated the last zinc finger, which also contains the nuclear localization signal (Hsu et al. 1992; Vesque and Charnay 1992; Matheny et al. 1994). The truncated CF2-encoding sequences (wt and A40) were then subcloned as EagI–Eco47III fragments into the NotI–StuI sites of pCaSpeR–hs vector as described above. A stop codon was regenerated immediately downstream of the fusion site with only one exogenous codon (CCC for proline) inserted.
Transgenic lines
Transforming vectors pCaSpeR–hs and pUAST containing various CF2-encoding sequences described above were used to transform y, w flies by use of standard procedures (Spradling 1986). For each transgene construct, four to five transgenic lines were generated and examined. The expression patterns and phenotypes described in this report are not attributable to individual insertion events.
Immunostaining
The polyclonal CF2 antisera were generated from rabbits injected with gel-purified full-length CF2 protein produced in E. coli cells (Gogos et al. 1992). The antibody was then purified by affinity chromatography against CF2 protein immobilized on CNBr-coupled Sepharose 4MB (from Pharmacia). Anti-CF2 antibodies were eluted with 0.1 m glycine (pH 2.5). Fractions containing immunoglobulin were identified by spectrophotometry, pooled, and dialyzed against PBS. The antisera were further concentrated by filtration. The final concentration factor is about 100×. Specificity and titer were determined by Western blotting assays. The antisera were diluted 50× for ovary staining and 200× for S2 cell staining. Preparation of ovarian samples and the subsequent staining procedures have been described (Hsu et al. 1996) except that biotin-conjugated goat anti-rabbit IgG was used as secondary antibody, and the egg chambers were then incubated with fluorescein-conjugated avidin (both reagents from Vector). Egg chambers were mounted in 50% glycerol/PBS for fluorescence microscopy.
Transfection assay
The expression plasmids pPac containing the actin 5C gene promoter and either Ras1V12 (encoding the constitutively active Ras1) or rlSem (encoding the constitutively active MAPK) were kindly provided by G. Rubin (University of California, Berkeley). The same vector containing either CF2wt or CF2A40 was constructed by standard cloning methods. Growth and transfection of Drosophila Schneider cell line S2 have been described (Cherbas et al. 1994). In cotransfection, the ratio of the CF2-containing plasmid versus the vector, Ras1-containing, or MAPK-containing plasmid is 1 : 7 (2.5 μg : 17.5 μg). Cells were incubated with the transfection plasmids for 24 hr, washed and incubated with fresh media, and harvested another 24 hr later. Immunofluorescence assay of transfected cells was performed as described previously (Fehon et al. 1990) with polyclonal anti-CF2 antibody (see above). After immunostaining, cells were dried on slides and mounted in 90% glycerol/PBS for fluorescence microscopy.
Detection of β-galactosidase activity in ovaries
Ovaries from w; P[w+, GAL4]55B/P[w+, UAS–lacZ]4-2-4B females were dissected in PBS and were fixed for 8 min in 4% paraformaldehyde (from Sigma) in PBS. Ovaries were then rinsed 3 times in PBS containing 0.5% Triton X-100 and stained at 37°C for 30 min in stain solution (5 mm K4[Fe(III)CN6], 5 mm K3[Fe(II)CN6], 0.3% Triton X-100, in PBS) containing 0.2% X-gal (from Sigma). The ovaries were then rinsed in PBS and mounted in PBS containing 50% glycerol for microscopic analysis.
RNA in situ hybridization
The hybridization probes used correspond to a 1.7-kb CF2 cDNA NarI–HindIII fragment encompassing the DNA-binding domain and most of the 3′-untranslated region, which was subcloned in the pBluescript II KS vector (Stratagene). Either the sense or the antisense RNA probes were synthesized and labeled with digoxigenin as described by the supplier (Boehringer-Mannheim Biochemicals). Newly eclosed females were conditioned for three days in the presence of live yeast at room temperature and then anesthetized on ice and ovaries dissected in Ringer’s solution. The staining and detection procedures have been described (Tautz and Pfeifle 1989; Hsu et al. 1993). For examination, ovaries were mounted in PBS/50% glycerol.
