Abstract
Lithium has been used clinically to treat bipolar disorder for over half a century, and remains a fundamental pharmacological therapy for patients with this illness. Although lithium’s therapeutic mechanisms are not fully understood, substantial in vitro and in vivo evidence suggests that it has neuroprotective/neurotrophic properties against various insults, and considerable clinical potential for the treatment of several neurodegenerative conditions. Evidence from pharmacological and gene manipulation studies support the notion that glycogen synthase kinase-3 inhibition and induction of brain-derived neurotrophic factor-mediated signaling are lithium’s main mechanisms of action, leading to enhanced cell survival pathways and alteration of a wide variety of downstream effectors. By inhibiting N-methyl-D-aspartate receptor-mediated calcium influx, lithium also contributes to calcium homeostasis and suppresses calcium-dependent activation of pro-apoptotic signaling pathways. In addition, lithium decreases inositol 1,4,5-trisphosphate by inhibiting phosphoinositol phosphatases, a process recently identified as a novel mechanism for inducing autophagy. Through these mechanisms, therapeutic doses of lithium have been demonstrated to defend neuronal cells against diverse forms of death insults and to improve behavioral as well as cognitive deficits in various animal models of neurodegenerative diseases, including stroke, amyotrophic lateral sclerosis, fragile X syndrome, as well as Huntington’s, Alzheimer’s, and Parkinson’s diseases, among others. Several clinical trials are also underway to assess the therapeutic effects of lithium for treating these disorders. This article reviews the most recent findings regarding the potential targets involved in lithium’s neuroprotective effects, and the implication of these findings for the treatment of a variety of diseases.
Keywords: apoptosis, brain-derived neurotrophic factor (BDNF), glycogen synthase kinase 3 (GSK-3), lithium, neurodegenerative diseases, neuroprotection
1. Introduction
Lithium, a monovalent cation, has been the standard pharmacological treatment for bipolar disorder (BD) for more than 60 years. It remains recommended by many treatment guidelines as the first-line treatment against acute mania, and prophylactically for recurrent manic and depressive episodes. Clinically, lithium has been used adjunctively with other mood stabilizers, antidepressants, and antipsychotic medications to facilitate, enhance, or prolong both treatment response and remission (Goodwin, 2003; Lin et al., 2006). Lithium also has strong anti-suicidal properties (Tondo & Baldessarini, 2009). The clearance of lithium is exclusively dependent on renal excretion as a free ion and is considered to be decreased with aging (Grandjean & Aubry, 2009). Although lithium has a narrow therapeutic margin and well-known adverse effects, it is safe to use in the therapeutic dose range. Several minor side effects may occur at serum levels of lithium ranging from 0.6 to 1.2 mEq/L that have been demonstrated to be efficacious in the treatment of BD (Moscovich, 1993; Speirs & Hirsch, 1978). Symptoms associated with serum levels above 1.5 mEq/L are generally mild, including tremor, nausea, diarrhea, vertigo, and confusion (American Psychiatric Association, 2002). Nonetheless, lithium levels at 1.2 mM or higher in the plasma rarely cause persistent neurological deficits (Chen et al., 2004b). Lithium does not appear to be carcinogenic or mutagenic, but may lead to renal and liver damage at prolonged exposures to serum levels of 2 mM or more (American Psychiatric Association, 2002; Gould et al., 2003; Mazlo et al., 1983). Patients may experience more severe neurological complications such as seizures, coma, cardiac dysrrhythmia, and permanent neurological impairment with plasma levels of lithium greater than 2.5 mEq/L (American Psychiatric Association, 2002). Therefore, regular monitoring of serum concentrations is essential, particularly in elderly patients with lower clearance or preexisting neurological illness, to ensure optimal clinical efficacy and minimal adverse effects.
Given its long history of clinical use (Jope, 1999a), multiple actions associated with lithium’s mood stabilizing effects have been recognized; nevertheless, the precise underlying biochemical mechanisms of this drug remain poorly defined. In addition, clinical use of lithium for the treatment of BD has declined in recent years due to its narrow therapeutic range and to the availability of alternative medications. However, the last decade has also seen significant attention focused on lithium’s neurotrophic and neuroprotective effects. Loosely defined, neurotrophic effects encompass therapeutic strategies intended to augment proliferation, differentiation, growth, and regeneration. In contrast, neuroprotective effects are defined as those that halt or slow the progression of neuronal atrophy or cell death following the onset of insult or disease. Indeed, due to lithium’s remarkable neuroprotective and neurotrophic properties, considerable research has been conducted on its efficacy as a novel therapeutic in various disease models.
Lithium is the lightest of all metals, with a density only half that of water. It induces multiple biochemical and molecular effects on neurotransmitter receptor-mediated signaling, signal transduction cascades, hormonal and circadian regulation, ion transport, and gene expression. These effects have been widely associated with the activation of neurotrophic pathways, and neuroprotection has been the most expected and replicated biological effect associated with lithium use in both human and preclinical studies. Growing evidence suggests that lithium has neuroprotective effects against a variety of insults, including glutamate-induced excitotoxicity, ischemia-induced neuronal damage, and other neurodegenerative conditions. Lithium’s beneficial effects normally require long-term treatment to become evident, and are not immediately reversed after discontinuation of the drug. Therefore, it has been suggested that the therapeutic actions of lithium may involve signaling pathway and gene expression alterations in the central nervous system (CNS). In fact, recent research has recognized prominent molecular and cellular targets associated with lithium’s neuroprotective effects. These include its ability to inhibit intracellular signaling kinases and phosphatases, to protect against apoptosis induced by a variety of insults in cultured neurons and neurally related cell lines, to affect transcriptional activity and gene expression, and to promote cell proliferation and likely neurogenesis in the CNS. This article reviews the most recent findings regarding these potential targets involved in lithium’s neuroprotective effects, and the implication of these findings for the treatment of a variety of diseases. Lithium is already FDA-approved for the treatment of BD; our conclusions support the notion that its clinical relevance can be expanded to include the treatment of several neurological and neurodegenerative-related diseases.
2. Mechanisms of lithium’s neuroprotective actions
2.1 Lithium as a multi-functional neuroprotectant
Initial hypotheses that lithium has neuroprotective and neurotrophic effects were based on observations that chronic lithium treatment at therapeutic concentrations increases m3-muscarinic acetylcholine receptor-mediated second messenger production and c-Fos and m3-receptor expression in cultured rat cerebellar granule cells (CGCs) (Gao et al., 1993). In addition, lithium also increases the activity of two prominent transcription factors—activator protein-1 (AP-1) and cyclic AMP-response element binding protein (CREB)—in the same CGC cultures and in distinct brain areas (e.g., frontal cortex, amygdala, hippocampus, and cerebellum) of rats (Ozaki & Chuang, 1997). Although AP-1 activation may be either anti-apoptotic or pro-apoptotic depending on the nature of AP-1 binding components and the target genes induced (Karin, 1998; Mummery, 1975), it has been suggested that m3-muscarinic receptors and the DNA binding activities of AP-1 and CREB play prominent roles in regulating cell viability. Earlier pioneering studies noted that lithium promotes survival of gamma aminobutyric acid-releasing (GABAergic) neurons (Volonte et al., 1994) and mature CGCs (D’Mello et al., 1994), and accumulating evidence from various laboratories supports the view that lithium protects against diverse forms of death insults, suggesting that it is a multifunctional neuroprotectant.
Glutamate-induced excitotoxicity in discrete brain areas has been implicated in a variety of neurodegenerative diseases such as stroke, Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), brain trauma, cerebellar degeneration, spinal cord injury, and possibly Alzheimer’s disease (AD) and Parkinson’s disease (PD) (Friedlander, 2003; Mattson & Kroemer, 2003; Yuan & Yankner, 2000). Therefore, the neuroprotective effects of lithium against glutamate-induced excitotoxicity have been extensively studied in various cellular and animal models. In addition to glutamate excitotoxicity, lithium can also protect against other forms of insults in CNS neurons and neurally related cell lines (Chuang, 2004; 2005; Chuang et al., 2002; Chuang & Priller, 2006; Rowe & Chuang, 2004; Rowe et al., 2007). For example, lithium also protects against endoplasmic reticulum (ER) stress (Chen et al., 1999; Hiroi et al., 2005). The ER is the primary site for protein synthesis, folding, and trafficking. It also acts as an intracellular calcium repository and regulates calcium signaling. The ER is highly sensitive to perturbation of its intralumenal environment, and ER dysfunction has been linked to impaired synaptic plasticity and pathophysiology of diseases such as BD (Hough et al., 1999), AD (Mattson et al., 2000), and cerebral ischemia (Mattson et al., 2000). Thus, the neuroprotective effects of mood stabilizers against ER stress may also be clinically relevant.
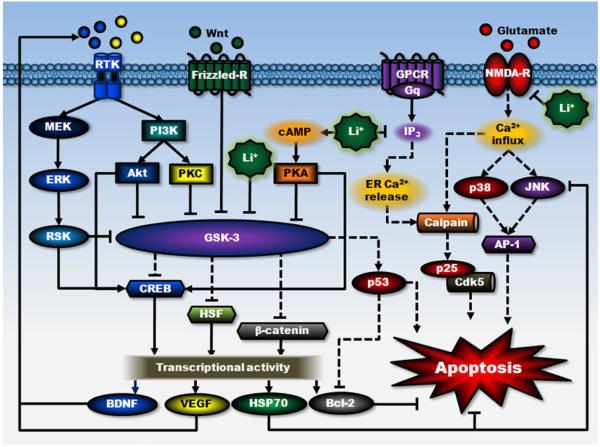
The pathogenesis of a number of diseases involves aberrant cell death processes (Chuang et al., 2005; Ekshyyan & Aw, 2004). Cell death can occur via two morphologically distinct processes: apoptosis and necrosis. Increasing lines of evidence support the notion that excessive cell death may be involved in certain forms of neuropsychiatric illness and multiple forms of neurodegenerative diseases, such as stroke, AD, HD, and PD (Mattson, 2006; Shacka & Roth, 2005). In cultured neurons and neurally related cell lines, lithium has been shown to have neuroprotective effects against apoptosis induced by a variety of insults such as growth factor withdrawal (Bhat et al., 2000), β-amyloid (Aβ) (Alvarez et al., 2002), colchicine (Jorda et al., 2004; 2005), high potassium deprivation (D’Mello et al., 1994), heat shock exposure (Bijur et al., 2000), and supra-therapeutic concentrations of anticonvulsants (phenytoin and carbamazepine) (Nonaka et al., 1998b), in addition to glutamate-induced excitotoxicity. Figure 1 illustrates the proposed multiple signaling pathways and mechanisms of actions involved in the neuroprotective effects of this drug.
Figure 1. An overview of proposed signaling mechanisms underlying lithium’s neuroprotective effects.
The neuroprotective effects of lithium against glutamate excitotoxicity are proposed to result from its interactions with cell survival and apoptotic machinery, as well as inhibition of receptor-mediated calcium entry. First, lithium can directly and indirectly reduce the activity of constitutively activated GSK-3 by multiple mechanisms, leading to disinhibition of several transcription factors, including CREB, HSF-1, and β-catenin, and subsequent induction of major cytoprotective proteins such as BDNF, VEGF, HSP70, and Bcl-2. GSK-3 is negatively regulated by Wnt-stimulated activation of the Frizzled receptor and decreased GSK-3 activity further reduces the activity of pro-apoptotic protein p53 and its downregulating effect on Bcl-2. Second, lithium-induced neurotrophic factors such as BDNF, in turn, activates its cell surface receptor and the downstream PI3K/Akt and MEK/ERK pathways. Both pathways are strongly associated with neuroprotective effects, which stimulate CREB and inhibit GSK-3. BDNF induction is an early and essential step for neuroprotection against glutamate excitotoxicity and may contribute to lithium-induced neurogenesis. Lithium also indirectly inhibits GSK-3 activity via PI3K-dependent activation of PKC and cAMP-dependent activation of PKA. Third, lithium inhibits NMDA receptor-mediated calcium influx, which in turn decreases subsequent activation of JNK, p38 kinase, and transcription factor AP-1. This NMDA receptor-mediated signaling plays a critical role in mediating glutamate-induced caspase activation and apoptosis. JNK activity is also inhibited by overexpression of HSP70. In addition, through inositol depletion, lithium reduces IP3-mediated calcium release from the ER. Inhibition of intracellular calcium increase not only suppresses cellular stress, but also reduces the activity of calpain and calpain-mediated activation of pro-apoptotic Cdk5/p25 kinase. Lines with solid arrows represent stimulatory connections; lines with flattened ends represent inhibitory connections. Dashed lines represent pathways with reduced activity as a result of lithium treatment. Frizzled-R, Frizzled receptor; GPCR, G protein-coupled receptor; NMDA-R, NMDA receptor; RTK, receptor tyrosine kinase.
2.2 Inositol depletion
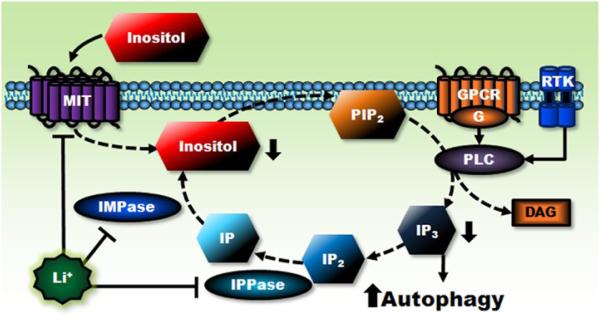
The cleavage of membrane inositol phospholipids, particularly phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylglycerol and inositol 1,4,5-trisphosphate (IP3), is necessary for cell surface G protein-coupled receptors, as well as certain receptor tyrosine kinases, to convey signals intracellularly (Berridge et al., 1989; Fisher et al., 1987; Phiel & Klein, 2001). At therapeutic concentrations, lithium is a powerful inhibitor of various phosphoinositol phosphatases involved in inositol phosphate metabolism, such as inositol polyphosphate 1-phosphatase (IPPase) and inositol monophosphatase (IMPase) (Berridge et al., 1989; Gould et al., 2004c; Quiroz et al., 2004; Sherman et al., 1986). Accordingly, lithium blocks the recycle of inositol for the re-synthesis of inositol phospholipids (Fig. 2). This inhibitory action of lithium on inositol phosphate metabolism has led to the inositol depletion hypothesis of lithium’s action, which posits that lithium’s therapeutic effects may result from interfering with IP3-mediated cell signaling caused by inositol depletion (Berridge et al., 1989). Via this inositol depletion mechanism, lithium was indeed found to inhibit the collapse of sensory growth cones in cultured sensory neurons and to increase these cone area (Williams et al., 2002); it was also found to suppress pilocarpine-induced reciprocal hind limb scratching in mice (Martinez & Raffa, 2002). Although several experiments found that the neuroprotective effects of lithium could not be mimicked by other potent IMPase inhibitors or prevented by excess inositol (Centeno et al., 1998; Nonaka et al., 1998a; 1998b), the ability of lithium to deplete free inositol was recently identified as a novel pathway for inducing autophagy (see section 2.11 below).
Figure 2. Lithium’s actions on inositol depletion and autophagy induction.
Extracellular signal binding to its cell surface receptor, either GPCR or RTK, activates phospholipase C (PLC), which hydrolyzes the phospholipid PIP2 to yield second messengers IP3 and diacylglycerol (DAG). IP3 is recycled by enzymes IPPase and IMPase and converted to inositol (mainly myo-inositol), which is required for PIP2 re-synthesis. Lithium decreases intracellular inositol levels by directly inhibiting IPPase, IMPase, and inositol transporter (MIT) that uptakes extracellular inositol. Decreased intracellular inositol levels are expected to subsequently reduce PIP2 and prevent the formation of IP3 and DAG, thus blocking transmembrane signaling and trigging the induction of autophagy. Lines with solid arrows represent stimulatory connections; lines with flattened ends represent inhibitory connections. Dashed lines represent pathways with reduced activity as a result of lithium treatment. DAG, diacylglycerol; IP, inositol monophosphate; IP2, inositol bisphosphate; MIT, inositol transporter; PLC, phospholipase C.
2.3 Inhibition of NMDA receptor-mediated signaling
Glutamate-induced excitotoxicity was found to be mediated by N-methyl-D-aspartate (NMDA) receptors, and was robustly reduced by chronic lithium treatment in cultured CNS neurons including rat CGCs, cerebral cortical, and hippocampal neurons (Nonaka et al., 1998a), partly via the inhibition of NMDA receptor-mediated calcium influx. This long-lasting neuroprotective effect, occurring at therapeutically relevant concentrations of lithium (EC50 ≈ 1 mM), is time-dependent and requires six to seven days of pretreatment for maximum efficacy. This neuroprotection is also specific to lithium, since other monovalent ions including rubidium and cesium, as well as classic antidepressants such as imipramine, desipramine, clomipramine, and fluoxetine are ineffective (Hashimoto et al., 2002a). Furthermore, these neuroprotective effects appears to occur independently of lithium’s inhibition of IMPase, given that co-addition of excessive myo-inositol fails to reverse lithium’s neuroprotective effects (Nonaka et al., 1998b).
Levels of Tyr1472-phosphorylation of the NR2B subunit are positively correlated with NMDA receptor-mediated synaptic activity and excitotoxicity. Studies indicate that the mechanism of action underlying lithium’s ability to inhibit NMDA receptor-mediated calcium influx results from the attenuation of constitutive phosphorylation at Tyr1472 of the NR2B subunit of the NMDA receptor, which is catalyzed by Fyn, a member of the Src tyrosine kinase family (Hashimoto et al., 2002a; 2003a). Neither total tyrosine protein kinase activity nor that of tyrosine protein phosphatase is affected by this drug, indicating the selectivity of the modulation and its contribution to neuroprotection. In addition, lithium has also been shown to reduce excitotoxicity-related brain damage following ischemic events. Brain ischemia increases Src-mediated tyrosine phosphorylation of NR2A (Liu et al., 2001; Takagi et al., 1997), and the interaction of NR2A with Src and Fyn, which is mediated by postsynaptic density protein 95 (PSD-95) (Hou et al., 2002). Lithium blocks both the ischemia-induced increases in NR2A phosphorylation and PSD-95 interaction (Ma & Zhang, 2003). This suggests that chronic lithium treatment may be beneficial in treating brain damage induced by hypoxic insult. However, glutamate-induced excitotoxicity in cultured cortical neurons, that is diminished by treatment with either lithium or MK-801, can be blocked only partially by a Src kinase inhibitor, SU6656 (Hashimoto et al., 2003a). Lithium’s neuroprotective effects against glutamate excitotoxicity also involve the blockade of apoptotic components and will be discussed in the following section (see section 2.4).
2.4 Anti-apoptotic actions
2.4.1 Suppression of the JNK/p38 MAP kinase pathways
The c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein (MAP) kinase are activated by site-specific phosphorylation in response to a variety of apoptotic insults (Mielke & Herdegen, 2000). These two kinases often act synergistically to enhance the DNA binding activity of AP-1, a dimeric transcription factor consisting of the Jun, Fos, CREB, and ATF subunits (Shaulian & Karin, 2002; Whitmarsh & Davis, 1996). AP-1 is also activated by a wide variety of stress factors and other cellular signals. Glutamate-induced, NMDA receptor-mediated apoptotic death in cultured rat CGCs requires the activation of both JNK and p38 MAP kinase, subsequently leading to a robust increase in AP-1 binding before apoptotic death (Chen et al., 2003). These glutamate-induced signaling events and apoptosis are prevented by long-term (seven days) treatment with therapeutic concentrations of lithium (0.5-2 mM) (Chen et al., 2003).
2.4.2 Downregulation of pro-apoptotic p53, Bax, caspase, and cytochrome c release
In cultured rat CGCs, glutamate-induced downregulation of the cytoprotective B-cell lymphoma/leukemia-2 (Bcl-2) protein and upregulation of pro-apoptotic proteins such as p53 and Bax are concentration-dependently reversed by long-term, but not acute, lithium pretreatment (Chen & Chuang, 1999). Studies using p53 inhibitors have implicated p53 in neuronal death induced by Aβ, ischemic and excitotoxic insults, and N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a neurotoxin commonly used to reproduce PD-like phenotypes in animals (Culmsee et al., 2001; Duan et al., 2002). Glutamate exposure also triggers the release of cytochrome c from the mitochondria into the cytosol. Lithium pretreatment blocks glutamate-induced cytochrome c release and cleavage of lamin B1, a nuclear substrate for caspase-3 (Chen & Chuang, 1999). These results suggest that lithium-induced downregulation of p53 and Bax plays a prominent role in neuroprotection against excitotoxicity.
2.4.3 Inhibition of calpain/Cdk5 pathway
Cyclin-dependent kinase 5 (Cdk5), a serine/threonine kinase essential for brain development, is the only cyclin-dependent kinase not related to cell cycle regulation (Nguyen et al., 2002). Cdk5 also regulates NMDA receptor-mediated signaling by direct phosphorylation of the NR2B subunit or indirectly through phosphorylation of PSD-95 (Morabito et al., 2004; Zhang et al., 2008). Cdk5 activity is primarily regulated by its co-activator p35. However, when it binds to p25, the product of calpain-mediated cleavage of p35, Cdk5 will become pro-apoptotic and its activity becomes dysregulated (Camins et al., 2006; Lee et al., 2000; Patrick et al., 1999). Calpain is a non-lysosomal calcium-dependent intracellular cysteine protease, and uncontrolled calpain activity has been implicated in an increasing number of pathological conditions including ischemic brain injury, AD, multiple sclerosis, and PD (Camins et al., 2009; Wang, 2000). Accordingly, p25 accumulation was observed in neurons in response to glutamate or oxidative stress, and in the brain of several animal models of neurodegenerative diseases. In addition, sustained activation of Cdk5 in neurons is believed to be involved in many neurodegenerative diseases (Cruz & Tsai, 2004; Dhariwala & Rajadhyaksha, 2008). In cultured rat CGCs, lithium pretreatment prevents colchicine-induced apoptosis and associated increases in Cdk5 expression and p35 to p25 fragmentation (Jorda et al., 2005).
In addition, intracellular calcium increase, calpain activity, Cdk5 activation, and cellular death induced by 3-nitropropionic acid (3-NP), a succinate dehydrogenase inhibitor used to induce striatal pathology similar to that observed in HD (Brouillet et al., 1999), are also reduced by pretreatment with lithium in cultured primary brain neurons (Crespo-Biel et al., 2009). In rats, lithium treatment reduces 3-NP-induced striatal neurodegeneration by preventing calpain activation and subsequent increases in Cdk5 activity (Crespo-Biel et al., 2009). These results indicate that calpain and Cdk5 are involved in lithium’s neuroprotective effects, and suggest that this drug is a good alternative for modulating calpain action in different pathological disorders (Camins et al., 2009).
2.5 Induction of survival pathways
Apoptosis is also regulated by survival signaling pathways. Stimulation of cell-surface trophic factor receptors such as tyrosine receptor kinase B (TrkB), the receptor of brain-derived neurotrophic factor (BDNF) (Huang & Reichardt, 2003), insulin, and other growth factors, activates multiple survival pathways including the phosphoinositide 3-kinases (PI3K)/Akt pathway (Brunet et al., 2001; Franke et al., 2003) and the MAP kinase kinase (MEK)/extracellular-signal regulated kinase (ERK) pathway (Chang et al., 2003; Segal & Greenberg, 1996).
2.5.1 The PI3K/Akt pathway
Akt is a serine/threonine kinase regulated by PI3K-mediated signaling. Activation of Akt involves phosphorylation at residues of Thr308 and Ser473 (Alessi & Cohen, 1998; Jacinto et al., 2006). In cultured rat CGCs, lithium treatment rapidly normalizes glutamate-induced inactivation of Akt by activating PI3K and subsequently increasing the phosphorylation of Akt at Ser473 (Chalecka-Franaszek & Chuang, 1999). In turn, activated Akt affects on several anti-apoptotic targets including Bcl-2 associated death promoter (BAD) (a Bcl-2 family member), CREB, members of the forkhead family, and procaspase 9 (Huang & Reichardt, 2003; Neri et al., 2002; Nicholson & Anderson, 2002). In addition, this pathway also mediates the indirect inhibitory effects of lithium on glycogen synthase kinase-3 (GSK-3) isoform α through enhanced Ser21 phosphorylation (Chalecka-Franaszek & Chuang, 1999) (see section 2.6 and Fig. 3). However, the effects of lithium on the PI3K/Akt pathway may be cell-type-specific and time-dependent, as some studies in certain cell lines detected no changes in Akt phosphorylation levels at specific time points following lithium application (De Sarno et al., 2002; Zhang et al., 2003a).
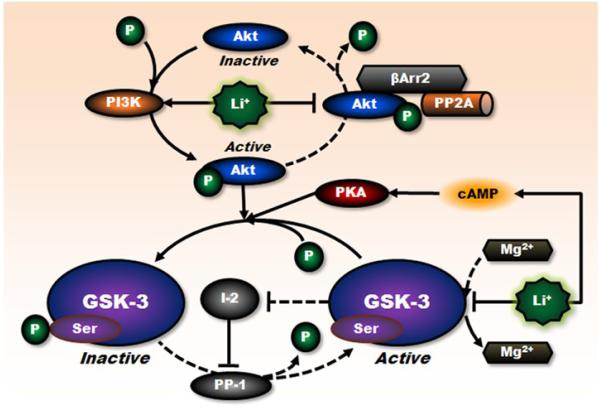
Figure 3. Inhibitory regulation of GSK-3 activity by lithium.
Lithium regulates the activity of constitutively activated GSK-3 through multiple mechanisms. First, lithium is a competitive inhibitor of magnesium that directly inhibits ATP-magnesium-dependent catalytic activity of GSK-3. Second, lithium can indirectly increase serine phosphorylation of GSK-3 through PI3K-mediated phosphorylation/activation of Akt. The activity of GSK-3 is reduced by phosphorylation at this specific serine residue. Third, lithium can also disrupt the formation of the βArr2/PP2A/Akt complex that dephosphorylates/inactivates Akt, thereby increasing the serine phosphorylation of GSK-3. In addition, lithium can negatively regulate GSK-3 activity through other protein kinases including cAMP-dependent activation of PKA and PI3K-mediated activation of PKC (not shown), and through other mechanisms including downregulation of GSK-3 (not shown). Moreover, direct inhibition of GSK-3 by lithium interrupts the auto-regulation of GSK-3, by disinhibiting the inhibitory action of inhibitor-2 (I-2) on protein phosphatase-l (PP-1) that dephosphorylates GSK-3 at serine residues, and further decreases GSK-3 activity. Lines with solid arrows represent stimulatory connections; lines with flattened ends represent inhibitory connections. Dashed lines represent pathways with reduced activity as a result of lithium treatment. I-2, inhibitor-2; PP-1, protein phosphatase-l.
