Abstract
The prevalence of vascular cognitive impairment (VCI) is likely to increase as the population ages and cardiovascular disease survival improves. We provide an overview of the definition and disease mechanisms of VCI and present a systematic literature review of the current evidence for the pharmacologic and nonpharmacologic therapies used to treat the VCI symptoms of cognitive dysfunction or to modify VCI through primary and secondary prevention. The Cochrane Database of Systematic Reviews was searched from 2005 to October 2010 using the keywords “vascular dementia” or “vascular cognitive impairment and therapy.” MEDLINE was searched for English-language articles published within the last 10 years using the combined Medical Subject Headings (MeSH) “therapeutics and dementia,” “vascular” or “vascular cognitive impairment.” Although cholinesterase inhibitors and memantine produce small cognitive improvements in patients with VCI, these drugs do not improve global clinical outcomes and have adverse effects and costs. Selective serotonin reuptake inhibitors and dihydropyridine calcium channel blockers may improve short-term cognitive function in patients with VCI. Anti-hypertensive therapy with an ACE inhibitor-based regimen and statins may prevent the major subtype of VCI known as poststroke cognitive decline. Clinical and effectiveness studies with long-term follow-up are needed to determine the benefits and risks of pharmacologic and nonpharmacologic therapies to prevent and treat VCI. Given its growing health, social, and economic burden, the prevention and treatment of VCI are critical priorities for clinical care and research.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-011-0047-z) contains supplementary material, which is available to authorized users.
Keywords: Vascular cognitive impairment, Vascular dementia, Mechanisms, Therapeutics
Introduction
Vascular dementia (VaD) is the second leading cause of dementia after Alzheimer’s disease (AD) [1, 2]. Coinciding with population aging and improved survival from cardiovascular diseases, including stroke, VaD is more frequent and will likely affect an increasing number of patients in the coming decades [3, 4]. Recently, the terms “vascular cognitive impairment” (VCI) was introduced to comprise the heterogeneous group of cognitive disorders that share a presumed vascular cause and to include both dementia and cognitive impairment without dementia [5–8]. VCI increases the morbidity, disability, and healthcare costs of the growing elderly population, and decreases their quality of life and survival [9–13]. Given the substantial health and economic burden of VCI, its prevention and treatment are critical research and clinical priorities.
This report focuses on VCI as a distinct clinical entity. Compared to AD, VCI, and particularly VaD, is associated with 50% lower median survival (6–7 years vs 3–4 years), greater healthcare costs, and higher rates of comorbidity, institutionalization, and caregiver use [9–11, 14–16]. However, VCI and AD mechanisms and pathology frequently coexist to cause mixed dementia [17, 18]. For example, stroke is a potent cause of VaD and worsens the cognitive effects of AD [10–24]. Most dementia cases in older adults have evidence of AD pathology (e.g., neuritic plaques and neurofibrillary tangles) and VCI pathology (e.g., cerebral or lacunar infarctions) [25, 26]. AD and VCI have the same vascular risk factors and vascular pathology may play a major role in the clinical expression of AD or VCI [27]. Despite this frequent overlap, VCI and AD are traditionally treated as unique clinical conditions and are studied separately [28]. Given this current approach to clinical practice and research, we discuss disease mechanisms of VCI and present the results of a systematic literature review of therapies used to treat the VCI symptoms of cognitive dysfunction or to modify VCI through primary and secondary prevention.
Defining Vascular Cognitive Impairment
The construct and diagnosis of VCI have evolved. Previous diagnostic criteria for VaD required the presence of memory loss and a severity of cognitive impairment sufficient to adversely affect independent functioning consistent with dementia [29–32]. However, these diagnostic criteria may not capture the executive dysfunction or less severe cognitive decline commonly observed in VCI [33, 34]. Recently, the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network published harmonization standards for VCI to address these potential limitations and to provide a first step toward developing diagnostic criteria for VCI [35]. Whether mixed dementia is included in VCI or AD remains controversial. Although the exact associations between CVD features (e.g., type, location, severity, volume) and cognitive impairment are not known, the general types of cerebrovascular injuries that occur or co-occur in VCI are large-vessel or small-vessel ischemia, hypoperfusion, hemorrhage, and vasculopathy [36]. For this report, we used the latest definition of VCI [37] for the overview of disease mechanisms, and we also used earlier VCI definitions [29–32] that were relevant during the study period (2000–2010) for the systematic literature review.
