Abstract
Deep eutectic solvents (DESs) consisting of mixtures of a choline salt (chloride or acetate form) and glycerol are prepared as easily accessible, biodegradable, and inexpensive alternatives to conventional aprotic cation-anion paired ionic liquids. These DES systems display excellent fluidity coupled with thermal stability to nearly 200 °C. In this work, the transesterification activities of cross-linked proteases (subtilisin and α-chymotrypsin), immobilized on chitosan, were individually examined in these novel DESs. In the 1:2 molar ratio mixture of choline chloride/glycerol containing 3% (v/v) water, cross-linked subtilisin exhibited an excellent activity (2.9 μmo l min−1 g−1) in conjunction with a selectivity of 98% in the transesterification reaction of N-acetyl-L-phenylalanine ethyl ester with 1-propanol. These highly encouraging results advocate more extensive exploration of DESs in protease-mediated biotransformations of additional polar substrates and use of DESs in biocatalysis more generally.
Keywords: Ionic liquid, Eutectic solvent, Protease, Subtilisin, Transesterification
1. Introduction
As potential environmentally-sustainable solvents, ionic liquids (ILs) have emerged as fascinating and customizable reaction media for various bio-applications, most notably enzyme catalysis [1–3]. One hindrance to broader development of these promising solvents in biocatalytic systems stems from the fact that archetypal cations employed in IL research overwhelmingly derive from prohibitively costly heterocycles, particularly substituted imidazoliums, and to a lesser extent, pyridiniums, pyrrolidiniums, and piperidiniums as well. Recently, Abbott et al. [4–6] pioneered the development of the so-called deep eutectic solvents (DESs) which are low-melting liquids derived from the mixture of a solid organic salt and a suitable organic complexant, typically a hydrogen-bond donating species such as a polyol. While the research community is ambivalent on whether DESs should be formally classified as ILs for the fact that they contain a significant molecular component, they certainly share a number of attractive solvent features which account for the popularity of their IL cousins. The exemplary DES is illustrated by the 1:2 molar ratio mixture of choline chloride (Tm = 302 °C, Scheme 1a) with urea (Tm = 133 °C), which yields a free-flowing fluid with a freezing point below ambient (Tf ≈12 °C) [4]. The foremost advantages of DESs are summarized by the following: (i) Low-cost quaternary ammonium salts and similarly inexpensive complexing agents such as amides, amines, carboxylic acids and alcohols can be combined to afford inexpensive DESs from widely available starting materials. (ii) Biodegradable ingredients can be employed, toward the development of truly green, sustainable DESs. For instance, a popular precursor salt in DES preparation, choline chloride (known colloquially as vitamin B4), is used in large quantities as an additive in chicken feed [7], as well as being implicated as an essential micronutrient for promoting proper human health [8]. (iii) DESs can dissolve a variety of typically water-soluble species like metal salts, amino and organic acids, and various polyols such as glucose which remain problematic solutes for conventional aprotic ILs [4, 5, 9–11]. As a result, there is serious practical interest toward developing cholinium-based and alternative low-toxicity DESs as designer biosolvents for performing catalysis.
Scheme 1.
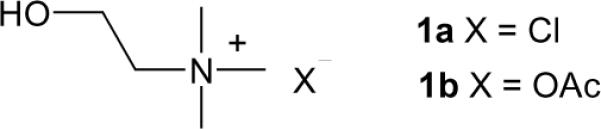
Structures of choline chloride (1a) and choline acetate (1b).
Recently, Gorke et al. [12] reported that a number of hydrolases, including Candida antarctica lipase B (CALB), Candida antarctica lipase A (CALA), and epoxide hydrolase, maintained high activities in DESs based on choline chloride or ethylammonium chloride paired with hydrogen-bond donor species such as acetamide, ethylene glycol, glycerol, urea, and malonic acid. As co-solvents, DESs were effectively shown to enhance the reaction rates and/or conversion efficiencies of these hydrolases. Another recent study [13] suggested that the 1:2 choline chloride/glycerol eutectic preserved the integrity and viability of encapsulated bacteria, signifying the possibility of performing whole-cell biocatalysis within DES media. More recently, our groups have developed biocompatible DESs such as 1:2 choline chloride/glycerol and 1:1.5 choline acetate/glycerol that permitted Novozym® 435, a commercially immobilized form of CALB, to retain high activity and stability, demonstrating high conversions of up to 97% in the enzymatic transesterification of Miglyol® oil 812 with methanol in a model biodiesel synthetic pathway [14]. Additionally, DESs derived from cholinium salts and glycerol tend to have substantially lower viscosities (~80 mPa s at 50 °C) relative to 1:2 choline chloride/urea, which has a viscosity near 120 mPa s at 50 °C [14], despite the fact that neat glycerol itself has a viscosity at room temperature in excess of 900 mPa s.