2.5.2 The MEK/ERK pathway
The second signaling pathway affected by lithium is the MEK/ERK pathway. ERK regulates several effector systems such as the NF-κB pathway, and ribosomal S6 kinase (RSK) that in turn activates CREB and inhibits GSK-3β (Chang et al., 2003; Steelman et al., 2004). CREB, a transcription factor involved in learning and memory, plays a major role in mediating adaptive responses at glutamatergic synapses and cell survival by promoting the expression of cell-protective proteins such as BDNF and Bcl-2 (Finkbeiner, 2000). In CGCs, toxic concentrations (≥ 50 μM) of glutamate induce a rapid increase in MEK activity and NMDA receptor-dependent decreases in CREB phosphorylation at Ser133, as well as CREB-driven transcriptional activity (Kopnisky et al., 2003); these latter processes are mediated by protein phosphatase 1. Commensurate with its neuroprotective effects, long-term but not acute lithium treatment potentiates glutamate-induced increases in MEK activity and suppresses glutamate-induced dephosphorylation of CREB, presumably by inhibiting protein phosphatase 1 as well as promoting MEK/ERK activity. It should be noted that lithium’s effects on the MEK/ERK pathway may also be cell-type-specific, as lithium has been reported to have opposite effects on this pathway in different types of neural cells (Pardo et al., 2003).
2.6 Inhibition of GSK-3
GSK-3, a serine/threonine protein kinase, is a down-stream mediator of signaling pathways induced by various stimuli and is involved in a diverse array of cellular functions. In general, GSK-3 is pro-apoptotic and considered to be constitutively active under non-stimulated basal conditions. Growing evidence in the literature also indicates that GSK-3 is a major regulator of inflammation. Dysfunction of this enzyme has been implicated in the pathophysiology of mood disorders, schizophrenia, AD, diabetes, cancer, inflammatory and autoimmune diseases, among others (Beurel et al., 2010; Huang & Klein, 2006; Jope et al., 2007; Meijer et al., 2004). GSK-3 is evolutionarily conserved, with α and β isoforms. The main structural differences between them lie in the N- and C-terminal regions, while their sequences within the kinase domain are highly homologous. GSK-3 inhibition has recently attracted widespread attention as one of the critical therapeutic targets whereby lithium exerts its effects on mood stabilization, neurogenesis, neurotrophicity, neuroprotection, anti-inflammation, and others (Beurel et al., 2010; Rowe & Chuang, 2004; Rowe et al., 2007).
GSK-3 activity is regulated by a wide variety of kinases and systems including Akt, protein kinase A (PKA), protein kinase C (PKC), MAP kinases, and the Wnt pathway. Phosphorylation of GSK-3 α/β at a specific 21/9 serine residue negatively regulates its activity, while phosphorylation at a specific 279/216 tyrosine residue positively regulates its activity (Jope, 2003; Yukimasa et al., 2001). As a competitive inhibitor of magnesium, lithium was found to directly inhibit the adenosine triphosphate (ATP)-magnesium-dependent catalytic activity of GSK-3 (Klein & Melton, 1996; Ryves & Harwood, 2001; Stambolic et al., 1996) (Fig. 3). At concentrations similar to those in human plasma after therapeutic administration, lithium also indirectly inhibits GSK-3 activity through enhanced phosphorylation of GSK-3α at Ser21 and GSK-3β at Ser9. Multiple mechanisms have been identified which contribute to this effect, including the involvement of the 3′,5′-cyclic adenosine monophosphate (cAMP)-dependent activation of PKA (Jope, 1999a; 1999b), PI3K-dependent activation of PKC (Kirshenboim et al., 2004), and Akt (Chalecka-Franaszek & Chuang, 1999), as well as the auto-regulation resulting from enhanced inhibition of protein phosphatase-l through the action of inhibitor-2 complex (Zhang et al., 2003a). In general, lithium raises basal levels, but suppresses stimulus-induced increases, in cAMP and PKA activity. This bimodal action has been proposed as the biochemical basis underlying lithium’s antidepressant and antimanic efficacy (Jope, 1999b).
Moreover, β-arrestin 2 (βArr2), a scaffolding protein associated with receptor desensitization and the termination of G protein-coupled receptor signaling (Ferguson et al., 1996; Lohse et al., 1989), is essential for regulating the Akt/GSK-3 signaling cascade (Beaulieu et al., 2005; 2007), and plays an important role in the expression of behavioral responses to drugs acting on dopamine neurotransmission (Beaulieu et al., 2005; Gainetdinov et al., 2004). In response to the stimulation of the dopamine D2 receptor, Akt can be dephosphorylated/inactivated by the formation of a complex composed with βArr2 and protein phosphatase 2A (PP2A), consequently resulting in the concomitant activation of GSK-3β (Beaulieu et al., 2005). By disrupting this βArr2 signaling complex, lithium was found to indirectly inhibit GSK-3 by activated Akt and induce behavioral changes associated with GSK-3 inhibition in mice (Beaulieu et al., 2008). Moreover, recent studies have discovered that lithium has novel effects on GSK-3 regulation. For example, GSK-3β transcription can be decreased by lithium treatment in vitro and in vivo (Mendes et al., 2009). In addition, the kinase activity of GSK-3 can be upregulated through its N-terminal cleavage by calpain (Goni-Oliver et al., 2007), a calcium dependent protease that is negatively regulated by lithium as described above.
Because GSK-3 activation has been linked to apoptotic cell death induced by multiple insults including glutamate excitotoxicity (Grimes & Jope, 2001), lithium’s inhibition of GSK-3 undoubtedly constitutes part of the molecular mechanisms underlying its neuroprotective effects. Isoform-specific small interfering RNAs (siRNAs) that selectively silence the expression of targeted GSK-3 have recently been designed to distinguish the functional and regulatory differences between these two GSK-3 isoforms (Liang & Chuang, 2007). In cultured rat cerebral cortical neurons, RNA interference-induced depletion of either isoform is sufficient to block glutamate-induced excitotoxicity. Moreover, transfection with isoform-specific dominant-negative mutants of GSK-3 or treatment with other non-selective pharmacological GSK-3 inhibitors mimics lithium-induced neuroprotection against glutamate excitotoxicity. Although GSK-3α and GSK-3β may have distinct roles in transcriptional regulation and cell survival (Liang & Chuang, 2006; 2007), these results strongly suggest that both isoforms of GSK-3 are involved in the execution of glutamate-induced neuronal death and that both isoforms are initial targets of lithium-induced neuroprotection. However, the development of GSK-3 isoform-specific inhibitors remains crucial for therapeutic interventions in GSK-3-related neuropathological conditions and reductions of unwanted side effects.
Substrates phosphorylated by GSK-3 include metabolic, signaling, and structural proteins as well as transcription factors. The DNA binding activity of AP-1 is also reduced by GSK-3β-dependent phosphorylation of c-Jun (Boyle et al., 1991). Dysfunction of GSK-3-mediated phosphorylation of transcription factors is believed to be related to the pathophysiology of various pathological conditions. Inhibition of GSK-3 leads to activation of cell-survival transcription factors such as CREB, AP-1, β-catenin, heat-shock factor-1 (HSF-1) (Bijur & Jope, 2000), and inhibition of pro-apoptotic factors such as p53 (Grimes & Jope, 2001; Jope, 2003; Jope & Roh, 2006). Many of these effector systems in turn regulate members of the Bcl-2 protein family, thus serving as an intersection between survival and death receptors. Pharmacological inhibition of GSK-3 is also likely involved in the antidepressant and antimanic effects of lithium observed in rodent models (Beaulieu et al., 2004; Gould et al., 2004b; Kaidanovich-Beilin et al., 2004; O’Brien et al., 2004), whereas overexpression of this kinase in mice produces behavioral correlates of hyperactivity and mania (Prickaerts et al., 2006). These findings suggest GSK-3 as the therapeutic target of lithium in BD treatment (see section 3.1).
2.7 Stabilizing β-catenin
The transcription factor β-catenin is a substrate of GSK-3 and is part of the Wnt pathway. Its cytoplasmic levels are negatively regulated by constitutively active GSK-3 as described above. After being phosphorylated by GSK-3, β-catenin undergoes proteasomal degradation (Jope & Johnson, 2004; Takahashi-Yanaga & Sasaguri, 2007). Increases in cytoplasmic accumulations of β-catenin facilitate its translocation into the nucleus and, in conjunction with T-cell-specific transcription factor (Tcf)/lymphoid enhancer binding factor (Lef), subsequently enhance the transcription of diverse genes such as growth factors (Silva et al., 2007; Sinha et al., 2005), and those involved in apoptotic inhibition (Feng, 1979; Huelsken & Behrens, 2002; Seidensticker & Behrens, 2000).
As a result, elevating β-catenin has been suggested as a novel therapeutic strategy for treating mood disorders. As expected, treatment with therapeutic concentrations of the GSK-3 inhibitor lithium increases β-catenin levels both in vitro (Chen et al., 1999; Stambolic et al., 1996) and in vivo (Gould et al., 2004a; O’Brien et al., 2004), and promotes β-catenin-dependent transcriptional events (Jope & Johnson, 2004; Marmol, 2008; O’Brien et al., 2004). In addition, overexpression of β-catenin in mouse brain mimics the antidepressant-like effects of lithium (Gould et al., 2007), whereas knockdown of this protein in mice results in a depression-like phenotype (Gould et al., 2008). These results indicate that lithium-induced accumulation of β-catenin could be relevant to its neuroprotective and therapeutic effects.
2.8 Negative regulation of Smad3/4-dependent transcription
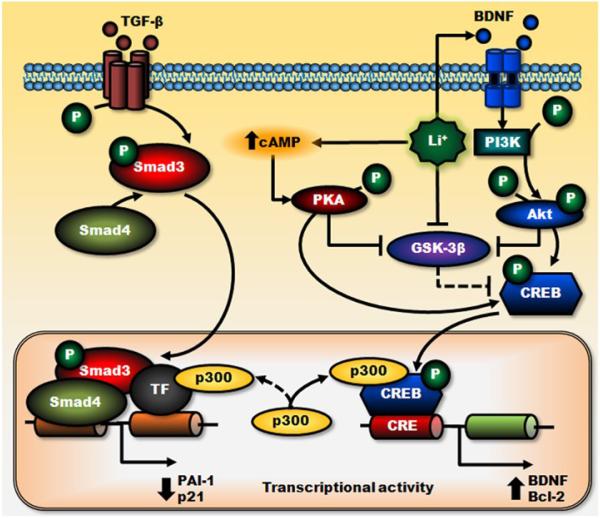
The transcription factor Smad3/4, a downstream mediator of the signaling pathway triggered by transforming growth factor-β (TGF-β) (Derynck & Zhang, 2003), plays a prominent role in regulating the expression of proteins involved in neuronal survival or death, differentiation, and synaptic plasticity (Gomes et al., 2005; Sanyal et al., 2004). The TGF-β signaling pathway has been implicated as a therapeutic target in neurodegeneration (Wyss-Coray, 2006). Smad3/4 was recently identified as a novel target for GSK-3 in neurons (Liang & Chuang, 2006). Using siRNA or dominant negative mutants specific to GSK-3 isoforms, GSK-3α inhibition was found to upregulate Smad3/4-dependent transactivation and protein levels of plasminogen activator inhibitor-1 (PAI-1), one of the Smad3/4-regulated protein targets (Liang et al., 2008); in contrast, GSK-3β inhibition downregulates the same processes (Liang & Chuang, 2006). Treating cultured cortical neurons with lithium at therapeutically relevant concentrations significantly decreases Smad3/4-dependent transactivation and protein levels of PAI-1 (Liang et al., 2008). Lithium’s effects on Smad3/4 likely result from the cross-talk between CRE-dependent, cAMP/PKA, and PI3K/Akt/GSK-3β signaling pathways and depend on increased CREB activation and subsequent complex formation with p300 (Liang et al., 2008) (Fig. 4). This suppression of Smad3/4-dependent transactivation by lithium may be beneficial, because elevated levels of PAI-1 have been associated with the pathogenesis of mood disorders (Eskandari et al., 2005; Higgins, 2006; Tsai, 2006).
Figure 4. Negative regulation of Smad3/4-dependent transcription by lithium.
Through stimulation of their cell surface receptors, TGF-β- and BDNF-triggered transcriptional activations are mediated by Smad3/4- and PI3K/Akt-dependent pathways, respectively. Lithium treatment reduces GSK-3β activity directly and indirectly via cAMP-dependent activation of PKA and BDNF-stimulated activation of PI3K/Akt pathways. These effects of lithium potentiate BDNF-induced phosphorylation/activation of CREB and increase cAMP response element (CRE)-mediated transactivation and expression of survival factors such as BDNF and Bcl-2. Increased BDNF-induced gene transcription causes sequestration of transcriptional co-activator p300, which suppresses Smad3/4-dependent transactivation and subsequently decreases the expression of TGF-β-responsive genes, PAI-1, and p21. Lines with solid arrows represent stimulatory connections; lines with flattened ends represent inhibitory connections. Dashed lines represent pathways with reduced activity as a result of lithium treatment. CRE, cAMP response element.
2.9 Altered AP-1 DNA binding activity
AP-1 activation can be either neuroprotective or neurodegenerative and in this regard plays a complex role in neuroprotection and apoptosis. As described above, DNA binding activity of the AP-1 complex can be positively or negatively regulated by the JNK/p38 pathway and GSK-3, respectively. Therefore, binding activity of AP-1 may be decreased via inhibition of the JNK/p38 pathway, and/or increased via inhibition of GSK-3 due to lithium treatment. Indeed, the fact that AP-1 can be either neuroprotective or neurodegenerative correlates well with evidence that lithium inhibits stimulus-induced AP-1 activity, but can also increase basal AP-1 activity (Asghari et al., 1998; Chen et al., 2003; Hiroi et al., 2005; Hongisto et al., 2003; Ozaki & Chuang, 1997; Song et al., 2002).
2.10 Induction of survival molecules
2.10.1 Bcl-2 upregulation
In contrast to pro-apoptotic proteins such as Bax and Bak, Bcl-2 is an anti-apoptotic protein that inhibits the release of cytochrome c from mitochondria by regulating the permeability of the mitochondrial outer membrane (Maiuri et al., 2007; Youle & Strasser, 2008). The ability to maintain calcium homeostasis in the ER is another cytoprotective action of Bcl-2 (He et al., 1997; Lam et al., 1994). Chronic lithium treatment has been found to induce Bcl-2 expression in the frontal cortex of rat brains (Chen et al., 1999) and in cultured CGCs (Chen et al., 1999; Chen & Chuang, 1999). In cultured neurons, lithium-induced Bcl-2 expression is paralleled by downregulation of the pro-apoptotic molecules p53 and Bax, and blockade of glutamate-induced cytochrome c release as well as caspase activation (Chen & Chuang, 1999). In PC12 cells, chronic lithium-induced upregulation of Bcl-2 is closely associated with the cytoprotective effects of this drug against Aβ peptide (Chen et al., 1999; Wei et al., 2000) and thapsigargin-induced ER stress (Chen et al., 1999; Hiroi et al., 2005). Notably, gene transfer-mediated Bcl-2 overexpression has been shown to protect against thapsigargin-induced apoptosis in neuronal cell lines (Wei et al., 1998) and increase neuronal survival in various animal models of neurodegenerative diseases such as stroke (Kitagawa et al., 1998), AD (Rohn et al., 2008), PD (Offen et al., 1998; Vila et al., 2001), and HD (Zhang et al., 2003c). These results strongly suggest that lithium-induced Bcl-2 upregulation plays a prominent role in lithium’s neuroprotective effects. It is interesting to note that chronic treatment with another mood stabilizing drug, valproate, a histone deacetylase inhibitor (Chuang et al., 2009; Gottlicher et al., 2001; Phiel et al., 2001) as well as an anticonvulsant drug that is often used in BD patients with poor response to lithium, also upregulates Bcl-2 in the brain of rats (Chen et al., 1999). MicroRNAs are 17-19 nucleotide non-protein coding RNAs that can inhibit the translation of their target genes. A recent study in SH-SY5Y cells shows that Bcl-2 translation is directly inhibited by the expression of a specific microRNA, miR-34a (Wang et al., 2009). Chronic treatment with lithium or valproate decreases the levels of several microRNAs in the hippocampus of rats as well as in the primary cultures of hippocampal neurons, including miR-34a (Zhou et al., 2009), suggesting a common regulator shared by these structurally dissimilar mood stabilizers and indicating a novel target for lithium’s effects.
2.10.2 BDNF upregulation
BDNF, one of the major neurotrophins, is essential for cortical development, synaptic plasticity, and neuronal survival, and is likely one of the mediators of the clinical efficacy of antidepressants and anxiolytics (Manji et al., 2003; Woo & Lu, 2006). The necessity of protracted lithium pretreatment in order for maximum neuroprotective effects to become apparent suggests the involvement of gene expression. Indeed, long-term treatment of cultured cortical neurons with lithium induces BDNF; this, in turn, activates its receptor, TrkB, by increasing phosphorylation at the Tyr490 residue (Hashimoto et al., 2002b). Without altering the expression of TrkB, chronic treatment of rats with lithium also increases protein levels of BDNF in various brain regions (Fukumoto et al., 2001; Jacobsen & Mork, 2004).
Recent studies in cultured cortical neurons further reveal that treatment with lithium or valproate at therapeutic concentrations for 48 hours selectively increases the levels of exon IV (formerly rat exon III)-containing BDNF mRNA, and the activity of BDNF promoter IV (Yasuda et al., 2009). Notably, this effect can be mimicked by pharmacological inhibition of GSK-3 or siRNA-mediated gene silencing of either the GSK-3α or GSK-3β isoform. In addition, lithium-induced neuroprotection against excitotoxicity can be prevented by a Trk tyrosine kinase inhibitor, K252a, or by a neutralizing antibody against BDNF (Hashimoto et al., 2002b). Lithium’s neuroprotective effects are also completely blocked by either heterozygous or homozygous knockout of the BDNF gene in cultured cortical neurons. Taken together, these results suggest that TrkB-stimulated upregulation of BDNF plays a central role in mediating many of the reported downstream effectors associated with lithium’s neuroprotective effects. This trophic action is likely involved in lithium-induced activation of survival PI3K/Akt and MEK/ERK pathways as described above. Through phosphorylation, activation of CREB, a common downstream target of both pathways, increases the expression of BDNF (Finkbeiner, 2000). Moreover, the findings that BDNF-induced antidepressant-like effects are blocked by either K252a or a MEK inhibitor, U0126 (Shirayama et al., 2002), further support the involvement of TrkB-mediated activation of the MEK/ERK pathway.
One of the BDNF-regulated transcription factors is forkhead box class O3a (FoxO3a). FoxO3a is a member of the mammalian FoxO family and plays a prominent role in regulating cell fate, differentiation, survival, and stress response (Greer & Brunet, 2005). BDNF-dependent activation of PI3K and Akt leads to phosphorylation of FoxO3a, resulting in redistribution of the transcription factor from the nucleus to the cytosol and a loss of transcriptional activity (Brunet et al., 1999). Levels of active FoxO3a are increased in the brain after ischemia (Fukunaga et al., 2005). Chronic lithium treatment of mice with a therapeutically-relevant dose decreases FoxO3a protein levels in the hippocampus (Mao et al., 2007). Interestingly, FoxO3a protein levels are decreased in both cytosol and nucleus to reduce FoxO3a transcriptional activity via an Akt-dependent mechanism. In light of a more recent report noting that FoxO3a-deficient mice display antidepressant-like behaviors (Polter et al., 2009), it is possible that lithium-induced downregulation of FoxO3a might contribute to both its neuroprotective and mood stabilizing effects.
2.10.3 VEGF upregulation
Lithium treatment also increases the expression of vascular endothelial growth factor (VEGF) in vitro and in vivo (Du et al., 2009; Guo et al., 2009; Kaga et al., 2006; Silva et al., 2007), an effect that most likely occurs via inhibition of GSK-3β and stabilization of β-catenin signaling. VEGF is widely expressed throughout the CNS and can function as a potent angiogenic/neurotrophic factor for multiple types of neurally related cells including astrocytes (Heine et al., 2005), neurons (Kutcher et al., 2004), and neuronal progenitor cells (Maurer et al., 2003). VEGF has been shown to promote cell proliferation (Jin et al., 2002), proneuronal differentiation of newly born cells (Meng et al., 2006), migration of immature neuroblasts (Zhang et al., 2003b), and neurovascular remodeling after stroke (Chen et al., 2005; Kaga et al., 2006). By upregulating VEGF, lithium treatment optimizes skeletal myoblast functions for cellular cardiomyoplasty in vitro (Du et al., 2009), prevents stress-induced reductions in VEGF levels (Silva et al., 2007), and promotes angiogenic and anti-apoptotic signaling in rat ischemic preconditioned myocardium (Kaga et al., 2006). These data support the notion that VEGF plays a role in the neuroprotective actions of lithium.
2.10.4 HSP70 upregulation
Heat shock proteins (HSPs) are a group of molecular chaperones that promote protein folding as well as refolding of misfolded proteins, inhibit aggregate formation, and facilitate degradation of abnormally folded proteins through the ubiquitin-proteasome system (Fink, 1999; Hartl & Hayer-Hartl, 2002; Hendrick & Hartl, 1993; Ma & Hendershot, 2001). Indeed, the induction of HSPs that help restore cellular homeostasis is one of the important regulators of cellular survival in response to stress or to the accumulation of misfolded proteins in cells (Lindquist, 1986). Studies have found that among HSPs, HSP70 exerts a wide variety of neuroprotective effects against apoptosis (Takayama et al., 2003). These occur by antagonizing apoptosis-inducing factors (Ravagnan et al., 2001), inhibiting the activation of NF-κB by stabilizing IκB protein (Feinstein et al., 1996; Yenari & Han, 2006), stabilizing Akt-1 protein (Gao & Newton, 2002), preventing mitochondrial cytochrome c release and caspase activation (Beere et al., 2000), and suppressing JNK activation (Mosser et al., 2000). In various animal models, overexpression of HSP70 has been recognized as a potential therapeutic target against ischemic neuronal injury (Hoehn et al., 2001; Majda et al., 2001; Rajdev et al., 2000; Tsuchiya et al., 2003).
The expression of HSP70 is regulated by HSF-1 (Bijur & Jope, 2000), a transcription factor negatively regulated by GSK-3β-dependent phosphorylation (Chu et al., 1996). Both DNA binding activity of HSF-1 and HSF-1-dependent transcription are negatively correlated with GSK-3β activity (Bijur & Jope, 2000; Xavier et al., 2000). In light of the fact that lithium’s inhibition of GSK-3 is associated with activation of HSF-1 (Bijur & Jope, 2000), upregulation of heat-shock response may be part of the neuroprotective mechanisms produced by lithium treatment. In fact, the neuroprotective effects of lithium in a stroke model are associated with a marked increase in the DNA binding activity of HSF-1 and subsequent elevations in the expression of HSP70 protein in the ischemic brain (Ren et al., 2003).
2.10.5 GRP78 upregulation
The 78 kDa glucose-regulated protein (GRP78) is a molecular chaperone of the HSP70 family that binds to calcium and protects cells from the deleterious effects of misfolded proteins in the ER (Katayama et al., 1999; Kaufman, 1999; Yu et al., 1999). Various apoptotic insults, including the ER calcium-ATPase inhibitor thapsigargin, induce the expression of GRP78 mRNA (Aoki et al., 1997; He et al., 2000). It has been suggested that transcription factor c-Fos is involved in the induction of GRP78 by thapsigargin, and that the c-Fos-dependent induction is likely triggered by thapsigargin-induced release of calcium from the ER (He et al., 2000). c-Fos associates with c-Jun to form a heterodimeric AP-1 transcription factor complex that regulates the expression of a large number of genes. However, regulation of GRP78 expression via the AP-1 complex seems to be indirect, given that no recognizable AP-1 interacting sequence motifs are present in the GRP78 promoter (He et al., 2000).
Lithium has been shown to induce c-Fos expression, AP-1 binding activity (Gao et al., 1993; Kalasapudi et al., 1990; Ozaki & Chuang, 1997), and upregulation of GRP78 (Hiroi et al., 2005, Shao et al., 2006; Wang et al., 2001). In PC12 cells, protracted lithium pretreatment is cytoprotective against thapsigargin-induced cytotoxicity resulting from ER stress (Hiroi et al., 2005). This protection is concomitant with attenuation of thapsigargin-triggered intracellular calcium release and upregulation of c-Fos and GRP78. Lithium alone does not affect basal calcium levels, suggesting that distinct mechanisms are likely involved in its ability to trigger c-Fos and GRP78 induction. Valproate pretreatment also upregulates this ER stress protein (Bown et al., 2000; Hiroi et al., 2005; Wang et al., 1999; 2001), and induces similar protective effects against ER stress in PC12 cells (Hiroi et al., 2005) and oxidative damages in primary cultured rat cerebrocortical cells (Wang et al., 2003). In addition, lithium pretreatment reverses thapsigargin-induced downregulation of the anti-apoptotic protein Bcl-2. Lithium’s cytoprotective effects against thapsigargin cytotoxicity are blocked by curcumin (Hiroi et al., 2005), an inhibitor of transcription factor AP-1; this suggests that induction of GRP78 and Bcl-2, as well as activation of AP-1, may contribute to lithium-induced protection against cytotoxicity resulting from ER stress.
2.10.6 tPA upregulation
Tissue-type plasminogen activator (tPA) is negatively regulated by PAI-1 (Wind et al., 2002), a Smad3/4-regulated protein target discussed in greater detail above (see section 2.8). The protein tPA is multifaceted, and believed to play multiple roles in the CNS, ranging from neural organization to the pathogenesis of brain disorders (Samson & Medcalf, 2006; Vivien & Ali, 2006). It is interesting to note that in the amygdala, tPA appears to be critical for stress-induced neuronal remodeling and for the development of anxiety-like behaviors that can subsequently be inhibited by PAI-1 (Pawlak et al., 2003). The tPA-plasminogen proteolytic cascade accelerates the clearance of fibrin and protects the brain from damage in stroke models, or when the blood-brain barrier breaks down (Akassoglou et al., 2003; Tabrizi et al., 1999); it also contributes to Aβ degradation (Melchor et al., 2003). These observations suggest that tPA may play a protective role in the neurodegenerative progression of AD.
In cultured cortical neurons, lithium increases protein levels of tPA by affecting PAI-1-dependent transcriptional suppression (Liang, M. H., & Chuang, D. M., unpublished observations). In addition, the neuroprotective effects of lithium against ER and oxidative stresses, induced respectively by thapsigargin and hydrogen peroxide, are also prevented by specific siRNA-induced tPA silencing. Conversely, exogenous or overexpression of tPA completely blocks the damaging effects of ER and oxidative stresses, but only weakly suppresses the neuronal death induced by glutamate or staurosporine (Liang, M. H., & Chuang, D. M., unpublished observations). Together, these data demonstrate that tPA overexpression is critical to lithium-induced neuroprotection, especially protection against ER and oxidative stresses.
2.11 Induction of autophagy
Macroautophagy, usually referred to as autophagy, is a key physiological process for the bulk degradation of cytoplasmic proteins or organelles, and has recently been recognized as one of the principal responses to cellular stress as well as one of the important regulators of neuronal survival and function. The process of autophagy is initiated with the formation of double-membrane structures called autophagosomes that fuse with lysosomes to form autolysosomes and ultimately degrade their contents by lysosomal hydrolytic enzymes (Rubinsztein et al., 2007). Autophagy is responsible for the recycling of cytosolic components during normal conditions, as well as the recycling of nutrients necessary for cell survival under starvation conditions (Maiuri et al., 2007; Melendez & Neufeld, 2008). The autophagy-lysosomal pathway and the ubiquitin–proteasome system (UPS) are two major intracellular mechanisms for protein clearance against abnormal protein accumulation and damage to cytoplasmic organelles in eukaryotic cells. In general, short-lived proteins are predominantly degraded by proteasomes, whereas aggregation-prone proteins with long half-lives appear to be better substrates for autophagic-lysosomal degradation (Klionsky & Emr, 2000; Levine & Kroemer, 2008). Accordingly, this quality control function of autophagy is believed to be beneficial in several neurodegenerative disorders characterized by the accumulation of misfolded disease-causing proteins; these include AD, PD, ALS, spinocerebellar ataxia type 3, and HD (Berger et al., 2006; Cuervo, 2004; Levine & Kroemer, 2008; Nixon, 2005; Ravikumar et al., 2002; Ravikumar et al., 2004; Rubinsztein et al., 2007; Shibata et al., 2006; Webb et al., 2003).
