Abstract
The sexually dimorphic characteristics of the reproductive tract in Drosophila require that cells of the gonad and the genital disc be assigned sex-specific fates. We report here that DWnt-2, a secreted glycoprotein related to wingless, is a signal required for cell fate determination and morphogenesis in the developing male reproductive tract. Testes from DWnt-2 null mutant flies lack the male-specific pigment cells of the reproductive tract sheath and the muscle precursors of the sheath fail to migrate normally. However, other cell types of the testis are unaffected. DWnt-2 is expressed in somatic cells of the gonad throughout development, implicating it as a signal that can influence pigment cell fate directly. Indeed, the ectopic expression of DWnt-2 in females results in the appearance of male-specific pigment cells in otherwise morphologically normal ovaries. Thus, the presence of pigment cells is a sexually dimorphic trait that is controlled by DWnt-2 expression. DWnt-2 is also expressed in regions of the male genital disc and gonad, which we have identified as sites of contact with muscle precursor cells, suggesting that secreted DWnt-2 protein is a signal for the migration or attachment of these cells.
Keywords: Drosophila, Wnt, male reproductive tract, sex determination, pigment cells, myoblast migration
The formation of the Drosophila reproductive tract during pupation illustrates several common developmental problems. For example, two separate tissues, the gonad and the genital disc, must grow toward each other, recognize, and fuse with one another. In the male, the gonads differentiate to form the testes and the genital disc differentiates to form the external genitalia and the internal structures that connect to the testes. The sheath of the male reproductive tract develops from two populations of cells, the pigment cells of the gonad and the precursors of smooth muscle cells. These cells migrate to form a bilayered sheath that covers both the mature gonad (the testis) and a portion of the genital disc (the seminal vesicle). Organogenesis of the male reproductive tract sheath depends on the proper specification and migration of cells, which may require either intrinsic cues or extracellular signals.
The existence of signaling molecules necessary for the development of the male reproductive tract was first postulated more than 60 years ago when researchers were studying two aspects of development, the morphogenesis of the testis and the sex-specific presence of testis pigment cells. It was noted that the male gonads of gynandromorphs, or sexual mosaics, that do not possess a male genital disc fail to undergo morphogenesis from an ovoid to a spiral shape (Dobzhansky 1931). In transplantation experiments, it was found that male gonads only undergo morphogenesis when attached to a male genital disc and that the extent of spiraling, a species-specific trait, depends on the species of the genital disc (Stern 1941a,b). These results led to the hypothesis that the male genital disc releases a signal, an inducer of morphogenesis, to which the gonad responds.
A second signal was proposed to explain the fact that a certain male-specific cell type can be induced in females. In gynandromorphs that contain ovaries and a male genital disc, some male-specific pigment cells are often present (Dobzhansky 1931). Through transplantation experiments, it was determined that these cells normally derive from the male gonad (Stern and Hadorn 1939), which raised the question of whether the female gonad is the source of the pigment cells seen in gynandromorphs. This appears to be the case, as transplanting a male genital disc into a female is sufficient for some cells of the ovary to acquire a pigment cell fate (Hadorn and Bertani 1948). Clearly, some signal emanating from male tissue can support the differentiation of female cells into male testis pigment cells. For many years, these signals, affectors of male development in the reproductive tract, were left unpursued.
In a screen for mutations in DWnt-2, a member of the Wnt family of genes (Russell et al. 1992), we have found it to be required for the development of the sheath of the male reproductive tract and testis morphogenesis. We have also found that DWnt-2, when expressed ectopically in females, results in the appearance of male-specific pigment cells. Pigment cells, the outer cells of the testis sheath, are absent in the mutants, indicating that DWnt-2 is required for their specification in males. The inner muscle layer of the testis sheath fails to develop in the male mutants and the testis does not undergo its normal morphogenesis. The muscle defect in the DWnt-2 mutant has led us to investigate the origin of the precursor cells of the muscle layer. From our observations we propose a model for the formation of the male reproductive tract and discuss the role of DWnt-2 in this process. We also discuss the relation of DWnt-2 to the signals proposed half a century ago to explain testis morphogenesis and the induction in females of male-specific pigment cells.
Results
Generation of mutations in DWnt-2
To determine the developmental requirement of DWnt-2 we created mutations in this gene. We first generated a deficiency chromosome by X-irradiating male flies of the 5R13A line, a homozygous viable P-element insertion at 45E (Kassis et al. 1992), the cytological location of DWnt-2 (Russell et al. 1992). We screened progeny of the irradiated flies for the loss of the P-element eye color marker ry+ and obtained a homozygous lethal deficiency Df(2R)11. The cytological band to which DWnt-2 maps is deleted in this deficiency and DWnt-2 expression is absent in embryos homozygous for the deficiency (data not shown), indicating that this deficiency lacks DWnt-2 sequences. We then performed a chemical mutagenesis and screened for mutations that failed to complement Df(2R)11 (see Materials and Methods). Four lethal complementation groups were recovered, as well as one adult viable complementation group that is male sterile and has the visible phenotype of held-out wings. (This wing phenotype will be described in detail elsewhere.) Three of the lethal complementation groups were eliminated from consideration as potential DWnt-2 mutants through complementation tests with the deficiency Df(2R)Np5: 44F10; 45D9-E1 (Konev et al. 1994) that overlaps Df(2R)11, but does not delete DWnt-2 sequences (data not shown).
The nine alleles of the two remaining complementation groups were used in single-strand conformational polymorphism (SSCP) analysis (see Materials and Methods). For each of the alleles in the viable male sterile complementation group, a polymorphism was detected in an exon of DWnt-2. Exons with polymorphisms were sequenced and found to have mutations in DWnt-2. The allele DWnt-280, which introduces a stop codon at W265, was used in a screen to generate seven other alleles of DWnt-2, all of which exhibit the same phenotype of male sterility and held out wings. For these additional seven alleles, PCR was used to amplify either the coding regions of the DWnt-2 gene or its cDNA and these were sequenced to identify mutations.
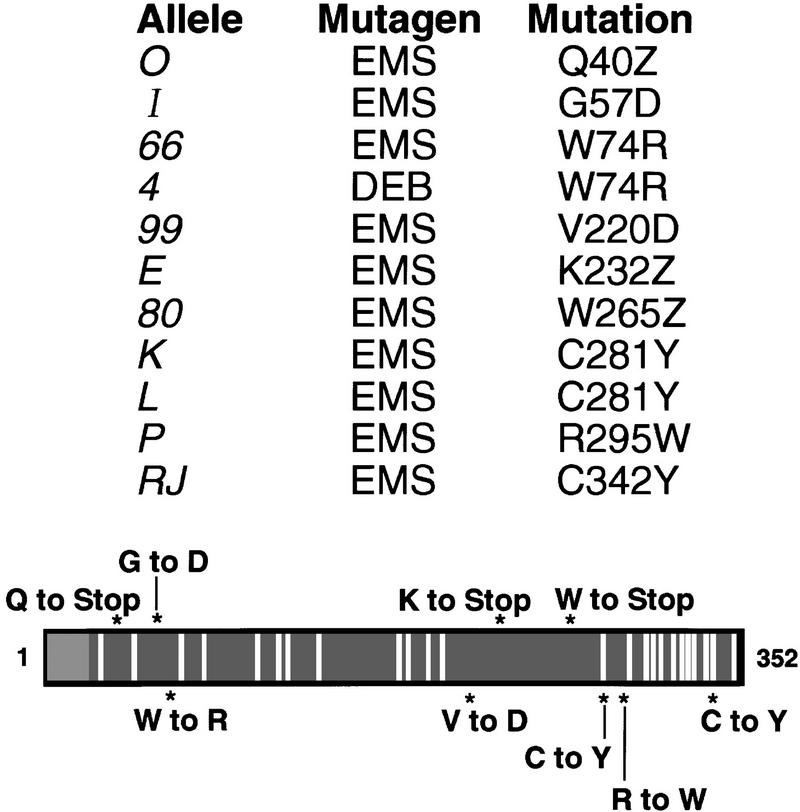
The molecular lesions of the DWnt-2 alleles are summarized in Figure 1. The pair of alleles DWnt-266 and DWnt-24 contain the same nucleotide change, although they were isolated independently. The alleles DWnt-2K and DWnt-2L also contain the same mutation, however, as they were generated in the same screen, may not represent independent events. Five alleles are missense mutations that cause either the gain or loss of a charged residue. Substitutions at conserved cysteine residues occur in three of the alleles. Stop codons are present in three of the alleles. DWnt-2O appears to be a null allele, as it is predicted to be truncated shortly after the signal sequence by a stop codon introduced at Q40.
Figure 1.
Mutations detected in DWnt-2. Schematic representation of the primary amino acid sequence of DWnt-2; vertical bars indicate cysteine residues.
All of the alleles exhibit the same phenotype, whether in a homozygous state or in heteroallelic combinations. The only exception is DWnt-2RJ, which has a less severe phenotype, as discussed below. The phenotype seen for all the other alleles cannot be exacerbated when in trans to Df(2R)11, defining these as amorphic alleles.
Analysis of the adult testis phenotype in DWnt-2 mutants
Flies with null mutations in DWnt-2 are male sterile. To determine the cause, testes were dissected from DWnt-2 mutant males and examined. With variable penetrance, testes from null mutant males are much smaller than wild-type, with the most severe examples having an abnormal oblong shape compared to the wild-type spiral. In addition, the null mutant testes do not possess the yellow pigmentation of the testis sheath (Fig. 2A,B). As noted above, males with the hypomorphic allele DWnt-2RJ have a less severe phenotype in which regions of the sheath produce yellow pigment (Fig. 2C).