Although proteases represent another significant category of hydrolases with valuable roles in many biocatalytic conversions [15], their catalytic performance in DESs has yet to be demonstrated. As part of our continuing research in the area, we have prepared inexpensive and biodegradable DESs derived from choline salts (chloride or acetate, Scheme 1b) mixed with glycerol, and examined their potential as solvent systems for conducting protease-catalyzed transformations using cross-linked subtilisin and α-chymotrypsin as biocatalysts.
2. Experimental
2.1. Materials
Calbiochem® protease from Bacillus licheniformis, commonly known as subtilisin Carslberg, was purchased from EMD Biosciences (product number 572909, ≥400 1.0 unit (U) corresponds to the quantity of enzyme that hydrolyzes 1.0 μmol of N-acetyl-L-tyrosine ethyl ester (ATEE) per min at pH 8.2 and 22 °C). The following materials were supplied by Sigma-Aldrich: choline chloride, anhydrous glycerol, α-chymotrypsin from bovine pancreas (Type II, lyophilized powder, ≥40 units/mg protein; 1.0 U hydrolyzes 1.0 μmol of N-benzoyl-L-tyrosine ethyl ester per min at pH 7.8 and 25 °C), and medium molecular weight chitosan (viscosity of 453 mPa s for a 1% (w/w) chitosan solution in 1% (v/v) acetic acid), 75–85% deacetylation; product # 448877, LOT # 07918TE).
2.2. Enzyme immobilization
A typical procedure for enzyme cross-linking followed methods found in the literature [16]. Briefly, after washing 1.0 g of chitosan with 2-propanol and water, respectively, the porous support was added to 20 mL of phosphate buffer (0.1 M, pH 7.4) containing 0.1 g of the desired protease. After stirring the suspension at room temperature for 2 h, 60 mL of 2-propanol was added. Following 5 min of gentle agitation, 1.5 mL of a 25% (w/w) glutaraldehyde solution was added into the suspension to give a 50 mM final concentration, and the mixture stirred for an additional hour at room temperature. The supernatant was finally decanted, the cross-linked/immobilized enzyme washed with water and diethyl ether, respectively, and then dried. The protein loading on the solid support was 10% (w/w) as determined by Bradford assay.
For cross-linked chitosan-immobilized subtilisin, the total yield (defined as the total mass of immobilized enzyme, including the solid support) was 1.29 g with an activity of 0.58 U/g protein. For immobilized α-chymotrypsin, the corresponding yield was 1.31 g with 0.60 U/g activity. The pure subtilisin showed an activity of 1.24 U/g. 1.0 U corresponding to the hydrolysis of 1.0 mM N-glutaryl-L-phenylalanine p-nitroanilide (GPANA) to produce 1.0 μmol of p-nitroaniline (molar extinction coefficient: 8900 M−1 cm−1 at 410 nm [17]) per min at pH 7.4 and 35 °C [18]. This assay was chosen because it represents a convenient and well-accepted method for quantifying a protease's hydrolytic activity, providing a unified activity scale for subtilisin and α-chymotrypsin, in contrast with divergent and non-standardized assay methods advocated by individual enzyme suppliers.
2.3. Preparation of eutectic ILs
Choline acetate was prepared in our laboratory based on a column anion-exchange method [19], with minor modifications as detailed in our recent report [14]. The synthesis of DESs followed simple thermal-mixing procedures [4, 6], which were also reported elsewhere [14]. The free-flowing DES products were dried over P2O5 at 45 °C for at least 2 weeks. The water content of the DESs was determined by Karl Fischer (KF) titration (Mettler Toledo C20X compact Coulometric; detection limit: 1 ppm water) at 20 °C, and was typically found to vary between 0.5 and 1.0% (w/w). Hydranal® Coulomat AG was used as anolyte for the KF titration.