Several signaling pathways and targets have been identified that regulate autophagy, including the mammalian target of rapamycin, mTOR, as a negative regulator. By inhibiting mTOR, rapamycin is currently the most suitable pharmacological agent for upregulating autophagy in mammalian cells, and has been shown to be beneficial in various models of neurodegenerative diseases (Berger et al., 2006; Ravikumar et al., 2004; Rubinsztein et al., 2007). In contrast to rapamycin which enhances autophagy, inhibition of GSK-3β downregulates autophagy by activating mTOR (Sarkar et al., 2008). Autophagy is also induced via mTOR-independent mechanisms. By inhibiting IMPase and inositol transporters (Phiel & Klein, 2001), the ability of lithium to deplete free inositol and subsequently decrease IP3 levels was recently identified as a novel mTOR-independent route for inducing autophagy (Sarkar et al., 2005; Sarkar & Rubinsztein, 2006). Other mood stabilizers that decrease IP3 levels, such as valproate and carbamazepine, can also induce autophagy (Sarkar et al., 2005).
Lithium independently inhibits both GSK-3β and IMPase. Interestingly, lithium inhibits IMPase at lower doses that induce autophagy (Ki ≈ 0.8 mM) (Sarkar et al., 2005); in contrast, it inhibits GSK-3β at higher doses that suppress autophagy (Ki ≈ 2 mM) (Stambolic et al., 1996). Nevertheless, induction of autophagy is considered a potential underlying mechanism of lithium that contributes to its neuroprotective effects. At therapeutic concentrations, lithium facilitates the clearance of known autophagy substrates such as mutant forms of huntingtin and α-synuclein (Sarkar et al., 2005), and induces clearance of protease-resistant prion protein in prion-infected cells (Heiseke et al., 2009). Lithium’s autophagy-inducing properties have also been hypothesized to contribute to its protective effects in ALS (Fornai et al., 2008), and its use in combination with rapamycin has been proposed as a rational therapy in various models of HD (Sarkar et al., 2008). Given that lithium has been used clinically for decades to treat BD and is known to pass the blood-brain barrier (Manji & Lenox, 1998), the autophagy induction properties of lithium—in addition to its other beneficial effects—underscore the potential clinical use of this drug in the treatment of neurodegenerative conditions associated with aggregate-prone proteins.
2.12 Induction of neurogenesis
Neurogenesis denotes the birth of progenitor cells that proliferate and differentiate into functional new neurons and, in turn, replace neurons in particular brain regions. This process is evident during development and continues into adulthood, and occurs particularly in the hippocampal dentate gyrus and olfactory bulb (Gage, 2000). Increasing evidence has indicated that several brain disorders are associated with reductions in volume of certain brain areas and the loss of neuronal cells. The proliferation of neuronal precursor cells is markedly enhanced by several growth factors, but attenuated by glutamate and glucocorticoids (Gage, 2000).
Lithium was found to stimulate progenitor proliferation in cultured brain neurons and to prevent the loss of proliferation induced by glutamate or glucocorticoids (Hashimoto et al., 2003b). In addition, chronic lithium treatment not only enhances neurogenesis in the hippocampus of normal mice (Chen et al., 2000), but also restores neurogenesis in the brain in an animal model of Down syndrome (Bianchi et al., 2009). Multiple mechanisms have been shown to mediate lithium’s effects on neurogenesis. In primary rat hippocampal progenitor cultures, long-term lithium treatment promotes the conversion of these progenitors into neurons through the GSK-3β inhibition/β-catenin activation pathway (Boku et al., 2009; Wexler et al., 2008). In a rat model of stroke, chronic lithium treatment upregulates the generation and survival of newborn cells in the hippocampus by the ERK pathway, and improves the behavioral performance of rats after transient global cerebral ischemia (Yan et al., 2007a). Chronic lithium-induced hippocampal neurogenesis in the dentate gyrus of rats occurs independently of long-term potentiation (LTP). However, chronic lithium treatment only enhances neurogenesis in the brains of adult (Son et al., 2003), but not aged, rats (Yu et al., 2003). One possible common downstream event related to neurogenesis is lithium-induced upregulation of BDNF, which is necessary for hippocampal neurogenesis (Rossi et al., 2006). This ability of lithium to promote neurogenesis and reduce proliferation deficits induced by various insults suggests that lithium has profound neurophysiological significance for the treatment of neurodegenerative and neuropathological conditions.
3. Lithium in models of CNS disorders and its clinical implications and applications
3.1 Bipolar disorder (BD)
BD is a common and chronic mental illness characterized by mood cycling between states of mania and depression (Manji & Lenox, 1998), and is one of the major causes of disability worldwide (Manji et al., 2003; Zarate, Jr. et al., 2006). Because lithium has been the mainstay of treatment for this disorder, its neuroprotective effects may provide novel insights into the potential causes of this disease. For instance, it is interesting to note that, with very few exceptions, neuroprotection is a common feature of drugs prescribed to treat BD (Ketter et al., 2003; Li et al., 2002; Manji & Duman, 2001). Consistent with this view, magnetic resonance imaging (MRI) studies indicate lower levels of N-acetyl-asparate (NAA), a neuronal integrity marker, in the prefrontal cortex of individuals with BD (Brambilla et al., 2004; 2005). In addition, patients with BD showed neuronal atrophy and reduced cellular density, as well as reduced grey matter volume, in various brain regions (Chang et al., 2005b; Sassi et al., 2004). Notably, chronic lithium treatment was found to reduce the decrease in NAA levels and the loss of grey matter volume in the brains of individuals with BD (Bearden et al., 2007; Chang et al., 2005a; 2005b; Chuang & Manji, 2007; Drevets, 2001; Manji et al., 2001; Moore et al., 2000a; 2000b). It should be noted that several species of microRNAs are common targets of lithium and valproate; these include let-7b, let-7c, miR-128a, miR-24a, miR-30c, miR-34a, miR-221, and miR-144 (Zhou et al., 2009). The predicted effectors of these microRNAs are involved in neurite outgrowth, neurogenesis, and signaling of ERK and Wnt/β-catenin pathways. Many of these effector-coding genes are also genetic risk factors for BD, suggesting that microRNAs and their predicted effectors are also targets of the action of mood stabilizers. These findings not only strongly imply that insufficient neuroprotection is relevant to the pathophysiology of BD, but also suggest that lithium’s neuroprotective effects contribute to the multiple mechanisms underlying its therapeutic efficacy in treating this disease.
As mentioned earlier in section 2.6, inhibition of GSK-3 is also likely involved in the antidepressant and antimanic effects of lithium. For example, both lithium (Beaulieu et al., 2004) and selective GSK-3 inhibitor (Gould et al., 2004b) reduce hyperactivity, while overexpression of GSK-3β produces hyperactivity and mania-like behaviors in mice (Prickaerts et al., 2006). On the other hand, both lithium (O’Brien et al., 2004) and GSK-3 inhibitors (Kaidanovich-Beilin et al., 2004; Rosa et al., 2008) produce antidepressant-like effects in mice, and target deletion of GSK-3β gene to produce heterozygous GSK-3β+/− mice induces behavioral effects similar to the antidepressant-like effects of lithium (O’Brien et al., 2004). Inactivation of GSK-3β, using pharmacological or genetic approaches, alleviates the depressive behaviors in mice expressing a mutant form of the brain serotonin synthesis enzyme (Beaulieu et al., 2008). Administration of lentiviral-mediated GSK-3β shRNA into the dentate gyrus causes antidepressant-like effects in mice subjected to chronic stress (Omata et al., 2010). In addition to GSK-3β, genetic inactivation of the GSK-3α in mice also produces antidepressant-like behaviors such as decreased immobility time and reduced aggressive behavior (Kaidanovich-Beilin et al., 2009). A recent study further reveals that mice deficient in the inhibitory serine-phosphorylation of GSK-3 increases susceptibility to mood disturbances, and serine-phosphorylation of GSK-3 is reduced during both stress-related behavioral responses in wild-type mouse brain and in blood cells from patients with BD (Polter et al., 2010). It is interesting to note that, in addition to lithium, other compounds used to treat BD such as valproate and lamotrigine, also enhance serine phosphorylation of GSK-3 (Jope, 2003; Rowe et al., 2007). These findings not only support the hypothesis that inhibition of GSK-3 is the therapeutic target of lithium in the treatment of BD, but also indicate that targeting GSK-3-linked pathways is a rational strategy for developing novel therapeutics to treat this disorder.
3.2 Stroke
Stroke, the most common form of acute brain trauma, is a major global cause of disability and mortality in adults and the third leading cause of death in the United States. Most stroke cases are caused by the interruption of blood supply to the brain, i.e. cerebral ischemia. It is becoming increasingly clear that a large proportion these injuries are induced by excessive increases in extracellular glutamate in the brain following ischemia and subsequent overstimulation of glutamate receptors, notably NMDA receptors (Lipton, 1999). In addition, intracellular mechanisms such as inflammation, oxidative stress, calcium overloading, and caspase activation have also been identified as contributing to the neural damage induced by ischemia (White et al., 2000). Stroke is frequently associated with vascular depression and dementia that are difficult to treat with conventional medications and, unfortunately, no satisfactory treatments are available for preventing neural cell death and associated long-term neurological deficits in stroke victims.
The therapeutic potential of lithium has been investigated in various animal models of stroke. Long-term pretreatment with lithium has been reported to decrease infarct volume and reduce neurological deficits not only in a model induced by permanent middle cerebral artery occlusion (MCAO) (Nonaka & Chuang, 1998), but also in transient MCAO models followed by reperfusion (Xu et al., 2003), which more closely approximates the pathophysiology of acute stroke. Mechanisms underlying lithium-induced neuroprotection are complex and may include inactivation of NMDA receptors (Ma & Zhang, 2003; Ma et al., 2004); reduction in apoptotic cell death (Xu et al., 2003) through downregulation of pro-apoptotic p53 but upregulation of anti-apoptotic Bcl-2 and HSP70 (Bian et al., 2007); activation of the PI3K/Akt cell survival pathway (Chalecka-Franaszek & Chuang, 1999); and inhibition of hypoxia-induced activation of GSK-3 (Roh et al., 2005). Studies in a gerbil model of global ischemia further demonstrate that the neuroprotection afforded by long-term lithium pretreatment is associated with an increase in viable cells and a decrease in apoptotic cells in the hippocampal CA1area, which in turn largely suppresses ischemia-induced behavior deficits and memory impairments (Bian et al., 2007). In rats after transient global cerebral ischemia, lithium facilitates hippocampal neurogenesis via the ERK pathway and improves the recovery of spatial learning as well as memory and behavioral deficits (Yan et al., 2007a; 2007b). Co-treatment with lithium also potentiates the anti-ischemic actions of prostaglandin A1 (Xu et al., 2006; 2007) and E1 (Han et al., 2008) by upregulating HSPs in rats subjected to permanent MCAO. Moreover, in an animal model of salt-loaded, stroke-prone spontaneously hypertensive rats, the addition of low-dose lithium to captopril prevents stroke and dramatically prolongs the effects of this angiotensin-converting enzyme inhibitor on survival through an effect independent of the reduction in blood pressure (Xu et al., 2005).
Two-day pretreatment with lithium was reported not to protect brain tissue against transient ischemia in gerbils (Yoshida et al., 1991), suggesting that long-term pretreatment is a prerequisite for lithium’s protective effects. However, post-insult treatment with therapeutic doses of lithium (~1.0 mEq/kg of body weight, subcutaneously), when administered up to three hours after the onset of ischemia, also markedly decreases infarct volume and suppresses neurological deficits measured by sensory, motor, and reflex tests in a rat model of transient MCAO (Ren et al., 2003). These beneficial effects are associated with HSF-1 activation and induction of the cytoprotective protein HSP70 in ischemic brain hemispheres. Recently, the neurohemodynamic aspects of lithium-induced recovery were further investigated in this transient MCAO model using functional MRI. This study found that delayed chronic lithium treatment—administered up to 12 hours after the onset of ischemia and followed by daily injections for two weeks—significantly improves blood oxygenation level dependence functional MRI response magnitude and influences vascular formation (Kim et al., 2008). The ability of lithium to affect neurovascular remodeling may be related to its ability to increase protein levels of matrix metallopeptidase 9 (MMP-9) and VEGF (Guo et al., 2009); the latter has been linked to angiogenesis, neurogenesis, and neuroprotection (Fan & Yang, 2007).
Taken together, the benefits of lithium observed in various animal models of stroke suggest that lithium pretreatment might be advantageous in controlling conditions that carry a high risk of predictable stroke, such as cardiac surgery, carotid endarterectomy, and transient ischemic attack. In addition, and of equal importance, is the finding that post-insult treatment with lithium is also effective within a time frame during which it could realistically be administered to human victims. These results raise the possibility that lithium has putative utility as a clinical tool for both prevention and treatment of patients with acute stroke.
3.3 Huntington’s disease (HD)
HD is an inherited autosomal dominant neurodegenerative disease characterized by progressive memory loss, cognitive decline, and psychiatric disturbances such as aggressiveness and depression, in addition to the impaired movement that is the main feature of this disease (Martin & Gusella, 1986). HD leads to death about 10 to 20 years after the initial symptoms occur (Vonsattel & DiFiglia, 1998), and currently there is no proven treatment to arrest or reverse the course of this disease. HD belongs to the polyglutamine disorders family and is caused by an abnormal expansion of a trinucleotide CAG repeat in the gene that encodes a polyQ stretch to more than 35 glutamines in the N-terminus of the disease-causing protein termed huntingtin (MacDonald et al., 1993). This abnormal expansion results in a selective loss of neurons in the brain, particularly the medium-sized spiny neurons in the striatum and, to a lesser extent, in the cortex (Friedlander, 2003; Hickey & Chesselet, 2003). Mutant huntingtin might cause neurotoxicity both through a toxic gain of function and a loss of wild-type huntingtin protein (Zuccato et al., 2001). Transcriptional dysregulation also plays a central role in the pathogenesis/pathophysiology of HD (Hodges et al., 2006; Sugars & Rubinsztein, 2003). HD pathogenesis is modeled frequently with the expression of mutant huntingtin that causes aggregate formation and toxicity in cell models and in vivo (Rubinsztein, 2002).
The protective properties of lithium against glutamate toxicity seem ideally suited to treat HD, particularly because supersensitivity or hyperactivation of NMDA receptors appears to contribute to the pathophysiology of HD (Taylor-Robinson et al., 1996). Initial research in animal models of HD found that lithium neuroprotection is mediated through Bcl-2 induction and GSK-3β inhibition. The striatal infusion of quinolinic acid (QA), a neuronal excitotoxin that causes the death of medium-sized spiny neurons by activating NMDA receptors, and produces many of the neuroanatomical changes found in HD, has been frequently used as an animal model for investigating this disease (Foster et al., 1983; Schwarcz & Whetsell, Jr., 1982). In this rat excitotoxic model of HD, lithium treatment at doses within the therapeutic range (0.5 and 1.0 mEq/kg) markedly reduces the size of QA-induced striatal lesions (Wei et al., 2001) and the loss of striatal medium-sized neurons (Senatorov et al., 2004). This lithium protection is correlated with upregulation of cytoprotective Bcl-2 and downregulation of caspase-3 activation.
Furthermore, the anti-apoptotic properties of lithium in HD may also be mediated through GSK-3β inhibition. For example, in a cell model of HD, the protective effects of lithium in reducing mutant huntingtin aggregates and cell death are mimicked by either treatment with a GSK-3β inhibitor or overexpression of a dominant-negative GSK-3β mutant (Carmichael et al., 2002). In Drosophila, lithium-induced protection against the toxicity of aggregate-prone proteins is mimicked by AR-A014418, a GSK-3β inhibitor (Berger et al., 2005). In addition to its ability to inhibit apoptosis, lithium pretreatment also stimulates the proliferation of striatal cells near the site of QA-induced injuries, and some of these replicating cells have the phenotype of neurons or astroglia (Senatorov et al., 2004). Thus it appears that both the cell-proliferating and anti-apoptotic properties of lithium may underlie its neuroprotective effects in HD models.
Abnormal proteolytic processing of mutant huntingtin has been implicated as a critical step in the onset of HD, and the cleavage of huntingtin in human HD tissue is believed to be mediated in part by calpain, a calcium-activated neutral protease whose activity has been shown to be elevated in the caudate of human HD tissues (Gafni & Ellerby, 2002). As mentioned above, the succinate dehydrogenase inhibitor 3-NP has been used to induce striatal pathology similar to that observed in HD (Brouillet et al., 1999). In a rat 3-NP model of HD, lithium treatment reduces striatal neurodegeneration by preventing calpain and, subsequently, Cdk5 activation (Crespo-Biel et al., 2009). Moreover, it was reported that eliminating mutant huntingtin expression not only halts symptomatic progression, but also leads to regression of disease-like symptoms (Yamamoto et al., 2000), suggesting that improving clearance of the mutant protein is expected to prevent cellular dysfunction and neurodegeneration in HD. In Drosophila and R6/2 mouse models of HD, inhibition of mTOR by systemic administration of rapamycin induces autophagy and reduces toxicity of polyglutamine expansions (Ravikumar et al., 2004). Because it is an autophagy inducer, lithium in combination with rapamycin shows greater protection against neurodegeneration than either pathway alone in cellular and Drosophila models of HD (Sarkar et al., 2008). Some behavioral benefits have been shown with lithium in transgenic mouse models of HD. R6/2 mice, the most frequently studied model of HD, carry a 145 CAG repeat expansion in huntingtin and show behavioral motor deficits as early as five to six weeks of age. In this model, post-, but not pre-symptomatic lithium treatment significantly improves rotarod performance but has no overall effect on survival (Wood & Morton, 2003). However, in the N171-82Q and YAC128 mouse models of HD, pre-symptomatic co-treatment with lithium and valproate, another mood stabilizer, produces more robust improvements in motor deficits and stronger anxiolytic as well as antidepressant-like effects than either drug alone (Chiu et al., 2009). Consistent with these results, synergistic neuroprotective effects of lithium and valproate have also been recently reported in vitro using rat CGCs exposed to glutamate (Leng et al., 2008). This neuroprotective synergy by combinatory treatment in CGCs is due, at least in part, to enhanced inhibition of GSK-3.
The clinical use of lithium in HD patients was explored decades ago, even before its neuroprotective properties were discovered. Lithium treatment of HD patients strikingly reduces chorea, and markedly improves voluntary movements (Dalen, 1973) and motor function (Mattsson, 1973). One study suggests that patients in the early disease stages might be more likely to benefit from lithium treatment (Foerster & Regli, 1977). Interestingly, some of these patients also experienced beneficial mood- and temper-stabilizing effects. Combined therapy with lithium and neuroleptics also proved beneficial in several HD patients (Anden et al., 1973; Leonard et al., 1974; 1975; Manyam & Bravo-Fernandez, 1973; Schenk & Leijnse-Ybema, 1974). However, some other reports showed that lithium exerted no beneficial effects in HD patients (Aminoff & Marshall, 1974; Vestergaard et al., 1977). In some instances, lithium treatment even worsened motor and cognitive performance, particularly when used as the sole therapeutic agent (Carman et al., 1974; Leonard et al., 1974). Nonetheless, it should be noted that the number of patients included in those trials was small, and the duration of lithium treatment was also too short to assess the potential benefit of this drug. Given that potential HD patients can be identified by genetic testing prior to the onset of symptoms, recent evidence of lithium’s neuroprotective properties in various models of HD still suggest its possible utility in treating HD, especially when administered in combination with other medications.
3.4 Alzheimer’s disease (AD)
In 2009, AD was the seventh leading cause of death in the US. Clinically, it is characterized by progressive memory loss and personality changes, ultimately leading to dementia. Although the pathogenesis of AD is not well understood, the neuropathological hallmarks of AD are an abnormal accumulation of Aβ resulting from an imbalance between Aβ production and clearance, and neurofibrillary tangles (tauopathies) resulting from hyper-phosphorylation of tau, a microtubule-binding protein (Selkoe, 2001). Accumulation of Aβ in the brain has been suggested as the primary cause of AD (Hardy & Selkoe, 2002). Hyper-phosphorylation of tau has also been implicated early in the development of the neurofibrillary pathology associated with AD and other neurodegenerative diseases (Lee et al., 2001; Planel et al., 2001). Therefore, Aβ and tau are considered as the primary targets in the treatment of AD.
Abnormal increases in GSK-3 levels and activity are associated with pathogenesis and neuronal death in the brain of individuals with AD (Bhat et al., 2004; Munoz-Montano et al., 1999), suggesting that lithium may have therapeutic benefits in treating this disorder (Huang & Klein, 2006). In fact, pioneering studies have demonstrated that lithium antagonizes AD toxicity by inhibiting GSK-3. For example, lithium reduces tau phosphorylation by inhibiting GSK-3 in vivo and in vitro (Hong et al., 1997; Munoz-Montano et al., 1997; Sang et al., 2001). In addition, levels of tau phosphorylation are also regulated by PP2A (Tanaka et al., 1998), and reduced PP2A activity has been reported in the brain of individuals with AD (Trojanowski & Lee, 1995). Inhibition of PP2A prevents tau dephosphorylation, a process that precedes and is required for its cleavage and degradation (Rametti et al., 2004). Cleavage and degradation of hyper-phosphorylated tau may reduce its accumulation and aggregation. Lithium treatment not only increases the activity of PP2A in the rat brain (Tsuji et al., 2003), but also decreases tau phosphorylation and in turn facilitates its destruction (Rametti et al., 2004), suggesting the involvement of PP2A in lithium’s action. This is supported by the finding that blockade of PP2A activity reverses lithium-induced downregulation of total tau proteins mediated by GSK-3β inhibition in cultured neurons (Martin et al., 2009). Furthermore, it was recently shown that lithium downregulates tau transcription in cultured cortical neurons (Rametti et al., 2008).
Chronic lithium treatment also blocks Aβ production through GSK-3 inhibition (Sun et al., 2002). Aβ peptide is derived from amyloid precursor protein (APP) by sequential secretase-dependent proteolytic processing. In the brains of mice overproducing APP, chronic lithium treatment blocks Aβ accumulation, presumably by interfering with the reaction of γ-secretase (Phiel et al., 2003). This effect of lithium in Chinese hamster ovarian cells is mimicked by transfection with siRNA of GSK-3α, but not GSK-3β (Phiel et al., 2003). However, the results from another study in HEK 293 cells show that GSK-3β inhibition can also mimic the ability of lithium or valproate to suppress the process of Aβ formation from APP (Su et al., 2004). In cultured neurons and neurally related cells, chronic lithium treatment largely suppresses exogenous Aβ-induced hyper-phosphorylation of tau, downregulation of Bcl-2, and neuronal death (Alvarez et al., 1999; 2002; Hong et al., 1997; Wei et al., 2000). In addition, lithium treatment prevents acetylcholinesterase-promoted Aβ toxicity and associated loss of function of Wnt signaling components in hippocampal slices (Inestrosa et al., 2000). It is interesting to note that the protein level of Bcl-2 in the brains of a mouse model of AD is inversely correlated with the expression of miR-34a (Wang et al., 2009), a microRNA that has recently emerged as common target for lithium and valproate (Zhou et al., 2009). These findings suggest a novel mechanism for lithium’s protective effects in AD by which it can upregulate Bcl-2 indirectly via downregulation of miR-34a.
Lithium was also found to have beneficial effects in various animal models of AD. In Drosophila models of tauopathies, lithium reverses axonal transport and locomotor deficits by inhibiting GSK-3β (Mudher et al., 2004). In mouse models of tauopathies, chronic lithium treatment not only inhibits GSK-3 mediated tau phosphorylation and neuronal degeneration (Noble et al., 2005), it also decreases tau lesions by promoting ubiquitination (Nakashima et al., 2005). In rat brains, chronic lithium treatment protects against Aβ-induced hippocampal neurodegeneration by activating the Wnt/β-catenin pathway (De Ferrari et al., 2003). It was found that induction of Dickkopf protein 1 (DKK1), a Wnt pathway inhibitor (Krupnik et al., 1999), is associated with neurodegeneration in the brains of individuals with AD (Caricasole et al., 2004). Accordingly, systemic administration of lithium reverses local infusion of DKK1-induced neuronal cell death and astrocytosis in the CA1 region of the rat hippocampus (Scali et al., 2006). In the hippocampus of rabbits, lithium prevents Aβ-induced ER stress and the subsequent activation of caspase-12 and -3, NF-κB activation, and GSK-3 nuclear translocation (Ghribi et al., 2003). This action of lithium against ER stress might be related to its ability to induce the chaperone protein GRP78 (Hiroi et al., 2005), or the tPA-plasminogen proteolytic cascade (Melchor et al., 2003) as described above (see sections 2.10.5 and 2.10.6). Chronic lithium treatment not only decreases mutant tau protein aggregation in a transgenic mouse model (Perez et al., 2003a), it also arrests the development of neurofibrillary tangles in mutant tau transgenic mice with advanced neurofibrillary pathology (Leroy et al., 2010).
With regards to its behavioral effects, chronic lithium treatment was found to improve learning and memory in normal rats (Nocjar et al., 2007), as well as improve spatial learning deficits in rats injected with preformed Aβ fibrils (De Ferrari et al., 2003). In transgenic mice overexpressing human APP, chronic (3-month) lithium treatment reduces the Aβ burden, tau hyper-phosphorylation, and neurodegeneration in the cortex and hippocampus, and normalizes deficits in water-maze performance by inhibiting GSK-3β signaling (Rockenstein et al., 2007). Taken together, these results from various cell and animal models of AD suggest a promising therapeutic role for lithium in the treatment of AD.
Lithium use in the treatment of AD has also been investigated clinically. One preliminary clinical study in individuals with BD found that a history of lithium treatment resulted in significantly better cognition and memory scores compared with individuals who had received other treatments (Terao et al., 2006). Chronic lithium treatment was further shown to reduce the prevalence of AD in elderly patients with BD (Nunes et al., 2007). A ten-year study in Denmark also found that continued lithium treatment of patients with dementia is associated with reduced rate of dementia to the same level as that for the general population (Kessing et al., 2008). Surprisingly, a German study found that lithium treatment of AD patients for 10 weeks significantly increases serum levels of BDNF, and that the diminution of cognitive impairment is inversely correlated with lithium serum concentrations (Leyhe et al., 2009). However, another trial of 33 AD patients reported no effect on GSK-3 activity or cognitive performance after 10 weeks of lithium treatment (Hampel et al., 2009). Similar reports with either small numbers of patients or short duration of treatment also indicate no therapeutic effect of lithium treatment in AD patients (Brinkman et al., 1984; Macdonald et al., 2008). Although lithium treatment in elderly people with AD was reported to have relatively few adverse effects (Macdonald et al., 2008), lithium levels in elderly patients still need to be carefully monitored due to reduced renal secretion of lithium (Williams, 1989). These preliminary results suggest that studies with longer treatment phases and larger patient groups are needed to observe the potential benefits of lithium in AD patients.