Figure 2.
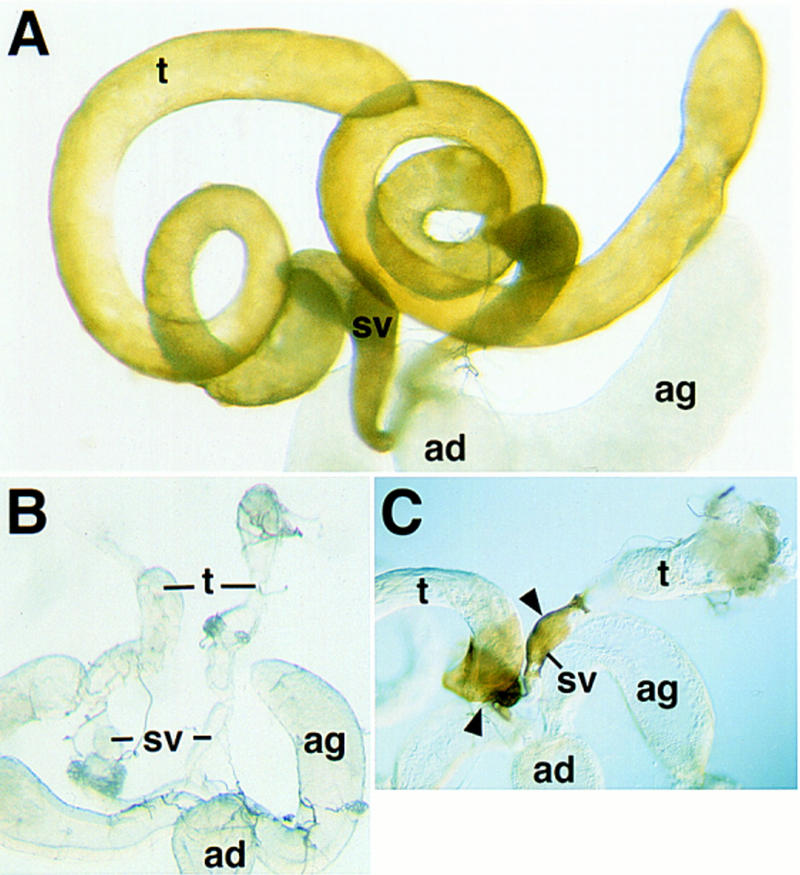
Morphology of the testes from DWnt-2 mutants. (A) Wild-type male reproductive tract. Testes are elongated and assume a helical spiral shape. (t) Testis; (sv) seminal vesicle; (ag) accessory gland; (ad) anterior ejaculatory duct. (B) DWnt-2 mutant male reproductive tract of the genotype DWnt-280/Df(2R)11. The internal genital disc-derived structures (sv, ag, ad) have a normal morphology, but the testes are small, abnormally shaped, and do not have the yellow pigmentation of wild type. (C) DWnt-2 mutant male reproductive tract of the genotype DWnt-2RJ/Df(2R)11. The male reproductive tract has some patches of cells that produce yellow pigment (arrowheads).
To determine whether the cell types necessary for spermatogenesis are present in the mutants, we assayed the expression of β-galactosidase from P-element enhancer trap lines specific for cells of the testis (Gönczy et al. 1992; Gönczy 1995). Expression from the germ-line-specific enhancer trap S346 (Gönczy 1995) clearly shows that a germ cell lineage is maintained in the mutants (Fig. 3A,B). All of the specialized somatic cells of the testis required for spermatogenesis (Fuller 1993) are also present in the mutants. These include the hub cells, cyst cells (Fig. 3C,D), and terminal epithelial cells (Fig. 3E,F), as assayed by expression from the markers I72 (Gönczy 1995) and 34 (Gönczy et al. 1992), respectively. The presence of these cell types, along with the observation that mutant testes occasionally possess motile sperm (data not shown), suggests that the male sterility of the mutants is not attributable to a failure in the process of spermatogenesis.
Figure 3.
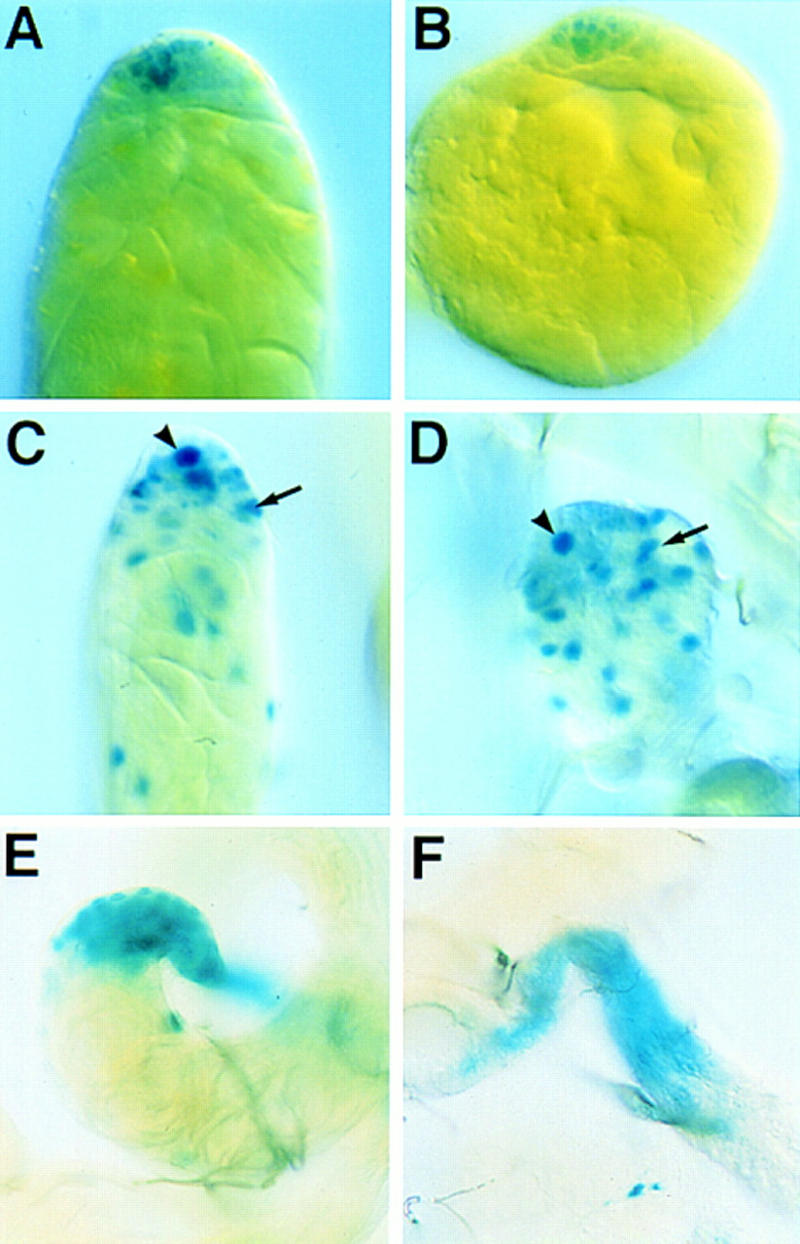
Specialized cells of the testis required for spermatogenesis are present in DWnt-2 mutants. All mutants were X-gal stained, showing β-galactosidase activity from P-element enhancer traps that mark specific cell types of the testis. Here and in Fig. 4, specific genotypes are noted for completeness, but no differences were seen when any of the following alleles were examined in trans to Df(2R)11: DWnt-266, DWnt-280, DWnt-299, and DWnt-24. (A) Wild-type testis; the marker line S346 is expressed in germ cells. (B) DWnt-24/Df(2R)11 mutant testis; S346 expression shows that germ cells are present. (C) Wild-type testis; the marker line I72 is expressed in cells of the hub (arrowhead) and cyst cells (arrow). (D) DWnt-266/Df(2R)11 mutant testis; I72 expression is normal, indicating the presence of a hub (arrowhead) and cyst cells (arrow). (E) Wild-type testis; the marker 34 labels cells of the terminal epithelium. (F) DWnt-266/Df(2R)11 testis; expression from the marker 34 shows that the mutant possesses a terminal epithelium.
The somatic cells necessary for the structure of the testis are affected in the mutants. These cells form a sheath composed of an outer pigment cell layer, an inner muscle cell layer, and their respective basal laminae (Bairati 1967). Assaying for expression from the pigment cell marker 365 (Gönczy 1995) shows that the testes from null mutant adults completely lack the outermost pigment cell layer, which normally covers both the testis and the seminal vesicles (Fig. 4A,B). Expression from the enhancer trap L44a (Fasano and Kerridge 1988), which marks muscle cells of the testis sheath (Gönczy 1995), reveals that the muscle cell layer is malformed or incomplete in these mutants (Fig. 4D). Cells in this morphologically aberrant tissue do have a muscle cell identity, as they express muscle myosin (Fig. 4F). The phenotype of the mutant testis muscle layer varies from a partially complete muscle layer to a tangle of muscle cells at the base of the testis.
Figure 4.