Mechanisms of Disease
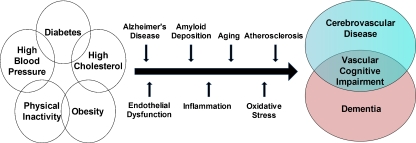
Shared mechanisms between cerebrovascular disease (CVD) and dementia may contribute to VCI. CVD and dementia share risk factors and neuropathology [28]. Vascular risk factors (hypertension, hyperlipidemia, diabetes) and behavioral factors (obesity, physical inactivity) are associated with both CVD and, particularly when present in mid-life dementia (Fig. 1) [37, 38]. Similarly, observational studies in middle-aged or older adults have found associations between VCI and hypertension [39, 40], hyperlipidemia [41], diabetes [27, 42], obesity [43], and physical inactivity [44], even when present later in life. Several pathogenic mechanisms including AD, amyloid deposition, aging, atherosclerosis, and hypertension may converge to cause CVD and dementia through pathways of inflammation and oxidative stress in blood vessels [45–48]. Vascular risk factors may lead to cerebrovascular dysfunction through pathways mediated by beta-amyloid and the enzyme nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, a major source of vascular oxidative stress [46]. Cerebrovascular dysfunction and blood brain barrier alterations may compromise the cerebral microenvironment and increase the vulnerability of regions critical for cognition (e.g., subcortical white matter, neocortex, hippocampus) to ischemic-hypoxic brain damage leading to neuronal dysfunction and cognitive deficits [46]. Also, insulin resistance, abdominal obesity, dysfunction of the cerebral small-vessel endothelium (i.e., the blood brain barrier) and chronic kidney disease may contribute to or accelerate VCI [48–51]. Whether due to shared or additive toxic vascular effects [52], CVD and dementia coexist frequently, particularly with increasing age [17, 18, 26].
Fig. 1.
Potential mechanisms between vascular risk factors, cerebrovascular disease, and dementia may lead to vascular cognitive impairment. Adapted from Middleton and Yaffe [48] in 2009
Hematologic and inflammatory factors may have etiological roles in VCI. Although atrial fibrillation is known to cause macroembolic complications, such as stroke, cardioembolic disorders may cause microembolic complications that lead to CVD and cognitive impairment [53] or accelerate cognitive and functional decline in VCI [54]. Also, recent data may implicate clot formation and micro-infarctions as mechanisms of VCI through hemostatic pathways. High levels of fibrinogen, factor VIII, or plasminogen activation inhibitor 1 have been associated with an increased risk of VCI [55, 56]. Moreover, observational studies suggest potential roles of inflammation in VCI. In a Japanese case-control study, elevated high-sensitivity C-reactive protein and antibodies for Chlamydia pneumoniae were more prevalent in VaD than AD [57]. A cross-sectional study found that high interleukin-6 plasma levels were associated with functional impairment in older adults with VaD, but not late-onset AD, independently of demographics and clinical factors, including previous stroke and cognitive function [58].
Genetic factors may influence the development or course of VCI. The apolipoprotein E epsilon 3 polymorphism [59] and the epsilon 4 polymorphism [60], particularly in persons with hypertension or diabetes [61], may be associated with increased VCI risk, but the data are not conclusive [62, 63]. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, a genetic form of subcortical ischemic VaD, is associated with Notch3 mutations whose location may differ by geography or demography [64–66]. The identification of quantifiable phenotypes that can be reliably and effectively determined in large samples of subjects is the most critical challenge for genetic studies of VCI [67].