Acknowledgments
We thank Norbert Perrimon, Celeste Berg, Hannele Ruohola-Baker, and Gerald Rubin for generous gifts of Drosophila strains and cDNA clones that are essential for this study. We also appreciate the unparalleled helpfulness of Kathy Matthews in providing us with fly strains from the Bloomington Stock Center. We are particularly grateful to Celeste Berg, Kay Meier, Maria Trojanowska, and an anonymous reviewer for their valuable comments on the manuscript. This work has been supported by a development grant from the Department of Energy; and by the Medical University of South Carolina Institutional Research Funds.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL hsut@musc.edu; FAX (803) 792-3940.
References
- Bier E, Jan LY, Jan YN. rhomboid, a gene required for dorsoventral axis establishment and peripheral nervous system development in Drosophila melanogaster. Genes & Dev. 1990;4:190–203. doi: 10.1101/gad.4.2.190. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- ————— Raf acts downstream of the EGF receptor to determine dorsoventral polarity during Drosophila oogenesis. Genes & Dev. 1994;8:629–639. doi: 10.1101/gad.8.5.629. [DOI] [PubMed] [Google Scholar]
- Brunner D, Oellers N, Szabad J, Biggs III WH, Zipursky SL, Hafen E. A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell. 1994;76:875–888. doi: 10.1016/0092-8674(94)90362-x. [DOI] [PubMed] [Google Scholar]
- Cherbas L, Moss R, Cherbas P. Transformation techniques for Drosophila cell lines. In: Goldstein LSB, Fyrberg EA, editors. Drosophila melanogaster: Practical uses in cell and molecular biology. San Diego, CA: Academic Press; 1994. pp. 161–183. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I, Sanghera JS, Pelech SL. Definition of a consensus sequence for peptide substrate recognition by p44mpk, the meiosis activated myelin basic protein kinase. J Biol Chem. 1991;266:15180–15184. [PubMed] [Google Scholar]
- Fehon RG, Kooh PJ, Rebay I, Regan CL, Xu T, Muskavitch MA, Artavanis-Tsakonas S. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell. 1990;61:523–534. doi: 10.1016/0092-8674(90)90534-l. [DOI] [PubMed] [Google Scholar]
- Fortini ME, Simon MA, Rubin GM. Signalling by the sevenless protein tyrosine kinase is mimicked by Ras1 activation. Nature. 1992;355:559–561. doi: 10.1038/355559a0. [DOI] [PubMed] [Google Scholar]
- Gabay L, Scholz H, Golembo M, Klaes A, Shilo B-Z, Klämbt K. EGF receptor signaling induces pointed P1 transcription and inactivates Yan protein in the Drosophila embryonic ventral ectoderm. Development. 1996;122:3355–3362. doi: 10.1242/dev.122.11.3355. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Hsu T, Bolton J, Kafatos FC. Sequence discrimination by alternatively spliced isoforms of a DNA binding zinc finger domain. Science. 1992;257:1951–1955. doi: 10.1126/science.1290524. [DOI] [PubMed] [Google Scholar]
- Golembo M, Raz E, Shilo B-Z. The Drosophila embryonic midline is the site of Spitz processing, and induced activation of the EGF receptor in the ventral ectoderm. Development. 1996;122:3363–3370. doi: 10.1242/dev.122.11.3363. [DOI] [PubMed] [Google Scholar]
- González-Reyes A, Elliott H, St. Johnston D. Polarization of both major body axes in Drosophila by gurken-torpedo signaling. Nature. 1995;375:654–658. doi: 10.1038/375654a0. [DOI] [PubMed] [Google Scholar]
- Goode S, Morgan M, Liang Y-P, Mahowald AP. brainiac encodes a novel, putative secreted protein that cooperates with Grk TGFα in the genesis of the follicular epithelium. Dev Biol. 1996;178:35–50. doi: 10.1006/dbio.1996.0196. [DOI] [PubMed] [Google Scholar]
- Hochstrasser M. Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Curr Opin Cell Biol. 1995;7:215–223. doi: 10.1016/0955-0674(95)80031-x. [DOI] [PubMed] [Google Scholar]
