Abstract
Tumor growth and metastasis depend on neovascularization, the growth of new blood vessels. Recent findings have revealed that tumor neovascularization is regulated in part by monocytes, which are myeloid lineage cells from the bone marrow. Tumors exhibit significant monocyte infiltrates, which are actively recruited to the tumor microenvironment. Upon tumor infiltration, monocytes can participate in tumor neovascularization. Monocytes can either differentiate into macrophages, which express proangiogenic growth factors, or into endothelial-like cells, which may directly participate in neovascularization. Preliminary studies in animals suggest that modulation of bone marrow derived cell trafficking into tumors will provide a useful new approach in cancer therapy.
1.1 Tumor angiogenesis
Neovascularization, the formation of blood vessels, plays important roles in development, inflammation, and wound repair. Mammalian cells require oxygen and nutrients for their survival and are therefore located within 100 to 200 μm of blood vessels, which is the diffusion limit of oxygen. Thirty-five years ago, Dr. Judah Folkman observed that neovascularization occurs around tumors and proposed that new blood vessel growth is necessary to supply nutrients and oxygen to tumor cells during exponential tumor growth [1]. These observations stimulated an intensive search for the mechanisms regulating tumor angiogenesis. It now known that new blood vessels originate from preexisting vessels by activation, proliferation and migration of endothelial cells through a process named “angiogenesis” [2]. Specific growth factors, such as vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) stimulate the proliferation and migration of naturally quiescent endothelial cells, resulting in the formation of new vessel structures during embryonic development and tumor growth [2,3]. Recent studies have also shown that “vasculogenesis” also occurs during tumor neovascularization [4, 5, 6]. Vasculogenesis is the coalescence of new blood vessels from individual endothelial cells or progenitor cells. The formation of the initial vascular tree during embryonic vascular development and the formation of new blood vessels by circulating endothelial progenitor in the adult animal are examples of vasculogenesis [4, 5, 6]. Importantly, current studies indicate that tumor angiogenesis and tumor vasculogenesis are both modulated by monocytes and myeloid progenitor cells.
1.2 Myeloid cell contributions to tumor neovascularization
Monocytes and macrophages belong to the myeloid cell lineage and derive from myeloid progenitor cells. These precursor cells are located in the bone marrow; upon maturation, monocytes are released into the bloodstream. Circulating blood monocytes migrate into tissues where they differentiate into resident tissue macrophages. The number of macrophages in tumor tissue is significantly greater than in most normal tissue [7, 8]. Importantly, this increase in macrophage content within tumor tissue appears to result from recruitment of circulating monocytes, rather than from expansion of tissue resident macrophages [9]. Tumor associated macrophages (TAMs) include M1 macrophages, which promote anti-tumor immunity, and M2 macrophages, which promote neovascularization [9]. In general. significantly more M2 macrophages than M1 macrophages are found in most tumors [9-13]. M1 macrophages express tumoricidal agents such as TNF alpha, IL-12 and iNOS [9]. In contrast, M2 TAMs release a number of potent pro-angiogenic cytokines, such as VEGF, VEGF-C, tumor necrosis factor -α (TNF-α), interleukin-8 (IL-8) and bFGF [10, 14-20]. Additionally, these TAMs also express a broad array of proteases known to play roles in the angiogenic process. These proteases include urokinase-type plasminogen activator (uPA), the matrix metalloproteinases MMP-2, MMP-7, MMP-9 and MMP12 and elastase [16, 21-24]. uPA and MMPs support angiogenesis by remodeling and breaking down the extracellular matrix (ECM). Degradation of ECM leads to the mobilization of growth factors and facilitates the migration of vascular cells into new environments [21-24]. Strong correlations are observed between TAM densities and vascular densities in many human tumor types, suggesting that TAMs regulate neovascularization [25-29]. Importantly, high TAM densities are indicative of poor prognoses in breast, prostate, ovarian and cervical cancers [25, 28]. TAMs can also directly stimulate tumor cell invasion by releasing chemotactic factors, thus contribution to tumor invasion and metastasis [30]. Other myeloid cells can also promote tumor angiogenesis; MMP9- and VEGF-expressing myeloid cells regulate the angiogenic switch in mice with pancreatic beta-islet cell carcinomas [16].