Evidence Acquisition
We first searched the Cochrane Database of Systematic Reviews for full systematic reviews from 2005 to October 2010 using the keywords vascular dementia or vascular cognitive impairment and therapy (i.e., the search terms used were: “cognitive,” “dementia,” “impairment,” “therapy,” and/or “vascular”). The title and abstracts of the 50 systematic reviews identified by this search were assessed for relevance to this report. Any review of therapy for vascular dementia, vascular cognitive impairment, or any type of dementia (n = 25) was retained. Then we searched MEDLINE for English language articles with human subjects published from December 15, 2000 to December 15, 2010. One author (D.A.L.) reviewed the title and abstracts of all 361 articles identified using the search terms “therapeutics” (MeSH Term) and “dementia, vascular” (MeSH Term) or “vascular cognitive impairment.” The searching and selection of articles for review is shown in Fig. 2. Randomized, double-blind, placebo-controlled trials (RCTs) with results reported as intention-to-treat analyses were considered to be the highest quality data and are the focus of this report. Large prospective cohort studies, meta-analyses, and systematic literature reviews were also included to supplement the RCT results.
Fig. 2.
The search and selection of articles for review. VCI = vascular cognitive impairment. *Alternative therapy includes acupuncture, Tai Chi, and light therapy
Evidence Synthesis
This report focuses on pharmacologic and nonpharmacologic therapies used to treat the VCI symptoms of cognitive dysfunction or to modify VCI through primary and secondary prevention. As of June 2010, no drugs were approved by the Food and Drug Administration specifically to treat VaD. Regulatory agency approval of drugs to treat dementia, including VaD, requires efficacy on cognition and clinical global or activities of daily living (ADL) measures [68]. However, medications approved for other indications (e.g., AD) may be efficacious in VCI. Currently, the cognitive dysfunction of VCI is treated with 3 drug classes: 1) cholinesterase inhibitors, 2) N-methyl-D-aspartate (NMDA) antagonists, and 3) anti-depressants. Primary and secondary prevention of VCI is approached generally with stroke prevention and vascular risk factor modification.
Cholinesterase Inhibitors
Acetylcholinesterase inhibitors inhibit the breakdown of acetylcholine and have been shown to improve cognitive function in adults with mild to moderate AD. Cholinergic deficits or reduced acetylcholine-mediated neurotransmission may play a role in VCI [69].
Donepezil
Some RCTs of donepezil in patients with probable or possible VaD have shown modest treatment benefits in cognition but inconsistent benefits in global functioning (Table 1) [70–72]. Interestingly, hippocampal size may modify the effect of donepezil on cognition [72]. A Cochrane review of donepezil for VCI based on two large-scale RCTs [70, 71] concluded that the drug is well-tolerated and has some efficacy in improving cognitive function, clinical global impression, and ADLs in patients with mild to moderate VCI after 6 months of treatment [73]. However, safety remains a concern because 1 donepezil trial found a markedly higher risk of death with treatment compared with placebo (1.7% vs 0%; odds ratio, 4.6; 95% confidence interval [CI], 1.3-16.1) [72, 74].
Table 1.
Randomized controlled trials of medication treatment for vascular dementia included in this review
| Source, Reference, Year | No. of Patients, Age | Medication | Trial Length in Weeks | Primary Outcomes |
|---|---|---|---|---|
| Wilkinson, et al. [71], 2003 | n = 616 Mean age, 75 years | Donepezil | 24 | 2.2-Point improvement on the ADAS-cog among treated patients (10 mg dose) vs a 0.1-point improvement for those receiving placebo (2.1-point treatment difference; p < 0.001); 1.8-point improvement on the ADAS-cog among treated patients (5 mg dose) vs placebo (1.7-point treatment difference; p < 0.01). Patients treated with 5 mg (39%) and those treated with 10 mg (32%) showed improvement on the CIBIC-plus vs 25% of those receiving placebo (p = 0.004 for 5 mg comparison, and p = 0.047 for 10 mg) |