3.5 Parkinson’s disease (PD)
PD is a prevalent neurodegenerative disease characterized by resting tremor, muscular rigidity, bradykinesia, and postural instability associated with a relatively selective loss of dopaminergic neurons, preferentially in the substantia nigra. Most cases of PD occur sporadically and primarily in older populations (age 65 or higher). PD is another neurodegenerative condition characterized by aggregates of mutant protein (Lewy bodies), mainly α-synuclein (Kruger et al., 1998; Polymeropoulos et al., 1997), a cytosolic protein essential for presynaptic regulatory function (Abeliovich et al., 2000). Although α-synuclein is degraded by both autophagy and the proteasome, the clearance of these mutant forms is retarded when autophagy is inhibited (Cuervo et al., 2004; Webb et al., 2003). Neurotoxins such as rotenone, 6-hydroxydopamine (6-OHDA), l-methyl-4-phenylpyridinium (MPP+), and the MPP+ precursor, MPTP, are widely used as parkinsonism-inducing neurotoxins to trigger PD-associated neurochemical changes in animal models.
Several lines of evidence support the therapeutic potential of lithium in PD. In cultured human neuroblastoma cells, caspase-3 activation induced by the mitochondrial complex I inhibitor rotenone or MPP+ is facilitated by GSK-3β activation, and inhibited by lithium treatment in a PI3K-dependent manner (King et al., 2001). GSK-3β activation in PD seems to depend on the presence of α-synuclein, since MPP+ or MPTP-induced tyrosine phosphorylation of GSK-3β is absent in cells lacking α-synuclein or from α-synuclein knockout mice (Duka et al., 2009). In cultured neurons, lithium prevents 6-OHDA (Chen et al., 2004a) and MPP+-induced (Kramer et al., 2010) neuronal death. In addition, chronic lithium treatment not only prevents MPTP-induced neurotoxicity in mice, it also normalizes the downregulation of Bcl-2 and upregulation of Bax elicited by MPTP in the striatum of the mouse brain (Youdim & Arraf, 2004). A recent study further indicates that lithium treatment, via GSK-β inhibition, prevents high concentrations of L-3,4-dihydroxyphenylalanine (L-DOPA)-induced neuronal cell death (Koh et al., 2008).
As with HD, clinical trials of lithium in PD patients were performed decades ago, before its neuroprotective properties were discovered. Lithium was used adjunctively to address the side effects of L-DOPA therapy, namely the on-off phenomenon (alternating periods of dyskinesia and akinesia) and L-DOPA-induced hyperkinesias associated with its early use. Despite experimental results suggesting that lithium may prevent striatal dopamine receptor desensitization, PD-like symptoms were reported in association with lithium treatment (Holroyd & Smith, 1995; Karcher et al., 1969). Case studies addressing the effects of lithium on L-DOPA-induced hyperkinesia remain contradictory (Karcher et al., 1969; McCaul & Stern, 1974; Van Woert & Ambani, 1973). On the other hand, lithium did prove beneficial in the management of the on-off phenomenon associated with L-DOPA therapy. In a double-blind crossover study and one case report, lithium was found to significantly reduce akinesia (Coffey et al., 1982; Ross et al., 1981), whereas lithium also increased dyskinesia (Coffey et al., 1982), or failed to improve the on-off phenomenon (Lieberman & Gopinathan, 1982). Nonetheless, the experimental evidence indicates that lithium is associated with protective properties that may encourage the use of this drug to treat PD patients; new, long-term trials are needed to fully explore this issue.
3.6 Retinal degeneration
Several hypotheses suggest that lithium alters circadian rhythms in individuals with BD by reducing sensitivity to light at the retinal level (Seggie, 1988). However, an early study of BD patients observed that chronic lithium use is not associated with differences in retinal light sensitivity, nor is there evidence for retinal toxicity after long-term lithium administration (Lam et al., 1997).
In contrast, in retina-brain slice co-cultures, lithium was found to support both the survival of retinal ganglion cells (RGCs) and regeneration of their axons through a Bcl-2-dependent mechanism (Huang et al., 2003). Lithium’s Bcl-2-dependent effects were later confirmed in vivo; in rats, lithium protects RGCs from partial optic nerve crush (Schuettauf et al., 2006). Co-application of lithium and astrotoxin, a glutamate analogue that selectively kills astrocytes with minimal effects on surrounding neurons, induces more robust optic nerve regeneration in adult mice (Cho & Chen, 2008). Lithium is also protective in cultured primary retinal neurocytes, where it promotes neurite outgrowth and DNA non-homologous end-joining following nutrient deprivation, an in vitro condition mimicking cell death in glaucoma (Zhuang et al., 2009). In addition, activation of the canonical Wnt pathway is necessary for the normal development of anterior eye structures (Liu et al., 2007), and plays a role in retinal development and homeostasis (Liu et al., 2006). In retinoblastoma, the cancer stem-like cell population is also regulated by the canonical Wnt pathway (Silva et al., 2010). All of these events are normalized by lithium treatment, suggesting that this drug may be beneficial for the treatment of RGC degeneration.
3.7 Fragile X syndrome (FXS)
FXS is the most common inherited single-gene disorder associated with mental retardation. It is caused by abnormal expansion of the trinucleotide (CGG) repeat-mediated transcriptional silencing of the fragile X mental retardation-1 (FMR1) gene (Crawford et al., 2002; Pieretti et al., 1991) that encodes the fragile X mental retardation protein (FMRP) (Garber et al., 2006). The discovery of the FMR1 gene, the first identified autism-related gene (Hagerman et al., 2005), led to the development of FMR1 gene knockout animals as models of FXS in order to improve our understanding of the pathophysiological mechanisms of autism and the development of effective therapeutics to treat this disorder. It has been suggested that the absence of FMRP causes enhanced metabotropic glutamate receptor (mGluR) signaling resulting from unchecked activation of neuronal mGluR5 and consequent enhanced mGluR-activated long-term depression in the brain of individuals with FXS (Bear et al., 2004; Dolen et al., 2007); this suggests that mGluR antagonists may ultimately be useful in treating this disorder. In addition, a recent study found that the inhibitory serine-phosphorylation of GSK-3 is impaired in FVB/NJ FMR1 knockout mice (Min et al., 2009), suggesting elevated GSK-3 activity in FXS. Recent studies demonstrate that lithium, which inhibits GSK-3, may be therapeutically useful for treating FXS (Berry-Kravis et al., 2008; Choi et al., 2009; McBride et al., 2005; Min et al., 2009; Yan et al., 2005).
In a Drosophila model of FXS, behavioral and cognitive deficits measured by the naive and conditioned courtship behaviors, respectively, are rescued by treatment with mGluR antogonists administered during development alone, during adulthood alone, or during both time periods (McBride et al., 2005). Lithium treatment in adulthood, presumably through modulating signaling in a manner similar to the downstream effects of mGluR antagonists, also increases naive courtship and restores short-term memory in FXS flies (McBride et al., 2005). A recent study further indicates that age-related cognitive impairments in the Drosophila model of FXS are also prevented by treatment with mGluR antagonists or lithium, and continuous treatment during aging effectively rescues all of these phenotypes (Choi et al., 2009). Mouse models of FXS display certain FXS- and autism-relevant behavioral phenotypes such as hyperactivity and impaired memory and social behavior (Liu & Smith, 2009; Qin et al., 2002; Spencer et al., 2005; The Dutch-Belgian Fragile X Consortium, 1994), and lithium treatment ameliorates several behavioral deficits in these mouse models of FXS (Min et al., 2009; Yuskaitis et al., 2010). In addition, the aberrant dendritic spine morphology, reduced anxiety levels, deficient social interactions, and impaired learning ability observed in FXS mice are also largely blocked by chronic lithium treatment (Liu, Z. H., Chuang, D. M., & Smith C. B., resubmitted for publication). These beneficial effects of lithium are associated with normalization of hypo-phosphorylation of GSK-3β at Ser9 in the brain of FXS mice. Activation of mGluR5 receptors inhibits GSK-3 activity in wild-type mice (Liu et al., 2005), whereas the levels of serine-phosphorylation of GSK-3 were found to be increased by 2-methyl-6-phenylethynyl-pyridine (MPEP), an mGluR5 antagonist, in the brain of FXS mice (Min et al., 2009; Yuskaitis et al., 2010). Moreover, the combination of an mGluR5 antagonist with a GSK-3 inhibitor does not produce additive therapeutic effects in FXS mice (Min et al., 2009). Collectively, these findings suggest that GSK-3 may be a fundamental component of the pathology of FXS, and support the therapeutic potential of lithium in FXS. Recent results from a pilot clinical study show that the effects of lithium are consistent with preclinical results; in FXS patients 6 to 23 years of age, lithium exerts positive effects on behavior, adaptive skills, and cognitive measures (Berry-Kravis et al., 2008).
3.8 Amyotrophic lateral sclerosis (ALS)
ALS is an adult-onset neurodegenerative disease characterized by progressive loss of motor neurons (MNs) in the brain, brain stem, and spinal cord, resulting in generalized weakness, muscle atrophy, paralysis, and eventual mortality within five years of disease onset (Rowland, 1994). Most ALS cases occur sporadically, with only about 10% of the patients categorized as having a familial form (Boillee et al., 2006; Wijesekera & Leigh, 2009). Of these, approximately 20% are attributed to gain-of-function mutations in the gene encoding Cu/Zn superoxide dismutase 1 (SOD1), a key antioxidant enzyme (Rosen et al., 1993). However, sporadic and familial forms of ALS produce similar pathological hallmarks. Mice expressing mutant Cu/Zn SOD1 exhibit ALS-like phenotypes, including the formation of intracellular aggregates of SOD1 in the brain and spinal cord, behavioral abnormalities, and premature death. In addition to MNs, damage to surrounding glial cells, muscle cells, interneurons, and Renshaw inhibitory neurons have also been reported in ALS (Boillee et al., 2006; Dobrowolny et al., 2008; Fornai et al., 2008).
Defective autophagy has been found in diseased MNs (Venkatachalam et al., 2008). In addition, upregulated GSK-3β (Yang et al., 2008), hyper-phosphorylated β-catenin (Yang et al., 2008), and downregulated VEGF and its receptors (Brockington et al., 2006) have also been observed in the postmortem tissue of ALS patients. VEGF prolongs survival in ALS mice (Wang et al., 2007) and protects MNs against excitotoxicity (Tolosa et al., 2008). These findings suggest that the neuroprotective mechanisms of lithium may be suitable for treating ALS. In fact, treatment with either lithium alone or in conjunction with an antioxidant has been shown to improve motor function and slow disease progression in a mouse model of ALS (Ferrucci et al., 2010; Fornai et al., 2008; Shin et al., 2007). In organotypic slice cultures of spinal cord, chronic treatment with lithium dose-dependently prevents excitotoxic cell death of MNs by inhibiting the GSK-3β signaling pathway (Caldero et al., 2010).
In addition, the autophagy-inducing properties of lithium are also believed to contribute to its protective effects in ALS (Fornai et al., 2008). A recent study found that combined treatment of ALS mice with lithium and valproate produces a greater and more consistent effect in delaying the onset of disease symptoms, decreasing neurological deficit scores, and prolonging life span than monotreatment with either drug (Feng et al., 2008). Moreover, a 15-month pilot clinical trial in randomized ALS patients found that lithium and riluzole cotreatment markedly reduced mortality when compared with matched control patients treated with riluzole alone (Fornai et al., 2008). However, it should be noted that inconsistent results have also been reported. In a sibling-matched, gender-balanced, investigator-blinded trial, chronic lithium treatment was found to be ineffective on any treatment measure (Gill et al., 2009). Another study also found no therapeutic or neuroprotective effects of lithium in female ALS mice (Pizzasegola et al., 2009). Moreover, a very recent study showed that lithium in combination with riluzole did not slow the progression of ALS more than riluzole alone (Aggarwal et al., 2010). Future studies are needed to clarify these discrepancies (Bedlack et al., 2008; Vanacore & Galeotti, 2008). Accordingly, an international effort is already underway to initiate larger, randomized trials to verify lithium’s effect in ALS patients (Meininger et al., 2008).
3.9 Multiple sclerosis (MS)
MS is the most common inflammatory demyelinating disease of the CNS, in which focal lymphocytic infiltration leads to damage of myelin sheaths around the axons of the brain and spinal cord, and results in demyelination and neurodegeneration with lesions predominantly in the white matter (Hafler, 2004; McFarland & Martin, 2007; Sospedra & Martin, 2005). MS is more prevalent in females and usually occurs in young adults in either relapsing or progressive form characterized by a broad spectrum of symptoms, including muscle weakness, motor incoordination, and cognitive impairments (Compston & Coles, 2008). Although MS has been widely thought to be an autoimmune disease, the etiology of this disease remains elusive and there is no cure at this time. Current therapeutic strategies for MS focus on slowing the disease progression and controlling the symptoms.
Experimental autoimmune encephalomyelitis (EAE) (Noseworthy et al., 2000) in animals is the most frequently used animal model of MS, and it has directly led to the development of three medications for treatment of MS, glatiramer acetate, mitoxantrone and natalizumab (Steinman & Zamvil, 2006). EAE recapitulates many of the clinical, immunological, and neuropathological aspects of human MS (Denic et al., 2010; Steinman & Zamvil, 2005), and it can be induced by systemic injection of an emulsion that contains synthetic peptides derived from myelin proteins, such as myelin oligodendrocyte glycoprotein (MOG), myelin basic protein, or proteolipid protein, in a variety of mammal species (Baxter, 2007). Activation of the immune system and subsequent infiltration of peripherally activated immune cells together with resident glia amplify neuroinflammation, and cause demyelination and loss of neuronal function in the CNS (Hafler, 2004; Kuchroo et al., 2002). A recent study demonstrates that EAE is more severe and develops more rapidly in knock-in mice expressing constitutively active GSK-3 (De Sarno et al., 2008), suggesting that this kinase is a potential therapeutic target for the treatment of MS.
Administration of GSK-3 inhibitors has been shown to control several inflammatory and immune conditions in both the periphery and the CNS (Beurel et al., 2010). For example, GSK-3 inhibition ameliorates arthritis, peritonitis, and colitis in rodents (Cuzzocrea et al., 2006; Dugo et al., 2005; Whittle et al., 2006), and increases the production of anti-inflammatory IL-10 by memory CD4+ T cells (Garcia et al., 2008) and B cells (Lambert & Martinez, 2007). Notably, lithium pretreatment at therapeutically relevant doses not only abolishes the onset of EAE, but also greatly reduces demyelination, microglia activation, and leukocyte infiltration in the spinal cord of mice (De Sarno et al., 2008). Although the disease rapidly relapses after lithium withdrawal, lithium also promotes recovery and prevents relapse episodes of EAE when administered postimmunization (after the first relapse episode). In addition, lithium treatment suppresses MOG peptide-induced immune responses in vitro and decreases the production of several proinflammatory cytokines by splenocytes stimulated with MOG peptide after isolation from EAE mice. These results suggest that GSK-3 is the most likely target mediating lithium’s beneficial effects in EAE, and lithium may be useful for therapeutic intervention in autoimmune and inflammatory diseases afflicting the CNS such as MS. However, it should be noted that numerous therapeutical approaches that showed promising results in EAE turned out to be either ineffective or even harmful in human MS (Sriram & Steiner, 2005). Therefore, the potential use of lithium as a novel therapeutic for this disease requires more studies to gain better insight into the mechanisms of action.
3.10 Other neurological conditions
3.10.1 Spinal cord injury
In a spinal cord injury model of rats induced by unilateral hemisection, combined lithium with chondroitinase was reported to have synergistic effects in increasing axonal regeneration of the rubrospinal tract and in improving forelimb movement (Yick et al., 2004). Intrathecal injection of lithium also reduces neuropathic pain responses in rats subjected to chronic constrictive injury to the sciatic nerve (Shimizu et al., 2000), raising its potential utility in neuropathic pain syndromes resulting from peripheral nerve injury. In addition, chronic lithium treatment, presumably through the secretion of BDNF (Su et al., 2009), enhances proliferation and neuronal differentiation of neural progenitor cells in vitro and after transplantation into the adult rat spinal cord with reduced activation of microglia and macrophages (Su et al., 2007). Systemic application of a therapeutic dose of lithium suppresses the activity of GSK-3 around the lesion sites of spinal cord-lesioned rats, and promotes axonal growth and recovery in rats with thoracic spinal cord transection or contusion injuries (Dill et al., 2008). Taken together, these preliminary results provide evidence that GSK-3 signaling is an important therapeutic target for promoting functional recovery, and that lithium may have therapeutic potential in cell replacement strategies for CNS injury due to its ability to suppress the host immune response.
3.10.2 HIV infection
Murine acquired immune deficiency syndrome (MAIDS), a mouse-related syndrome with extensive similarities to human immunodeficiency virus (HIV) infection, is caused by a defective murine leukemia virus. Lithium has been shown to have immune-enhancing properties in peripheral blood mononuclear cells obtained from normal subjects and patients with AIDS-related complex (ARC) (Sztein et al., 1987). MAIDS mice treated with lithium show a marked reduction in the development of lymphadenopathy and splenomegaly (Gallicchio et al., 1993). In rat CGCs, lithium abolishes HIV-1 transactivator protein (Tat)-induced increases in GSK-3β activity and enhances neuronal survival following exposure to Tat or prenatal platelet activating factor (PAF), a protein produced from HIV-1-infected brain-resident macrophages (Maggirwar et al., 1999). Lithium also reverses PAF-induced GSK-3β overactivation, as well as cell migration and death in cultured CGCs (Tong et al., 2001). In addition, noncytotoxic/noncytostatic concentrations of lithium inhibit the replication of HIV in a Wnt/β-catenin-dependent manner (Kumar et al., 2008). A study notes that lithium pretreatment protects the hippocampus of mice from HIV-gp120-induced toxicity (Everall et al., 2002). Similarly, pre- but not post-exposure of human neuroblastoma cells to lithium reduces gp120-induced neurotoxicity, and this protective effect is blocked by a PI3K inhibitor. The neuroprotective mechanisms of lithium in murine HIV-1 encephalitis identified in vivo, appear to be mediated through the classic PI3K/Akt and GSK-3β pathways (Dou et al., 2005). These results are compatible with the view that prophylactic treatment with lithium may prevent or delay the onset and progression of HIV-associated cognitive impairments. Indeed, lithium has already been safely used to improve HIV-associated neurocognitive impairment in diagnosed patients (Letendre et al., 2006; Schifitto et al., 2009).
3.10.3 Prion diseases
Transmissible spongiform encephalopathies, also known as prion diseases, are a group of unusual, fatal, neurodegenerative disorders. Prion diseases are caused by aggregates of a proteinase-resistant, abnormal isoform of the native prion protein (PrP) that induces neuronal cell death and glial proliferation (Collinge, 2001; Prusiner, 1998). Creutzfeldt-Jakob disease is the most prevalent human prion disease with possible sporadic, genetic, and acquired etiologies. Several studies indicate that serotonergic dysregulation are likely to associate with high frequency of neuropsychiatric symptoms in prion diseases, and that prion-mediated cytotoxicity may be blocked by NMDA receptor antagonists (Appleby, 2009). Notably, PrP-induced neuronal cell death in both primary neuronal cultures and neuroblastoma cells is mediated through a pathway involving GSK-3 and is blocked by lithium (Perez et al., 2003b). Lithium significantly reduces the amount of pathological PrP in prion-infected neuronal and cultured non-neuronal cells by inducing autophagy (Fornai et al., 2006; Heiseke et al., 2009). Co-treatment with lithium and rapamycin has an additive effect on pathological PrP clearance compared to treatment with either drug alone (Heiseke et al., 2009). These data indicate that lithium has potential utility in treating these diseases.
3.10.4 Alcohol-induced neurodegeneration
The developing nervous system is vulnerable to ethanol toxicity. Lithium protects cultured CGCs from ethanol-induced apoptosis and caspase-3/9 activation, but neither ethanol nor lithium significantly affects the phosphorylation of Akt at ser473 or GSK-3β at ser9 (Zhong et al., 2006). Since lithium-induced activation of Akt in CGCs is transient (Chalecka-Franaszek & Chuang, 1999), these data cannot exclude the involvement of the Akt/GSK-3β pathway. Lithium also protects infant mice against ethanol-induced apoptotic cell death as well as caspase-3 activation in the brain (Chakraborty et al., 2008; Zhong et al., 2006). Notably, Akt, GSK-3β, and 5′ adenosine monophosphate-activated protein kinase (AMPK) have been shown to be involved in ethanol-induced neurodegeneration and the neuroprotective effects of lithium in an in vivo study (Chakraborty et al., 2008).
3.10.5 Down syndrome (DS)
DS is a high-incidence genetic disorder in which an extra portion of chromosome 21 leads to brain hypoplasia, mental retardation, and high prevalence of dementia. Segmental trisomy Ts65Dn mice, the most widely used model for DS, have elevated levels of brain myo-inositol, one of the metabolites that has been investigated in relation to the mental retardation associated with DS. Studies found that this elevated myo-inositol in the brain of DS mice is decreased by lithium treatment (Huang et al., 2000). In addition, emerging evidence suggests that reduced neurogenesis may be a major determinant of brain underdevelopment in DS. The Ts65Dn mice exhibit severe proliferation impairment and lithium restores neurogenesis in the subventricular zone of these DS mice (Bianchi et al., 2009). These data suggest that lithium may potentially improve neurogenesis in patients with DS.
3.10.6 Spinocerebellar ataxia type 1 (SCA1)
Spinocerebellar ataxia type 1 (SCA1), one of the nine polyglutamine disorders, is caused by the expansion of a CAG repeat that encodes polyglutamine in the respective disease protein ataxin-1 (Gatchel & Zoghbi, 2005). SCA1 is a dominantly inherited neurodegenerative disorder characterized by progressive motor and cognitive dysfunction. The pathogenesis of this disease includes the misfolding of ataxin-1 and consequent transcriptional dysregulation, and is characterized by degeneration of cerebellar Purkinje cells and brain stem neurons (Burk et al., 2003; Zoghbi & Orr, 1995). One study in a knock-in mouse model of SCA1 found that dietary lithium supplementation attenuates the reduction of dendritic branching in mutant hippocampal pyramidal neurons, and results in improvement of motor coordination, learning, and memory in these SCA1 mice. Importantly, motor improvement is achieved by either pre- or post-symptomatic lithium treatment (Watase et al., 2007). These results suggest that lithium is an excellent treatment candidate for human SCA1 patients. A clinical trial assessing the side effects and tolerability of lithium in SCA1 patients (18 to 65 years of age) has recently been completed by the National Institutes of Health, USA, and data analyses are ongoing.
3.10.7 Tardive dyskinesia (TD)
TD is clinically characterized by involuntary choreiform movements—mostly of the mouth, tongue, and face—that disappear during sleep. TD is hypothesized to result from dopaminergic supersensitivity associated with the prolonged use of typical antipsychotic medications. Early studies using animal models of TD found that lithium treatment prevents supersensitivity to apomorphine, a non-selective dopamine receptor agonist (Klawans et al., 1977; Pert et al., 1978), underscoring its potential prophylactic and therapeutic usefulness in TD. Numerous case reports also suggest that the lithium salt is beneficial in the treatment of drug-induced TD, particularly in combination with antidepressants (Dalen, 1973; Pickar & Davies, 1978; Reda et al., 1975; Rosenbaum et al., 1977; Simpson et al., 1976). However, other studies indicate that lithium only improves a subpopulation of patients (Gerlach et al., 1975; Jus et al., 1978), or fails to show a therapeutic effect in older TD patients (Simpson et al., 1976). Although a previous Cochrane review did not recommend lithium for everyday use in TD (Soares-Weiser & Joy, 2003), a recent nine-year follow-up study suggests a beneficial effect of lithium on TD in some patients using long-term antipsychotics (van Harten et al., 2008).
3.10.8 Schizophrenia
Based on the co-administration of second generation antipsychotics with mood stabilizers for BD, mood stabilizers such as lithium or anticonvulsants have been used together with antipsychotics as adjunctive medication for the treatment of schizophrenia, for the same purpose of producing potential synergistic or enhanced responses (Citrome et al., 2005; Johannessen, 2008). Although adjunctive lithium treatment in early studies seemed to be somewhat beneficial (Carman et al., 1981; Kellams et al., 1976; Small et al., 1975), later, well-designed trials found that this treatment strategy did not produce robust evidence of improvement. Therefore, this application has not been approved by regulatory agencies. While the reasons underlying lithium’s lack of conclusive effects in individuals with schizophrenia are unclear, it is interesting to note that the expression of GSK-3β protein and mRNA are decreased in both schizophrenic patients and in a rat model of this disease (Kozlovsky et al., 2005).
4. Conclusion and future directions
In recent years, accumulating evidence has focused on the neuroprotective and neurotrophic properties of lithium, rekindling interest in this drug for the treatment of a wide variety of disorders. Studies from various laboratories support the notion that lithium has robust neuroprotective effects in a vast number of cellular and animal models of brain disorders. Indeed, neuroprotection is the most consistent biological outcome associated with lithium treatment in both preclinical and clinical experimental settings. It is becoming clear that GSK-3 hyperactivity is involved in the cell death and pathophysiology of many neurodegenerative conditions, and that GSK-3 inhibition is an important target for lithium to induce neuroprotective and neurotrophic actions.
GSK-3 is inhibited by lithium via multiple mechanisms, and this GSK-3 inhibition plays a prominent role in activating a wide spectrum of signaling pathways, and inducing various anti-apoptotic and neurotrophic proteins. Furthermore, lithium-induced inhibition of phosphoinositide metabolism and IP3 production appears to be involved in upregulation of autophagy, and lithium’s effects on this process may be critical to the clearance of protein aggregates associated with neurodegenerative diseases. Emerging evidence suggests that specific microRNAs are targets of lithium and another mood stabilizer, valproate, and are involved in the regulation of expression of anti-apoptotic proteins and could have a role in the pathophysiology of some brain disorders. Further investigations in the area of microRNA research may provide new insights into the etiology of diseases and mechanisms of action of lithium.
As reviewed above, lithium treatment has been shown to be beneficial in an increasing list of animal models of CNS disorders. Decreased neurodegeneration, enhanced neurogenesis, improved behavioral performance and cognitive function, and prolonged survival time have all been reported in many preclinical studies. In addition, co-treatment of lithium with other drugs such as valproate has sometimes been found to produce more robust and consistent neuroprotective effects and behavioral improvements in vitro and in vivo. Thus, combinatory treatment to enhance the beneficial effects of lithium seems to be warranted. As a result of these promising preclinical evidences, and because of its long history of safe use in humans, a number of clinical trials using lithium to treat a variety of brain disorders are now underway. While some clinical studies show promising positive results, others indicate no improvement or response after lithium treatment. Large-scale clinical trials with long treatment durations are necessary to resolve these discrepancies. In light of the results from recently completed preclinical studies, combinatory treatment with lithium and other neuroprotective drug(s) is also recommended for future clinical investigation.
acknowledgements
This work was supported by the Intramural Research Program of the NIMH, NIH. The authors thank Dr. Joshua Hunsberger, Peter Leeds, and Ioline Henter of the Mood and Anxiety Disorder Program, NIMH, NIH, for critical review and editorial assistance of this manuscript.