1.3 Endothelial cell progenitor roles in tumor neovasculature
Recent studies indicate that postnatal bone marrow-derived precursor cells also play roles in neovascularization of tumors and ischemic tissues. Progenitor cells have been shown to home to ischemic or hypoxic tissues (angiogenic niches), where they incorporate into sprouting blood vessels at low, but measurable frequencies. The exact nature of this cell type remains unclear, however. In the first report to describe endothelial progenitor cells, Isner and colleagues isolated mononuclear cells from human peripheral blood that were enriched for expression of the hematopoietic stem cell marker CD34 [5]. Upon culture in endothelial growth media, these cells expressed endothelial lineage markers, such as CD31, Tie2 and VEGFR2, and incorporated into blood vessels in ischemic tissues [5]. These cells were therefore described as bone marrow derived endothelial progenitor cells, or “hemangioblasts”. Subsequent studies described a VEGFR2 and AC133 expressing subpopulation of these CD34 positive circulating cells that could form endothelial colonies in vitro [31-32].
Since these initial reports, other studies have shown that mononuclear progenitor cells expressing monocyte markers such as CD11b or CD14 also give rise to endothelial cell-like colonies in vitro and in vivo [33-38]. Importantly, Hildbrand and colleagues described a CD34+CD11b+ cell population from cord blood that gave rise to endothelial cell colonies in vitro [37]. However, CD34-/CD14+ human peripheral blood monocytes have also been shown to express endothelial cell markers, such as von Willebrand factor and VE-cadherin, after several weeks in culture in the presence of endothelial growth media [38]. Taken together, these findings suggest that endothelial-like cells may arise from bone marrow-derived hematopoietic progenitor cell populations (CD34+ AC133+ cells) or myeloid cell populations (CD14+ or CD11b+ cells). Alternatively, endothelial cells and monocytes cells may share a common precursor.
Further evidence that myeloid cells give rise to new blood vessels came from recent studies by Charles Lin and colleagues, who identified a novel Gr+ CD11b+ bone marrow derived cell population that contributes to neovascularization [39]. These myeloid immune suppressor cells comprised 5% of the total cell population within colorectal carcinoma and Lewis lung carcinoma tumors. In fact, tumor angiogenesis was significantly increased in the presence of Gr+CD11b+ cells, which also exhibited high levels of MMP9. Importantly, deletion of MMP9 abolished the angiogenesis-promoting activity. Tumor derived Gr+Cd11b+ cells also expressed the endothelial cell markers VE-cadherin and VEGFR2 and incorporated into tumor endothelium. These data support the concept that subsets of myeloid cells contribute to tumor neovascularization by differentiating into endothelial cells.
Dendritic precursor cells may also be recruited to tumor blood vessels, whereupon they adopt endothelial cell characteristics [40]. CD11c+ CD45+ CD8+ myeloid dendritic precursor cells accumulated in xenograft ovarian carcinomas, in response to VEGF and β-defensin expression. Eighty percent of these CD11c+ CD45+ CD8+ cells expressed progenitor cell markers such as CD31 and CD34, and forty percent of these cells expressed endothelial cell specific markers such as VE-cadherin and CD146 [40]. These CD11c+ dendritic precursor cells home to tumor vessels, undergo endothelial cell-like differentiation and assembled neovessels. These studies suggest common link between monocyte-derived dendritic cells and microvascular endothelial cells and further support the idea that tumor vasculogenesis occurs through the recruitment of bone marrow-derived endothelial precursor cells, which may include subsets of myeloid lineage cells. Continued characterization of the bone marrow derived cells that contribute to neovascularization and analysis of the mechanisms by which these cells modulate neovascularization will clarify the cells types that promote vasculogenesis. Together, these studies suggest that myeloid lineage bone marrow derived cells give rise to vascular endothelium and that several lineage markers, including Gr, CD11c and CD11b, identify putative endothelial progenitor cells.