| Black, et al. [70], 2003 | n = 603 Mean age, 74 years | Donepezil | 24 | 2.0-Point improvement on the ADAS-cog among treated patients (10 mg dose) vs a 0.3-point decrease for those receiving placebo (2.3-point treatment difference; P < 0.001); 1.6-point improvement on the ADAS-cog among treated patients (5 mg dose) vs placebo (1.9-point treatment difference; p < 0.01). Patients treated with 5 mg (38%) and those treated with 10 mg (31%) showed improvement on the CIBIC-plus vs 32% of those receiving placebo (p < 0.05 for 5 mg comparison, but not significant for 10 mg comparison) |
| Roman, et al. [72], 2010 | n = 974, Mean age, 73 years | Donepezil | 24 | 1.0-Point improvement on the V-ADAS-cog among treated patients (5 mg dose) vs a 0.1-point decrease for those receiving placebo (1.2-point treatment difference; P < 0.01); no significant difference in the CIBIC-plus score |
| Auchus, et al. [79], 2007 | n = 788 Mean age, 72 years | Galantamine | 26 | 1.8-Point improvement on the ADAS-cog among treated patients vs a 0.3-point improvement for those receiving placebo (1.5-point treatment difference; p < 0.001); no significant difference in the ADCS-ADL score |
| Ballard, et al. [82], 2008 | n = 710 Mean age, 73 years | Rivastigmine | 24 | 0.7-Point improvement on the V-ADAS-cog among treated patients vs a 0.6-point decrease for those receiving placebo (1.3-point treatment difference; p = 0.028); no significant difference in the ADCS-CGIC score |
| Orgogozo, et al. [86], 2002 | n = 321 Mean age, 76 years | Memantine | 28 | 0.4-Point improvement on the ADAS-cog among treated patients vs a 1.6-point decline for those receiving placebo (2.0-point treatment difference; p = 0.002); no significant difference in the CIBIC-plus score |
| Wilcock, et al. [87], 2002 | n = 579 Mean age, 77 years | Memantine | 28 | 0.5-Point decline on the ADAS-cog among treated patients vs a 2.3-point decline for those receiving placebo (1.8-point treatment difference; p < 0.001); no significant difference in the CGIC score. |
Vascular dementia (VaD) was determined by the National Institute of Neurological Disorders and Stroke (NINDS) and the Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN) criteria for vascular dementia and computed tomography or magnetic resonance imaging evidence in all trials. The Vascular-Alzheimer’s Disease Assessment Scale-cognitive subscale (V-ADAS-cog) comprises the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog) plus the Maze and Number Cancellation test (NCT) to assess executive function
ADCS-ADL = Alzheimer’s Disease Cooperative Study-Activities of Daily Living inventory total score; ADCS-CGIC = Alzheimer’s Disease Cooperative Study-Clinician’s Global Impression of Change; CGIC = Clinical Global Impression of Change; CIBIC-plus = Clinician’s Interview-Based Impression of Change plus Caregiver Input
Because trials may include VaD patients with heterogeneous CVD pathology (e.g., differing prevalence rates of white matter disease or cortical lesions) [74], it has been questioned whether there are subtypes of VaD defined by a unique pathology that may be targets for greater treatment efficacy [68]. Specifically, cholinergic deficits may vary by VaD subtype. However, a recent trial of donepezil in 168 patients (mean age, 55 years) with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy and cognitive impairment found no significant difference in the primary cognitive endpoint after 18 weeks of treatment. Donepezil treatment had modest improvements on several measures of executive function but the clinical relevance of these findings is not clear [75].
Galantamine
Galantamine is an acetylcholinesterase inhibitor that also modulates nicotinic receptors [76, 77]. One trial found benefits of galantamine treatment (24 mg/day for 6 months) for cognitive function and global functioning in the VaD subgroup (n = 188) that was of similar magnitude, but not statistically significant, to those observed in the overall group (VaD or AD with CVD) and the AD subgroup [78]. A second trial found small benefits of galantamine treatment for cognition, including executive function, but not for independent performance of ADLs or global functioning in a sample of patients with VaD [79]. A Cochrane review of galantamine for VCI concluded that the evidence was inconsistent for its efficacy, but consistently showed a higher risk of adverse gastrointestinal events (nausea and vomiting) [80].