Abbreviations
- 3-NP
3-nitropropionic acid
- Aβ
β-amyloid
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- AP-1
activator protein-1
- βArr2
β-arrestin 2
- Bcl-2
B-cell lymphoma/leukemia-2 protein
- BD
bipolar disorder
- BDNF
brain-derived neurotrophic factor
- Cdk5
cyclin-dependent kinase 5
- CGCs
cerebellar granule cells
- CNS
central nervous system
- CREB
cyclic AMP-response element binding protein
- DS
Down syndrome
- EAE
experimental autoimmune encephalomyelitis
- ER
endoplasmic reticulum
- ERK
extracellular-signal regulated kinase
- FoxO3a
forkhead box class O3a
- FXS
fragile X syndrome
- GRP78
glucose-regulated protein 78
- GSK-3
glycogen synthase kinase-3
- HD
Huntington’s disease
- HIV
human immunodeficiency virus
- HSF-1
heat-shock factor-1
- HSP
heat shock protein
- IMPase
inositol monophosphatase
- IP3
inositol 1,4,5-trisphosphate
- JNK
c-Jun N-terminal kinase
- L-DOPA
L-3,4-dihydroxyphenylalanine
- MAP kinase
mitogen-activated protein kinase
- MCAO
middle cerebral artery occlusion
- MEK
MAP kinase kinase
- mGluR
metabotropic glutamate receptor
- MNs
motor neurons
- MPTP
N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MS
multiple sclerosis
- mTOR
the mammalian target of rapamycin
- NMDA
N-methyl-D-aspartate
- PAI-1
plasminogen activator inhibitor-1
- PD
Parkinson’s disease
- PI3K
phosphoinositide 3-kinase
- PKA
protein kinase A
- PKC
protein kinase C
- PP2A
protein phosphatase 2A
- tPA
tissue-type plasminogen activator
- SCA1
spinocerebellar ataxia type 1
- siRNA
small interfering RNA
- TD
tardive dyskinesia
- TrkB
tyrosine receptor kinase B
- VEGF
vascular endothelial growth factor
Footnotes
Conflict of interest The authors have no conflicts of interest, financial or otherwise, to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- Aggarwal SP, Zinman L, Simpson E, McKinley J, Jackson KE, Pinto H, et al. Safety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9:481–488. doi: 10.1016/S1474-4422(10)70068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akassoglou K, Akpinar P, Murray S, Strickland S. Fibrin is a regulator of Schwann cell migration after sciatic nerve injury in mice. Neurosci.Lett. 2003;338:185–188. doi: 10.1016/s0304-3940(02)01387-3. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr.Opin.Genet.Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- Alvarez G, Munoz-Montano JR, Satrustegui J, Avila J, Bogonez E, Diaz-Nido J. Lithium protects cultured neurons against beta-amyloid-induced neurodegeneration. FEBS Lett. 1999;453:260–264. doi: 10.1016/s0014-5793(99)00685-7. [DOI] [PubMed] [Google Scholar]
- Alvarez G, Munoz-Montano JR, Satrustegui J, Avila J, Bogonez E, Diaz-Nido J. Regulation of tau phosphorylation and protection against beta-amyloid-induced neurodegeneration by lithium. Possible implications for Alzheimer’s disease. Bipolar.Disord. 2002;4:153–165. doi: 10.1034/j.1399-5618.2002.01150.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association Practice guideline for the treatment of patients with bipolar disorder (revision) Am.J.Psychiatry. 2002;159:1–50. [PubMed] [Google Scholar]
- Aminoff MJ, Marshall J. Treatment of Huntington’s chorea with lithium carbonate. A double-blind trial. Lancet. 1974;1:107–109. doi: 10.1016/s0140-6736(74)92339-3. [DOI] [PubMed] [Google Scholar]
- Anden NE, Dalen P, Johansson B. Baclofen and lithium in Huntington’s chorea. Lancet. 1973;2:93. doi: 10.1016/s0140-6736(73)93285-6. [DOI] [PubMed] [Google Scholar]
- Aoki T, Koike T, Nakano T, Shibahara K, Kondo S, Kikuchi H, et al. Induction of Bip mRNA upon programmed cell death of differentiated PC12 cells as well as rat sympathetic neurons. J.Biochem. 1997;121:122–127. doi: 10.1093/oxfordjournals.jbchem.a021554. [DOI] [PubMed] [Google Scholar]
- Appleby BS. Psychotropic medications and the treatment of human prion diseases. CNS.Neurol.Disord.Drug Targets. 2009;8:353–362. doi: 10.2174/187152709789541961. [DOI] [PubMed] [Google Scholar]
- Asghari V, Wang JF, Reiach JS, Young LT. Differential effects of mood stabilizers on Fos/Jun proteins and AP-1 DNA binding activity in human neuroblastoma SH-SY5Y cells. Brain Res.Mol.Brain Res. 1998;58:95–102. doi: 10.1016/s0169-328x(98)00107-7. [DOI] [PubMed] [Google Scholar]
- Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nat.Rev.Immunol. 2007;7:904–912. doi: 10.1038/nri2190. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Thompson PM, Dalwani M, Hayashi KM, Lee AD, Nicoletti M, et al. Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biol.Psychiatry. 2007;62:7–16. doi: 10.1016/j.biopsych.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol.Sci. 2007;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, et al. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc.Natl.Acad.Sci.U.S.A. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, et al. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc.Natl.Acad.Sci.U.S.A. 2008;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedlack RS, Maragakis N, Heiman-Patterson T. Lithium may slow progression of amyotrophic lateral sclerosis, but further study is needed. Proc.Natl.Acad.Sci.U.S.A. 2008;105:E17. doi: 10.1073/pnas.0801762105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat.Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- Berger Z, Ravikumar B, Menzies FM, Oroz LG, Underwood BR, Pangalos MN, et al. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum.Mol.Genet. 2006;15:433–442. doi: 10.1093/hmg/ddi458. [DOI] [PubMed] [Google Scholar]
- Berger Z, Ttofi EK, Michel CH, Pasco MY, Tenant S, Rubinsztein DC, et al. Lithium rescues toxicity of aggregate-prone proteins in Drosophila by perturbing Wnt pathway. Hum.Mol.Genet. 2005;14:3003–3011. doi: 10.1093/hmg/ddi331. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Downes CP, Hanley MR. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989;59:411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Sumis A, Hervey C, Nelson M, Porges SW, Weng N, et al. Open-label treatment trial of lithium to target the underlying defect in fragile X syndrome. J.Dev.Behav.Pediatr. 2008;29:293–302. doi: 10.1097/DBP.0b013e31817dc447. [DOI] [PubMed] [Google Scholar]
- Beurel E, Michalek SM, Jope RS. Innate and adaptive immune responses regulated by glycogen synthase kinase-3 (GSK3) Trends Immunol. 2010;31:24–31. doi: 10.1016/j.it.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat RV, Haeberlein S. L. Budd, Avila J. Glycogen synthase kinase 3: a drug target for CNS therapies. J.Neurochem. 2004;89:1313–1317. doi: 10.1111/j.1471-4159.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Shanley J, Correll MP, Fieles WE, Keith RA, Scott CW, et al. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc.Natl.Acad.Sci.U.S.A. 2000;97:11074–11079. doi: 10.1073/pnas.190297597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian Q, Shi T, Chuang DM, Qian Y. Lithium reduces ischemia-induced hippocampal CA1 damage and behavioral deficits in gerbils. Brain Res. 2007;1184:270–276. doi: 10.1016/j.brainres.2007.09.054. [DOI] [PubMed] [Google Scholar]
- Bianchi P, Ciani E, Contestabile A, Guidi S, Bartesaghi R. Lithium Restores Neurogenesis in the Subventricular Zone of the Ts65Dn Mouse, a Model for Down Syndrome. Brain Pathol. 2009;20:106–118. doi: 10.1111/j.1750-3639.2008.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijur GN, De SP, Jope RS. Glycogen synthase kinase-3beta facilitates staurosporine- and heat shock-induced apoptosis. Protection by lithium. J.Biol.Chem. 2000;275:7583–7590. doi: 10.1074/jbc.275.11.7583. [DOI] [PubMed] [Google Scholar]
- Bijur GN, Jope RS. Opposing actions of phosphatidylinositol 3-kinase and glycogen synthase kinase-3beta in the regulation of HSF-1 activity. J.Neurochem. 2000;75:2401–2408. doi: 10.1046/j.1471-4159.2000.0752401.x. [DOI] [PubMed] [Google Scholar]
- Boku S, Nakagawa S, Masuda T, Nishikawa H, Kato A, Kitaichi Y, et al. Glucocorticoids and lithium reciprocally regulate the proliferation of adult dentate gyrus-derived neural precursor cells through GSK-3beta and beta-catenin/TCF pathway. Neuropsychopharmacology. 2009;34:805–815. doi: 10.1038/npp.2008.198. [DOI] [PubMed] [Google Scholar]
- Boillee S, Vande VC, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Bown CD, Wang JF, Young LT. Increased expression of endoplasmic reticulum stress proteins following chronic valproate treatment of rat C6 glioma cells. Neuropharmacology. 2000;39:2162–2169. doi: 10.1016/s0028-3908(00)00029-0. [DOI] [PubMed] [Google Scholar]
- Boyle WJ, Smeal T, Defize LH, Angel P, Woodgett JR, Karin M, et al. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991;64:573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Stanley JA, Nicoletti MA, Sassi RB, Mallinger AG, Frank E, et al. 1H magnetic resonance spectroscopy investigation of the dorsolateral prefrontal cortex in bipolar disorder patients. J.Affect.Disord. 2005;86:61–67. doi: 10.1016/j.jad.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Stanley JA, Sassi RB, Nicoletti MA, Mallinger AG, Keshavan MS, et al. 1H MRS study of dorsolateral prefrontal cortex in healthy individuals before and after lithium administration. Neuropsychopharmacology. 2004;29:1918–1924. doi: 10.1038/sj.npp.1300520. [DOI] [PubMed] [Google Scholar]
- Brinkman SD, Pomara N, Barnett N, Block R, Domino EF, Gershon S. Lithium-induced increases in red blood cell choline and memory performance in Alzheimer-type dementia. Biol.Psychiatry. 1984;19:157–164. [PubMed] [Google Scholar]
- Brockington A, Wharton SB, Fernando M, Gelsthorpe CH, Baxter L, Ince PG, et al. Expression of vascular endothelial growth factor and its receptors in the central nervous system in amyotrophic lateral sclerosis. J.Neuropathol.Exp.Neurol. 2006;65:26–36. doi: 10.1097/01.jnen.0000196134.51217.74. [DOI] [PubMed] [Google Scholar]
- Brouillet E, Conde F, Beal MF, Hantraye P. Replicating Huntington’s disease phenotype in experimental animals. Prog.Neurobiol. 1999;59:427–468. doi: 10.1016/s0301-0082(99)00005-2. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr.Opin.Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Burk K, Globas C, Bosch S, Klockgether T, Zuhlke C, Daum I, et al. Cognitive deficits in spinocerebellar ataxia type 1, 2, and 3. J.Neurol. 2003;250:207–211. doi: 10.1007/s00415-003-0976-5. [DOI] [PubMed] [Google Scholar]
- Caldero J, Brunet N, Tarabal O, Piedrafita L, Hereu M, Ayala V, et al. Lithium prevents excitotoxic cell death of motoneurons in organotypic slice cultures of spinal cord. Neuroscience. 2010;165:1353–1369. doi: 10.1016/j.neuroscience.2009.11.034. [DOI] [PubMed] [Google Scholar]
- Camins A, Crespo-Biel N, Junyent F, Verdaguer E, Canudas AM, Pallas M. Calpains as a target for therapy of neurodegenerative diseases: putative role of lithium. Curr.Drug Metab. 2009;10:433–447. doi: 10.2174/138920009788898028. [DOI] [PubMed] [Google Scholar]
- Camins A, Verdaguer E, Folch J, Canudas AM, Pallas M. The role of CDK5/P25 formation/inhibition in neurodegeneration. Drug News Perspect. 2006;19:453–460. doi: 10.1358/dnp.2006.19.8.1043961. [DOI] [PubMed] [Google Scholar]
- Caricasole A, Copani A, Caraci F, Aronica E, Rozemuller AJ, Caruso A, et al. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer’s brain. J.Neurosci. 2004;24:6021–6027. doi: 10.1523/JNEUROSCI.1381-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman JS, Bigelow LB, Wyatt RJ. Lithium combined with neuroleptics in chronic schizophrenic and schizoaffective patients. J.Clin.Psychiatry. 1981;42:124–128. [PubMed] [Google Scholar]
- Carman JS, Shoulson I, Chase TN. Letter: Huntington’s chorea treated with lithium carbonate. Lancet. 1974;1:811. doi: 10.1016/s0140-6736(74)92881-5. [DOI] [PubMed] [Google Scholar]
- Carmichael J, Sugars KL, Bao YP, Rubinsztein DC. Glycogen synthase kinase-3beta inhibitors prevent cellular polyglutamine toxicity caused by the Huntington’s disease mutation. J.Biol.Chem. 2002;277:33791–33798. doi: 10.1074/jbc.M204861200. [DOI] [PubMed] [Google Scholar]
- Centeno F, Mora A, Fuentes JM, Soler G, Claro E. Partial lithium-associated protection against apoptosis induced by C2-ceramide in cerebellar granule neurons. Neuroreport. 1998;9:4199–4203. doi: 10.1097/00001756-199812210-00036. [DOI] [PubMed] [Google Scholar]
- Chakraborty G, Saito M, Mao RF, Wang R, Vadasz C, Saito M. Lithium blocks ethanol-induced modulation of protein kinases in the developing brain. Biochem.Biophys.Res.Commun. 2008;367:597–602. doi: 10.1016/j.bbrc.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalecka-Franaszek E, Chuang DM. Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc.Natl.Acad.Sci.U.S.A. 1999;96:8745–8750. doi: 10.1073/pnas.96.15.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Steelman LS, Shelton JG, Lee JT, Navolanic PM, Blalock WL, et al. Regulation of cell cycle progression and apoptosis by the Ras/Raf/MEK/ERK pathway (Review) Int.J.Oncol. 2003;22:469–480. [PubMed] [Google Scholar]
- Chang K, Barnea-Goraly N, Karchemskiy A, Simeonova DI, Barnes P, Ketter T, et al. Cortical magnetic resonance imaging findings in familial pediatric bipolar disorder. Biol.Psychiatry. 2005a;58:197–203. doi: 10.1016/j.biopsych.2005.03.039. [DOI] [PubMed] [Google Scholar]
- Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. J.Am.Acad.Child Adolesc.Psychiatry. 2005b;44:565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- Chen G, Bower KA, Ma C, Fang S, Thiele CJ, Luo J. Glycogen synthase kinase 3beta (GSK3beta) mediates 6-hydroxydopamine-induced neuronal death. FASEB J. 2004a;18:1162–1164. doi: 10.1096/fj.04-1551fje. [DOI] [PubMed] [Google Scholar]
- Chen G, Huang LD, Jiang YM, Manji HK. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J.Neurochem. 1999;72:1327–1330. doi: 10.1046/j.1471-4159.2000.0721327.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK. Enhancement of hippocampal neurogenesis by lithium. J.Neurochem. 2000;75:1729–1734. doi: 10.1046/j.1471-4159.2000.0751729.x. [DOI] [PubMed] [Google Scholar]
- Chen G, Zeng WZ, Yuan PX, Huang LD, Jiang YM, Zhao ZH, et al. The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J.Neurochem. 1999;72:879–882. doi: 10.1046/j.1471-4159.1999.720879.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J.Cereb.Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KP, Shen WW, Lu ML. Implication of serum concentration monitoring in patients with lithium intoxication. Psychiatry Clin.Neurosci. 2004b;58:25–29. doi: 10.1111/j.1440-1819.2004.01188.x. [DOI] [PubMed] [Google Scholar]
- Chen RW, Chuang DM. Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression. A prominent role in neuroprotection against excitotoxicity. J.Biol.Chem. 1999;274:6039–6042. doi: 10.1074/jbc.274.10.6039. [DOI] [PubMed] [Google Scholar]
- Chen RW, Qin ZH, Ren M, Kanai H, Chalecka-Franaszek E, Leeds P, et al. Regulation of c-Jun N-terminal kinase, p38 kinase and AP-1 DNA binding in cultured brain neurons: roles in glutamate excitotoxicity and lithium neuroprotection. J.Neurochem. 2003;84:566–575. doi: 10.1046/j.1471-4159.2003.01548.x. [DOI] [PubMed] [Google Scholar]
- Chiu CT, Liu G, Leeds P, Chuang DM. A comparative behavioral study of the beneficial effects of mood stabilizing drugs lithium and valproate in transgenic mouse models of Huntington’s disease. Program No. 240.23. The 39th Annual Meeting of Society for Neuroscience.2009. [Google Scholar]
- Cho KS, Chen DF. Promoting optic nerve regeneration in adult mice with pharmaceutical approach. Neurochem.Res. 2008;33:2126–2133. doi: 10.1007/s11064-008-9736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CH, McBride SM, Schoenfeld BP, Liebelt DA, Ferreiro D, Ferrick NJ, et al. Age-dependent cognitive impairment in a Drosophila Fragile X model and its pharmacological rescue. Biogerontology. 2009;11:347–362. doi: 10.1007/s10522-009-9259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B, Soncin F, Price BD, Stevenson MA, Calderwood SK. Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor-1. J.Biol.Chem. 1996;271:30847–30857. doi: 10.1074/jbc.271.48.30847. [DOI] [PubMed] [Google Scholar]
- Chuang DM. Neuroprotective and neurotrophic actions of the mood stabilizer lithium: can it be used to treat neurodegenerative diseases? Crit Rev.Neurobiol. 2004;16:83–90. doi: 10.1615/critrevneurobiol.v16.i12.90. [DOI] [PubMed] [Google Scholar]
- Chuang DM. The antiapoptotic actions of mood stabilizers: molecular mechanisms and therapeutic potentials. Ann.N.Y.Acad.Sci. 2005;1053:195–204. doi: 10.1196/annals.1344.018. [DOI] [PubMed] [Google Scholar]
- Chuang DM, Chen RW, Chalecka-Franaszek E, Ren M, Hashimoto R, Senatorov V, et al. Neuroprotective effects of lithium in cultured cells and animal models of diseases. Bipolar.Disord. 2002;4:129–136. doi: 10.1034/j.1399-5618.2002.01179.x. [DOI] [PubMed] [Google Scholar]
- Chuang DM, Hough C, Senatorov VV. Glyceraldehyde-3-phosphate dehydrogenase, apoptosis, and neurodegenerative diseases. Annu.Rev.Pharmacol.Toxicol. 2005;45:269–290. doi: 10.1146/annurev.pharmtox.45.120403.095902. [DOI] [PubMed] [Google Scholar]
- Chuang DM, Leng Y, Marinova Z, Kim HJ, Chiu CT. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009;32:591–601. doi: 10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang DM, Manji HK. In search of the Holy Grail for the treatment of neurodegenerative disorders: has a simple cation been overlooked? Biol.Psychiatry. 2007;62:4–6. doi: 10.1016/j.biopsych.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang DM, Priller J. Potential use of lithium in neurodegenerative disorders. In: Bauer M, Grof P, Müller-Oerlinghausen B, editors. Lithium in Neuropsychiatry: The Comprehensive Guide. Informa Health Care; Abingdon, Oxfordshire, United Kingdom: 2006. pp. 381–397. [Google Scholar]
- Citrome L, Goldberg JF, Stahl SM. Toward convergence in the medication treatment of bipolar disorder and schizophrenia. Harv.Rev.Psychiatry. 2005;13:28–42. doi: 10.1080/10673220590923164. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Ross DR, Ferren EL, Sullivan JL, Olanow CW. Treatment of the “on-off” phenomenon in Parkinsonism with lithium carbonate. Ann.Neurol. 1982;12:375–379. doi: 10.1002/ana.410120410. [DOI] [PubMed] [Google Scholar]
- Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu.Rev.Neurosci. 2001;24:519–550. doi: 10.1146/annurev.neuro.24.1.519. [DOI] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Crawford DC, Meadows KL, Newman JL, Taft LF, Scott E, Leslie M, et al. Prevalence of the fragile X syndrome in African-Americans. Am.J.Med.Genet. 2002;110:226–233. doi: 10.1002/ajmg.10427. [DOI] [PubMed] [Google Scholar]
- Crespo-Biel N, Camins A, Pallas M, Canudas AM. Evidence of calpain/cdk5 pathway inhibition by lithium in 3-nitropropionic acid toxicity in vivo and in vitro. Neuropharmacology. 2009;56:422–428. doi: 10.1016/j.neuropharm.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Cruz JC, Tsai LH. A Jekyll and Hyde kinase: roles for Cdk5 in brain development and disease. Curr.Opin.Neurobiol. 2004;14:390–394. doi: 10.1016/j.conb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Cuervo AM. Autophagy: many paths to the same end. Mol.Cell Biochem. 2004;263:55–72. doi: 10.1023/B:MCBI.0000041848.57020.57. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Zhu X, Yu QS, Chan SL, Camandola S, Guo Z, et al. A synthetic inhibitor of p53 protects neurons against death induced by ischemic and excitotoxic insults, and amyloid beta-peptide. J.Neurochem. 2001;77:220–228. doi: 10.1046/j.1471-4159.2001.t01-1-00220.x. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Mazzon E, Di PR, Muia C, Crisafulli C, Dugo L, et al. Glycogen synthase kinase-3beta inhibition attenuates the degree of arthritis caused by type II collagen in the mouse. Clin.Immunol. 2006;120:57–67. doi: 10.1016/j.clim.2006.03.005. [DOI] [PubMed] [Google Scholar]
- D’Mello SR, Anelli R, Calissano P. Lithium induces apoptosis in immature cerebellar granule cells but promotes survival of mature neurons. Exp.Cell Res. 1994;211:332–338. doi: 10.1006/excr.1994.1095. [DOI] [PubMed] [Google Scholar]
- Dalen P. Lithium therapy in Huntington’s chorea and tardive dyskinesia. Lancet. 1973;1:107–108. doi: 10.1016/s0140-6736(73)90510-2. [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Chacon MA, Barria MI, Garrido JL, Godoy JA, Olivares G, et al. Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by beta-amyloid fibrils. Mol.Psychiatry. 2003;8:195–208. doi: 10.1038/sj.mp.4001208. [DOI] [PubMed] [Google Scholar]
- De Sarno P, Axtell RC, Raman C, Roth KA, Alessi DR, Jope RS. Lithium prevents and ameliorates experimental autoimmune encephalomyelitis. J.Immunol. 2008;181:338–345. doi: 10.4049/jimmunol.181.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sarno P, Li X, Jope RS. Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology. 2002;43:1158–1164. doi: 10.1016/s0028-3908(02)00215-0. [DOI] [PubMed] [Google Scholar]
- Denic A, Johnson AJ, Bieber AJ, Warrington AE, Rodriguez M, Pirko I. The relevance of animal models in multiple sclerosis research. Pathophysiology. 2010 doi: 10.1016/j.pathophys.2010.04.004. Published online: May 25, 2010. doi:10.1016/j.pathophys.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Dhariwala FA, Rajadhyaksha MS. An unusual member of the Cdk family: Cdk5. Cell Mol.Neurobiol. 2008;28:351–369. doi: 10.1007/s10571-007-9242-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill J, Wang H, Zhou F, Li S. Inactivation of glycogen synthase kinase 3 promotes axonal growth and recovery in the CNS. J.Neurosci. 2008;28:8914–8928. doi: 10.1523/JNEUROSCI.1178-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolny G, Aucello M, Rizzuto E, Beccafico S, Mammucari C, Boncompagni S, et al. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab. 2008;8:425–436. doi: 10.1016/j.cmet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Dolen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, et al. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou H, Ellison B, Bradley J, Kasiyanov A, Poluektova LY, Xiong H, et al. Neuroprotective mechanisms of lithium in murine human immunodeficiency virus-1 encephalitis. J.Neurosci. 2005;25:8375–8385. doi: 10.1523/JNEUROSCI.2164-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr.Opin.Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Du WJ, Li JK, Wang QY, Hou JB, Yu B. Lithium chloride preconditioning optimizes skeletal myoblast functions for cellular cardiomyoplasty in vitro via glycogen synthase kinase-3beta/beta-catenin signaling. Cells Tissues.Organs. 2009;190:11–19. doi: 10.1159/000167699. [DOI] [PubMed] [Google Scholar]
- Duan W, Zhu X, Ladenheim B, Yu QS, Guo Z, Oyler J, et al. p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann.Neurol. 2002;52:597–606. doi: 10.1002/ana.10350. [DOI] [PubMed] [Google Scholar]
- Dugo L, Collin M, Allen DA, Patel NS, Bauer I, Mervaala EM, et al. GSK-3beta inhibitors attenuate the organ injury/dysfunction caused by endotoxemia in the rat. Crit Care Med. 2005;33:1903–1912. doi: 10.1097/01.ccm.0000178350.21839.44. [DOI] [PubMed] [Google Scholar]
- Duka T, Duka V, Joyce JN, Sidhu A. Alpha-Synuclein contributes to GSK-3beta-catalyzed Tau phosphorylation in Parkinson’s disease models. FASEB J. 2009;23:2820–2830. doi: 10.1096/fj.08-120410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekshyyan O, Aw TY. Apoptosis in acute and chronic neurological disorders. Front Biosci. 2004;9:1567–1576. doi: 10.2741/1357. [DOI] [PubMed] [Google Scholar]
- Eskandari F, Mistry S, Martinez PE, Torvik S, Kotila C, Sebring N, et al. Younger, premenopausal women with major depressive disorder have more abdominal fat and increased serum levels of prothrombotic factors: implications for greater cardiovascular risk. Metabolism. 2005;54:918–924. doi: 10.1016/j.metabol.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Everall IP, Bell C, Mallory M, Langford D, Adame A, Rockestein E, et al. Lithium ameliorates HIV-gp120-mediated neurotoxicity. Mol.Cell Neurosci. 2002;21:493–501. doi: 10.1006/mcne.2002.1196. [DOI] [PubMed] [Google Scholar]
- Fan Y, Yang GY. Therapeutic angiogenesis for brain ischemia: a brief review. J.Neuroimmune.Pharmacol. 2007;2:284–289. doi: 10.1007/s11481-007-9073-3. [DOI] [PubMed] [Google Scholar]
- Feinstein DL, Galea E, Aquino DA, Li GC, Xu H, Reis DJ. Heat shock protein 70 suppresses astroglial-inducible nitric-oxide synthase expression by decreasing NFkappaB activation. J.Biol.Chem. 1996;271:17724–17732. doi: 10.1074/jbc.271.30.17724. [DOI] [PubMed] [Google Scholar]
- Feng HL, Leng Y, Ma CH, Zhang J, Ren M, Chuang DM. Combined lithium and valproate treatment delays disease onset, reduces neurological deficits and prolongs survival in an amyotrophic lateral sclerosis mouse model. Neuroscience. 2008;155:567–572. doi: 10.1016/j.neuroscience.2008.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng YT. The necessity for a collection development policy statement. Libr.Resour.Tech.Serv. 1979;23:39–44. [PubMed] [Google Scholar]
- Ferguson SS, Downey WE, III, Colapietro AM, Barak LS, Menard L, Caron MG. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- Ferrucci M, Spalloni A, Bartalucci A, Cantafora E, Fulceri F, Nutini M, et al. A systematic study of brainstem motor nuclei in a mouse model of ALS, the effects of lithium. Neurobiol.Dis. 2010;37:370–383. doi: 10.1016/j.nbd.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–449. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000;25:11–14. doi: 10.1016/s0896-6273(00)80866-1. [DOI] [PubMed] [Google Scholar]