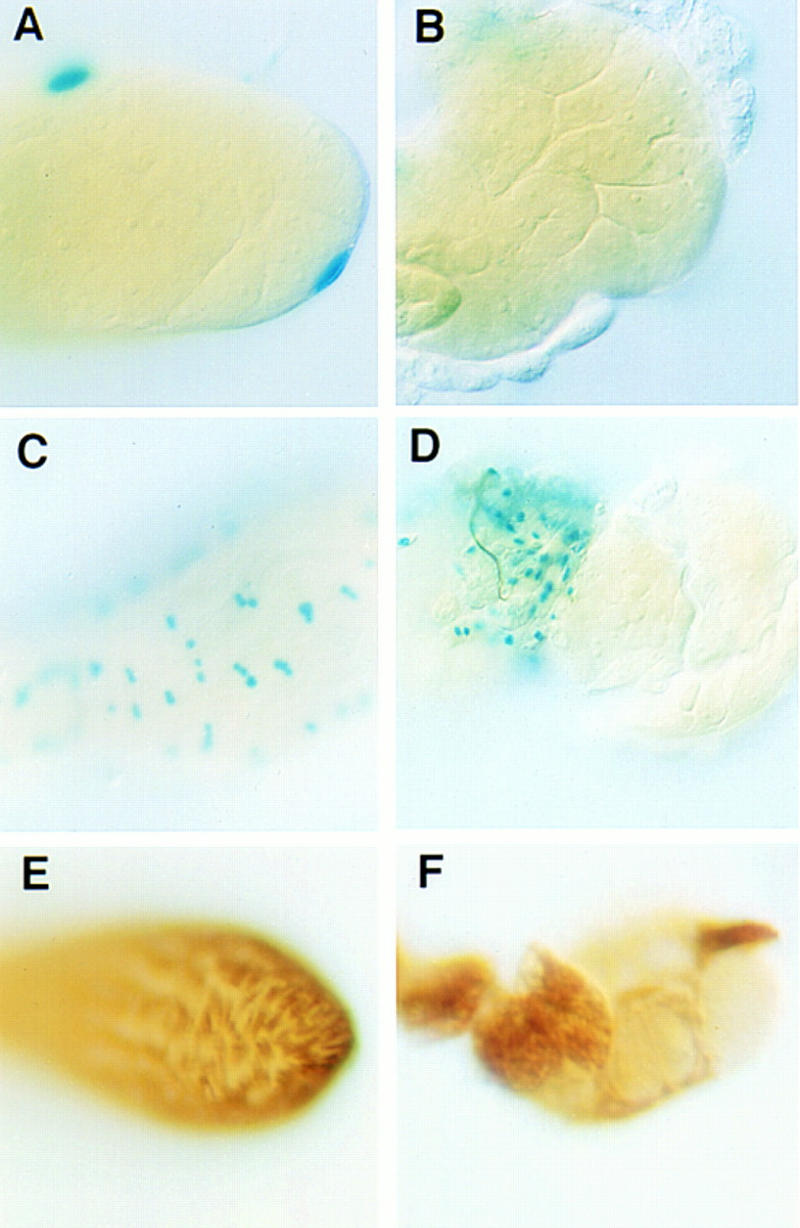
Abnormalities in the sheath of DWnt-2 mutant testes. (A–D) Mutants are X-gal stained for β-galactosidase activity from marker lines; (E,F) mutants are immunostained for muscle myosin. (A) Wild-type testis; β-galactosidase expression from the pigment cell marker 365. β-Galactosidase activity is targeted to the nuclei of the pigment cells that form the outer layer of the testis. (B) DWnt-280/Df(2R)11 mutant testis; a lack of β-galactosidase activity from the marker 365 shows that no pigment cells are present. The testes in A and B may appear slightly yellow, but this is caused by the fixation procedure, not the presence of yellow pigment. The 365 marker is present in a white genotype background, which eliminates the yellow pigment in both the wild-type and the mutant. (C) Wild-type testis; β-galactosidase is expressed from marker line L44a in the cells of the muscle layer. (D) DWnt-24/Df(2R)11 mutant testis; β-galactosidase expression from the marker line L44a shows muscle tissue that does not ensheathe the testis. (E) Wild-type testis; immunostaining with an antibody to muscle myosin illustrates the continuity of the testis muscle layer. (F) DWnt-24/Df(2R)11 mutant testis; immunostaining for muscle myosin shows that myosin-expressing tissue is present but does not form a continuous layer over the testis.
The testis muscle layer derives from the genital disc
To understand how DWnt-2 affects the formation of a complete muscle layer, we have studied the development of this tissue in wild-type animals during pupation. An early study of testis morphogenesis had concluded that the inner cell layer of the testis sheath derived from somatic cells at the base of the gonad and that these either grew or stretched after the genital disc-derived seminal vesicles had contacted the gonad (Stern 1941a). At that time, the inner cell layer was not recognized as being muscle tissue.
To determine the origin of the muscle layer we have used Twist expression as a marker for myoblasts. Expression of Twist is initiated in the mesoderm during embryogenesis (Thisse et al. 1988), persists in adult muscle precursors (Bate et al. 1991), and is required at high levels for myogenesis (Baylies and Bate 1996). To determine the location of the testis muscle precursors we dissected gonads and genital discs before the formation of a connection between them and stained them with an anti-Twist antibody. Twist is expressed in the adepithelial cells that are closely associated with the male genital disc, but not in any cells of the gonad (Fig. 5). The lack of Twist expression in cells of the pupal gonad strongly argues against the possibility that they contribute to the testis muscle layer and supports the conclusion that precursors of the testis muscle sheath reside in the genital disc.
Figure 5.
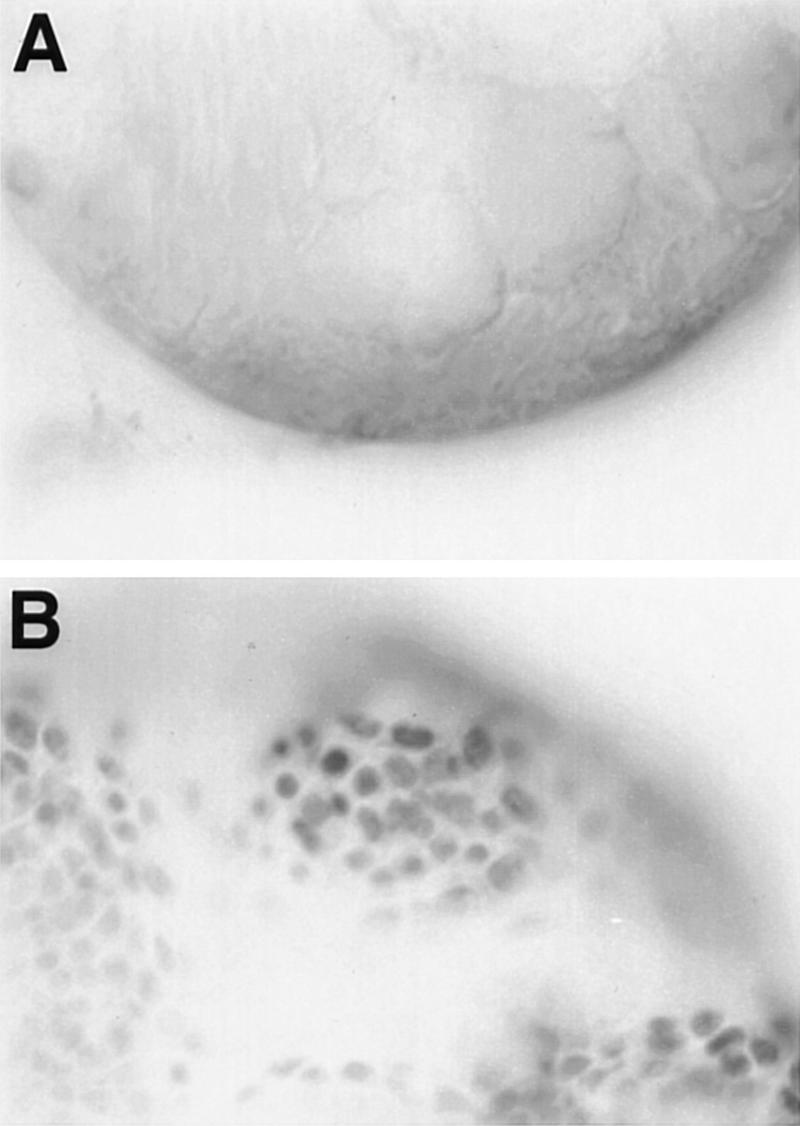
Expression of Twist in the male pupal gonad and genital disc. Wild-type male gonad (A) and genital disc (B) were dissected 24 hr after pupal formation and were immunostained with anti-Twist antibody. There are many Twist-expressing adepithelial cells associated with the genital disc, but none are in the gonad or are associated with it.
To determine the fate of the adepithelial cells of the male genital disc, we have made use of the testis muscle marker L44a, from which we have also seen expression earlier in development. The gonads and genital disc were dissected from individual pupae of the L44a marker line at various stages after puparium formation and were stained for β-galactosidase activity to visualize the muscle precursor cells (Fig. 6A–C). During development, muscle precursor cells are present first on the genital disc as adepithelial cells (Fig. 6A,A′), then contact the base of the gonad (Fig. 6B,B′) and migrate to ensheathe it (Fig. 6C,C′). Pseudopodial extensions from cell bodies are seen during this process (Fig. 6D), which are indicative of cell migration.
Figure 6.