2.4. Transesterification reaction
Fifty microliters of a 1-propanol solution containing N-acetyl-L-phenylalanine ethyl ester (N-Ac-L-Phe-OEt) were mixed with DES or organic solvent to make a 1.0 mL solution with final concentrations in N-Ac-L-Phe-OEt and 1-propanol of 20 mM and 0.67 M, respectively. The reaction was initiated by addition of 20 mg of immobilized protease or 2.0 mg of free protease. The reaction vial was capped and held at 50 °C under constant stirring. At regular intervals, 50 μL was taken and diluted with 100 μL of methanol. After centrifugation, the clear supernatant was injected into a LC-20AT Shimadzu HPLC using a Phenomenex® Kinetex C18 column (100 mm × 4.6 mm, particle size: 2.6 μm) and eluted with 1:1 (v/v) methanol/aqueous acetate buffer (0.05 M, pH 4.5) at 1 mL min−1. The HPLC was equipped with an SPD-20A UV-visible dual wavelength detector, with eluate monitored at 258 nm. All experiments were run in duplicate. Standard curves of N-Ac-L-Phe and N-Ac-L-Phe-OEt at 258 nm were nearly identical, allowing us to use their average to establish a standard curve for N-Ac-L-Phe-OPr, a compound not available commercially. Laszlo and Compton [20] similarly did not differentiate the UV-responses of these three compounds at 258 nm. Initial reaction rates were derived from plots of product concentration versus reaction time, expressed in the unit of μmol min−1 g−1 protein, based on the 10% (w/w) protein loading on solid carrier determined earlier. The reaction selectivity was obtained by dividing the yield of N-Ac-L-Phe-OPr by the conversion of N-Ac-L-Phe-OEt (each determined by HPLC analysis); N-Ac-L-Phe-OPr formation proceeds in competition with substrate hydrolysis which yields the major by-product N-Ac-L-Phe, as shown in Scheme 2. The selectivity was determined for the same reaction period during which the initial reaction rate was determined, typically within the first hour of reaction when the substrate conversion was less than 5%.
Scheme 2.
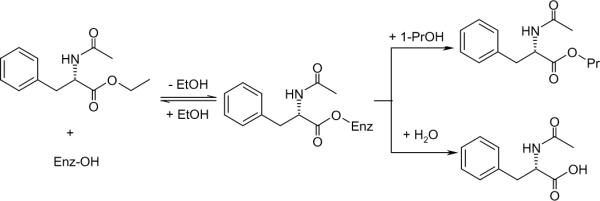
Protease-catalyzed transesterification of N-acetyl-L-phenylalanine ethyl ester with 1-propanol in competition with the hydrolysis reaction.
2.5. Equilibration of water activity
The solvent in question (e.g., t-butanol) was equilibrated over a saturated salt solution in a closed vessel for 7 days at 25 °C. The hygrostats used to achieve various thermodynamic water activities were based on these salts: LiCl (aw = 0.11), MgCl2 (aw = 0.33), NaCl (aw = 0.75), and KNO3 (aw = 0.94) [21–23]. The analytical water contents present in the solvent were then measured using KF coulometric titration conducted at 25 °C, with details as specified above.
3. Results and discussion
3.1. Physical properties of eutectic ILs
DESs selected for investigation include 1:2 choline chloride/glycerol (herein, all DES ratios denote molar ratios), and 1:1.5 or 1:2 choline acetate/glycerol. Recent studies [11, 24] based on the visual inspection of freezing point (Tf) suggest the eutectic composition of choline chloride/glycerol to be 1:2, coincident with a Tf near −40 °C. Recent differential scanning calorimetry (DSC) melting point determinations indicate a eutectic composition close to 1:1.5 for the choline acetate/glycerol DES, however [14]. Beyond the advantages of low cost and low toxicity, these glycerol-based DESs also reveal lower viscosities than urea-based eutectics such as 1:2 choline chloride/urea [14]. In addition, glycerol-based DESs are now known to be compatible with hydrolases such as CALB, CALA, and epoxide hydrolase [12, 14], making them a logical choice for initiating studies of protease activity within DESs.
The thermal stabilities of these DESs were determined by thermogravimetric analysis (TGA), using as a quantitative measure the decomposition temperature (Tdcp), which signifies the temperature for which 90% of the initial mass remains for a given ramp rate during the TGA scanning experiment, in this case a constant heating rate of 10 °C min−1. As shown in Fig 1, these DESs are generally stable up to nearly 200 °C, sufficient for nearly any biocatalytic investigation conceivable. The Tdcp values for 1:2 choline chloride/urea, 1:2 choline chloride/glycerol, 1:1.5 choline acetate/glycerol, and 1:2 choline acetate/glycerol all fall in the range from 205 to 216 °C.