Rivastigmine
Rivastigmine, a dual inhibitor of acetylcholinesterase and butyrylcholinesterase, may have significant, sustained efficacy on cognition and daily function in VCI [81]. The Vascular Dementia Trial studying Exelon (VantagE) found modest benefits of rivastigmine treatment on 3 measures of cognitive performance, but not on global performance, ADLs, or neuropsychiatric symptoms. In addition, the rivastigmine group had higher rates of nausea and vomiting than the placebo group [82]. Pre-specified exploratory subgroup analyses indicated that older patients (≥75 years) had greater efficacy on cognitive outcomes and safety, but younger patients showed no efficacy and possible harm (elevated blood pressure, stroke, and death) [82]. A Cochrane review of rivastigmine for VCI was unable to perform a meta-analysis given the absence of suitable unconfounded RCTs, and the conclusion of the review was no evidence of benefit [83].
NMDA Antagonists
Memantine
Cerebral ischemia may lead to excessive release of glutamate that activates postsynaptic NMDA receptors [84]. Memantine, a noncompetitive NMDA receptor antagonist, may have neuroprotective properties and improve cognition [85]. Two RCTs of memantine, MMM300 and MMM500, have shown modest treatment benefits on cognition, but not global functioning for patients with mild to moderate VaD (Table 1) [86, 87]. A Cochrane review that pooled data from the 2 RCTs indicated a small beneficial effect of memantine (20 mg/day) on cognition (1.9-point improvement on Alzheimer’s Disease Assessment Scale-cognitive subscale [ADAS-cog], 95% CI, 0.9-2.8; p < 0.001), but this was not supported by clinical global measures [88]. Memantine appeared to be well tolerated.
Summary of Cholinesterase Inhibitors and Memantine in Vascular Dementia
Kavirajan and Schneider [74] recently published a meta-analysis of RCTs of cholinesterase inhibitors and memantine in VaD that included the 3 donepezil, 2 galantamine, 1 rivastigmine, and 2 memantine trials listed in Table 1. In patients with mild to moderate VaD, the authors found small benefits in cognition of uncertain clinical significance (Table 2). There was evidence of clinical global improvement for 5 mg daily donepezil only and no behavioral or functional benefits were observed, except for a −1.0-point difference (95% CI, −1.7- − 0.2) with 10 mg daily donepezil on the Alzheimer’s Disease Functional Assessment and Change Scale. Cholinesterase inhibitors, but not memantine, were associated with greater odds of dropouts and adverse events (Table 2). The validity and mechanisms of the lower risk of stroke for memantine are unknown.
Table 2.
Efficacy and adverse events of cholinesterase inhibitors and memantine in vascular dementia: summary of results of a meta-analysis of randomized controlled trials*
| Cognitive Outcomes | Global Change Outcomes | All-Cause Dropouts | Adverse Events | |
|---|---|---|---|---|
| Treatment | Change in ADAS-cog subscale from baseline, WMD† (95% CI) | Change in CIBIC-plus or CGIC‡, Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI)§ |
| Donepezil 5 mg vs placebo | −1.2 (−1.7– − 0.6) | 1.5 (1.1–2.1) | 1.2 (0.9–1.8) | Anorexia, 2.0 (1.0–3.8) |
| Insomnia, 2.2 (1.0–4.7) | ||||
| Diarrhea, 1.5 (1.0–2.3) | ||||
| Donepezil 10 mg vs placebo | −2.2 (−3.0– − 1.4) | 1.2 (0.9–1.6) | 1.9 (1.4–2.7) | Anorexia, 2.5 (1.3–4.7) |
| Nausea, 2.5 (1.6–3.9) | ||||
| Diarrhea, 1.8 (1.2–2.7) | ||||
| Galantamine 24 mg vs placebo | −1.6 (−2.4– − 0.8) | 1.1 (0.8–1.4) | 1.7 (1.3–2.2) | Anorexia, 4.5 (2.0–10.1) |
| Nausea, 3.6 (2.4–5.4) | ||||
| Diarrhea, 1.6 (1.0–2.4) | ||||
| Vomiting, 3.1 (1.8–5.1) | ||||
| Insomnia, 3.3 (1.6–7.0) | ||||
| Rivastigmine 12 mg vs placebo | −1.1 (−2.2– − 0.1) | 1.0 (0.8–1.4) | 2.0 (1.4–3.0) | Anorexia, 3.1 (1.2–7.9) |
| Nausea, 8.9 (4.9–16.2) | ||||
| Diarrhea, 2.2 (1.2–4.1) | ||||
| Vomiting, 11.9 (5.6–25.0) | ||||
| Memantine 20 mg vs placebo | −1.9 (−2.8– − 0.9) | 1.3 (0.9–2.2) | 1.1 (0.8–1.5) | Stroke, 0.4 (0.2–0.9) |
In all trials and the meta-analyses, primary outcomes were determined using intention-to-treat analyses with last observation carried forward sample. The outcomes and numbers of patients in each randomization group were statistically combined using fixed-effects models.