Some studies suggest that monocyte precursors of M2 macrophages, rather than endothelial progenitor cells, are the key bone marrow derived cell type that contributes to adult neovascularization. Studies by de Palma and colleagues identified a Tie2+ subpopulation of CD11b+tumor associated myeloid cells that promotes angiogenesis in tumors [41]. While stimulating angiogenesis, these cells do not actively incorporate into blood vessels. Further studies from the same group showed that Tie2 expressing monocytes are specifically recruited to spontaneously arising mouse pancreatic tumors and to orthotopic xenografts of human gliomas [42]. These studies suggest that pro-angiogenic Tie2+ monocytes, rather bone marrow-derived endothelial progenitor cells, promote tumor neovascularization. From these many studies, it is clear that myeloid cells play key roles in tumor angiogenesis; importantly, these cells include endothelial progenitor cells, macrophages and other myeloid lineage cells.
1.4 Recruitment of monocytes into tumors
Substantial evidence indicates that myeloid cells and their precursors promote neovascularization in tumors and inflammatory tissues. These cells are actively recruited to the tumor microenvironment from the bloodstream. Immune cell trafficking in vivo is regulated by chemokines and by members of the integrin, immunoglobulin superfamily and selectin adhesion molecule families [43-44]. Hypoxia, as well as chemokines and their receptors, stimulates homing of circulating monocytes to tissues [45-48]. Tissues respond to hypoxia by upregulating expression of hypoxia responsive proteins such as VEGF, MMPs, interleukins and chemokines. A number of chemokines, including CCL2, CCL5, CXCL8/IL-8, SDF-1 and others attract monocytes to invade tissues. Monocyte chemoattractant protein-1 (MCP-1, or CCL2) and RANTES (CCL5) increased the infiltration of TAMs into primary tumors, including breast and ovarian carcinomas, melanoma, and glioblastoma [49-53]. Furthermore, CCL2 and CCL5 stimulated the secretion of matrix-degrading enzymes, such as MMP9 and MMP12 by macrophages [49-53].
Interleukin-8/CXCL8 (IL-8) also serves as a monocyte chemoattractant. This chemokine is also a pro-angiogenic factor and an autocrine growth factor for several human tumor cell types [54-56]. IL-8 stimulates the adhesion of monocytes, which express low levels of the IL-8 receptors CXCR1 and CXCR2, to vascular endothelium under flow conditions [56]. These studies indicate that IL-8 and CXCR-1/2 interactions play roles in monocyte recruitment.
Several cytokines and growth factors, including colony stimulating factor-1 (CSF-1), vascular endothelial growth factor (VEGF), and platelet derived growth factor (PlGF) have been implicated in the recruitment of monocytes into tumors [57-60]. CSF-1 is produced by various types of human tumors and is a potent chemoattractant for macrophages. Coordinated expression of CSF-1 in macrophages and epidermal growth factor (EGF) in mammary tumor cells resulted in increased myeloid cell invasion into mammary tumors [59].
IL-1β, another myeloid cell chemokine, increased infiltration of neutrophils and macrophages in a mouse model of corneal neovascularization. In contrast, deletion of monocytes by genetic approaches or by use of toxins significantly suppressed IL-1β induced angiogenesis. Importantly, CD11b+ F4/80+ cells that infiltrated sites of corneal neovascularization were COX-2+, while macrophages that remained in the limbal vessel did not express COX-2. As signaling molecules downstream of COX-2, such as PGE2 and thromboxane A2, have been shown to enhance the production of various angiogenesis-related factors like VEGF, MMPs, and chemokines, these studies indicate that COX2 expressing macrophages regulate angiogenesis [60].
β-defensin may also serve as a recruitment factor for myeloid lineage cells. β-defensin is a chemoattractant factor for dendritic cells. Conjeo-Garcia and colleagues found that the recruitment of dendritic precursor cells into tumors required the presence of β-defensin. Depletion of β-defensin or inhibition of its receptor CCR6 using function-blocking antibodies abolished the infiltration of dendritic precursor cells into tumors. These studies indicate that the ligand/receptor pair β-defensin/CCR6 is essential for dendritic precursor cell recruitment [40].