Fig. 1.
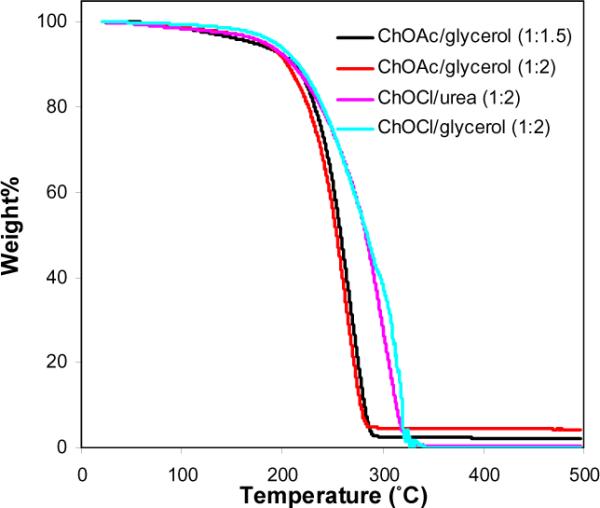
Thermogravimetric analysis of DESs conducted on a TA Instruments 2950. DES samples (35–50 mg) were micropipetted into open platinum pans and then heated from ambient temperature up to 500 °C at a heating rate of 10 °C min−1 under a protective atmosphere of nitrogen gas (50 mL min−1 flow rate) (ChOAc for choline acetate; ChOCl for choline chloride).
3.2. Transesterification activity and selectivity in eutectic ILs
Proteases typically remain active in hydrophobic ILs, such as those based on the bis(trifluoromethylsulfonyl)imide [Tf2N−] or hexafluorophosphate [PF6−] anions, in the presence of suitable amounts of water, but are reportedly inactive in most hydrophilic ILs, unless in dilute aqueous solution in which case the hydrolytic activity naturally predominates [1–3]. For this reason, it remains a considerable challenge to perform enzymatic transformations on polar substrates such as sugars, which typically only have nominal solubility in conventional ILs [25, 26]. Although recent studies have reported high activities for lipase in DESs [12, 14], it is known that lipases are generally far more robust than proteases within the non-aqueous milieu. Indeed, lipases have even been shown to maintain their catalytic properties in some hydrophilic ILs [1, 3]. On the other hand, the catalytic behavior of proteases within DESs remains completely unexplored. In order to address this deficiency, this study examines the synthetic activities of two of the most well-studied proteases, subtilisin and α-chymotrypsin, in glycerol-based DESs using the standard transesterification reaction of N-Ac-L-Phe-OEt with 1-propanol. Scheme 2 outlines this model reaction, showing the fast formation of an acyl-enzyme intermediate, followed by the competition between esterification and hydrolysis reactions.
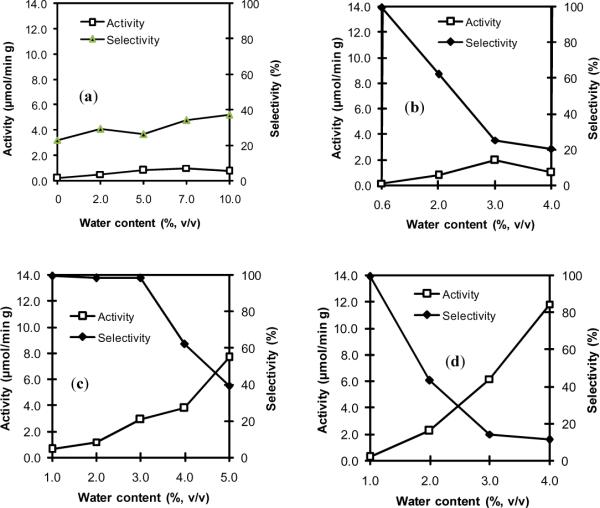
The transesterification activities of free and cross-linked (immobilized) subtilisin were examined in t-butanol alongside several DESs, as summarized in Table 1 and Fig 2. The reaction temperature of 50 °C was elected on the basis of two considerations: (a) proteases have been previously shown to retain their activity at this temperature [27, 28]; and (b) DESs have relatively low viscosities at this temperature [14]. Our experimental data suggest the following trends for subtilisin activity in DESs: (1) Immobilized subtilisin activity and selectivity remain poor in t-butanol containing up to 10% (v/v) water (Fig 2a). As Fig 3 makes clear, the thermodynamic water activity (aw) in t-butanol is correlated with the water content, with 7.6% (v/v) water corresponding to an aw above 0.9 at 25 °C. (2) Conversely, as can be seen in Fig 2b, activities close to 2.0 μmol min−1 g−1 are achieved in 1:2 choline acetate/glycerol, but only at the expense of selectivity which falls to 25% at 