- Hsu J-C, Perrimon N. A temperature-sensitive MEK mutation demonstrates the conservation of the signaling pathways activated by receptor tyrosine kinases. Genes & Dev. 1994;8:2176–2187. doi: 10.1101/gad.8.18.2176. [DOI] [PubMed] [Google Scholar]
- Hsu T, Gogos JA, Kirsh SA, Kafatos FC. Multiple zinc finger forms resulting from developmentally regulated alternative splicing of a transcription factor gene. Science. 1992;257:1946–1950. doi: 10.1126/science.1411512. [DOI] [PubMed] [Google Scholar]
- Hsu T, King DL, LaBonne C, Kafatos FC. A Drosophila single-stranded DNA/RNA-binding factor contains a high-mobility-group box and is enriched in the nucleolus. Proc Natl Acad Sci. 1993;90:6488–6492. doi: 10.1073/pnas.90.14.6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu T, Bagni C, Sutherland JD, Kafatos FC. The transcription factor CF2 is a mediator of EGF-R-activated dorsoventral patterning in Drosophila oogenesis. Genes & Dev. 1996;10:1411–1421. doi: 10.1101/gad.10.11.1411. [DOI] [PubMed] [Google Scholar]
- Karin M. Signal transduction from the cell surface to the nucleus through the phosphorylation of transcription factors. Curr Opin Cell Biol. 1994;6:415–424. doi: 10.1016/0955-0674(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Li S, Li Y, Carthew RW, Lai Z-C. Photoreceptor cell differentiation requires regulated proteolysis of the transcriptional repressor Tramtrack. Cell. 1997;90:469–478. doi: 10.1016/s0092-8674(00)80507-3. [DOI] [PubMed] [Google Scholar]
- Lu X, Melnick MB, Hsu J-C, Perrimon N. Genetic and molecular analyses of mutations involved in Drosophila raf signal transduction. EMBO J. 1994;13:2592–2599. doi: 10.1002/j.1460-2075.1994.tb06549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheny C, Day MC, Milbrandt J. The nuclear localization signal of NGF1-A is located within the zinc finger binding domain. J Biol Chem. 1994;269:8176–8181. [PubMed] [Google Scholar]
- Morimoto AM, Jordan KC, Tietze K, Britton JS, O’Neill EM, Ruohola-Baker H. Pointed, an ETS domain transcription factor, negatively regulates the EGF receptor pathway in Drosophila oogenesis. Development. 1996;122:3745–3754. doi: 10.1242/dev.122.12.3745. [DOI] [PubMed] [Google Scholar]
- Musti AM, Treier M, Bohmann D. Reduced ubiquitin-dependent degradation of c-Jun after phoshporylation by MAP kinases. Science. 1997;275:400–402. doi: 10.1126/science.275.5298.400. [DOI] [PubMed] [Google Scholar]
- Neuman-Silberberg FS, Schüpbach T. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGFα-like protein. Cell. 1993;75:165–174. [PubMed] [Google Scholar]
- Palombella VJ, Rando OJ, Goldberg AL, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- Perrimon N, Perkins LA. There must be 50 ways to rule the signal: The case of the Drosophila EGF receptor. Cell. 1997;89:13–16. doi: 10.1016/s0092-8674(00)80177-4. [DOI] [PubMed] [Google Scholar]
- Price JV, Clifford RJ, Schüpbach T. The maternal ventralizing locus torpedo is allelic to faint little ball, an embryonic lethal, and encodes the Drosophila EGF receptor homologue. Cell. 1989;56:1085–1092. doi: 10.1016/0092-8674(89)90641-7. [DOI] [PubMed] [Google Scholar]
- Ray RP, Schüpbach T. Intercellular signaling and the polarization of body axes during Drosophila oogenesis. Genes & Dev. 1996;10:1711–1723. doi: 10.1101/gad.10.14.1711. [DOI] [PubMed] [Google Scholar]
- Rebay I, Rubin GM. Yan functions as a general inhibitor of differentiation and is negatively regulated by activation of the Ras1/MAPK pathway. Cell. 1996;81:857–866. doi: 10.1016/0092-8674(95)90006-3. [DOI] [PubMed] [Google Scholar]