A key role for stromal derived factor (SDF-1) in progenitor cell recruitment was recently described [61]. Syngeneic tumors transplanted into thrombocytopenic mice (such as Thpo-/- and Mpl-/- mice) exhibited impaired neovascularization and reduced release of the chemokine stromal-derived factor-1 (SDF-1). Further studies demonstrated that hematopoietic cytokines including soluble Kit-ligand and thrombopoietin trigger the release of SDF-1 from platelets, which results in the mobilization of unique subset of hemangiogenic progenitor cells (CXCR4+ VEGF+) to neoangiogenic niches. These studies demonstrate the complexity of cytokines that control the mobilization of bone marrow-derived precursor cells.
Thus, a variety of inflammatory stimuli recruit monocytes to invade tumor tissue. Once recruited, monocytes may differentiate into endothelial cells or into macrophages. While IL-10 stimulates M2 macrophage differentiation from monocytes, it is not yet clear which factors are required for endothelial cell differentiation from circulating cell types [9]. It is, however, likely that vascular endothelial growth factor, a key endothelial cell mitogen, plays a key role in this process.
1.5 Integrins roles in bone marrow derived cell trafficking to neovascular tissues
Until recently, the specific mechanisms by which myeloid and endothelial precursor cells adhere to tumor endothelium and extravasate into tumor tissue were not well studied. Myeloid cells express a number of functional integrins (α2β1, α4β1, α5β1, αvβ3, αvβ5, αMβ2 (CD11b) and αXβ2 (CD11c) that may play roles in of these cells within neovascular microenvironments [6-7].
Our studies on the roles of integrins in circulating cell trafficking revealed important functions for integrin α4β1 [6]. We found that integrin α4β1, a receptor for Vascular Cell Adhesion Molecule (VCAM) and fibronectin, selectively promotes the homing of both endothelial progenitor cells [62] and monocytes [7] to neovascular tissue and that this integrin is essential for the participation of these cells in angiogenesis and tumor growth. Human CD34+ and murine Lin-Sca1+ progenitor cells as well as bone marrow-derived myeloid cells (CD14+ CD11b+) adhered to endothelial cells in vitro and tumor endothelium in vivo via integrin α4β1. Treatment of mice bearing Lewis lung carcinoma tumors with antagonists of integrin α4β1 significantly suppressed the number of monocytes/endothelial progenitor cells within tumors and reduced blood vessel density. Our studies suggest that suppression of monocyte/macrophage homing to tumors by the application of an integrin α4β1 antagonist could be a useful supplementary approach to suppress tumor angiogenesis and growth.
Other integrins have been described to mediate homing of precursor cells to sites of ischemia [35]. Dimmeler and coworkers reported that adhesion of putative circulating endothelial precursor cells to endothelial monolayers depends on β2 integrins. Hematopoietic progenitor cells (Sca+ Lin- cells) from β2-integrin deficient mice exhibited reduced homing to sites of ischemia and reduced neovascularization [35]. Together, these studies identify key functions for integrins during precursor cell/monocyte homing to angiogenic sites.
1.6 Therapeutic opportunities
Emerging evidence indicates that monocytes and bone marrow-derived progenitor cells contribute to postnatal neovascularization. Investigation of the potential of adoptive transfer of monocytes and progenitor cells to restore blood flow and increase capillary density in peripheral artery disease and myocardial infarction is underway [5, 65-66]. Similarly, therapeutic opportunities to suppress M2 macrophage and/or endothelial progenitor cell roles in tumor growth are under investigation.
A variety of approaches to suppress macrophage/progenitor cell content in tumors have dramatically suppressed tumor neovascularization and tumor growth. For example, clodronate liposomes have proven effective at depleting tissues of macrophages by inducing macrophage apoptosis. Treatment with clodronate encapsulated in liposomes (clodrolip) efficiently depleted macrophages from F9 teratocarcinoma and A673 rhabdomyosarcoma tumors, resulting in significant inhibition of tumor growth, blood vessel density and F4/80+/CD11b+ cell content in tumors [67]. In addition, the density of CD11c+ cells, which have been shown to potentially differentiate into endothelial-like cells, were also reduced in clodronate treated tumors [67].