3% (v/v) water. (3) Low activities of 0.42–0.90 μmol min−1 g−1 paired with selectivities near 99% were found for the cross-linked subtilisin in 1:1.5 choline acetate/glycerol (Table 1). (4) As shown in Fig 2c, a high activity of 2.9 μmol min−1 g−1 with simultaneously excellent selectivity (98%) is attained in 1:2 choline chloride/glycerol containing 3% (v/v) water. (5) Free subtilisin exhibits superb synthetic activity in 1:2 choline chloride/glycerol at high water contents (Fig 2d), however, the selectivity falls precipitously as water content increases and is only 14% at 3% (v/v) water. The covalent cross-linking of free proteases onto a solid carrier clearly results in some losses in transesterification activity, a result typical of enzyme immobilization [29], however, our data clearly point to seriously improved selectivity for this model transesterification reaction in DESs upon subtilisin immobilization, making the cross-linked enzyme a far superior choice for application in DESs.
Table 1.
Activities of subtilisin and α-chymotrypsin in DESs
| Solvent | Protease | Water content (v/v) (%) | Activity (μmol min−1 g−1) | Selectivity (%) |
|---|---|---|---|---|
| t-butanol | subtilisin on chitosan | 2 | 0.50 | 29 |
| choline acetate /glycerol (1:1.5) | subtilisin on chitosan | 2 | 0.42 | 99 |
| choline acetate/glycerol (1:1.5) | subtilisin on chitosan | 3 | 0.40 | 99 |
| choline acetate/glycerol (1:1.5) | subtilisin on chitosan | 4 | 0.90 | 99 |
| choline chloride/glycerol (1:2) | free α-chymotrypsin | 2 | 0.028 | 99 |
| choline chloride/glycerol (1:2) | α-chymotrypsin on chitosan | 2 | 0.031 | 99 |
| choline chloride/glycerol (1:2) | α-chymotrypsin on chitosan | 3 | 0.75 | 99 |
| choline chloride/glycerol (1:2) | subtilisin on chitosan | 3 | 2.9 | 98 |
Notes: The transesterification between N-Ac-L-Phe-OEt (20 mM) and 1-propanol (0.67 M) was catalyzed by 20 mg of immobilized protease or 2.0 mg of the free enzyme in 1.0 mL of solvent at 50 °C. The reaction mixture was periodically analyzed by HPLC. The initial reaction rate (i.e., activity) was calculated from the rate of product formation and the selectivity calculated from the ratio of production yield over substrate conversion.
Fig. 2.
Protease activity and selectivity in different solvents at various water contents (%, v/v): (a) cross-linked (immobilized) subtilisin in t-butanol; (b) immobilized subtilisin in 1:2 choline acetate/glycerol; (c) immobilized subtilisin in 1:2 choline chloride/glycerol; (d) free subtilisin in 1:2 choline chloride/glycerol. The transesterification between N-Ac-L-Phe-OEt (20 mM) and 1-propanol (0.67 M) was catalyzed by 20 mg of immobilized protease (or 2.0 mg of the corresponding free protease) in 1.0 mL of solvent at 50 °C.
Fig. 3.
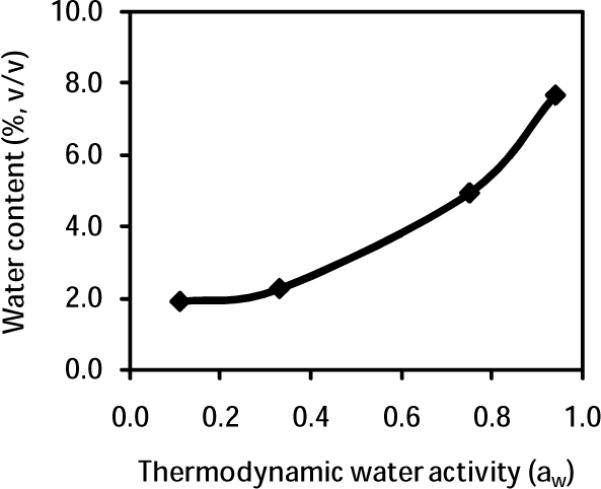
The relationship between water content and thermodynamic water activity (aw) of t-butanol at 25 °C.