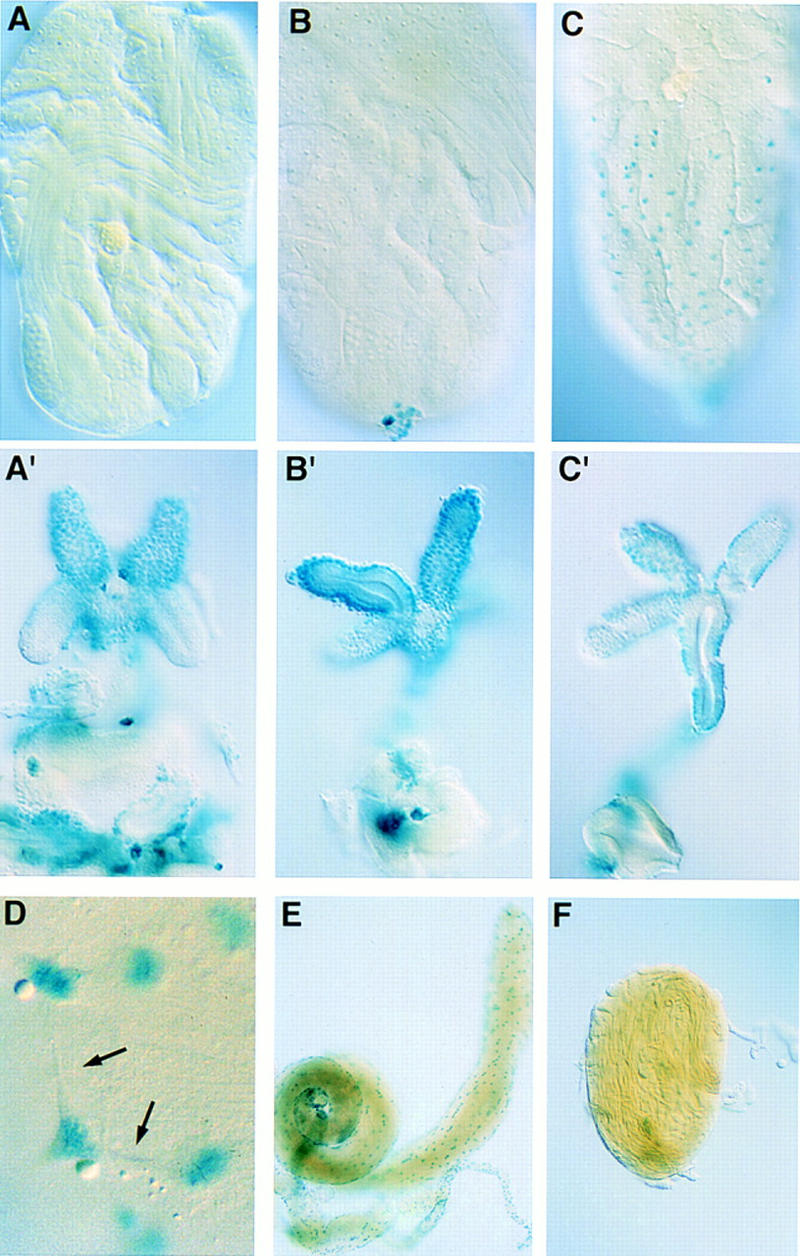
Testis muscle cells originate in the genital disc. (A–D). Male larvae of the muscle-specific marker line L44a were allowed to pupate and the genital disc and gonads from individual pupae were dissected and stained with X-gal at increasing times after pupal formation (APF). (A) gonad ∼24 hr APF (A′) genital disc from same pupa as A; (B) gonad ∼30 hr APF (B′) genital disc from same pupa as B; (C) gonad ∼40 hr APF (C′) genital disc from same pupa as C; the connection between the two broke during dissection. (D) Muscle precursors show cellular extensions as they migrate over testis (arrows) (higher magnification than in other panels). (E) A rudimentary genital disc and attached testis from an In(1)sx male was stained for β-galactosidase activity from the L44a enhancer trap. Expression from this marker shows that the testis has acquired a muscle layer. (F) An unattached gonad from an adult In(1)sx male was stained for β-galactosidase expression from the L44a muscle marker. No muscle cells are associated with it.
Having observed the appearance of muscle precursors on the testis after its connection to the genital disc, we asked how a failure of the genital disc to contact the testis would affect muscle development. For this purpose we have used the male sterile mutant sexcombless (sx). In sx/Y males, internal structures of the reproductive tract are missing or partially formed and consequently, one or both testes remain unattached to the seminal vesicles (Stern 1941a). If the hypothesis that the testis muscle derives from the genital disc is correct, then one would predict that the failure of the gonad to contact the genital disc would abolish testis muscle formation. Expression from the L44a enhancer trap in testes dissected from adult sx/Y males shows that the muscle layer forms when a rudimentary genital disc has contacted the testis but does not form in a free unattached testis (Fig. 6E,F). Together these findings support the conclusion that the testis muscle layer derives from muscle precursor cells of the genital disc.
The DWnt-2 mutant phenotype results from defects in development
On the basis of our conclusion that muscle precursor cells must migrate to form the testis sheath, the muscle phenotype in mutant adults indicates a thwarted attempt at the normal developmental process. The myoblasts in DWnt-2 mutants begin migration over the testis, but fail to complete it (see Fig. 4D). The lack of pigment cells in adult mutants, however, could indicate that either these cells are not specified or they degenerate for lack of contact with a normal muscle layer. To address this, we examined expression of the 365 pigment cell marker in DWnt-2 mutant gonads before any contact with the muscle precursor cells from the genital disc. At 24 hr after puparium formation, 6 hr before the genital disc contacts the gonad (Stern 1941a), mutants already lack the pigment cell layer that covers the wild-type gonad (Fig. 7). Even at the late third instar larval stage, the earliest that expression can be detected from the pigment cell marker line, the mutants lack pigment cells (data not shown). This rules out the possibility that the absence of pigment cells in the mutants is attributable to a degenerative process related to the muscle aberrations. Rather it appears that the pigment cells are never specified in the mutants or die before the morphogenesis of the reproductive tract occurs.
Figure 7.
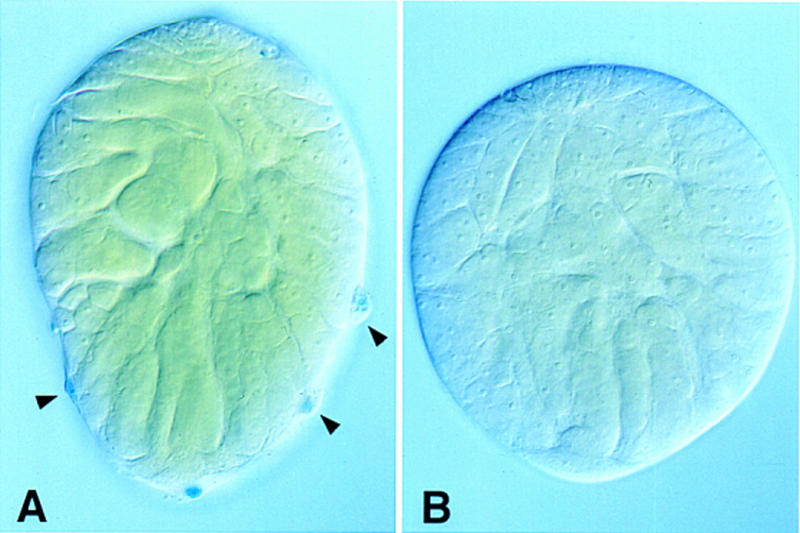
DWnt-2 and the specification of pigment cells. Gonads were dissected from male pupae at 24 hr APF and X-gal stained for β-galactosidase activity from the 365 marker. (A) Wild-type male gonad. The bulging nuclei of the pigment cells (arrowheads) are distinct from other cell types. (B) Male gonad from a DWnt-24/Df(2R)11 mutant. The mutant completely lacks cells with the distinctive pigment cell morphology.
DWnt-2 expression in the developing reproductive tract
To determine how DWnt-2 might affect male reproductive tract development, we have identified sites of DWnt-2 expression by in situ hybridization. DWnt-2 is expressed in all mesodermal cells of the gonad, before the mesoderm and germ cells have condensed to form a compact gonad (Fig. 8A,B). As previously reported, DWnt-2 expression is limited to the posterior mesodermal cells of the gonad late in embryogenesis (Fig. 8C; Russell et al. 1992). This late pattern of expression is apparently maintained in the male, as DWnt-2 is expressed at the posterior of the pupal gonad in the cells that will become the terminal epithelia (Fig. 8D). In the male pupal genital disc, DWnt-2 is expressed in the epithelial cells at the apical tip of each developing seminal vesicle (Fig. 8E). This is a refinement of expression that was present earlier in the third instar larval disc (Fig. 8F) in the male genital primordium (Epper and Nothiger 1982). DWnt-2 is not expressed in the genital disc of the female third instar larva (Fig. 8H), nor is it expressed during pupation in the developing oviducts, the female structures analogous to the developing seminal vesicles (Fig. 8G). Thus, DWnt-2 expression occurs in sexually dimorphic patterns.
Figure 8.