- Fisher SK, Agranoff BW, Quiroz JA, Gould TD, Manji HK. Receptor activation and inositol lipid hydrolysis in neural tissues Molecular effects of lithium. J.Neurochem. 1987;48:999–1017. doi: 10.1111/j.1471-4159.1987.tb05618.x. [DOI] [PubMed] [Google Scholar]
- Foerster K, Regli F. Lithium therapy of extrapyramidal movement disorders-an attempt (author’s transl)] Nervenarzt. 1977;48:228–232. [PubMed] [Google Scholar]
- Fornai F, Ferrucci M, Gesi M, Bandettini di PA, Giorgi FS, Biagioni F, et al. A hypothesis on prion disorders: are infectious, inherited, and sporadic causes so distinct? Brain Res.Bull. 2006;69:95–100. doi: 10.1016/j.brainresbull.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Fornai F, Longone P, Cafaro L, Kastsiuchenka O, Ferrucci M, Manca ML, et al. Lithium delays progression of amyotrophic lateral sclerosis. Proc.Natl.Acad.Sci.U.S.A. 2008;105:2052–2057. doi: 10.1073/pnas.0708022105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster AC, Collins JF, Schwarcz R. On the excitotoxic properties of quinolinic acid, 2,3-piperidine dicarboxylic acids and structurally related compounds. Neuropharmacology. 1983;22:1331–1342. doi: 10.1016/0028-3908(83)90221-6. [DOI] [PubMed] [Google Scholar]
- Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- Friedlander RM. Apoptosis and caspases in neurodegenerative diseases. N.Engl.J.Med. 2003;348:1365–1375. doi: 10.1056/NEJMra022366. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Morinobu S, Okamoto Y, Kagaya A, Yamawaki S. Chronic lithium treatment increases the expression of brain-derived neurotrophic factor in the rat brain. Psychopharmacology (Berl) 2001;158:100–106. doi: 10.1007/s002130100871. [DOI] [PubMed] [Google Scholar]
- Fukunaga K, Ishigami T, Kawano T. Transcriptional regulation of neuronal genes and its effect on neural functions: expression and function of forkhead transcription factors in neurons. J.Pharmacol.Sci. 2005;98:205–211. doi: 10.1254/jphs.fmj05001x3. [DOI] [PubMed] [Google Scholar]
- Gafni J, Ellerby LM. Calpain activation in Huntington’s disease. J.Neurosci. 2002;22:4842–4849. doi: 10.1523/JNEUROSCI.22-12-04842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu.Rev.Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Gallicchio VS, Cibull ML, Hughes NK, Tse KF. Effect of lithium in murine immunodeficiency virus infected animals. Pathobiology. 1993;61:216–221. doi: 10.1159/000163797. [DOI] [PubMed] [Google Scholar]
- Gao T, Newton AC. The turn motif is a phosphorylation switch that regulates the binding of Hsp70 to protein kinase C. J.Biol.Chem. 2002;277:31585–31592. doi: 10.1074/jbc.M204335200. [DOI] [PubMed] [Google Scholar]
- Gao XM, Fukamauchi F, Chuang DM. Long-term biphasic effects of lithium treatment on phospholipase C-coupled M3-muscarinic acetylcholine receptors in cultured cerebellar granule cells. Neurochem.Int. 1993;22:395–403. doi: 10.1016/0197-0186(93)90021-v. [DOI] [PubMed] [Google Scholar]
- Garber K, Smith KT, Reines D, Warren ST. Transcription, translation and fragile X syndrome. Curr.Opin.Genet.Dev. 2006;16:270–275. doi: 10.1016/j.gde.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Garcia CA, Benakanakere MR, Alard P, Kosiewicz MM, Kinane DF, Martin M. Antigenic experience dictates functional role of glycogen synthase kinase-3 in human CD4+ T cell responses. J.Immunol. 2008;181:8363–8371. doi: 10.4049/jimmunol.181.12.8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatchel JR, Zoghbi HY. Diseases of unstable repeat expansion: mechanisms and common principles. Nat.Rev.Genet. 2005;6:743–755. doi: 10.1038/nrg1691. [DOI] [PubMed] [Google Scholar]
- Gerlach J, Thorsen K, Munkvad I. Effect of lithium on neuroleptic-induced tardive dyskinesia compared with placebo in a double-blind cross-over trial. Pharmakopsychiatr.Neuropsychopharmakol. 1975;8:51–56. doi: 10.1055/s-0028-1094443. [DOI] [PubMed] [Google Scholar]
- Ghribi O, Herman MM, Savory J. Lithium inhibits Abeta-induced stress in endoplasmic reticulum of rabbit hippocampus but does not prevent oxidative damage and tau phosphorylation. J.Neurosci.Res. 2003;71:853–862. doi: 10.1002/jnr.10511. [DOI] [PubMed] [Google Scholar]
- Gill A, Kidd J, Vieira F, Thompson K, Perrin S. No benefit from chronic lithium dosing in a sibling-matched, gender balanced, investigator-blinded trial using a standard mouse model of familial ALS. PLoS.One. 2009;4:e6489. doi: 10.1371/journal.pone.0006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FC, Sousa VO, Romao L. Emerging roles for TGF-beta1 in nervous system development. Int.J.Dev.Neurosci. 2005;23:413–424. doi: 10.1016/j.ijdevneu.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Goni-Oliver P, Lucas JJ, Avila J, Hernandez F. N-terminal cleavage of GSK-3 by calpain: a new form of GSK-3 regulation. J.Biol.Chem. 2007;282:22406–22413. doi: 10.1074/jbc.M702793200. [DOI] [PubMed] [Google Scholar]
- Goodwin FK. Rationale for using lithium in combination with other mood stabilizers in the management of bipolar disorder. J.Clin.Psychiatry. 2003;64(Suppl 5):18–24. [PubMed] [Google Scholar]
- Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, Chen G, Manji HK. In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology. 2004a;29:32–38. doi: 10.1038/sj.npp.1300283. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H, Bhat R, Manji HK. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int.J.Neuropsychopharmacol. 2004b;7:387–390. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H, O’Donnell KC, Picchini AM, Schloesser RJ, Manji HK. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007;32:2173–2183. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- Gould TD, Gray NA, Manji HK. Effects of a glycogen synthase kinase-3 inhibitor, lithium, in adenomatous polyposis coli mutant mice. Pharmacol.Res. 2003;48:49–53. [PubMed] [Google Scholar]
- Gould TD, O’Donnell KC, Picchini AM, Dow ER, Chen G, Manji HK. Generation and behavioral characterization of beta-catenin forebrain-specific conditional knock-out mice. Behav.Brain Res. 2008;189:117–125. doi: 10.1016/j.bbr.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, Quiroz JA, Singh J, Zarate CA, Manji HK. Emerging experimental therapeutics for bipolar disorder: insights from the molecular and cellular actions of current mood stabilizers. Mol.Psychiatry. 2004c;9:734–755. doi: 10.1038/sj.mp.4001518. [DOI] [PubMed] [Google Scholar]
- Grandjean EM, Aubry JM. Lithium: updated human knowledge using an evidence-based approach. Part II: Clinical pharmacology and therapeutic monitoring. CNS.Drugs. 2009;23:331–349. doi: 10.2165/00023210-200923040-00005. [DOI] [PubMed] [Google Scholar]
- Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog.Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Guo S, Arai K, Stins MF, Chuang DM, Lo EH. Lithium upregulates vascular endothelial growth factor in brain endothelial cells and astrocytes. Stroke. 2009;40:652–655. doi: 10.1161/STROKEAHA.108.524504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafler DA. Multiple sclerosis. J.Clin.Invest. 2004;113:788–794. doi: 10.1172/JCI21357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Ono MY, Hagerman PJ. Recent advances in fragile X: a model for autism and neurodegeneration. Curr.Opin.Psychiatry. 2005;18:490–496. doi: 10.1097/01.yco.0000179485.39520.b0. [DOI] [PubMed] [Google Scholar]
- Hampel H, Ewers M, Burger K, Annas P, Mortberg A, Bogstedt A, et al. Lithium trial in Alzheimer’s disease: a randomized, single-blind, placebo-controlled, multicenter 10-week study. J.Clin.Psychiatry. 2009;70:922–931. [PubMed] [Google Scholar]
- Han R, Gao B, Sheng R, Zhang LS, Zhang HL, Gu ZL, et al. Synergistic effects of prostaglandin E1 and lithium in a rat model of cerebral ischemia. Acta Pharmacol.Sin. 2008;29:1141–1149. doi: 10.1111/j.1745-7254.2008.00873.x. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Hough C, Nakazawa T, Yamamoto T, Chuang DM. Lithium protection against glutamate excitotoxicity in rat cerebral cortical neurons: involvement of NMDA receptor inhibition possibly by decreasing NR2B tyrosine phosphorylation. J.Neurochem. 2002a;80:589–597. doi: 10.1046/j.0022-3042.2001.00728.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Fujimaki K, Jeong MR, Christ L, Chuang DM. Lithium-induced inhibition of Src tyrosine kinase in rat cerebral cortical neurons: a role in neuroprotection against N-methyl-D-aspartate receptor-mediated excitotoxicity. FEBS Lett. 2003a;538:145–148. doi: 10.1016/s0014-5793(03)00167-4. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Senatorov V, Kanai H, Leeds P, Chuang DM. Lithium stimulates progenitor proliferation in cultured brain neurons. Neuroscience. 2003b;117:55–61. doi: 10.1016/s0306-4522(02)00577-8. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Takei N, Shimazu K, Christ L, Lu B, Chuang DM. Lithium induces brain-derived neurotrophic factor and activates TrkB in rodent cortical neurons: an essential step for neuroprotection against glutamate excitotoxicity. Neuropharmacology. 2002b;43:1173–1179. doi: 10.1016/s0028-3908(02)00217-4. [DOI] [PubMed] [Google Scholar]
- He H, Lam M, McCormick TS, Distelhorst CW. Maintenance of calcium homeostasis in the endoplasmic reticulum by Bcl-2. J.Cell Biol. 1997;138:1219–1228. doi: 10.1083/jcb.138.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, McColl K, Distelhorst CW. Involvement of c-Fos in signaling grp78 induction following ER calcium release. Oncogene. 2000;19:5936–5943. doi: 10.1038/sj.onc.1203994. [DOI] [PubMed] [Google Scholar]
- Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur.J.Neurosci. 2005;21:1304–1314. doi: 10.1111/j.1460-9568.2005.03951.x. [DOI] [PubMed] [Google Scholar]
- Heiseke A, Aguib Y, Riemer C, Baier M, Schatzl HM. Lithium induces clearance of protease resistant prion protein in prion-infected cells by induction of autophagy. J.Neurochem. 2009;109:25–34. doi: 10.1111/j.1471-4159.2009.05906.x. [DOI] [PubMed] [Google Scholar]
- Hendrick JP, Hartl FU. Molecular chaperone functions of heat-shock proteins. Annu.Rev.Biochem. 1993;62:349–384. doi: 10.1146/annurev.bi.62.070193.002025. [DOI] [PubMed] [Google Scholar]
- Hickey MA, Chesselet MF. Apoptosis in Huntington’s disease. Prog.Neuropsychopharmacol.Biol.Psychiatry. 2003;27:255–265. doi: 10.1016/S0278-5846(03)00021-6. [DOI] [PubMed] [Google Scholar]
- Higgins PJ. The TGF-beta1/Upstream Stimulatory Factor-Regulated PAI-1 Gene: Potential Involvement and a Therapeutic Target in Alzheimer’s Disease. J.Biomed.Biotechnol. 2006;2006:15792. doi: 10.1155/JBB/2006/15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi T, Wei H, Hough C, Leeds P, Chuang DM. Protracted lithium treatment protects against the ER stress elicited by thapsigargin in rat PC12 cells: roles of intracellular calcium, GRP78 and Bcl-2. Pharmacogenomics.J. 2005;5:102–111. doi: 10.1038/sj.tpj.6500296. [DOI] [PubMed] [Google Scholar]
- Hodges A, Strand AD, Aragaki AK, Kuhn A, Sengstag T, Hughes G, et al. Regional and cellular gene expression changes in human Huntington’s disease brain. Hum.Mol.Genet. 2006;15:965–977. doi: 10.1093/hmg/ddl013. [DOI] [PubMed] [Google Scholar]
- Hoehn B, Ringer TM, Xu L, Giffard RG, Sapolsky RM, Steinberg GK, et al. Overexpression of HSP72 after induction of experimental stroke protects neurons from ischemic damage. J.Cereb.Blood Flow Metab. 2001;21:1303–1309. doi: 10.1097/00004647-200111000-00006. [DOI] [PubMed] [Google Scholar]
- Holroyd S, Smith D. Disabling parkinsonism due to lithium: a case report. J.Geriatr.Psychiatry Neurol. 1995;8:118–119. doi: 10.1177/089198879500800208. [DOI] [PubMed] [Google Scholar]
- Hong M, Chen DC, Klein PS, Lee VM. Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J.Biol.Chem. 1997;272:25326–25332. doi: 10.1074/jbc.272.40.25326. [DOI] [PubMed] [Google Scholar]
- Hongisto V, Smeds N, Brecht S, Herdegen T, Courtney MJ, Coffey ET. Lithium blocks the c-Jun stress response and protects neurons via its action on glycogen synthase kinase 3. Mol.Cell Biol. 2003;23:6027–6036. doi: 10.1128/MCB.23.17.6027-6036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou XY, Zhang GY, Yan JZ, Chen M, Liu Y. Activation of NMDA receptors and L-type voltage-gated calcium channels mediates enhanced formation of Fyn-PSD95-NR2A complex after transient brain ischemia. Brain Res. 2002;955:123–132. doi: 10.1016/s0006-8993(02)03376-0. [DOI] [PubMed] [Google Scholar]
- Hough C, Lu SJ, Davis CL, Chuang DM, Post RM. Elevated basal and thapsigargin-stimulated intracellular calcium of platelets and lymphocytes from bipolar affective disorder patients measured by a fluorometric microassay. Biol.Psychiatry. 1999;46:247–255. doi: 10.1016/s0006-3223(98)00308-4. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu.Rev.Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Huang HC, Klein PS. Multiple roles for glycogen synthase kinase-3 as a drug target in Alzheimer’s disease. Curr.Drug Targets. 2006;7:1389–1397. doi: 10.2174/1389450110607011389. [DOI] [PubMed] [Google Scholar]
- Huang W, Galdzicki Z, van GP, Balbo A, Chikhale EG, Schapiro MB, et al. Brain myo-inositol level is elevated in Ts65Dn mouse and reduced after lithium treatment. Neuroreport. 2000;11:445–448. doi: 10.1097/00001756-200002280-00004. [DOI] [PubMed] [Google Scholar]
- Huang X, Wu DY, Chen G, Manji H, Chen DF. Support of retinal ganglion cell survival and axon regeneration by lithium through a Bcl-2-dependent mechanism. Invest Ophthalmol.Vis.Sci. 2003;44:347–354. doi: 10.1167/iovs.02-0198. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Behrens J. The Wnt signalling pathway. J.Cell Sci. 2002;115:3977–3978. doi: 10.1242/jcs.00089. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Alvarez A, Godoy J, Reyes A, De Ferrari GV. Acetylcholinesterase-amyloid-beta-peptide interaction and Wnt signaling involvement in Abeta neurotoxicity. Acta Neurol.Scand.Suppl. 2000;176:53–59. doi: 10.1034/j.1600-0404.2000.00308.x. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Jacobsen JP, Mork A. The effect of escitalopram, desipramine, electroconvulsive seizures and lithium on brain-derived neurotrophic factor mRNA and protein expression in the rat brain and the correlation to 5-HT and 5-HIAA levels. Brain Res. 2004;1024:183–192. doi: 10.1016/j.brainres.2004.07.065. [DOI] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc.Natl.Acad.Sci.U.S.A. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen LC. Antiepileptic drugs in non-epilepsy disorders: relations between mechanisms of action and clinical efficacy. CNS.Drugs. 2008;22:27–47. doi: 10.2165/00023210-200822010-00003. [DOI] [PubMed] [Google Scholar]
- Jope RS. Anti-bipolar therapy: mechanism of action of lithium. Mol.Psychiatry. 1999a;4:117–128. doi: 10.1038/sj.mp.4000494. [DOI] [PubMed] [Google Scholar]
- Jope RS. A bimodal model of the mechanism of action of lithium. Mol.Psychiatry. 1999b;4:21–25. doi: 10.1038/sj.mp.4000444. [DOI] [PubMed] [Google Scholar]
- Jope RS. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol.Sci. 2003;24:441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem.Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Jope RS, Roh MS. Glycogen synthase kinase-3 (GSK3) in psychiatric diseases and therapeutic interventions. Curr.Drug Targets. 2006;7:1421–1434. doi: 10.2174/1389450110607011421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem.Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorda EG, Verdaguer E, Canudas AM, Jimenez A, Garcia de AS, Allgaier C, et al. Implication of cyclin-dependent kinase 5 in the neuroprotective properties of lithium. Neuroscience. 2005;134:1001–1011. doi: 10.1016/j.neuroscience.2005.04.061. [DOI] [PubMed] [Google Scholar]
- Jorda EG, Verdaguer E, Morano A, Jimenez A, Canudas AM, Camins A, et al. Lithium prevents colchicine-induced apoptosis in rat cerebellar granule neurons. Bipolar.Disord. 2004;6:144–149. doi: 10.1046/j.1399-5618.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- Jus A, Villeneuve A, Gautier J, Jus K, Villeneuve C, Pires P, et al. Deanol, lithium and placebo in the treatment of tardive dyskinesia. A double-blind crossover study. Neuropsychobiology. 1978;4:140–149. doi: 10.1159/000117629. [DOI] [PubMed] [Google Scholar]
- Kaga S, Zhan L, Altaf E, Maulik N. Glycogen synthase kinase-3beta/beta-catenin promotes angiogenic and anti-apoptotic signaling through the induction of VEGF, Bcl-2 and survivin expression in rat ischemic preconditioned myocardium. J.Mol.Cell Cardiol. 2006;40:138–147. doi: 10.1016/j.yjmcc.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Lipina TV, Takao K, van EM, Hattori S, Laliberte C, et al. Abnormalities in brain structure and behavior in GSK-3alpha mutant mice. Mol.Brain. 2009;2:35. doi: 10.1186/1756-6606-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H. Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol.Psychiatry. 2004;55:781–784. doi: 10.1016/j.biopsych.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Kalasapudi VD, Sheftel G, Divish MM, Papolos DF, Lachman HM. Lithium augments fos protoonocogene expression in PC12 pheochromocytoma cells: implications for therapeutic action of lithium. Brain Res. 1990;521:47–54. doi: 10.1016/0006-8993(90)91523-j. [DOI] [PubMed] [Google Scholar]
- Karcher D, Chamoles N, Zeman W, Lowenthal A. [Hydrosoluble proteins of the nervous system of man and the animal] Acta Zool.Pathol.Antverp. 1969;48:73–76. [PubMed] [Google Scholar]
- Karin M. Mitogen-activated protein kinase cascades as regulators of stress responses. Ann.N.Y.Acad.Sci. 1998;851:139–146. doi: 10.1111/j.1749-6632.1998.tb08987.x. [DOI] [PubMed] [Google Scholar]
- Katayama T, Imaizumi K, Sato N, Miyoshi K, Kudo T, Hitomi J, et al. Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat.Cell Biol. 1999;1:479–485. doi: 10.1038/70265. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- Kellams JJ, Small JG, Milstein V, Perex HC. Lithium combined with neuroleptics in the treatment of chronic schizophrenia. Psychopharmacol.Bull. 1976;12:27–30. [PubMed] [Google Scholar]
- Kessing LV, Sondergard L, Forman JL, Andersen PK. Lithium treatment and risk of dementia. Arch.Gen.Psychiatry. 2008;65:1331–1335. doi: 10.1001/archpsyc.65.11.1331. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Manji HK, Post RM. Potential mechanisms of action of lamotrigine in the treatment of bipolar disorders. J.Clin.Psychopharmacol. 2003;23:484–495. doi: 10.1097/01.jcp.0000088915.02635.e8. [DOI] [PubMed] [Google Scholar]
- Kim YR, van Meer MP, Tejima E, Murata Y, Mandeville JB, Dai G, et al. Functional MRI of delayed chronic lithium treatment in rat focal cerebral ischemia. Stroke. 2008;39:439–447. doi: 10.1161/STROKEAHA.107.492215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King TD, Bijur GN, Jope RS. Caspase-3 activation induced by inhibition of mitochondrial complex I is facilitated by glycogen synthase kinase-3beta and attenuated by lithium. Brain Res. 2001;919:106–114. doi: 10.1016/s0006-8993(01)03005-0. [DOI] [PubMed] [Google Scholar]
- Kirshenboim N, Plotkin B, Shlomo SB, Kaidanovich-Beilin O, Eldar-Finkelman H. Lithium-mediated phosphorylation of glycogen synthase kinase-3beta involves PI3 kinase-dependent activation of protein kinase C-alpha. J.Mol.Neurosci. 2004;24:237–245. doi: 10.1385/JMN:24:2:237. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Tsujimoto Y, Ohtsuki T, Kuwabara K, Matsushita K, et al. Amelioration of hippocampal neuronal damage after global ischemia by neuronal overexpression of BCL-2 in transgenic mice. Stroke. 1998;29:2616–2621. doi: 10.1161/01.str.29.12.2616. [DOI] [PubMed] [Google Scholar]
- Klawans HL, Weiner WJ, Nausieda PA. The effect of lithium on an animal model of tardive dyskinesia. Prog.Neuropsychopharmacol. 1977;1:53–60. doi: 10.1016/0364-7722(77)90027-3. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc.Natl.Acad.Sci.U.S.A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SH, Song C, Noh MY, Kim HY, Lee KY, Lee YJ, et al. Inhibition of glycogen synthase kinase-3 reduces L-DOPA-induced neurotoxicity. Toxicology. 2008;247:112–118. doi: 10.1016/j.tox.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Kopnisky KL, Chalecka-Franaszek E, Gonzalez-Zulueta M, Chuang DM. Chronic lithium treatment antagonizes glutamate-induced decrease of phosphorylated CREB in neurons via reducing protein phosphatase 1 and increasing MEK activities. Neuroscience. 2003;116:425–435. doi: 10.1016/s0306-4522(02)00573-0. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Nadri C, Agam G. Low GSK-3beta in schizophrenia as a consequence of neurodevelopmental insult. Eur.Neuropsychopharmacol. 2005;15:1–11. doi: 10.1016/j.euroneuro.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Kramer M, Dang J, Baertling F, Denecke B, Clarner T, Kirsch C, et al. TTC staining of damaged brain areas after MCA occlusion in the rat does not constrict quantitative gene and protein analyses. J.Neurosci.Methods. 2010;187:84–89. doi: 10.1016/j.jneumeth.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, et al. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nat.Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Krupnik VE, Sharp JD, Jiang C, Robison K, Chickering TW, Amaravadi L, et al. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238:301–313. doi: 10.1016/s0378-1119(99)00365-0. [DOI] [PubMed] [Google Scholar]
- Kuchroo VK, Anderson AC, Waldner H, Munder M, Bettelli E, Nicholson LB. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu.Rev.Immunol. 2002;20:101–123. doi: 10.1146/annurev.immunol.20.081701.141316. [DOI] [PubMed] [Google Scholar]
- Kumar A, Zloza A, Moon RT, Watts J, Tenorio AR, Al-Harthi L. Active beta-catenin signaling is an inhibitory pathway for human immunodeficiency virus replication in peripheral blood mononuclear cells. J.Virol. 2008;82:2813–2820. doi: 10.1128/JVI.02498-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutcher ME, Klagsbrun M, Mamluk R. VEGF is required for the maintenance of dorsal root ganglia blood vessels but not neurons during development. FASEB J. 2004;18:1952–1954. doi: 10.1096/fj.04-2320fje. [DOI] [PubMed] [Google Scholar]
- Lam M, Dubyak G, Chen L, Nunez G, Miesfeld RL, Distelhorst CW. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc.Natl.Acad.Sci.U.S.A. 1994;91:6569–6573. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam RW, Allain S, Sullivan K, Beattie CW, Remick RA, Zis AP. Effects of chronic lithium treatment on retinal electrophysiologic function. Biol.Psychiatry. 1997;41:737–742. doi: 10.1016/S0006-3223(96)00004-2. [DOI] [PubMed] [Google Scholar]
- Lambert SL, Martinez OM. Latent membrane protein 1 of EBV activates phosphatidylinositol 3-kinase to induce production of IL-10. J.Immunol. 2007;179:8225–8234. doi: 10.4049/jimmunol.179.12.8225. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- Lee VM, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu.Rev.Neurosci. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- Leng Y, Liang MH, Ren M, Marinova Z, Leeds P, Chuang DM. Synergistic neuroprotective effects of lithium and valproic acid or other histone deacetylase inhibitors in neurons: roles of glycogen synthase kinase-3 inhibition. J.Neurosci. 2008;28:2576–2588. doi: 10.1523/JNEUROSCI.5467-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard DP, Kidson MA, Brown JG, Shannon PJ, Taryan S. A double blind trial of lithium carbonate and haloperidol in Huntington’s chorea. Aust.N.Z.J.Psychiatry. 1975;9:115–118. doi: 10.3109/00048677509159834. [DOI] [PubMed] [Google Scholar]
- Leonard DP, Kidson MA, Shannon PJ, Brown J. Letter: Double-blind trial of lithium carbonate and haloperidol in Huntington’s chorea. Lancet. 1974;2:1208–1209. doi: 10.1016/s0140-6736(74)90847-2. [DOI] [PubMed] [Google Scholar]
- Leroy K, Ando K, Heraud C, Yilmaz Z, Authelet M, Boeynaems JM, et al. Lithium treatment arrests the development of neurofibrillary tangles in mutant tau transgenic mice with advanced neurofibrillary pathology. J.Alzheimers.Dis. 2010;19:705–719. doi: 10.3233/JAD-2010-1276. [DOI] [PubMed] [Google Scholar]
- Letendre SL, Woods SP, Ellis RJ, Atkinson JH, Masliah E, van den Brande G, et al. Lithium improves HIV-associated neurocognitive impairment. AIDS. 2006;20:1885–1888. doi: 10.1097/01.aids.0000244208.49123.1b. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyhe T, Eschweiler GW, Stransky E, Gasser T, Annas P, Basun H, et al. Increase of BDNF serum concentration in lithium treated patients with early Alzheimer’s disease. J.Alzheimers.Dis. 2009;16:649–656. doi: 10.3233/JAD-2009-1004. [DOI] [PubMed] [Google Scholar]
- Li X, Ketter TA, Frye MA. Synaptic, intracellular, and neuroprotective mechanisms of anticonvulsants: are they relevant for the treatment and course of bipolar disorders? J.Affect.Disord. 2002;69:1–14. doi: 10.1016/s0165-0327(00)00361-x. [DOI] [PubMed] [Google Scholar]
- Liang MH, Chuang DM. Differential roles of glycogen synthase kinase-3 isoforms in the regulation of transcriptional activation. J.Biol.Chem. 2006;281:30479–30484. doi: 10.1074/jbc.M607468200. [DOI] [PubMed] [Google Scholar]
- Liang MH, Chuang DM. Regulation and function of glycogen synthase kinase-3 isoforms in neuronal survival. J.Biol.Chem. 2007;282:3904–3917. doi: 10.1074/jbc.M605178200. [DOI] [PubMed] [Google Scholar]