Recent studies from the Xiang and Reisfeld groups at the Scripps Research Institutes indicated that anti-tumor vaccines targeting macrophages potently inhibit tumor angiogenesis and growth. A legumain-based vaccine dramatically reduced macrophage densities in tumor tissues and resulted in a significant decrease in secretion of proangiogenic factors by TAMs, including TGF-beta, TNF-alpha, MMP-9, and VEGF [68]. Legumain, a member of the asparaginyl endopeptidase family is strongly overexpressed by TAMs. This legumain-based DNA vaccine induced a strong CD8+ T cell response against TAMs, leading to suppression of both tumor angiogenesis and tumor growth and metastasis [68].
A variety of other approaches to suppress myeloid/endothelial progenitor cell recruitment to tumors using integrin and chemokine antagonists are also under preclinical investigation [7, 62].
Efforts have begun to stimulate angiogenesis by the adoptive transfer of endothelial progenitor cells. In the Phase II clinical trial “Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI)”, patients received infusions of bone marrow–derived mononuclear cells or endothelial progenitor cells approximately 4 days after myocardial infarction. Patients receiving either population of cells exhibited improved ejection fraction and end-systolic volumes, indicating improved cardiac function. Other early phase clinical studies to promote vascularization of ischemic tissues showed similar effects [65-66].
In contrast, an alternative, proposed therapeutic use of progenitor cells/monocytes is based on the observation that progenitor cells specifically traffic to the tumor neovasculature. Thus, bone marrow-derived cells may serve as potential vectors for selective gene/imaging agent delivery to neovessels. De Palma and colleagues showed that ex vivo transduced bone marrow derived cells could be used to deliver secreted therapeutics at the tumor site [41-42].
1.7 Conclusions
Links between chronic inflammation and cancer have been recognized for several decades [69-70]. Studies to understand the recruitment of myeloid cells and endothelial progenitor cells and their contributions to angiogenesis are ongoing. New studies suggest that several myeloid subpopulations may play roles during neovascularization of tumors. These cells may form vessels, secrete growth factors or support angiogenesis in other ways. Such properties define myeloid cells/progenitor cells as putative targets for anti-cancer therapies. Suppression of cell homing to the tumor microenvironment offers a new strategy to inhibit tumor neovascularization, while stimulation of homing may promote tissue recovery from ischemia.
Figure 1. Bone marrow derived myeloid cells contribute to tumor angiogenesis.
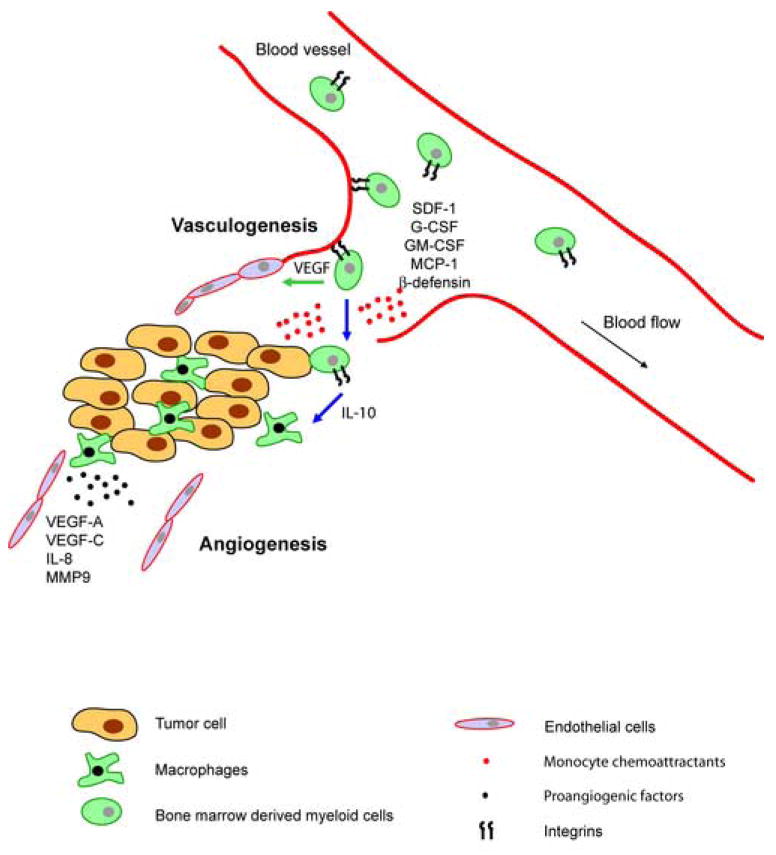
Myeloid precursor cells adhere to angiogenic endothelium via activated α4β1 or β2 integrins. Stromal derived growth factor 1 (SDF-1), colony stimulating factors (GM-CSF and others) or β-defensin mobilize specific subsets of myeloid precursor cells. In the presence of specific cytokines and growth factors, these cells differentiate either into endothelial-like cells, which are directly incorporated into new blood vessels (green arrow), or into monocytes / macrophages, which support tumor growth in a indirect manner (blue arrow). While interleukin-10 promotes differentiation of monocytes into M2 macrophages, VEGF and other factors promote endothelial cell differentiation from progenitor cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Folkman J, Merler E, Abernathy C, Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971;2:275–288. doi: 10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;428:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 3.