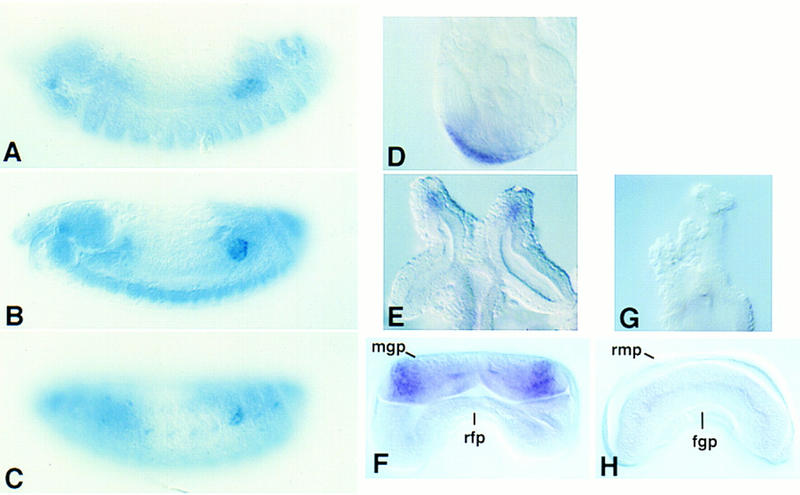
Expression of DWnt-2 in primordia of the male reproductive tract. (A–C) Gonads were hybridized with a DWnt-2 DNA probe. (D–H) Gonads were hybridized with an antisense DWnt-2 RNA probe. (A) Stage 13 embryo; DWnt-2 is expressed in mesodermal cells that will coalesce to form the somatic mesoderm of the gonad. (B) Stage 15 embryo; DWnt-2 is expressed in all mesodermal cells of the newly formed gonad. (C) Stage 16 embryo; DWnt-2 expression is limited to the posterior mesodermal cells of the gonad. (D) Testis dissected at 24 hr APF shows DWnt-2 expression in the terminal epithelia. (E) Male genital disc dissected at 24 hr APF shows expression in the primordia of the seminal vesicles. (F) Male genital disc from a late third instar larva shows DWnt-2 expression in the male genital primordium (mgp) but not in the repressed female primordium (rfp). (G) A pupal female genital disc dissected at 24 hr APF shows no DWnt-2 expression in the oviducts. (H) Female genital disc from a late third instar larva shows no DWnt-2 expression in the repressed male primordium (rmp), nor in the female genital primordium (fgp).
DWnt-2 is sufficient for the induction of pigment cells in the female
Although the pigment cells are a sexually dimorphic cell type found only in the male, it has been shown that the ovary possesses some cells that can differentiate into pigment cells if they have been exposed to a male genital disc during development (Hadorn and Bertani 1948). Our finding that DWnt-2 is expressed only in the male third instar larval genital disc suggested that DWnt-2 is the signal provided by a transplanted male genital disc to induce pigment cell fate in females. To test this hypothesis, we expressed DWnt-2 ectopically in clones during female development. This was accomplished by using a heatshock-driven Flp recombinase to remove an interruption cassette from an actin>cd2>gal4 transgene. The ensuing clonal Gal4 activity allows for expression from a UAS transgene (Pignoni and Zipursky 1997). We induced clones expressing Gal4 in a genetic background containing either UAS:DWnt-2 and the enhancer trap 365, or UAS:DWnt-2 and a wild type copy of the white gene, which is necessary for the yellow pigment of the testis to be produced (Lindsley and Zimm 1992). When clones were induced in third instar female larvae, all of the resulting adults have ectopic pigment cells, as assessed by either their production of yellow pigment (Fig. 9B,D), or the expression of β-galactosidase from the 365 pigment cell enhancer trap (Fig. 9F).
Figure 9.
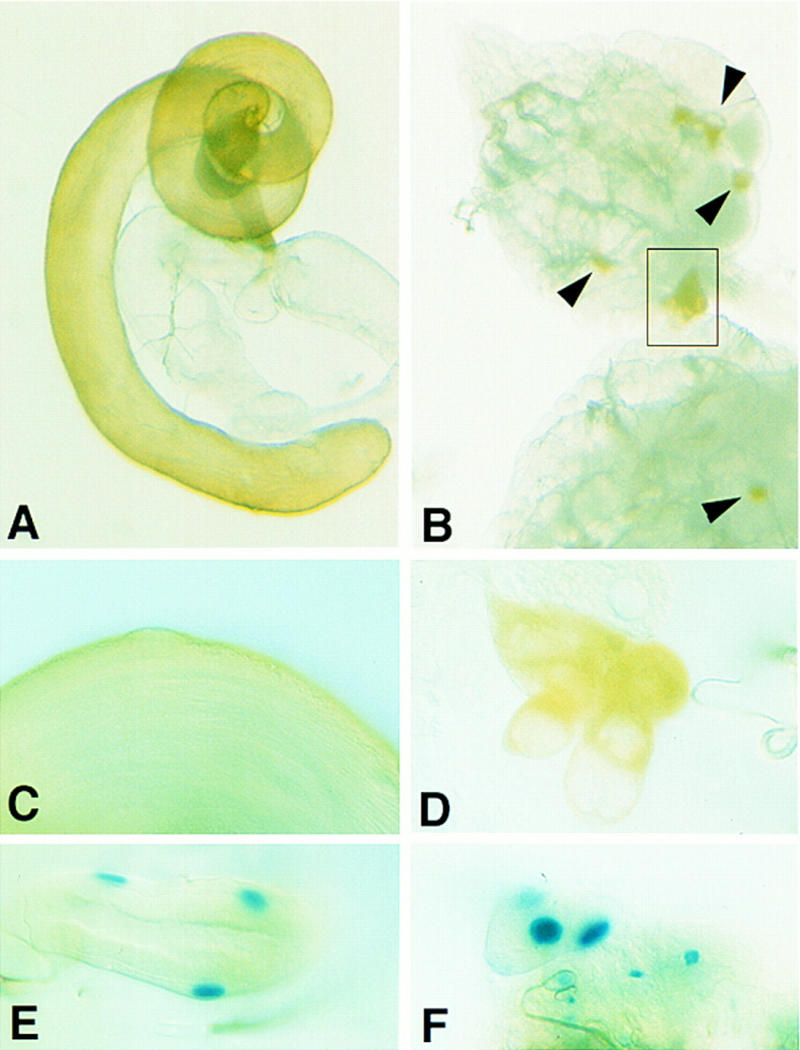
Ectopic expression of DWnt-2 in females induces pigment cells. (A) Wild-type male reproductive tract; testes and seminal vesicles possess yellow pigmentation. (B) Ovaries dissected from adults of the genotype white+/yw, actin<cd2<gal4; UAS-DWnt-2/+; hsFLP, Sb/+. Some cells in close association with the ovary are producing yellow pigment (arrowheads). As third instar larvae, these animals had been heatshocked for 20 min to initiate clonal expression of DWnt-2. The boxed area of the whole mount ovary in B is the same subject as the slide preparation in D, which is shown at an enlargement of 4× relative to B and rotated 90° counterclockwise. C–F are all presented at the same magnification. (C) Pigment cells of a wild-type testis. (E) Wild-type testis; β-galactosidase expression from the 365 marker. The pigment cells shown are those that cover the seminal vesicle. (F) Ovaries dissected from adults of the genotype: yw,actin<cd2<gal4/UAS-DWnt-2; 365/+; hsFLP, Sb/+, that had been heat-shocked as third instar larvae, as in B; cells associated with the ovaries are expressing β-galactosidase from the pigment cell marker 365.
Discussion
Mutations in the DWnt-2 gene of Drosophila result in a male sterile phenotype. Mutants exhibit structural abnormalities in the muscle layer of the testis sheath and lack the pigment cells of the testis. From these defects we conclude that DWnt-2 functions to determine the fate of somatic cells of the male gonad and aid the migration or attachment of muscle precursors, by either a direct or an indirect mechanism. Ectopic expression of DWnt-2 in females results in the formation of ectopic pigment cells. This misexpression of a male cell fate implicates DWnt-2 as a signal that controls a sexually dimorphic trait.
DWnt-2 is required for the formation of the male reproductive tract
Males mutant for DWnt-2 are sterile. Testes from DWnt-2 null mutants have an abnormal morphology and are moderately to severely reduced in size. There is also variation in the extent to which testis muscle cell migration has proceeded. One invariant aspect of the null phenotype is the lack of an outer pigment cell layer. DWnt-2 is required for the differentiation or survival of this sex-specific cell type in the male. The germ cells and the somatic cell types that support spermatogenesis are present in the mutant testes, indicating that sterility is caused by structural and mechanical defects of the reproductive tract.
A new model for the development of the sheath of the testis
The sheath of the reproductive tract in the adult male includes a continuous layer of smooth muscle that, for the testis and seminal vesicles, is covered by a layer of pigment cells. The pigment cells that cover the seminal vesicle have migrated to that position from the gonad (Stern and Hadorn 1939). Using a Twist antibody to identify cells that are fated to become muscle (Bate et al. 1991; Baylies and Bate 1996), we found that muscle precursors originate in the genital disc, not as cells of the gonad, as was believed previously (Stern 1941a). We propose a model in which the two events, muscle migration from the genital disc and pigment cell migration from the gonad, occur in a concerted fashion (Fig. 10). This would stabilize the connection between the genital disc and the testis, facilitating the fusion of the seminal vesicle epithelial cell layer with the testis terminal epithelial cells. In this scenario, one might predict pigment and muscle cell migration to be interdependent, requiring that each cell type recognize the other by their membrane surface proteins or extracellular matrix components.
Figure 10.
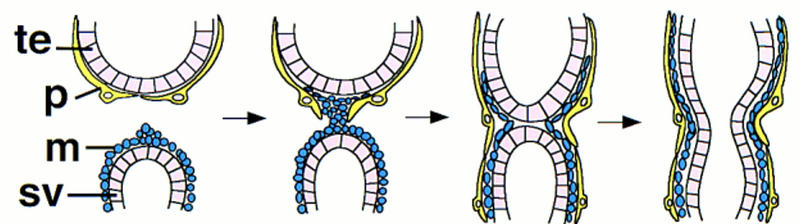
A model for the migration of pigment and muscle precursors cells. The first cells of the genital disc to contact the gonad are the muscle precursor cells, which must gain access to the basal surface of the pigment cells. Migration of muscle and pigment cells then proceeds in opposite directions until the gonad and the seminal vesicle have each acquired an inner layer of muscle tissue and an outer layer of pigment cells. (te) Terminal epithelial cells of the testis; (p) pigment cell; (m) muscle precursor cell; (sv) epithelial cells of the seminal vesicle.