- Liang MH, Wendland JR, Chuang DM. Lithium inhibits Smad3/4 transactivation via increased CREB activity induced by enhanced PKA and AKT signaling. Mol.Cell Neurosci. 2008;37:440–453. doi: 10.1016/j.mcn.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman A, Gopinathan G. Treatment of “on-off” phenomena with lithium. Ann.Neurol. 1982;12:402. doi: 10.1002/ana.410120416. [DOI] [PubMed] [Google Scholar]
- Lin D, Mok H, Yatham LN. Polytherapy in bipolar disorder. CNS.Drugs. 2006;20:29–42. doi: 10.2165/00023210-200620010-00003. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu.Rev.Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Liu F, Gong X, Zhang G, Marquis K, Reinhart P, Andree TH. The inhibition of glycogen synthase kinase 3beta by a metabotropic glutamate receptor 5 mediated pathway confers neuroprotection to Abeta peptides. J.Neurochem. 2005;95:1363–1372. doi: 10.1111/j.1471-4159.2005.03474.x. [DOI] [PubMed] [Google Scholar]
- Liu H, Thurig S, Mohamed O, Dufort D, Wallace VA. Mapping canonical Wnt signaling in the developing and adult retina. Invest Ophthalmol.Vis.Sci. 2006;47:5088–5097. doi: 10.1167/iovs.06-0403. [DOI] [PubMed] [Google Scholar]
- Liu H, Xu S, Wang Y, Mazerolle C, Thurig S, Coles BL, et al. Ciliary margin transdifferentiation from neural retina is controlled by canonical Wnt signaling. Dev.Biol. 2007;308:54–67. doi: 10.1016/j.ydbio.2007.04.052. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang G, Gao C, Hou X. NMDA receptor activation results in tyrosine phosphorylation of NMDA receptor subunit 2A(NR2A) and interaction of Pyk2 and Src with NR2A after transient cerebral ischemia and reperfusion. Brain Res. 2001;909:51–58. doi: 10.1016/s0006-8993(01)02619-1. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Smith CB. Dissociation of social and nonsocial anxiety in a mouse model of fragile X syndrome. Neurosci.Lett. 2009;454:62–66. doi: 10.1016/j.neulet.2009.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse MJ, Lefkowitz RJ, Caron MG, Benovic JL. Inhibition of beta-adrenergic receptor kinase prevents rapid homologous desensitization of beta 2-adrenergic receptors. Proc.Natl.Acad.Sci.U.S.A. 1989;86:3011–3015. doi: 10.1073/pnas.86.9.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Zhang GY. Lithium reduced N-methyl-D-aspartate receptor subunit 2A tyrosine phosphorylation and its interactions with Src and Fyn mediated by PSD-95 in rat hippocampus following cerebral ischemia. Neurosci.Lett. 2003;348:185–189. doi: 10.1016/s0304-3940(03)00784-5. [DOI] [PubMed] [Google Scholar]
- Ma J, Zhang GY, Liu Y, Yan JZ, Hao ZB. Lithium suppressed Tyr-402 phosphorylation of proline-rich tyrosine kinase (Pyk2) and interactions of Pyk2 and PSD-95 with NR2A in rat hippocampus following cerebral ischemia. Neurosci.Res. 2004;49:357–362. doi: 10.1016/j.neures.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM. The unfolding tale of the unfolded protein response. Cell. 2001;107:827–830. doi: 10.1016/s0092-8674(01)00623-7. [DOI] [PubMed] [Google Scholar]
- Macdonald A, Briggs K, Poppe M, Higgins A, Velayudhan L, Lovestone S. A feasibility and tolerability study of lithium in Alzheimer’s disease. Int.J.Geriatr.Psychiatry. 2008;23:704–711. doi: 10.1002/gps.1964. [DOI] [PubMed] [Google Scholar]
- MacDonald ME, Ambrose CM, Duyao MP, Myers RH, Lin C, Srinidhi L, et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Maggirwar SB, Tong N, Ramirez S, Gelbard HA, Dewhurst S. HIV-1 Tat-mediated activation of glycogen synthase kinase-3beta contributes to Tat-mediated neurotoxicity. J.Neurochem. 1999;73:578–586. doi: 10.1046/j.1471-4159.1999.0730578.x. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat.Rev.Mol.Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- Majda BT, Meloni BP, Rixon N, Knuckey NW. Suppression subtraction hybridization and northern analysis reveal upregulation of heat shock, trkB, and sodium calcium exchanger genes following global cerebral ischemia in the rat. Brain Res.Mol.Brain Res. 2001;93:173–179. doi: 10.1016/s0169-328x(01)00203-0. [DOI] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat.Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Manji HK, Duman RS. Impairments of neuroplasticity and cellular resilience in severe mood disorders: implications for the development of novel therapeutics. Psychopharmacol.Bull. 2001;35:5–49. [PubMed] [Google Scholar]
- Manji HK, Lenox RH. Lithium: a molecular transducer of mood-stabilization in the treatment of bipolar disorder. Neuropsychopharmacology. 1998;19:161–166. doi: 10.1016/S0893-133X(98)00021-9. [DOI] [PubMed] [Google Scholar]
- Manji HK, Quiroz JA, Sporn J, Payne JL, Denicoff K, Gray A, et al. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol.Psychiatry. 2003;53:707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- Manyam NV, Bravo-Fernandez E. Lithium carbonate in Huntington’s chorea. Lancet. 1973;1:1010. doi: 10.1016/s0140-6736(73)91664-4. [DOI] [PubMed] [Google Scholar]
- Mao Z, Liu L, Zhang R, Li X. Lithium reduces FoxO3a transcriptional activity by decreasing its intracellular content. Biol.Psychiatry. 2007;62:1423–1430. doi: 10.1016/j.biopsych.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Marmol F. Lithium: bipolar disorder and neurodegenerative diseases Possible cellular mechanisms of the therapeutic effects of lithium. Prog.Neuropsychopharmacol.Biol.Psychiatry. 2008;32:1761–1771. doi: 10.1016/j.pnpbp.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Martin JB, Gusella JF. Huntington’s disease. Pathogenesis and management. N.Engl.J.Med. 1986;315:1267–1276. doi: 10.1056/NEJM198611133152006. [DOI] [PubMed] [Google Scholar]
- Martin L, Magnaudeix A, Esclaire F, Yardin C, Terro F. Inhibition of glycogen synthase kinase-3beta downregulates total tau proteins in cultured neurons and its reversal by the blockade of protein phosphatase-2A. Brain Res. 2009;1252:66–75. doi: 10.1016/j.brainres.2008.11.057. [DOI] [PubMed] [Google Scholar]
- Martinez RP, Raffa RB. LiCl attenuates M(1)AChR-mediated intrathecal pilocarpine-induced reciprocal hindlimb scratching in mice. Pharmacology. 2002;65:210–214. doi: 10.1159/000064346. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Neuronal life-and-death signaling, apoptosis, and neurodegenerative disorders. Antioxid.Redox.Signal. 2006;8:1997–2006. doi: 10.1089/ars.2006.8.1997. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Kroemer G. Mitochondria in cell death: novel targets for neuroprotection and cardioprotection. Trends Mol.Med. 2003;9:196–205. doi: 10.1016/s1471-4914(03)00046-7. [DOI] [PubMed] [Google Scholar]
- Mattson MP, LaFerla FM, Chan SL, Leissring MA, Shepel PN, Geiger JD. Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2000;23:222–229. doi: 10.1016/s0166-2236(00)01548-4. [DOI] [PubMed] [Google Scholar]
- Mattsson B. Huntington’s chorea and lithium therapy. Lancet. 1973;1:718–719. doi: 10.1016/s0140-6736(73)91500-6. [DOI] [PubMed] [Google Scholar]
- Maurer MH, Tripps WK, Feldmann RE, Jr., Kuschinsky W. Expression of vascular endothelial growth factor and its receptors in rat neural stem cells. Neurosci.Lett. 2003;344:165–168. doi: 10.1016/s0304-3940(03)00407-5. [DOI] [PubMed] [Google Scholar]
- Mazlo M, Pataky I, Szucs R. Morphological study of the kidneys of lithium treated rats. Acta Morphol.Hung. 1983;31:309–314. [PubMed] [Google Scholar]
- McBride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45:753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- McCaul JA, Stern GM. Letter: Lithium in Parkinson’s disease. Lancet. 1974;1:1117. doi: 10.1016/s0140-6736(74)90602-3. [DOI] [PubMed] [Google Scholar]
- McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat.Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- Meijer L, Flajolet M, Greengard P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol.Sci. 2004;25:471–480. doi: 10.1016/j.tips.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Meininger V, Shefner J, Cudkowicz M. Lithium therapy in ALS. Amyotroph.Lateral.Scler. 2008;9:122. doi: 10.1080/17482960802028247. [DOI] [PubMed] [Google Scholar]
- Melchor JP, Pawlak R, Strickland S. The tissue plasminogen activator-plasminogen proteolytic cascade accelerates amyloid-beta (Abeta) degradation and inhibits Abeta-induced neurodegeneration. J.Neurosci. 2003;23:8867–8871. doi: 10.1523/JNEUROSCI.23-26-08867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez A, Neufeld TP. The cell biology of autophagy in metazoans: a developing story. Development. 2008;135:2347–2360. doi: 10.1242/dev.016105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes CT, Mury FB, de Sa ME, Alberto FL, Forlenza OV, Dias-Neto E, et al. Lithium reduces Gsk3b mRNA levels: implications for Alzheimer Disease. Eur.Arch.Psychiatry Clin.Neurosci. 2009;259:16–22. doi: 10.1007/s00406-008-0828-5. [DOI] [PubMed] [Google Scholar]
- Meng H, Zhang Z, Zhang R, Liu X, Wang L, Robin AM, et al. Biphasic effects of exogenous VEGF on VEGF expression of adult neural progenitors. Neurosci.Lett. 2006;393:97–101. doi: 10.1016/j.neulet.2005.09.044. [DOI] [PubMed] [Google Scholar]
- Mielke K, Herdegen T. JNK and p38 stresskinases--degenerative effectors of signal-transduction-cascades in the nervous system. Prog.Neurobiol. 2000;61:45–60. doi: 10.1016/s0301-0082(99)00042-8. [DOI] [PubMed] [Google Scholar]
- Min WW, Yuskaitis CJ, Yan Q, Sikorski C, Chen S, Jope RS, et al. Elevated glycogen synthase kinase-3 activity in Fragile X mice: key metabolic regulator with evidence for treatment potential. Neuropharmacology. 2009;56:463–472. doi: 10.1016/j.neuropharm.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GJ, Bebchuk JM, Hasanat K, Chen G, Seraji-Bozorgzad N, Wilds IB, et al. Lithium increases N-acetyl-aspartate in the human brain: in vivo evidence in support of bcl-2’s neurotrophic effects? Biol.Psychiatry. 2000a;48:1–8. doi: 10.1016/s0006-3223(00)00252-3. [DOI] [PubMed] [Google Scholar]
- Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK. Lithium-induced increase in human brain grey matter. Lancet. 2000b;356:1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- Morabito MA, Sheng M, Tsai LH. Cyclin-dependent kinase 5 phosphorylates the N-terminal domain of the postsynaptic density protein PSD-95 in neurons. J.Neurosci. 2004;24:865–876. doi: 10.1523/JNEUROSCI.4582-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovich DG. Lithium neurotoxicity at normal therapeutic levels. Br.J.Psychiatry. 1993;163:410–412. doi: 10.1192/bjp.163.3.410. [DOI] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, et al. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol.Cell Biol. 2000;20:7146–7159. doi: 10.1128/mcb.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudher A, Shepherd D, Newman TA, Mildren P, Jukes JP, Squire A, et al. GSK-3beta inhibition reverses axonal transport defects and behavioural phenotypes in Drosophila. Mol.Psychiatry. 2004;9:522–530. doi: 10.1038/sj.mp.4001483. [DOI] [PubMed] [Google Scholar]
- Mummery RV. Letter: Caries under composite restorations. Br.Dent.J. 1975;139:263. doi: 10.1038/sj.bdj.4803583. [DOI] [PubMed] [Google Scholar]
- Munoz-Montano JR, Lim F, Moreno FJ, Avila J, Diaz-Nido J. Glycogen Synthase Kinase-3 Modulates Neurite Outgrowth in Cultured Neurons: Possible Implications for Neurite Pathology in Alzheimer’s Disease. J.Alzheimers.Dis. 1999;1:361–378. doi: 10.3233/jad-1999-1602. [DOI] [PubMed] [Google Scholar]
- Munoz-Montano JR, Moreno FJ, Avila J, Diaz-Nido J. Lithium inhibits Alzheimer’s disease-like tau protein phosphorylation in neurons. FEBS Lett. 1997;411:183–188. doi: 10.1016/s0014-5793(97)00688-1. [DOI] [PubMed] [Google Scholar]
- Nakashima H, Ishihara T, Suguimoto P, Yokota O, Oshima E, Kugo A, et al. Chronic lithium treatment decreases tau lesions by promoting ubiquitination in a mouse model of tauopathies. Acta Neuropathol. 2005;110:547–556. doi: 10.1007/s00401-005-1087-4. [DOI] [PubMed] [Google Scholar]
- Neri LM, Borgatti P, Capitani S, Martelli AM. The nuclear phosphoinositide 3-kinase/AKT pathway: a new second messenger system. Biochim.Biophys.Acta. 2002;1584:73–80. doi: 10.1016/s1388-1981(02)00300-1. [DOI] [PubMed] [Google Scholar]
- Nguyen KC, Rosales JL, Barboza M, Lee KY. Controversies over p25 in Alzheimer’s disease. J.Alzheimers.Dis. 2002;4:123–126. doi: 10.3233/jad-2002-4207. [DOI] [PubMed] [Google Scholar]
- Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- Nixon RA. Endosome function and dysfunction in Alzheimer’s disease and other neurodegenerative diseases. Neurobiol.Aging. 2005;26:373–382. doi: 10.1016/j.neurobiolaging.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, et al. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc.Natl.Acad.Sci.U.S.A. 2005;102:6990–6995. doi: 10.1073/pnas.0500466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocjar C, Hammonds MD, Shim SS. Chronic lithium treatment magnifies learning in rats. Neuroscience. 2007;150:774–788. doi: 10.1016/j.neuroscience.2007.09.063. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Chuang DM. Neuroprotective effects of chronic lithium on focal cerebral ischemia in rats. Neuroreport. 1998;9:2081–2084. doi: 10.1097/00001756-199806220-00031. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Hough CJ, Chuang DM. Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influx. Proc.Natl.Acad.Sci.U.S.A. 1998a;95:2642–2647. doi: 10.1073/pnas.95.5.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S, Katsube N, Chuang DM. Lithium protects rat cerebellar granule cells against apoptosis induced by anticonvulsants, phenytoin and carbamazepine. J.Pharmacol.Exp.Ther. 1998b;286:539–547. [PubMed] [Google Scholar]
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N.Engl.J.Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- Nunes PV, Forlenza OV, Gattaz WF. Lithium and risk for Alzheimer’s disease in elderly patients with bipolar disorder. Br.J.Psychiatry. 2007;190:359–360. doi: 10.1192/bjp.bp.106.029868. [DOI] [PubMed] [Google Scholar]
- O’Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S, et al. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J.Neurosci. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offen D, Beart PM, Cheung NS, Pascoe CJ, Hochman A, Gorodin S, et al. Transgenic mice expressing human Bcl-2 in their neurons are resistant to 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine neurotoxicity. Proc.Natl.Acad.Sci.U.S.A. 1998;95:5789–5794. doi: 10.1073/pnas.95.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata N, Chiu CT, Moya PR, Leng Y, Wang Z, Hunsberger JG, et al. Lentiviral-mediated GSK-3β silencing in the hippocampal dentate gyrus induces antidepressant-like effects in stressed mice. Int.J.Neuropsychopharmacol. 2010 doi: 10.1017/S1461145710000726. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki N, Chuang DM. Lithium increases transcription factor binding to AP-1 and cyclic AMP-responsive element in cultured neurons and rat brain. J.Neurochem. 1997;69:2336–2344. doi: 10.1046/j.1471-4159.1997.69062336.x. [DOI] [PubMed] [Google Scholar]
- Pardo R, Andreolotti AG, Ramos B, Picatoste F, Claro E. Opposed effects of lithium on the MEK-ERK pathway in neural cells: inhibition in astrocytes and stimulation in neurons by GSK3 independent mechanisms. J.Neurochem. 2003;87:417–426. doi: 10.1046/j.1471-4159.2003.02015.x. [DOI] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat.Neurosci. 2003;6:168–174. doi: 10.1038/nn998. [DOI] [PubMed] [Google Scholar]
- Perez M, Hernandez F, Lim F, Diaz-Nido J, Avila J. Chronic lithium treatment decreases mutant tau protein aggregation in a transgenic mouse model. J.Alzheimers.Dis. 2003a;5:301–308. doi: 10.3233/jad-2003-5405. [DOI] [PubMed] [Google Scholar]
- Perez M, Rojo AI, Wandosell F, Diaz-Nido J, Avila J. Prion peptide induces neuronal cell death through a pathway involving glycogen synthase kinase 3. Biochem.J. 2003b;372:129–136. doi: 10.1042/BJ20021596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pert A, Rosenblatt JE, Sivit C, Pert CB, Bunney WE., Jr. Long-term treatment with lithium prevents the development of dopamine receptor supersensitivity. Science. 1978;201:171–173. doi: 10.1126/science.566468. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Klein PS. Molecular targets of lithium action. Annu.Rev.Pharmacol.Toxicol. 2001;41:789–813. doi: 10.1146/annurev.pharmtox.41.1.789. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J.Biol.Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- Pickar D, Davies RK. Tardive dyskinesia in younger patients. Am.J.Psychiatry. 1978;135:385–386. doi: 10.1176/ajp.135.3.aj1353385. [DOI] [PubMed] [Google Scholar]
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT, et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Pizzasegola C, Caron I, Daleno C, Ronchi A, Minoia C, Carri MT, et al. Treatment with lithium carbonate does not improve disease progression in two different strains of SOD1 mutant mice. Amyotroph.Lateral.Scler. 2009;10:221–228. doi: 10.1080/17482960902803440. [DOI] [PubMed] [Google Scholar]
- Planel E, Yasutake K, Fujita SC, Ishiguro K. Inhibition of protein phosphatase 2A overrides tau protein kinase I/glycogen synthase kinase 3 beta and cyclin-dependent kinase 5 inhibition and results in tau hyperphosphorylation in the hippocampus of starved mouse. J.Biol.Chem. 2001;276:34298–34306. doi: 10.1074/jbc.M102780200. [DOI] [PubMed] [Google Scholar]
- Polter A, Beurel E, Yang S, Garner R, Song L, Miller CA, et al. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology. 2010;35:1761–1774. doi: 10.1038/npp.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polter A, Yang S, Zmijewska AA, van GT, Paik JH, Depinho RA, et al. Forkhead box, class O transcription factors in brain: regulation and behavioral manifestation. Biol.Psychiatry. 2009;65:150–159. doi: 10.1016/j.biopsych.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, Moechars D, Cryns K, Lenaerts I, van CH, Goris I, et al. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J.Neurosci. 2006;26:9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner SB. Prions. Proc.Natl.Acad.Sci.U.S.A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M, Kang J, Smith CB. Increased rates of cerebral glucose metabolism in a mouse model of fragile X mental retardation. Proc.Natl.Acad.Sci.U.S.A. 2002;99:15758–15763. doi: 10.1073/pnas.242377399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroz JA, Gould TD, Manji HK. Molecular effects of lithium. Mol.Interv. 2004;4:259–272. doi: 10.1124/mi.4.5.6. [DOI] [PubMed] [Google Scholar]
- Rajdev S, Hara K, Kokubo Y, Mestril R, Dillmann W, Weinstein PR, et al. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann.Neurol. 2000;47:782–791. [PubMed] [Google Scholar]
- Rametti A, Esclaire F, Yardin C, Cogne N, Terro F. Lithium down-regulates tau in cultured cortical neurons: a possible mechanism of neuroprotection. Neurosci.Lett. 2008;434:93–98. doi: 10.1016/j.neulet.2008.01.034. [DOI] [PubMed] [Google Scholar]
- Rametti A, Esclaire F, Yardin C, Terro F. Linking alterations in tau phosphorylation and cleavage during neuronal apoptosis. J.Biol.Chem. 2004;279:54518–54528. doi: 10.1074/jbc.M408186200. [DOI] [PubMed] [Google Scholar]
- Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat.Cell Biol. 2001;3:839–843. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Duden R, Rubinsztein DC. Aggregate-prone proteins with polyglutamine and polyalanine expansions are degraded by autophagy. Hum.Mol.Genet. 2002;11:1107–1117. doi: 10.1093/hmg/11.9.1107. [DOI] [PubMed] [Google Scholar]
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat.Genet. 2004;36:585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- Reda FA, Escobar JI, Scanlan JM. Lithium carbonate in the treatment of tardive dyskinesia. Am.J.Psychiatry. 1975;132:560–562. doi: 10.1176/ajp.132.5.560. [DOI] [PubMed] [Google Scholar]
- Ren M, Senatorov VV, Chen RW, Chuang DM. Postinsult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model. Proc.Natl.Acad.Sci.U.S.A. 2003;100:6210–6215. doi: 10.1073/pnas.0937423100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Torrance M, Adame A, Mante M, Bar-on P, Rose JB, et al. Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer’s disease are associated with reduced amyloid precursor protein phosphorylation. J.Neurosci. 2007;27:1981–1991. doi: 10.1523/JNEUROSCI.4321-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh MS, Eom TY, Zmijewska AA, De SP, Roth KA, Jope RS. Hypoxia activates glycogen synthase kinase-3 in mouse brain in vivo: protection by mood stabilizers and imipramine. Biol.Psychiatry. 2005;57:278–286. doi: 10.1016/j.biopsych.2004.10.039. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Vyas V, Hernandez-Estrada T, Nichol KE, Christie LA, Head E. Lack of pathology in a triple transgenic mouse model of Alzheimer’s disease after overexpression of the anti-apoptotic protein Bcl-2. J.Neurosci. 2008;28:3051–3059. doi: 10.1523/JNEUROSCI.5620-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa AO, Kaster MP, Binfare RW, Morales S, Martin-Aparicio E, Navarro-Rico ML, et al. Antidepressant-like effect of the novel thiadiazolidinone NP031115 in mice. Prog.Neuropsychopharmacol.Biol.Psychiatry. 2008;32:1549–1556. doi: 10.1016/j.pnpbp.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rosenbaum AH, Niven RG, Hanson NP, Swanson DW. Tardive dyskinesia: relationship with a primary affective disorder. Dis.Nerv.Syst. 1977;38:423–427. [PubMed] [Google Scholar]
- Ross DR, Coffey CE, Ferren EL, Walker JI, Olanow CW. “On-off” syndrome treated with lithium carbonate: a case report. Am.J.Psychiatry. 1981;138:1626–1627. doi: 10.1176/ajp.138.12.1626. [DOI] [PubMed] [Google Scholar]
- Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur.J.Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- Rowe MK, Chuang DM. Lithium neuroprotection: molecular mechanisms and clinical implications. Expert.Rev.Mol.Med. 2004;6:1–18. doi: 10.1017/S1462399404008385. [DOI] [PubMed] [Google Scholar]
- Rowe MK, Wiest C, Chuang DM. GSK-3 is a viable potential target for therapeutic intervention in bipolar disorder. Neurosci.Biobehav.Rev. 2007;31:920–931. doi: 10.1016/j.neubiorev.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LP. Amyotrophic lateral sclerosis. Curr.Opin.Neurol. 1994;7:310–315. doi: 10.1097/00019052-199408000-00006. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC. Lessons from animal models of Huntington’s disease. Trends Genet. 2002;18:202–209. doi: 10.1016/s0168-9525(01)02625-7. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Nat.Rev.Drug Discov. 2007;6:304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- Ryves WJ, Harwood AJ. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem.Biophys.Res.Commun. 2001;280:720–725. doi: 10.1006/bbrc.2000.4169. [DOI] [PubMed] [Google Scholar]
- Samson AL, Medcalf RL. Tissue-type plasminogen activator: a multifaceted modulator of neurotransmission and synaptic plasticity. Neuron. 2006;50:673–678. doi: 10.1016/j.neuron.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Sang H, Lu Z, Li Y, Ru B, Wang W, Chen J. Phosphorylation of tau by glycogen synthase kinase 3beta in intact mammalian cells influences the stability of microtubules. Neurosci.Lett. 2001;312:141–144. doi: 10.1016/s0304-3940(01)02206-6. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Kim SM, Ramaswami M. Retrograde regulation in the CNS; neuron-specific interpretations of TGF-beta signaling. Neuron. 2004;41:845–848. doi: 10.1016/s0896-6273(04)00152-7. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, et al. Lithium induces autophagy by inhibiting inositol monophosphatase. J.Cell Biol. 2005;170:1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Krishna G, Imarisio S, Saiki S, O’Kane CJ, Rubinsztein DC. A rational mechanism for combination treatment of Huntington’s disease using lithium and rapamycin. Hum.Mol.Genet. 2008;17:170–178. doi: 10.1093/hmg/ddm294. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Rubinsztein DC. Inositol and IP3 levels regulate autophagy: biology and therapeutic speculations. Autophagy. 2006;2:132–134. doi: 10.4161/auto.2387. [DOI] [PubMed] [Google Scholar]
- Sassi RB, Brambilla P, Hatch JP, Nicoletti MA, Mallinger AG, Frank E, et al. Reduced left anterior cingulate volumes in untreated bipolar patients. Biol.Psychiatry. 2004;56:467–475. doi: 10.1016/j.biopsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Scali C, Caraci F, Gianfriddo M, Diodato E, Roncarati R, Pollio G, et al. Inhibition of Wnt signaling, modulation of Tau phosphorylation and induction of neuronal cell death by DKK1. Neurobiol.Dis. 2006;24:254–265. doi: 10.1016/j.nbd.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Schenk G, Leijnse-Ybema HJ. Letter: Huntington’s chorea and levodopa. Lancet. 1974;1:364. doi: 10.1016/s0140-6736(74)93130-4. [DOI] [PubMed] [Google Scholar]
- Schifitto G, Zhong J, Gill D, Peterson DR, Gaugh MD, Zhu T, et al. Lithium therapy for human immunodeficiency virus type 1-associated neurocognitive impairment. J.Neurovirol. 2009;15:176–186. doi: 10.1080/13550280902758973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettauf F, Rejdak R, Thaler S, Bolz S, Lehaci C, Mankowska A, et al. Citicoline and lithium rescue retinal ganglion cells following partial optic nerve crush in the rat. Exp.Eye Res. 2006;83:1128–1134. doi: 10.1016/j.exer.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu.Rev.Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- Seggie J. Lithium and the retina. Prog.Neuropsychopharmacol.Biol.Psychiatry. 1988;12:241–253. doi: 10.1016/0278-5846(88)90041-3. [DOI] [PubMed] [Google Scholar]