- Rogers S, Wells R, Rechsteiner M. Aminoacid sequences common to rapidly degraded proteins: The PEST hypothesis. Science. 1986;234:364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Roth S, Stein D, Nüsslein-Volhard C. A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell. 1989;59:1189–1202. doi: 10.1016/0092-8674(89)90774-5. [DOI] [PubMed] [Google Scholar]
- Roth S, Neuman-Silberberg FS, Barcelo G, Schüpbach T. Cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell. 1995;81:1967–978. doi: 10.1016/0092-8674(95)90016-0. [DOI] [PubMed] [Google Scholar]
- Ruohola-Baker H, Grell E, Chou T-B, Baker D, Jan LY, Jan YN. Spatially-localized rhomboid is required for establishment of the dorsal-ventral axis in Drosophila oogenesis. Cell. 1993;73:953–965. doi: 10.1016/0092-8674(93)90273-s. [DOI] [PubMed] [Google Scholar]
- Schejter ED, Shilo B-Z. The Drosophila EGF homolog (DER) gene is allelic to faint little ball, a locus essential for embryonic development. Cell. 1989;56:1093–1104. doi: 10.1016/0092-8674(89)90642-9. [DOI] [PubMed] [Google Scholar]
- Schnorr JD, Berg CA. Differential activity of Ras1 during patterning of the Drosophila dorsoventral axis. Genetics. 1996;144:1545–1557. doi: 10.1093/genetics/144.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüpbach T. Germ line and soma cooperate during oogenesis to establish the dorsoventral pattern of egg shell and embryo in Drosophila melanogaster. Cell. 1987;49:699–707. doi: 10.1016/0092-8674(87)90546-0. [DOI] [PubMed] [Google Scholar]
- Schüpbach T, Clifford RJ, Manseau LJ, Price JV. Dorsoventral signaling processes in Drosophila oogenesis. In: Gerhart J, editor. Cell–cell interactions in early development. New York, NY: Wiley-Liss; 1991. pp. 163–174. [Google Scholar]
- Schweitzer R, Shilo B-Z. A thousand and one roles for the Drosophila EGF receptor. Trends Genet. 1997;13:191–196. doi: 10.1016/s0168-9525(97)01091-3. [DOI] [PubMed] [Google Scholar]
- Schweitzer R, Howes R, Smith R, Shilo B-Z, Freeman M. Inhibition of Drosophila EGF receptor activation by the secreted protein Argos. Nature. 1995;376:699–702. doi: 10.1038/376699a0. [DOI] [PubMed] [Google Scholar]
- Shea MJ, King DL, Conboy MJ, Mariani BD, Kafatos FC. Proteins that bind to Drosophila chorion cis-regulatory elements: A new C2H2 zinc finger protein and a C2C2 steroid receptor-like component. Genes & Dev. 1990;4:1128–1140. doi: 10.1101/gad.4.7.1128. [DOI] [PubMed] [Google Scholar]
- Spradling A. P-element-mediated transformation. In: Roberts DB, editor. Drosophila, a practical approach. New York, NY: IRL Press; 1986. pp. 175–198. [Google Scholar]
- Sturtevant MA, Roark M, Bier E. The Drosophila rhomboid gene mediates the localized formation of wing veins and interacts genetically with components of the EGF-R signaling pathway. Genes & Dev. 1993;7:961–973. doi: 10.1101/gad.7.6.961. [DOI] [PubMed] [Google Scholar]
- Tang AH, Neufeld TP, Kwan E, Rubin GM. PHYL acts to down-regulate TTK88, a transcription repressor of neuronal cell fates, by a SINA-dependent mechanism. Cell. 1997;90:459–467. doi: 10.1016/s0092-8674(00)80506-1. [DOI] [PubMed] [Google Scholar]
- Tautz D, Pfeifle C. A nonradioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals a translational control of the segmentation gene hunchback. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Vandromme M, Gauthier-Rouviére C, Lamb N, Fernandez A. Regulation of transcription factor localization: Fine-tuning of gene expression. Trends Biochem Sci. 1996;21:59–64. [PubMed] [Google Scholar]
- Vesque C, Charnay P. Mapping functional regions of the segment-specific transcription factor Krox-20. Nucleic Acids Res. 1992;20:2485–2492. doi: 10.1093/nar/20.10.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]