At first glance, the requirement of DWnt-2 for the development of the sheath of the male reproductive tract would seem to fulfill the early prediction that there is a signal produced by the genital disc that induces testis morphogenesis. However, this signal was proposed at a time when it was understood that the inner layer of the sheath is required for testis morphogenesis, but when it was falsely assumed that cells of the gonad contribute to the inner layer. When transplantation experiments showed that contact with a genital disc is required for morphogenesis, it was proposed that cells of the gonad respond to a genital disc signal by forming the inner layer of the sheath (Stern 1941a,b). Our current finding that the muscle precursors of the sheath originate in the genital disc and migrate to cover the testis has led us to reevaluate the proposed morphogenetic signal. We conclude that the signal can be redefined as the muscle precursors themselves, which are supplied by the genital disc and induce testis morphogenesis. This signal is not equivalent to DWnt-2 activity, which is required for morphogenesis because either it directly or indirectly affects the migration or adherence of the muscle precursors.
Our model is in agreement with a study of the female reproductive tract in which it was observed that cells associated with the genital disc make the first contact with the ovary, migrate over its surface, and form the muscle layer covering each ovariole (Babcock 1971). We have examined the muscle tissue of ovaries from DWnt-2 mutants for defects, but found none and no loss of fertility (K. Kozopas and R. Nusse, unpubl.). This is perhaps not surprising, given the sex-specific expression of DWnt-2 (Fig. 8). It appears that the migration of ovary and testis muscle precursors are influenced by independent factors.
DWnt-2 affects muscle migration
The role of DWnt-2 in testis muscle cell development, as revealed by the mutant phenotype, is to ensure the proper morphogenesis of the muscle precursor cells. This is different from the role of wingless (wg) in determining muscle cell fate in the embryonic mesoderm (Baylies et al. 1995; Ranganayakulu et al. 1996), and from the conserved function of Wnt-1 and Wnt-3, which induce myogenesis in the somites of chick explants (Munsterberg et al. 1995; Stern et al. 1995). In the case of the testis muscle layer, DWnt-2 appears not to be required to specify muscle cell fate, as myosin-expressing testis muscle tissue is present (Fig. 4F) and covers mutant testes to variable degrees.
Instead of initiating cell differentiation, DWnt-2 contributes to either the migration of the muscle precursors away from the genital disc or to their adherence and migration over the testis. This contribution may be a direct one, in which DWnt-2 regulates motility or adherence, or an indirect one, in which the role of DWnt-2 is to specify the pigment cell substrate. The variation in penetrance of the muscle phenotype, seen for all alleles, indicates that DWnt-2 is not essential for muscle precursor migration and may argue that the loss of pigment cells is the primary reason why the muscle fails to develop.
However, DWnt-2 is expressed in the seminal vesicle and the terminal epithelium of the gonad before the contact of the genital disc and the gonad (Fig. 8D,E). This expression pattern strongly suggests that DWnt-2 may have a function in the recognition or migration events that occur when the myoblasts of the seminal vesicle contact the pigment cells of the gonad. At this stage, DWnt-2 may be a signal required by either or both of the migrating cell types. Because of the absence of the pigment cells in the mutants we have not been able to determine whether this late expression of DWnt-2 affects pigment cell migration. Likewise, we have not yet separated effects attributable to a lack of pigment cells from any direct requirement of DWnt-2 for muscle cell migration.
DWnt-2 is necessary and sufficient to specify pigment cell fate
DWnt-2 activity is required in males for the presence of pigment cells. Although the pigment cells are a male specific cell type, this cell fate can be induced in females into which has been transplanted a male genital disc. By expressing DWnt-2 ectopically, we have also induced this pigment cell fate in females (Fig. 9). This result and our observation that DWnt-2 is expressed only in the male genital disc (Fig. 8E,F) suggest that DWnt-2 is the activity responsible for the ectopic pigment cells seen in transplant experiments (Hadorn and Bertani 1948).
DWnt-2 is necessary and sufficient for the differentiation of the male-specific pigment cell fate, at least for a certain population of cells. It is not clear what cell type of the female gives rise to the pigment-producing cells in our experiments. In a commentary on the origin of the pigment cells produced in female recipients of transplanted male discs, King (1970) has speculated that they derive from the colorless sheath cells of the ovary. An alternative explanation is that an external cell type of the ovary, referred to as a lamellocyte and seen only in prepupae (King 1970), may be the cell type with the potential to become a pigment cell.
Because females do not have pigment cells, yet are capable of producing them, DWnt-2 must be regulated transcriptionally so that it is either repressed in females or only active in males. This regulation could be controlled by the sex determination hierarchy or by tissue-specific factors. Male-specific pigment cells have been found in ovaries that were treated with colchicine at the third instar larval stage and allowed to develop in an untreated host (Hadorn 1946; Hadorn and Niggli 1946). One explanation offered for this result was that colchicine damages the ovary and alters its physiology, allowing a male cell type to develop (Hadorn 1946). Although we do not understand the mechanism by which colchicine can generate pigment cells, we speculate that it may cause misregulation of DWnt-2 expression, perhaps through nondisjunctional events that change the dosage of a repressor.
A comparison of the role of DWnt-2 and wg in the development of the reproductive tract reveals major differences. wg has a general requirement in both males and females for the formation of the somatic cells of the gonad (Warrior 1994; Boyle et al. 1997) and for structures derived from the genital disc (Chen and Baker 1997). In contrast, DWnt-2 is specifically active in males, regulating the appearance of a sexually dimorphic trait.
Possible mechanisms of DWnt-2 signal transduction
Our results indicate that DWnt-2 has the ability to direct the differentiation of a particular cell fate. We presume that as an instructive signal for the differentiation of pigment cells DWnt-2 affects changes in gene transcription. It is known that wg affects transcription by stabilizing Armadillo (van Leeuwen et al. 1994), a β-catenin homolog (Peifer and Wieschaus 1990; McCrea et al. 1991). When Armadillo is bound to pangolin, a Tcf DNA-binding protein, the complex acts as a transcriptional activator (Brunner et al. 1977; van de Wetering et al. 1997). It will be interesting to determine whether DWnt-2 also uses the same signaling components. We might predict that this will be true; however, there are examples of Wnt functions that are not mediated by transcriptional regulation through β-catenin and Tcf. This is the case for the Caenorhabditis elegans Wnt gene mom-2, which controls orientation of the mitotic spindle, a cytoskeletal effect that occurs in the absence of transcription (Rocheleau et al. 1997; Thorpe et al. 1997).
The potentially direct affects of DWnt-2 on muscle precursor cell migration could be mediated in several ways. DWnt-2 might affect motility through the regulation of cytoskeletal elements. Alternatively, the migration defects seen in DWnt-2 mutants might not be attributable to a loss of motility, but rather a loss of the ability of cells to determine where their final position should be. This specific function is implicated for the C. elegens gene lin-17, a Wnt receptor homolog required for the migration of certain neuronal cells to their proper position (Harris et al. 1996). A third possible explanation for the affect of DWnt-2 on myoblast migration is through alterations in adhesion. Wingless has a positive effect on E-cadherin transcription in vitro (Yanagawa et al. 1997), suggesting that DWnt-2 may also affect adhesiveness at the transcriptional level. The phenotype of the DWnt-2 mutant raises many questions about how this signal functions to specify cell fate and promote the morphogenetic process of migration. Further studies of the DWnt-2 signaling mechanism should greatly advance our understanding of how Wnt genes control development.
Materials and methods
Fly stocks
The 5R13A stock was obtained from Judith Kassis (Food and Drug Administration, Washington, D.C.). The deficiency stock w; In(2LR)w45-32n, cn, Df(2R)Np5/CyO was obtained from the Umeå stock center. The P-element enhancer traps S346, I72, 34, 365, and L44a, which are expressed in specific cells of the testis, were obtained from Steve DiNardo (Rockefeller University, New York, NY). The sexcombless stock of the genotype In(1)sx, sc, ec, cv, ct, v/sc, ec, cv, ct, v, g, f was obtained from the Bloomington stock center. The fly stock with the actin<cd2<gal4 and hsFLP transgenes was obtained from Larry Zipursky (University of California, Los Angeles) and the hsFLP transgene on the MKRS chromosome (Chou and Perrimon 1992) was obtained from the Bloomington stock center. To create the UAS:DWnt-2 transgenic stock, the DWnt-2 cDNA was cloned into the pUAST plasmid (Brand and Perrimon 1993) and this was injected into white 118 embryos. Wild-type flies used for in situs and immunohistochemistry were of the Canton-S stock. Mutant larvae and pupae were identified through the use of an attached SM5-TM6,Tb balancer.
X-ray mutagenesis
Males of the line 5R13A (P[ry+], cn) were irradiated with 3500 rads in a cabinet X-ray machine and crossed to females of the genotype Sco/CyO, cn; ry. Approximately 22,700 progeny were screened for the loss of the P[ry+] marker. Flies that were cn/CyO; ry were crossed to flies of the genotype Sco/CyO; ry. Progeny of the genotype cn/CyO; ry were crossed among one another to establish a stock and to determine whether the loss of the P-element had created a lethal deficiency. In this manner, deficiency Df(2R)11 was created. By examining polytene chromosomes from salivary glands of third instar larvae of the genotype Df(2R)11/+, we have mapped the approximate breakpoints of this deficiency as 45C6; 45E1.