*Kavirajan and Schneider [74].
†Change in Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-cog) from baseline calculated as a weighted mean differences (WMD) with 95% confidence interval (CI)
‡Change in clinical global improvement measured by Clinical Global Impression of Change (CGIC) for 1 memantine trial (MMM500) or measured by Clinician's Interview-Based Impression of Change plus Caregiver Input (CIBIC-plus) for all other trials
§Odds Ratios dervied from Yousef-Peto fixed effects models for dichotomous clinical outcomes, dropouts, and adverse events
ADAS-cog = Alzheimer’s Disease Assessment Scale-cognitive subscale
Although cholinesterase inhibitors and memantine produce small cognitive improvements compared to placebo, these drugs have not been shown to improve global clinical outcomes and have adverse effects and costs [89]. In 2010, the British Association for Psychopharmacology recommended against prescribing cholinesterase inhibitors or memantine to patients with VaD, although those with mixed VaD and AD may benefit [90]. RCTs of cholinesterase inhibitors and memantine in VaD have been limited by heterogeneity in the type, location, and severity of CVD, use of predominantly white cohorts, variability in the requisite diagnostic imaging, small sample size precluding analyses of subgroups with greater (or less) benefit, short-treatment duration, and lack of VaD specific outcomes [74]. In addition, all trials were sponsored by the drugs’ manufacturers and subsequent cost-effectiveness analyses used a short (i.e., 24–28 week) time horizon [91]. Moreover, effectiveness studies that determine the effect on clinical outcomes (e.g., cognition, function, neuropsychiatric symptoms, and behaviors), the real-world tolerability and the adherence of VCI treatments in unselected, nontrial patients are warranted. These effectiveness data would help inform patients, caregivers, and clinicians’ treatment decisions in often medically-complex VCI patients.
Cardiovascular Agents
Antihypertensives
Longitudinal studies have found that high blood pressure (BP), in mid-life [92] or late-life [39], is associated with late-life VaD. Moreover, antihypertensive medication use is associated with a decreased risk of VaD [93], with each year of treatment associated with a 5% reduction in VaD risk [94]. For secondary prevention, one cohort study (n = 52) of hypertensive patients with VaD or mixed dementia showed improved or stabilized cognitive scores with control of systolic BP in the upper limits of normal range (135 to 150 mm Hg), even after controlling for age, cerebral blood flow, diastolic and mean arterial BP, and pulse pressure; interestingly, systolic BP levels below 135 mm Hg were associated with steeper cognitive declines [95]. Few large-scale RCTs have assessed whether BP control influences the course of VCI.