Compared with subtilisin, α-chymotrypsin was much less active in 1:2 choline chloride/glycerol in both free and immobilized forms. As shown in Table 1, at 2% (v/v) water, the activities of free and cross-linked α-chymotrypsin were only 0.028 and 0.031 μmol min−1 g−1, respectively; even at a 3% (v/v) water level, the immobilized α-chymotrypsin remained about four-fold less active than the similarly-immobilized subtilisin (0.75 versus 2.9 μmol min−1 g−1).
The high subtilisin activity in DESs can be rationalized by a number of factors. (1) Although these DESs contain enzyme-denaturing anions like chloride and acetate, their molar concentrations are notably reduced by the addition of 1.5–2 molar equivalents of glycerol which tends to form hydrogen bonds with these anions. Therefore, the tendency of these anions to negatively impact the protease is greatly diminished. As a result, the denaturing tendency of these DESs is much lower than for common hydrophilic ILs carrying denaturing anions. (2) We also suggest that the hydrophilic nature of the DESs allows them to structurally “tie up” water molecules, limiting their availability as nucleophiles in the competing hydrolysis. Consequently, the actual thermodynamic water activity (aw) of DESs is anticipated to be lower than that of conventional hydrophobic organic solvents and ILs. This postulate has been experimentally validated by a number of enzymatic studies carried out in hydrophilic ILs [28, 30, 31]. It is also well established that the enzyme's activity is far more dependent upon aw than on the analytical water content per se [32]. (3) In the present case, the enzyme-host (chitosan) additionally contains hydroxyl groups, which allow the support to absorb water molecules via hydrogen-bond interactions, further reducing aw in the reaction system. Therefore, a higher selectivity might reasonably be expected for the chitosan-immobilized proteases than for freely dissolved enzyme.
4. Conclusions
Glycerol-based DESs derived from choline chloride (or acetate) are shown to possess relatively low viscosities and high thermal stability. Furthermore, these eutectic melts can be tailored as effective solvents for carrying out protease-mediated synthesis. In particular, cross-linked subtilisin exhibited a high activity and selectivity in 1:2 choline chloride/glycerol in the presence of 3% (v/v) water whereas free subtilisin gave poor selectivity in the same DES. Subtilisin was also found to be more active in 1:2 choline chloride/glycerol than in choline acetate/glycerol mixtures, and subtilisin was also more compatible with DESs than was α-chymotrypsin. The excellent suitability of DESs for subtilisin biocatalysis is thought to be tied to the low molar concentration of the denaturing anion (relative to their IL peers), reducing the propensity for hydrogen bond formation between these detrimental anions and the protein itself, and the hydrophilic features of the chitosan host which aid in controlling the local water activity in the vicinity of the enzyme.
Acknowledgements
This research was supported by the National Institutes of Health under a RIMI grant (5P20MD003941) subproject to HZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].van Rantwijk F, Sheldon RA. Chem. Rev. 2007;107:2757. doi: 10.1021/cr050946x. [DOI] [PubMed] [Google Scholar]
- [2].Zhao H. J. Chem. Tech. Biotechnol. 2010;85:891. [Google Scholar]
- [3].Moniruzzaman M, Nakashima K, Kamiya N, Goto M. Biochem. Eng. J. 2010;48:295. [Google Scholar]