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:927–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 4.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;11:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;5302:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 6.Garmy-Susini B, Varner J. Circulating endothelial progenitor cells. Br J Cancer. 2005;93:855–858. doi: 10.1038/sj.bjc.6602808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin H, Su J, Garmy-Susini B, Kleeman J, Varner J. Integrin alpha4beta1 promotes monocyte trafficking and angiogenesis in tumors. Cancer Res. 2006;4:2146–2152. doi: 10.1158/0008-5472.CAN-05-2704. [DOI] [PubMed] [Google Scholar]
- 8.Gouon-Evans V, Lin EY, Pollard JW. Requirement of macrophages and eosinophils and their cytokines/chemokines for mammary gland development. Breast Cancer Res. 2002;4:155–164. doi: 10.1186/bcr441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in Immunology. 2004;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 10.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Sironi M, Martinez F, D’Ambrosio D, Gattorno M, Polentarutti N, Locati M, Gregorio A, et al. Differential regulation of chemokine production by Fc{gamma} receptor engagement in human monocytes: association of CCL1 with a distinct form of M2 monocyte activation (M2b, Type 2) J Leukoc Biol. 2006;80:342–349. doi: 10.1189/jlb.1005586. [DOI] [PubMed] [Google Scholar]
- 12.Lewis C, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 14.Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 15.Murdoch C, Muthana M, Lewis C. Hypoxia regulates macrophage functions in inflammation. J Immunol. 2005;175:6257–6263. doi: 10.4049/jimmunol.175.10.6257. [DOI] [PubMed] [Google Scholar]
- 16.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan A, Chen J, Yao P, Yang P. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci. 2005;10:853–865. doi: 10.2741/1579. [DOI] [PubMed] [Google Scholar]
- 18.Schoppmann S, Fenzl A, Nagy K, Unger S, Bayer G, Geleff S, et al. VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival. Surgery. 2006;139:839–46. doi: 10.1016/j.surg.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Yamashiro S, Takeya M, Nishi T, Kuratsu J, Yoshimura T, Ushio Y, et al. Tumor-derived monocyte chemoattractant protein-1 induces intratumoral infiltration of monocyte-derived macrophage subpopulation in transplanted rat tumors. Am J Pathol. 1994;4:856–867. [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;2:150–158. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 21.Houghton A, Grisolano J, Baumann M, Kobayashi D, Hautamaki R, Nehring L, et al. Macrophage elastase (matrix metalloproteinase-12) suppresses growth of lung metastases. Cancer Res. 2006;66:6149–6155. doi: 10.1158/0008-5472.CAN-04-0297. [DOI] [PubMed] [Google Scholar]
- 22.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;5:623–633. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hildenbrand R, Dilger I, Horlin A, Stutte HJ. Urokinase and macrophages in tumour angiogenesis. Br J Cancer. 1995;4:818–823. doi: 10.1038/bjc.1995.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang S, Van Arsdall M, Tedjarati S, McCarty M, Wu W, Langley R, et al. Contributions of stromal metalloproteinase-9 to angiogenesis and growth of human ovarian carcinoma in mice. J Natl Cancer Inst. 2002;15:1134–1142. doi: 10.1093/jnci/94.15.1134. [DOI] [PubMed] [Google Scholar]
- 25.Oosterling SJ, van der Bij GJ, Meijer GA, Tuk CW, van Garderen E, van Rooijen N, Meijer S, van der Sijp JR, Beelen RH, van Egmond M. Macrophages direct tumour histology and clinical outcome in a colon cancer model. J Pathol. 2005 Oct;207(2):147–55. doi: 10.1002/path.1830. [DOI] [PubMed] [Google Scholar]