Chemical mutagenesis
Wild-type males with an isogenized second chromosome (isoII) were mutagenized with ethylmethanesulfonate (EMS) or diepoxybutane (DEB) according to standard methods (Grigliatti 1986) and were crossed with Sco/CyO, cn females. Single F1 males with the mutagenized isoII/CyO, cn were crossed at 29°C to females of the genotype Df(2R)11, cn/CyO, cn. The F2 generation was screened for lethality of isoII/Df(2R)11, cn by the absence of straight-winged flies. Flies with isoII carrying a lethal mutation over CyO were crossed among one another to establish a stock. Of the chromosomes screened, 3186 were mutagenized with 24 mm EMS, 1678 were mutagenized with 30 mm EMS, and 1102 were mutagenized with 5 mm DEB. Sixteen independent mutations were recovered, representing five complementation groups.
The DWnt-2 mutations in trans to Df(2R)11 are adult viable and have the visible phenotype of held-out wings, which allowed them to be identified in a screen for lethals. However, all chromosomes bearing the original alleles (4, 66, 80, 99) of the DWnt-2 complementation group are lethal in any heteroallelic combination. This is apparently a case of synthetic lethality caused by a genetic locus or loci present on the isogenized chromosome used for the mutagenesis. This was revealed when the DWnt-2 alleles were allowed to recombine with a recessively marked chromosome. Recombination events that exchanged genetic material distal to the gene curved and proximal to the gene plexus, resulted in chromosomes carrying the DWnt-2 alleles that were no longer lethal in trans to each other.
Once the DWnt-2 complementation group was identified, more alleles were generated by the following procedure. Males of the genotype yw; isoIIa (a more recently isogenized second chromosome) were mutagenized with EMS and crossed to yw; Sco/CyO females. Single males of the genotype yw; isoIIa/CyO were crossed at 29°C to females carrying a P[w+] marker on the same chromosome arm as the DWnt-280 allele (DWnt-280, P[w+]47A/CyO). Crosses in which all the straight winged flies had held-out wings were screened for defects in the male reproductive tract. Flies of the genotype isoIIa/CyO were mated to create stocks of new alleles. Of the chromosomes screened, 931 were mutagenized with 30 mm EMS and 1727 were mutagenized with 25 mm EMS. Seven alleles were isolated independently (E, I, K, L, O, P, RJ) and when sequenced all had mutations in DWnt-2.
SSCP analysis
Primers to intronic sequences of DWnt-2 were used in PCR with genomic DNA from flies of the following genotypes: mutant/Df(2R)11, mutant/CyO, isoII/isoII, and Df(2R)11/CyO, the latter two serving as controls. Reactions were labeled by including the following in a 25-μl reaction: 2.5 μl 10× Hot Tub buffer (Amersham), 400 ng of genomic DNA template, 40 pmoles of each primer, 200 nmoles each of dATP, dCTP, dGTP, and dTTP, 0.25–0.375 μl [32P]dCTP (3000 Ci/nmole, 10 μCi/μl), and 1.25 units of Taq DNA polymerase. Amplification was carried out for 30 cycles at temperatures optimized for each primer set. One microliter of each PCR reaction was diluted with 5 μl of stop solution (95% formamide, 20 nm EDTA, 0.05% bromophenol blue, 0.05% xylene cyanol). The samples were denatured at 95°C for 3 min, placed on ice, and loaded on a 0.5× Hydrolink MDE (mutation detection enhancement) gel (AT Biochem) in 0.6× TBE. Electrophoresis was carried out at a constant 8 W for 12–16 hr at room temperature. Gels were dried on filter paper and exposed to film for 1-112 hr. For some PCR reactions the sizes of the DNA fragments were reduced by restriction enzyme digestion to enhance the ability to detect polymorphisms. In these cases, PCR samples were phenol/chloroform extracted, ethanol precipitated, and then resuspended in 15 μl of 1× restriction digestion buffer with enzyme. One microliter of this reaction was diluted with 5 μl of stop solution and electrophoresed as above. Exons of alleles in which a polymorphism was detected were either subcloned for sequencing or were sequenced directly from PCR template. Each of the original DWnt-2 alleles gave rise to either the gain or loss of a restriction enzyme site, which was used to confirm each mutation by checking for the corresponding restriction fragment polymorphism on Southern blots.
PCR amplification of mutant alleles
For the alleles generated by failure to complement DWnt-280, the coding sequences of DWnt-2 were cloned and sequenced. This was accomplished either by amplifying genomic exons of DWnt-2, as mentioned above for SSCP analysis, or by reverse transcription PCR (RT–PCR) from RNA isolated from homozygous mutant adults. RT–PCR was carried out with the Superscript Preamplification System (GIBCO-BRL) according to the manufacturer’s directions. PCR fragments were then cloned in Bluescript plasmid and sequenced.
X-gal staining of tissues
Testes from adults, and gonads and genital discs from larval and pupal stages were dissected in PBS with 0.1% Tween (PBT). Tissues were fixed in PBT with 1% glutaraldehyde for 30 min then washed three times with PBT. Tissues were placed in staining buffer [7.2 mm Na2HPO4, 2.8 mm NaH2PO4, 1 mm MgCl2, 0.15 m NaCl, 5 mm K4Fe(CN)6, 5 mm K3Fe(CN)6 and 0.2% X-gal (5-bromo-4-chloro-3-indolyl-β-d galactopyranoside)] and incubated at 37°C for 12–24 hr. Tissues were washed with PBT and mounted in 50% glycerol.
In situ hybridization
Hybridization with DNA probes was as described previously (Russell et al. 1992). Hybridization with RNA probes was as follows. RNA probe was labeled with digoxigenin (DIG; Boehringer Mannheim) using linearized DNA template according to the manufacturer’s directions. Reactions were phenol/chloroform extracted, then ethanol precipitated. Probe was then resuspended in 20 μl of hydrolysis buffer [(40 mm NaHCO3, 60 mm Na2CO3 (pH 10)] and incubated at 60°C for 60 min. To this, 10 μl of 1 m Tris (pH 7.5) and 300 μl hybridization solution (50% formamide, 5× SSC, 100 μg/ml salmon sperm DNA, 100 μg/ml tRNA, 50 μg/ml heparin, 0.1% Tween) were added.
Testes and genital discs from larval and pupal stages were dissected in PBS and fixed in 4% paraformaldehyde in PBS for 15 min on ice. Deoxycholate and Triton X-100 were each added to a concentration of 0.1% and fixation was continued at room temperature for 15 min. The fixative was then removed and the tissues washed twice in 0.3 m NH4OAc. An equal volume of ethanol was then added dropwise. Tissues were washed in ethanol and stored at −20°C, if necessary. Tissues to be hybridized were then washed in 50% ethanol/50% xylene for 10 min. The tissues were then washed three times in ethanol, twice in methanol, once in 50% methanol/50% PBT + 4% formaldehyde (PBTF). All washes were for 2 min. The tissues were then fixed for 20 min in PBTF. The tissues were washed five times for 5 min in PBT and then once in 50% PBT/50% hybridization solution. Tissues were incubated in hybridization solution at 56°C for 1–4 hr. DIG-labeled RNA probe prepared as described above was added at a 1:1000 dilution and incubated at 56°C for 12–16 hr. Tissues were washed at 56°C for 30 min each in the following washes: once in hybridization solution, once in 50% hybridization solution/50% PBT, then five times in PBT. The hybridized tissues were then incubated overnight at 4°C with anti-DIG antibody conjugated to alkaline phosphatase (Boehringer Mannheim) at a final dilution of 1:2000. The tissues were then washed four times for 30 min in PBT, washed twice in staining buffer [0.1 m NaCl, 50 mm MgCl2, 0.1 m Tris-HCl (pH 9.5), and 0.1% Tween 20], and put into 1 ml of staining buffer plus 4.5 μl of nitroblue tetroleum and 3.5 μl of X-phosphate (Boehringer Mannheim). The staining reaction was allowed to proceed in the dark for 30 min to 2 hr. Tissues were postfixed in PBTF for 30 min, washed in PBT, and mounted in 50% glycerol.
Immunohistochemistry
Tissues were dissected in PBS with 0.1% Triton X-100 (PBX), fixed in PBX plus 4% formaldehyde for 30 min at room temperature, and washed in 2% H2O2 in methanol for 20 min to block endogenous peroxidase activity. The tissues were incubated in PBT with 1% bovine serum albumin and 0.01% azide (BBT) with a primary antibody at a 1:1000 dilution overnight at 4°C. Tissues were washed in BBT three times for 30 min, then blocked with 2% normal serum in BBT for 1 hr, washed in BBT for 30 min, and incubated with 1:500 biotin-conjugated secondary antibody (Vector Laboratories) in BBT overnight at 4°C. Tissues were washed five times in PBX, then incubated with a biotinylated horseradish peroxidase-avidin complex (Vector Laboratories) and washed three times for 20 min with PBX. Samples were then incubated in PBX with 0.5 mg/ml diaminobenzidine and 0.03% nickel chloride. Staining was stopped by washing tissues in BBT. Tissues were mounted in 50% glycerol.
The antibodies used were rabbit anti-muscle myosin (Kiehart and Feghali 1986) at a 1:500 dilution and rabbit anti-Twist (Roth et al. 1989) at a 1:1000 dilution.
Acknowledgments
For discussions and comments on the manuscript we thank Ken Cadigan, Ronald Johnson, and Eric Rulifson. We also thank Minx Fuller and members of her laboratory for discussions and protocols. Antibodies were gifts from Siegfried Roth and Dan Kiehart. We are indebted to Matt Fish for microinjection of the pUAST–DWnt-2 construct and we are grateful for the fly stocks that were sent by Steve DiNardo and Judith Kassis. K.M.K. was supported by an National Research Service Award (NRSA) training grant (GM08404-02), the Howard Hughes Medical Institute, and an NRSA fellowship (HD0779809-02). R. N. is an investigator with the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL rnusse@cmgm.stanford.edu; FAX (650) 723-1399.