- Seidensticker MJ, Behrens J. Biochemical interactions in the wnt pathway. Biochim.Biophys.Acta. 2000;1495:168–182. doi: 10.1016/s0167-4889(99)00158-5. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Senatorov VV, Ren M, Kanai H, Wei H, Chuang DM. Short-term lithium treatment promotes neuronal survival and proliferation in rat striatum infused with quinolinic acid, an excitotoxic model of Huntington’s disease. Mol.Psychiatry. 2004;9:371–385. doi: 10.1038/sj.mp.4001463. [DOI] [PubMed] [Google Scholar]
- Shacka JJ, Roth KA. Regulation of neuronal cell death and neurodegeneration by members of the Bcl-2 family: therapeutic implications. Curr.Drug Targets.CNS.Neurol.Disord. 2005;4:25–39. doi: 10.2174/1568007053005127. [DOI] [PubMed] [Google Scholar]
- Shao L, Sun X, Xu L, Young LT, Wang JF. Mood stabilizing drug lithium increases expression of endoplasmic reticulum stress proteins in primary cultured rat cerebral cortical cells. Life Sci. 2006;78:1317–1323. doi: 10.1016/j.lfs.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat.Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- Sherman WR, Gish BG, Honchar MP, Munsell LY. Effects of lithium on phosphoinositide metabolism in vivo. Fed.Proc. 1986;45:2639–2646. [PubMed] [Google Scholar]
- Shibata M, Lu T, Furuya T, Degterev A, Mizushima N, Yoshimori T, et al. Regulation of intracellular accumulation of mutant Huntingtin by Beclin 1. J.Biol.Chem. 2006;281:14474–14485. doi: 10.1074/jbc.M600364200. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Shibata M, Wakisaka S, Inoue T, Mashimo T, Yoshiya I. Intrathecal lithium reduces neuropathic pain responses in a rat model of peripheral neuropathy. Pain. 2000;85:59–64. doi: 10.1016/s0304-3959(99)00249-3. [DOI] [PubMed] [Google Scholar]
- Shin JH, Cho SI, Lim HR, Lee JK, Lee YA, Noh JS, et al. Concurrent administration of Neu2000 and lithium produces marked improvement of motor neuron survival, motor function, and mortality in a mouse model of amyotrophic lateral sclerosis. Mol.Pharmacol. 2007;71:965–975. doi: 10.1124/mol.106.030676. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J.Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Whetsell WO., Jr. Post-mortem high affinity glutamate uptake in human brain. Neuroscience. 1982;7:1771–1778. doi: 10.1016/0306-4522(82)90034-3. [DOI] [PubMed] [Google Scholar]
- Silva AK, Yi H, Hayes SH, Seigel GM, Hackam AS. Lithium chloride regulates the proliferation of stem-like cells in retinoblastoma cell lines: a potential role for the canonical Wnt signaling pathway. Mol.Vis. 2010;16:36–45. [PMC free article] [PubMed] [Google Scholar]
- Silva R, Martins L, Longatto-Filho A, Almeida OF, Sousa N. Lithium prevents stress-induced reduction of vascular endothelium growth factor levels. Neurosci.Lett. 2007;429:33–38. doi: 10.1016/j.neulet.2007.09.062. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Branchey MH, Lee JH, Voitashevsky A, Zoubok B. Lithium in tardive dyskinesia. Pharmakopsychiatr.Neuropsychopharmakol. 1976;9:76–80. doi: 10.1055/s-0028-1094481. [DOI] [PubMed] [Google Scholar]
- Sinha D, Wang Z, Ruchalski KL, Levine JS, Krishnan S, Lieberthal W, et al. Lithium activates the Wnt and phosphatidylinositol 3-kinase Akt signaling pathways to promote cell survival in the absence of soluble survival factors. Am.J.Physiol Renal Physiol. 2005;288:F703–F713. doi: 10.1152/ajprenal.00189.2004. [DOI] [PubMed] [Google Scholar]
- Small JG, Kellams JJ, Milstein V, Moore J. A placebo-controlled study of lithium combined with neuroleptics in chronic schizophrenic patients. Am.J.Psychiatry. 1975;132:1315–1317. doi: 10.1176/ajp.132.12.1315. [DOI] [PubMed] [Google Scholar]
- Soares-Weiser KV, Joy C. Miscellaneous treatments for neuroleptic-induced tardive dyskinesia. Cochrane.Database.Syst.Rev. 2003 doi: 10.1002/14651858.CD000208. CD000208. [DOI] [PubMed] [Google Scholar]
- Son H, Yu IT, Hwang SJ, Kim JS, Lee SH, Lee YS, et al. Lithium enhances long-term potentiation independently of hippocampal neurogenesis in the rat dentate gyrus. J.Neurochem. 2003;85:872–881. doi: 10.1046/j.1471-4159.2003.01725.x. [DOI] [PubMed] [Google Scholar]
- Song L, De SP, Jope RS. Central role of glycogen synthase kinase-3beta in endoplasmic reticulum stress-induced caspase-3 activation. J.Biol.Chem. 2002;277:44701–44708. doi: 10.1074/jbc.M206047200. [DOI] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu.Rev.Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Speirs J, Hirsch SR. Severe lithium toxicity with “normal” serum concentrations. Br.Med.J. 1978;1:815–816. doi: 10.1136/bmj.1.6116.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4:420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- Sriram S, Steiner I. Experimental allergic encephalomyelitis: a misleading model of multiple sclerosis. Ann.Neurol. 2005;58:939–945. doi: 10.1002/ana.20743. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr.Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18:189–218. doi: 10.1038/sj.leu.2403241. [DOI] [PubMed] [Google Scholar]
- Steinman L, Zamvil SS. Virtues and pitfalls of EAE for the development of therapies for multiple sclerosis. Trends Immunol. 2005;26:565–571. doi: 10.1016/j.it.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Steinman L, Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann.Neurol. 2006;60:12–21. doi: 10.1002/ana.20913. [DOI] [PubMed] [Google Scholar]
- Su H, Chu TH, Wu W. Lithium enhances proliferation and neuronal differentiation of neural progenitor cells in vitro and after transplantation into the adult rat spinal cord. Exp.Neurol. 2007;206:296–307. doi: 10.1016/j.expneurol.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Su H, Zhang W, Guo J, Guo A, Yuan Q, Wu W. Lithium enhances the neuronal differentiation of neural progenitor cells in vitro and after transplantation into the avulsed ventral horn of adult rats through the secretion of brain-derived neurotrophic factor. J.Neurochem. 2009;108:1385–1398. doi: 10.1111/j.1471-4159.2009.05902.x. [DOI] [PubMed] [Google Scholar]
- Su Y, Ryder J, Li B, Wu X, Fox N, Solenberg P, et al. Lithium, a common drug for bipolar disorder treatment, regulates amyloid-beta precursor protein processing. Biochemistry. 2004;43:6899–6908. doi: 10.1021/bi035627j. [DOI] [PubMed] [Google Scholar]
- Sugars KL, Rubinsztein DC. Transcriptional abnormalities in Huntington disease. Trends Genet. 2003;19:233–238. doi: 10.1016/S0168-9525(03)00074-X. [DOI] [PubMed] [Google Scholar]
- Sun X, Sato S, Murayama O, Murayama M, Park JM, Yamaguchi H, et al. Lithium inhibits amyloid secretion in COS7 cells transfected with amyloid precursor protein C100. Neurosci.Lett. 2002;321:61–64. doi: 10.1016/s0304-3940(01)02583-6. [DOI] [PubMed] [Google Scholar]
- Sztein MB, Simon GL, Parenti DM, Scheib R, Goldstein AL, Goodman R, et al. In vitro effects of thymosin and lithium on lymphoproliferative responses of normal donors and HIV seropositive male homosexuals with AIDS-related complex. Clin.Immunol.Immunopathol. 1987;44:51–62. doi: 10.1016/0090-1229(87)90051-1. [DOI] [PubMed] [Google Scholar]
- Tabrizi P, Wang L, Seeds N, McComb JG, Yamada S, Griffin JH, et al. Tissue plasminogen activator (tPA) deficiency exacerbates cerebrovascular fibrin deposition and brain injury in a murine stroke model: studies in tPA-deficient mice and wild-type mice on a matched genetic background. Arterioscler.Thromb.Vasc.Biol. 1999;19:2801–2806. doi: 10.1161/01.atv.19.11.2801. [DOI] [PubMed] [Google Scholar]
- Takagi N, Shinno K, Teves L, Bissoon N, Wallace MC, Gurd JW. Transient ischemia differentially increases tyrosine phosphorylation of NMDA receptor subunits 2A and 2B. J.Neurochem. 1997;69:1060–1065. doi: 10.1046/j.1471-4159.1997.69031060.x. [DOI] [PubMed] [Google Scholar]
- Takahashi-Yanaga F, Sasaguri T. The Wnt/beta-catenin signaling pathway as a target in drug discovery. J.Pharmacol.Sci. 2007;104:293–302. doi: 10.1254/jphs.cr0070024. [DOI] [PubMed] [Google Scholar]
- Takayama S, Reed JC, Homma S. Heat-shock proteins as regulators of apoptosis. Oncogene. 2003;22:9041–9047. doi: 10.1038/sj.onc.1207114. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Zhong J, Iqbal K, Trenkner E, Grundke-Iqbal I. The regulation of phosphorylation of tau in SY5Y neuroblastoma cells: the role of protein phosphatases. FEBS Lett. 1998;426:248–254. doi: 10.1016/s0014-5793(98)00346-9. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson SD, Weeks RA, Bryant DJ, Sargentoni J, Marcus CD, Harding AE, et al. Proton magnetic resonance spectroscopy in Huntington’s disease: evidence in favour of the glutamate excitotoxic theory. Mov Disord. 1996;11:167–173. doi: 10.1002/mds.870110209. [DOI] [PubMed] [Google Scholar]
- Terao T, Nakano H, Inoue Y, Okamoto T, Nakamura J, Iwata N. Lithium and dementia: a preliminary study. Prog.Neuropsychopharmacol.Biol.Psychiatry. 2006;30:1125–1128. doi: 10.1016/j.pnpbp.2006.04.020. [DOI] [PubMed] [Google Scholar]
- The Dutch-Belgian Fragile X Consortium Fmr1 knockout mice: A model to study fragile X mental retardation. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- Tolosa L, Mir M, Asensio VJ, Olmos G, Llado J. Vascular endothelial growth factor protects spinal cord motoneurons against glutamate-induced excitotoxicity via phosphatidylinositol 3-kinase. J.Neurochem. 2008;105:1080–1090. doi: 10.1111/j.1471-4159.2007.05206.x. [DOI] [PubMed] [Google Scholar]
- Tondo L, Baldessarini RJ. Long-term lithium treatment in the prevention of suicidal behavior in bipolar disorder patients. Epidemiol.Psichiatr.Soc. 2009;18:179–183. doi: 10.1017/s1121189x00000439. [DOI] [PubMed] [Google Scholar]
- Tong N, Sanchez JF, Maggirwar SB, Ramirez SH, Guo H, Dewhurst S, et al. Activation of glycogen synthase kinase 3 beta (GSK-3beta) by platelet activating factor mediates migration and cell death in cerebellar granule neurons. Eur.J.Neurosci. 2001;13:1913–1922. doi: 10.1046/j.0953-816x.2001.01572.x. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Lee VM. Phosphorylation of paired helical filament tau in Alzheimer’s disease neurofibrillary lesions: focusing on phosphatases. FASEB J. 1995;9:1570–1576. doi: 10.1096/fasebj.9.15.8529836. [DOI] [PubMed] [Google Scholar]
- Tsai SJ. The possible role of tissue-type plasminogen activator and the plasminogen system in the pathogenesis of major depression. Med.Hypotheses. 2006;66:319–322. doi: 10.1016/j.mehy.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Tsuchiya D, Hong S, Matsumori Y, Shiina H, Kayama T, Swanson RA, et al. Overexpression of rat heat shock protein 70 is associated with reduction of early mitochondrial cytochrome C release and subsequent DNA fragmentation after permanent focal ischemia. J.Cereb.Blood Flow Metab. 2003;23:718–727. doi: 10.1097/01.WCB.0000054756.97390.F7. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Morinobu S, Tanaka K, Kawano K, Yamawaki S. Lithium, but not valproate, induces the serine/threonine phosphatase activity of protein phosphatase 2A in the rat brain, without affecting its expression. J.Neural Transm. 2003;110:413–425. doi: 10.1007/s00702-002-0798-0. [DOI] [PubMed] [Google Scholar]
- van Harten PN, Hoek HW, Matroos GE, van OJ. Evidence that lithium protects against tardive dyskinesia: the Curacao Extrapyramidal syndromes study VI. Eur.Neuropsychopharmacol. 2008;18:152–155. doi: 10.1016/j.euroneuro.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Van Woert MH, Ambani LM. Lithium and levodopa in parkinsonism. Lancet. 1973;1:1390–1391. doi: 10.1016/s0140-6736(73)91717-0. [DOI] [PubMed] [Google Scholar]
- Vanacore N, Galeotti F. A clinical specification for a randomized clinical trial on lithium in amyotrophic lateral sclerosis. Proc.Natl.Acad.Sci.U.S.A. 2008;105:E35. doi: 10.1073/pnas.0801837105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Long AA, Elsaesser R, Nikolaeva D, Broadie K, Montell C. Motor deficit in a Drosophila model of mucolipidosis type IV due to defective clearance of apoptotic cells. Cell. 2008;135:838–851. doi: 10.1016/j.cell.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard P, Baastrup PC, Petersson H. Lithium treatment of Huntington’s chorea. A placebo-controlled clinical trial. Acta Psychiatr.Scand. 1977;56:183–188. doi: 10.1111/j.1600-0447.1977.tb03561.x. [DOI] [PubMed] [Google Scholar]
- Vila M, Jackson-Lewis V, Vukosavic S, Djaldetti R, Liberatore G, Offen D, et al. Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Proc.Natl.Acad.Sci.U.S.A. 2001;98:2837–2842. doi: 10.1073/pnas.051633998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivien D, Ali C. Transforming growth factor-beta signalling in brain disorders. Cytokine Growth Factor Rev. 2006;17:121–128. doi: 10.1016/j.cytogfr.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Volonte C, Ciotti MT, Merlo D. LiCl promotes survival of GABAergic neurons from cerebellum and cerebral cortex: LiCl induces survival of GABAergic neurons. Neurosci.Lett. 1994;172:6–10. doi: 10.1016/0304-3940(94)90649-1. [DOI] [PubMed] [Google Scholar]
- Vonsattel JP, DiFiglia M. Huntington disease. J.Neuropathol.Exp.Neurol. 1998;57:369–384. doi: 10.1097/00005072-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Wang JF, Azzam JE, Young LT. Valproate inhibits oxidative damage to lipid and protein in primary cultured rat cerebrocortical cells. Neuroscience. 2003;116:485–489. doi: 10.1016/s0306-4522(02)00655-3. [DOI] [PubMed] [Google Scholar]
- Wang JF, Bown CD, Chen B, Young LT. Identification of mood stabilizer-regulated genes by differential-display PCR. Int.J.Neuropsychopharmacol. 2001;4:65–74. doi: 10.1017/S1461145701002231. [DOI] [PubMed] [Google Scholar]
- Wang JF, Bown C, Young LT. Differential display PCR reveals novel targets for the mood-stabilizing drug valproate including the molecular chaperone GRP78. Mol.Pharmacol. 1999;55:521–527. [PubMed] [Google Scholar]
- Wang KK. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:20–26. doi: 10.1016/s0166-2236(99)01536-2. [DOI] [PubMed] [Google Scholar]
- Wang X, Liu P, Zhu H, Xu Y, Ma C, Dai X, et al. miR-34a, a microRNA upregulated in a double transgenic mouse model of Alzheimer’s disease, inhibits bcl2 translation. Brain Res.Bull. 2009;80:268–273. doi: 10.1016/j.brainresbull.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA, et al. Vascular endothelial growth factor overexpression delays neurodegeneration and prolongs survival in amyotrophic lateral sclerosis mice. J.Neurosci. 2007;27:304–307. doi: 10.1523/JNEUROSCI.4433-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watase K, Gatchel JR, Sun Y, Emamian E, Atkinson R, Richman R, et al. Lithium therapy improves neurological function and hippocampal dendritic arborization in a spinocerebellar ataxia type 1 mouse model. PLoS.Med. 2007;4:e182. doi: 10.1371/journal.pmed.0040182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. Alpha-Synuclein is degraded by both autophagy and the proteasome. J.Biol.Chem. 2003;278:25009–25013. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- Wei H, Leeds PR, Qian Y, Wei W, Chen R, Chuang D. beta-amyloid peptide-induced death of PC 12 cells and cerebellar granule cell neurons is inhibited by long-term lithium treatment. Eur.J.Pharmacol. 2000;392:117–123. doi: 10.1016/s0014-2999(00)00127-8. [DOI] [PubMed] [Google Scholar]
- Wei H, Qin ZH, Senatorov VV, Wei W, Wang Y, Qian Y, et al. Lithium suppresses excitotoxicity-induced striatal lesions in a rat model of Huntington’s disease. Neuroscience. 2001;106:603–612. doi: 10.1016/s0306-4522(01)00311-6. [DOI] [PubMed] [Google Scholar]
- Wei H, Wei W, Bredesen DE, Perry DC. Bcl-2 protects against apoptosis in neuronal cell line caused by thapsigargin-induced depletion of intracellular calcium stores. J.Neurochem. 1998;70:2305–2314. doi: 10.1046/j.1471-4159.1998.70062305.x. [DOI] [PubMed] [Google Scholar]
- Wexler EM, Geschwind DH, Palmer TD. Lithium regulates adult hippocampal progenitor development through canonical Wnt pathway activation. Mol.Psychiatry. 2008;13:285–292. doi: 10.1038/sj.mp.4002093. [DOI] [PubMed] [Google Scholar]
- White BC, Sullivan JM, DeGracia DJ, O’Neil BJ, Neumar RW, Grossman LI, et al. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J.Neurol.Sci. 2000;179:1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J.Mol.Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- Whittle BJ, Varga C, Posa A, Molnar A, Collin M, Thiemermann C. Reduction of experimental colitis in the rat by inhibitors of glycogen synthase kinase-3beta. Br.J.Pharmacol. 2006;147:575–582. doi: 10.1038/sj.bjp.0706509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijesekera LC, Leigh PN. Amyotrophic lateral sclerosis. Orphanet.J.Rare.Dis. 2009;4:3. doi: 10.1186/1750-1172-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GO. Management of depression in the elderly. Prim.Care. 1989;16:451–474. [PubMed] [Google Scholar]
- Williams RS, Cheng L, Mudge AW, Harwood AJ. A common mechanism of action for three mood-stabilizing drugs. Nature. 2002;417:292–295. doi: 10.1038/417292a. [DOI] [PubMed] [Google Scholar]
- Wind T, Hansen M, Jensen JK, Andreasen PA. The molecular basis for anti-proteolytic and non-proteolytic functions of plasminogen activator inhibitor type-1: roles of the reactive centre loop, the shutter region, the flexible joint region and the small serpin fragment. Biol.Chem. 2002;383:21–36. doi: 10.1515/BC.2002.003. [DOI] [PubMed] [Google Scholar]
- Woo NH, Lu B. Regulation of cortical interneurons by neurotrophins: from development to cognitive disorders. Neuroscientist. 2006;12:43–56. doi: 10.1177/1073858405284360. [DOI] [PubMed] [Google Scholar]
- Wood NI, Morton AJ. Chronic lithium chloride treatment has variable effects on motor behaviour and survival of mice transgenic for the Huntington’s disease mutation. Brain Res.Bull. 2003;61:375–383. doi: 10.1016/s0361-9230(03)00141-2. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T. Tgf-Beta pathway as a potential target in neurodegeneration and Alzheimer’s. Curr.Alzheimer Res. 2006;3:191–195. doi: 10.2174/156720506777632916. [DOI] [PubMed] [Google Scholar]
- Xavier IJ, Mercier PA, McLoughlin CM, Ali A, Woodgett JR, Ovsenek N. Glycogen synthase kinase 3beta negatively regulates both DNA-binding and transcriptional activities of heat shock factor 1. J.Biol.Chem. 2000;275:29147–29152. doi: 10.1074/jbc.M002169200. [DOI] [PubMed] [Google Scholar]
- Xu J, Culman J, Blume A, Brecht S, Gohlke P. Chronic treatment with a low dose of lithium protects the brain against ischemic injury by reducing apoptotic death. Stroke. 2003;34:1287–1292. doi: 10.1161/01.STR.0000066308.25088.64. [DOI] [PubMed] [Google Scholar]
- Xu J, Scholz A, Rosch N, Blume A, Unger T, Kreutz R, et al. Low-dose lithium combined with captopril prevents stroke and improves survival in salt-loaded, stroke-prone spontaneously hypertensive rats. J.Hypertens. 2005;23:2277–2285. doi: 10.1097/01.hjh.0000189868.48290.d8. [DOI] [PubMed] [Google Scholar]
- Xu XH, Hua YN, Zhang HL, Wu JC, Miao YZ, Han R, et al. Greater stress protein expression enhanced by combined prostaglandin A1 and lithium in a rat model of focal ischemia. Acta Pharmacol.Sin. 2007;28:1097–1104. doi: 10.1111/j.1745-7254.2007.00624.x. [DOI] [PubMed] [Google Scholar]
- Xu XH, Zhang HL, Han R, Gu ZL, Qin ZH. Enhancement of neuroprotection and heat shock protein induction by combined prostaglandin A1 and lithium in rodent models of focal ischemia. Brain Res. 2006;1102:154–162. doi: 10.1016/j.brainres.2006.04.111. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell. 2000;101:57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]
- Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major Fragile X Syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Yan XB, Hou HL, Wu LM, Liu J, Zhou JN. Lithium regulates hippocampal neurogenesis by ERK pathway and facilitates recovery of spatial learning and memory in rats after transient global cerebral ischemia. Neuropharmacology. 2007a;53:487–495. doi: 10.1016/j.neuropharm.2007.06.020. [DOI] [PubMed] [Google Scholar]
- Yan XB, Wang SS, Hou HL, Ji R, Zhou JN. Lithium improves the behavioral disorder in rats subjected to transient global cerebral ischemia. Behav.Brain Res. 2007b;177:282–289. doi: 10.1016/j.bbr.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Yang W, Leystra-Lantz C, Strong MJ. Upregulation of GSK3beta expression in frontal and temporal cortex in ALS with cognitive impairment (ALSci) Brain Res. 2008;1196:131–139. doi: 10.1016/j.brainres.2007.12.031. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol.Psychiatry. 2009;14:51–59. doi: 10.1038/sj.mp.4002099. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Han HS. Influence of hypothermia on post-ischemic inflammation: role of nuclear factor kappa B (NFkappaB) Neurochem.Int. 2006;49:164–169. doi: 10.1016/j.neuint.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Yick LW, So KF, Cheung PT, Wu WT. Lithium chloride reinforces the regeneration-promoting effect of chondroitinase ABC on rubrospinal neurons after spinal cord injury. J.Neurotrauma. 2004;21:932–943. doi: 10.1089/neu.2004.21.932. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Kirino T, Tamura A, Basugi N, Sano K. Lithium ion does not protect brain against transient ischemia in gerbils. Stroke. 1991;22:84–89. doi: 10.1161/01.str.22.1.84. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Arraf Z. Prevention of MPTP (N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) dopaminergic neurotoxicity in mice by chronic lithium: involvements of Bcl-2 and Bax. Neuropharmacology. 2004;46:1130–1140. doi: 10.1016/j.neuropharm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat.Rev.Mol.Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Yu IT, Kim JS, Lee SH, Lee YS, Son H. Chronic lithium enhances hippocampal long-term potentiation, but not neurogenesis, in the aged rat dentate gyrus. Biochem.Biophys.Res.Commun. 2003;303:1193–1198. doi: 10.1016/s0006-291x(03)00494-7. [DOI] [PubMed] [Google Scholar]
- Yu Z, Luo H, Fu W, Mattson MP. The endoplasmic reticulum stress-responsive protein GRP78 protects neurons against excitotoxicity and apoptosis: suppression of oxidative stress and stabilization of calcium homeostasis. Exp.Neurol. 1999;155:302–314. doi: 10.1006/exnr.1998.7002. [DOI] [PubMed] [Google Scholar]
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- Yukimasa N, Yoshida K, Ohkushi H, Tanabe S, Fukasawa K, Kanou M, et al. [Hepatitis C virus genotyping by restriction fragment length polymorphism of polymerase chain reaction products generated with a HCV detection kit] Rinsho Byori. 2001;49:711–715. [PubMed] [Google Scholar]
- Yuskaitis CJ, Mines MA, King MK, Sweatt JD, Miller CA, Jope RS. Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndrome. Biochem.Pharmacol. 2010;79:632–646. doi: 10.1016/j.bcp.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr., Singh J, Manji HK. Cellular plasticity cascades: targets for the development of novel therapeutics for bipolar disorder. Biol.Psychiatry. 2006;59:1006–1020. doi: 10.1016/j.biopsych.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Zhang F, Phiel CJ, Spece L, Gurvich N, Klein PS. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Evidence for autoregulation of GSK-3. J.Biol.Chem. 2003a;278:33067–33077. doi: 10.1074/jbc.M212635200. [DOI] [PubMed] [Google Scholar]
- Zhang H, Vutskits L, Pepper MS, Kiss JZ. VEGF is a chemoattractant for FGF-2-stimulated neural progenitors. J.Cell Biol. 2003b;163:1375–1384. doi: 10.1083/jcb.200308040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Edelmann L, Liu J, Crandall JE, Morabito MA. Cdk5 regulates the phosphorylation of tyrosine 1472 NR2B and the surface expression of NMDA receptors. J.Neurosci. 2008;28:415–424. doi: 10.1523/JNEUROSCI.1900-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ona VO, Li M, Drozda M, Dubois-Dauphin M, Przedborski S, et al. Sequential activation of individual caspases, and of alterations in Bcl-2 proapoptotic signals in a mouse model of Huntington’s disease. J.Neurochem. 2003c;87:1184–1192. doi: 10.1046/j.1471-4159.2003.02105.x. [DOI] [PubMed] [Google Scholar]
- Zhong J, Yang X, Yao W, Lee W. Lithium protects ethanol-induced neuronal apoptosis. Biochem.Biophys.Res.Commun. 2006;350:905–910. doi: 10.1016/j.bbrc.2006.09.138. [DOI] [PubMed] [Google Scholar]
- Zhou R, Yuan P, Wang Y, Hunsberger JG, Elkahloun A, Wei Y, et al. Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers. Neuropsychopharmacology. 2009;34:1395–1405. doi: 10.1038/npp.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang J, Li F, Liu X, Liu Z, Lin J, Ge Y, et al. Lithium chloride protects retinal neurocytes from nutrient deprivation by promoting DNA non-homologous end-joining. Biochem.Biophys.Res.Commun. 2009;380:650–654. doi: 10.1016/j.bbrc.2009.01.162. [DOI] [PubMed] [Google Scholar]
- Zoghbi HY, Orr HT. Spinocerebellar ataxia type 1. Semin.Cell Biol. 1995;6:29–35. doi: 10.1016/1043-4682(95)90012-8. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science. 2001;293:493–498. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]