- 26.Esposito I, Menicagli M, Funel N, Bergmann F, Boggi U, Mosca F, et al. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J Clin Pathol. 2004;6:630–636. doi: 10.1136/jcp.2003.014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polverini PJ, Leibovich SJ. Effect of macrophage depletion on growth and neovascularization of hamster buccal pouch carcinomas. J Oral Pathol. 1987;9:436–441. doi: 10.1111/j.1600-0714.1987.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 28.Leek RD, Harris AL. Tumor-associated macrophages in breast cancer. J Mammary Gland Biol Neoplasia. 2002;2:177–189. doi: 10.1023/a:1020304003704. [DOI] [PubMed] [Google Scholar]
- 29.Nishie A, Ono M, Shono T, Fukushi J, Otsubo M, Onoue H, et al. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res. 1999;5:1107–1113. [PubMed] [Google Scholar]
- 30.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–236. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;3:952–958. [PubMed] [Google Scholar]
- 32.Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, et al. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;2:167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 33.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;8:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 34.Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG, et al. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;11:1023–1025. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 35.Chavakis E, Aicher A, Heeschen C, Sasaki K, Kaiser R, El Makhfi N, et al. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;1:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urbich C, Heeschen C, Aicher A, Sasaki K, Bruhl T, Farhadi MR, et al. Cathepsin L is required for endothelial progenitor cell-induced neovascularization. Nat Med. 2005;2:206–213. doi: 10.1038/nm1182. [DOI] [PubMed] [Google Scholar]
- 37.Hildbrand P, Cirulli V, Prinsen RC, Smith KA, Torbett BE, Salomon DR, et al. The role of angiopoietins in the development of endothelial cells from cord blood CD34+ progenitors. Blood. 2004;7:2010–2019. doi: 10.1182/blood-2003-12-4219. [DOI] [PubMed] [Google Scholar]
- 38.Schmeisser A, Garlichs CD, Zhang H, Eskafi S, Graffy C, Ludwig J, et al. Monocytes coexpress endothelial and macrophagocytic lineage markers and form cord-like structures in Matrigel under angiogenic conditions. Cardiovasc Res. 2001;3:671–680. doi: 10.1016/s0008-6363(00)00270-4. [DOI] [PubMed] [Google Scholar]
- 39.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;4:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 40.Conejo-Garcia JR, Benencia F, Courreges MC, Kang E, Mohamed-Hadley A, Buckanovich RJ, et al. Tumor-infiltrating dendritic cell precursors recruited by a beta-defensin contribute to vasculogenesis under the influence of Vegf-A. Nat Med. 2004;9:950–958. doi: 10.1038/nm1097. [DOI] [PubMed] [Google Scholar]
- 41.De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;6:789–795. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- 42.De Palma M, Venneri MA, Galli R, Sergi LS, Politi LS, Sampaolesi M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;3:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Weber C, Koenen RR. Fine-tuning leukocyte responses: towards a chemokine ‘interactome’. Trends Immunol. 2006;6:268–273. doi: 10.1016/j.it.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;12:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 45.Oda T, Hirota K, Nishi K, Takabuchi S, Oda S, Yamada H. Activation of hypoxia-inducible factor 1 during macrophage differentiation. Am J Physiol Cell Physiol. 2006;291:C104–113. doi: 10.1152/ajpcell.00614.2005. [DOI] [PubMed] [Google Scholar]