References
- Babcock MB. Oviduct development in Drosophila. II. Metamorphic events in normal and ovarectomized females. Wilhelm Roux’s Arch Dev Biol. 1971;167:24–63. doi: 10.1007/BF00576328. [DOI] [PubMed] [Google Scholar]
- Bairati A. The structure and ultrastructure of the male genital apparatus of the Drosophila melanogaster Meig. 1. The testis. Z Zellforsch Mikrosk Anat. 1967;76:56–99. [PubMed] [Google Scholar]
- Bate M, Rushton E, Currie DA. Cells with persistent twist expression are the embryonic precursors of adult muscles in Drosophila. Development. 1991;113:79–89. doi: 10.1242/dev.113.1.79. [DOI] [PubMed] [Google Scholar]
- Baylies MK, Bate M. twist: A myogenic switch in Drosophila. Science. 1996;272:1481–1484. doi: 10.1126/science.272.5267.1481. [DOI] [PubMed] [Google Scholar]
- Baylies MK, Martinez Arias A, Bate M. wingless is required for the formation of a subset of muscle founder cells during Drosophila embryogenesis. Development. 1995;121:3829–3837. doi: 10.1242/dev.121.11.3829. [DOI] [PubMed] [Google Scholar]
- Boyle M, Bonini N, DiNardo S. Expression and function of clift in the development of somatic gonadal precursors within the Drosophila mesoderm. Development. 1997;124:971–982. doi: 10.1242/dev.124.5.971. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brunner E, Peter O, Schweizer L, Basler K. pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature. 1997;385:829–833. doi: 10.1038/385829a0. [DOI] [PubMed] [Google Scholar]
- Chen EH, Baker BS. Compartmental organization of the Drosophila genital imaginal discs. Development. 1997;124:205–218. doi: 10.1242/dev.124.1.205. [DOI] [PubMed] [Google Scholar]
- Chou T-B, Perrimon N. Use of a yeast site-specific recombinase to produce female germline chimeras in Drosophila. Genetics. 1992;131:643–653. doi: 10.1093/genetics/131.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T. Interactions between female and male parts in gynandromorphs of Drosophila simulans. Wilhelm Roux’s Arch Dev Biol. 1931;123:719–746. doi: 10.1007/BF01380651. [DOI] [PubMed] [Google Scholar]
- Epper F, Nothiger R. Genetic and developmental evidence for a repressed genital primordium in Drosophila melanogaster. Dev Biol. 1982;94:163–175. doi: 10.1016/0012-1606(82)90079-3. [DOI] [PubMed] [Google Scholar]
- Fasano L, Kerridge S. Monitoring positional information during oogenesis in adult Drosophila. Development. 1988;104:245–253. doi: 10.1242/dev.104.Supplement.245. [DOI] [PubMed] [Google Scholar]
- Fuller MT. Spermatogenesis. In: Bate M, Martinez Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 71–147. [Google Scholar]
- Gönczy P. “Towards a molecular genetic analysis of spermatogenesis in Drosophila.” Ph.D. thesis. New York, NY: The Rockefeller University; 1995. [Google Scholar]
- Gönczy P, Viswanathan S, DiNardo S. Probing spermatogenesis in Drosophila with P-element enhancer detectors. Development. 1992;114:89–98. doi: 10.1242/dev.114.1.89. [DOI] [PubMed] [Google Scholar]
- Grigliatti T. Mutagenesis. In: Roberts DB, editor. Drosophila, a practical approach. Oxford, UK: IRL Press; 1986. pp. 39–42. [Google Scholar]
- Hadorn E. Mutationsversuche mit Chemikalien an Drosophila. I. Wirkung von Colchicin auf transplantierte Larven-Ovarien nach Behandlung in vitro. Rev Suisse Zool. 1946;53:486–494. [Google Scholar]
- Hadorn E, Bertani G. Induktion mannlicher Pigmentierung in somatischen Zellen von Drosophila-Ovarien. Rev Suisse Zool. 1948;55:232–248. [Google Scholar]
- Hadorn E, Niggli H. Mutations in Drosophila after chemical treatment of gonads in vitro. Nature. 1946;157:162–163. doi: 10.1038/157162a0. [DOI] [PubMed] [Google Scholar]
- Harris J, Honigberg L, Robinson N, Kenyon C. Neuronal cell migration in C. elegans: Regulation of Hox gene expression and cell position. Development. 1996;122:3117–3131. doi: 10.1242/dev.122.10.3117. [DOI] [PubMed] [Google Scholar]
- Kassis JA, Noll E, VanSickle EP, Odenwald WF, Perrimon N. Altering the insertional specificity of a Drosophila transposable element. Proc Natl Acad Sci. 1992;89:1919–1923. doi: 10.1073/pnas.89.5.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehart DP, Feghali R. Cytoplasmic myosin from Drosophila melanogaster. J Cell Biol. 1986;103:1517–1525. doi: 10.1083/jcb.103.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. Ovarian development in Drosophila melanogaster. New York, NY: Academic Press; 1970. pp. 1–17. [Google Scholar]
- Konev AY, Varentsova ER, Khromykh YM. Cytogenetic analysis of the chromosome region containing the radiosensitivity gene in Drosophila. II. A study of vital loci of the 44F-45C region of chromosome 2. Russian J Genet. 1994;30:181–190. [PubMed] [Google Scholar]
- Lindsley DL, Zimm GG. The genome of Drosophila melanogaster. San Diego, CA: Academic Press; 1992. [Google Scholar]
- McCrea PD, Turck CW, Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science. 1991;254:1359–1361. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- Munsterberg AE, Kitajewski J, Bumcrot DA, McMahon AP, Lassar AB. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Gene & Dev. 1995;9:2911–2922. doi: 10.1101/gad.9.23.2911. [DOI] [PubMed] [Google Scholar]
- Peifer M, Wieschaus E. The segment polarity gene armadillo encodes a functionally modular protein that is the Drosophila homolog of human plakoglobin. Cell. 1990;63:1167–1176. doi: 10.1016/0092-8674(90)90413-9. [DOI] [PubMed] [Google Scholar]
- Pignoni F, Zipursky SL. Induction of Drosophila eye development by decapentaplegic. Development. 1997;124:271–278. doi: 10.1242/dev.124.2.271. [DOI] [PubMed] [Google Scholar]
- Ranganayakulu G, Schulz RA, Olson EN. Wingless signaling induces nautilus expression in the ventral mesoderm of the Drosophila embryo. Dev Biol. 1996;176:143–148. doi: 10.1006/dbio.1996.9987. [DOI] [PubMed] [Google Scholar]
- Rocheleau CE, Downs WD, Lin R, Wittmann C, Bei Y, Cha YH, Ali M, Priess JR, Mello CC. Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell. 1997;90:707–716. doi: 10.1016/s0092-8674(00)80531-0. [DOI] [PubMed] [Google Scholar]
- Roth S, Stein D, Nüsslein-Volhard C. A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell. 1989;59:1189–1202. doi: 10.1016/0092-8674(89)90774-5. [DOI] [PubMed] [Google Scholar]
- Russell J, Gennissen A, Nusse R. Isolation and expression of two novel Wnt/wingless gene homologues in Drosophila. Development. 1992;115:475–485. doi: 10.1242/dev.115.2.475. [DOI] [PubMed] [Google Scholar]
- Stern C. The growth of the testes in Drosophila. I. The relation between vas deferens and testis within various species. J Exper Zool. 1941a;87:113–158. [Google Scholar]
- ————— The growth of the testes in Drosophila. II. The nature of interspecific differences. J Exper Zool. 1941b;87:159–180. [Google Scholar]
- Stern C, Hadorn E. The relation between the color of testes and vasa efferentia in Drosophila. Genetics. 1939;24:162–179. doi: 10.1093/genetics/24.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern HM, Brown AMC, Hauschka SD. Myogenesis in paraxial mesoderm: Preferential induction by dorsal neural tube and by cells expressing Wnt-1. Development. 1995;121:3675–3686. doi: 10.1242/dev.121.11.3675. [DOI] [PubMed] [Google Scholar]
- Thisse B, Stoetzel C, Gorostiza-Thisse C, Perrin-Schmitt F. Sequence of the twist gene and nuclear localization of its protein in endomesodermal cells of early Drosophila embryos. EMBO J. 1988;7:2175–2183. doi: 10.1002/j.1460-2075.1988.tb03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe CJ, Schlesinger A, Carter JC, Bowerman B. Wnt signaling polarizes an early C. elegans blastomere to distinguish endoderm from mesoderm. Cell. 1997;90:695–705. doi: 10.1016/s0092-8674(00)80530-9. [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- van Leeuwen F, Samos CH, Nusse R. Biological activity of soluble wingless protein in cultured Drosophila imaginal disc cells. Nature. 1994;368:342–344. doi: 10.1038/368342a0. [DOI] [PubMed] [Google Scholar]
- Warrior R. Primordial germ cell migration and the assembly of the Drosophila embryonic gonad. Dev Biol. 1994;166:180–194. doi: 10.1006/dbio.1994.1306. [DOI] [PubMed] [Google Scholar]
- Yanagawa S, Lee JS, Haruna T, Oda H, Uemura T, Takeichi M, Ishimoto A. Accumulation of Armadillo induced by Wingless, Dishevelled, and dominant-negative Zeste-White 3 leads to elevated DE-cadherin in Drosophila clone 8 wing disc cells. J Biol Chem. 1997;272:25243–25251. doi: 10.1074/jbc.272.40.25243. [DOI] [PubMed] [Google Scholar]