- [4].Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V. Chem. Commun. 2003:70. doi: 10.1039/b210714g. [DOI] [PubMed] [Google Scholar]
- [5].Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK. J. Am. Chem. Soc. 2004;126:9142. doi: 10.1021/ja048266j. [DOI] [PubMed] [Google Scholar]
- [6].Abbott AP, Capper G, Gray S. ChemPhysChem. 2006;7:803. doi: 10.1002/cphc.200500489. [DOI] [PubMed] [Google Scholar]
- [7].Boethling RS, Sommer E, DiFiore D. Chem. Rev. 2007;107:2207–2227. doi: 10.1021/cr050952t. [DOI] [PubMed] [Google Scholar]
- [8].Blusztajn JK. Science. 1998;281:794. doi: 10.1126/science.281.5378.794. [DOI] [PubMed] [Google Scholar]
- [9].Abbott AP, Capper G, Swain BG, Wheeler DA. Trans. Inst. Metal Finishing. 2005;83:51. [Google Scholar]
- [10].Hou Y, Gu Y, Zhang S, Yang F, Ding H, Shan Y. J. Mol. Liq. 2008;143:154. [Google Scholar]
- [11].Abbott AP, Cullis PM, Gibson MJ, Harris RC, Raven E. Green Chem. 2007;9:868. [Google Scholar]
- [12].Gorke JT, Srienc F, Kazlauskas RJ. Chem. Commun. 2008:1235. doi: 10.1039/b716317g. [DOI] [PubMed] [Google Scholar]
- [13].Gutierrez MC, Ferrer ML, Yuste L, Rojo F, del Monte F. Angew. Chem. Int. Ed. 2010;49:2158. doi: 10.1002/anie.200905212. [DOI] [PubMed] [Google Scholar]
- [14].Zhao H, Baker GA, Holmes S. Org. Biomol. Chem. 2011;9:1908. doi: 10.1039/c0ob01011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bornscheuer UT, Kazlauskas RJ. Hydrolases in Organic Synthesis. Wiley-VCH; Weinheim: 2006. [Google Scholar]
- [16].Toral AR, de los Ríos AP, Hernández FJ, Janssen MHA, Schoevaart R, van Rantwijk F, Sheldon RA. Enzyme Microb. Technol. 2007;40:1095. [Google Scholar]
- [17].Lyublinskaya LA, Belyaev SV, Strongin AY, Matyash LF, Levin ED, Stepanov VM. Anal. Biochem. 1974;62:371. doi: 10.1016/0003-2697(74)90169-9. [DOI] [PubMed] [Google Scholar]
- [18].Erlanger BF, Cooper AG, Bendich AJ. Biochemistry. 1964;3:1880. doi: 10.1021/bi00900a015. [DOI] [PubMed] [Google Scholar]
- [19].Dinarès I, de Miguel CG, Ibáñez A, Mesquida N, Alcalde E. Green Chem. 2009;11:1507. [Google Scholar]
- [20].Laszlo JA, Compton DL. Biotechnol. Bioeng. 2001;75:181–186. doi: 10.1002/bit.1177. [DOI] [PubMed] [Google Scholar]
- [21].Eckstein M, Sesing M, Kragl U, Adlercreutz P. Biotechnol. Lett. 2002;24:867. [Google Scholar]
- [22].Eckstein M, Wasserscheid P, Kragl U. Biotechnol. Lett. 2002;24:763–767. [Google Scholar]
- [23].Ulbert O, Bélafi-Bakó K, Tonova K, Gubicza L. Biocatalysis Biotransform. 2005;23:177. [Google Scholar]
- [24].Abbott AP, Harris RC, Ryder KS, D'Agostino C, Gladden LF, Mantle MD. Green Chem. 2011;13:82. doi: 10.1039/c1cp22554e. [DOI] [PubMed] [Google Scholar]
- [25].Zhao H, Baker GA, Song Z, Olubajo O, Crittle T, Peters D. Green Chem. 2008;10:696. [Google Scholar]
- [26].Zhao H, Jones CL, Cowins JV. Green Chem. 2009;11:1128. [Google Scholar]
- [27].Roy I, Gupta MN. Tetrahedron. 2003;59:5431. [Google Scholar]
- [28].Zhao H, Song Z, Olubajo O. Biotechnol. Lett. 2010;32:1109. doi: 10.1007/s10529-010-0262-4. [DOI] [PubMed] [Google Scholar]
- [29].Guisan JM. Immobilization of Enzymes and Cells. Humana Press Inc.; Totowa: 2006. [Google Scholar]
- [30].De Diego T, Lozano P, Abad MA, Steffensky K, Vaultier M, Iborra JL. J. Biotechnol. 2009;140:234. doi: 10.1016/j.jbiotec.2009.01.012. [DOI] [PubMed] [Google Scholar]
- [31].Vafiadi C, Topakas E, Nahmias VR, Faulds CB, Christakopoulos P. J. Biotechnol. 2009;139:124. doi: 10.1016/j.jbiotec.2008.08.008. [DOI] [PubMed] [Google Scholar]
- [32].Bell G, Halling PJ, Moore BD, Partridge J, Rees DG. Trends Biotechnol. 1995;13:468. [Google Scholar]