- 46.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 47.Lewis C, Murdoch C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol. 2005;167:627–635. doi: 10.1016/S0002-9440(10)62038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murdoch C, Lewis CE. Macrophage migration and gene expression in response to tumor hypoxia. Int J Cancer. 2005;117:701–708. doi: 10.1002/ijc.21422. [DOI] [PubMed] [Google Scholar]
- 49.Kuroda T, Kitadai Y, Tanaka S, Yang X, Mukaida N, Yoshihara M, Chayama K. Monocyte chemoattractant protein-1 transfection induces angiogenesis and tumorigenesis of gastric carcinoma in nude mice via macrophage recruitment. Clin Cancer Res. 2005;11:7629–7636. doi: 10.1158/1078-0432.CCR-05-0798. [DOI] [PubMed] [Google Scholar]
- 50.Varney ML, Johansson SL, Singh RK. Tumour-associated macrophage infiltration, neovascularization and aggressiveness in malignant melanoma: role of monocyte chemotactic protein-1 and vascular endothelial growth factor-A. Melanoma Res. 2005;15:417–425. doi: 10.1097/00008390-200510000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res. 2000;8:3282–3289. [PubMed] [Google Scholar]
- 52.Niwa Y, Akamatsu H, Niwa H, Sumi H, Ozaki Y, Abe A. Correlation of tissue and plasma RANTES levels with disease course in patients with breast or cervical cancer. Clin Cancer Res. 2001;2:285–289. [PubMed] [Google Scholar]
- 53.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;6:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;8:2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- 55.Zhu YM, Webster SJ, Flower D, Woll PJ. Interleukin-8/CXCL8 is a growth factor for human lung cancer cells. Br J Cancer. 2004;11:1970–1976. doi: 10.1038/sj.bjc.6602227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;6729:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 57.Uutela M, Wirzenius M, Paavonen K, Rajantie I, He Y, Karpanen T, et al. PDGF-D induces macrophage recruitment, increased interstitial pressure, and blood vessel maturation during angiogenesis. Blood. 2004;10:3198–3204. doi: 10.1182/blood-2004-04-1485. [DOI] [PubMed] [Google Scholar]
- 58.Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;8:3336–3343. [PubMed] [Google Scholar]
- 59.Goswami S, Sahai E, Wyckoff JB, Cammer M, Cox D, Pixley FJ, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;12:5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [DOI] [PubMed] [Google Scholar]
- 60.Nakao S, Kuwano T, Tsutsumi-Miyahara C, Ueda S, Kimura YN, Hamano S, et al. Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. J Clin Invest. 2005;11:2979–2991. doi: 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4(+) hemangiocytes. Nat Med. 2006;5:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin H, Aiyer A, Su J, Borgstrom P, Stupack D, Friedlander M, et al. A homing mechanism for bone marrow-derived progenitor cell recruitment to the neovasculature. J Clin Invest. 2006;3:652–662. doi: 10.1172/JCI24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, et al. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;7:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H, et al. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;10:1311–1325. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 65.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation. 2004;21:2487–2491. doi: 10.1161/01.CIR.0000128595.79378.FA. [DOI] [PubMed] [Google Scholar]
- 66.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation. 2004;22:2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 67.Zeisberger S, Odermatt B, Marty C, Zehnder-Fjallman A, Ballmer-Hofer K, Schwendener R. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo Y, Zhou H, Krueger J, Kaplan C, Lee S, Dolman C, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;116:2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;1:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]