In the Systolic Hypertension in Europe (Syst-Eur) trial, treatment of isolated systolic hypertension with a regimen based on the calcium-channel blocker nitrendipine, with the addition of the ACE inhibitor enalapril or hydrochlorothiazide, if necessary, reduced the incidence of dementia by 50% (3.8 vs 7.7 cases per 1000 patient-years; p = 0.05) for a 2–year span and 55% during extended follow-up [96, 97]. The small number of dementia cases (n = 32; only 2 cases of VaD) precluded an analysis of incident VaD. Among patients with CVD, the Perindopril Protection against Recurrent Stroke Study (PROGRESS) trial demonstrated that antihypertensive treatment with perindopril ± indapamide did not reduce the risk of dementia, a secondary endpoint, but did reduce the risk of recurrent stroke, and dementia or cognitive decline associated with recurrent stroke [98, 99]. These findings are consistent with the data from The Heart Outcomes Prevention Evaluation (HOPE) RCT showing that treatment with the angiotensin-converting enzyme inhibitor ramipril decreased stroke incidence and the risk of stroke-related cognitive decline in patients with, or at high-risk for, vascular disease [100]. Conversely, the Hypertension in the Very Elderly Trial (HYVET) found that treatment with indapamine ± perindopril did not reduce the risk of VaD (unadjusted hazard ratio, 0.87; 95% CI, 0.57-1.34), cognitive decline or total dementia in patients ages 80 years or older with hypertension [101]. The treatment targets for BP (150/80 mm Hg) may have been too high for optimal cognitive benefits, a potential explanation applicable to the PROGRESS trial as well. Two recent RCTs reported no benefit of angiotensin II receptor blocker treatment on cognition or dementia in patients with hypertension [102] or ischemic stroke [103].
Meta-analyses have found conflicting evidence that BP lowering in late life prevents cognitive decline or dementia with positive results if the PROGRESS trial is included [101], and negative results if the PROGRESS trial is excluded [104], suggesting greater efficacy in populations with CVD. Previous RCTs were limited by measurement of secondary study outcomes, few VCI cases, premature discontinuation of the study, imprecision regarding effect estimates, differential drop-outs, and contamination (i.e., placebo patients receiving active therapy or premature discontinuation of study treatment) [105]. Another challenge is that age may modify the relationship between BP, CVD, and cognition [105, 106]. Taken together, the PROGRESS and HOPE trials suggest that BP lowering or other possible mechanisms (e.g., renin-angiotensin-aldosterone system blockade) may reduce the risk of a major VCI subtype, poststroke cognitive decline, but not other cognitive outcomes; these findings were consistent with other recent clinical studies [107]. Future research is needed to determine whether long-acting dihydropyridine calcium channel blockers have efficacy in the primary prevention of VCI.
In regard to secondary prevention, a Cochrane review of nimodipine for patients with dementia identified 10 trials that included patients with VaD. When VaD trials were pooled separately, significant efficacy was noted for clinical global impression and cognitive function, but not on scales assessing ADL for the 90 mg/day dose of nimodipine at 12 weeks [108]. Although nimodipine had evidence of short-term benefit and was well tolerated with a low rate of adverse events, the meta-analyses were based only on participants who completed the study and were not intention-to-treat analyses. One small, randomized prospective trial compared rivastigmine to nimodipine for 14 months among older adults (n = 100) stratified by VCI subtype. Although rivastigmine improved behavioral symptoms compared to nimodipine, there were no differences in clinical dementia or cognitive scores between treatments [109]. These data suggest that long-acting dihydropyridine calcium channel blockers may have efficacy in the secondary prevention of VCI, but trials with long-term follow-up are needed.
Aspirin
Aspirin may prevent or reduce VCI through several potential biological mechanisms including reduced platelet aggregation, vasodilatation, decreased circulating β-amyloid production (derived from platelets), and fewer superoxide radicals [110]. However, a Canadian observational study of older adults showed an association between aspirin use and an increased risk of incident VaD [60]. Confounding by indication may bias investigations of the aspirin-VCI association because older adults with existing vascular disease or vascular risk factors (and possible subclinical CVD) are more likely to be prescribed aspirin. In a small, randomized clinical trial (n = 70; mean follow-up, 15 months), cognition, cerebral blood flow, and functional status improved in multi-infarct patients (mean age, 67 years) treated with daily aspirin (325 mg); however, differences in recurrent stroke may have explained these findings [111]. To date, no RCTs of aspirin have been reported for the treatment of VCI [112].
Statins
Observational studies have found associations between elevated levels of serum total cholesterol, low-density lipoprotein (LDL) cholesterol, and nonhigh-density lipoprotein, and decreased levels of high-density lipoprotein and risk of VaD [41, 113, 114]. In a prospective longitudinal community-based study spanning a 7-year period (1991–1998), LDL cholesterol levels were associated with an increased risk of dementia with stroke [115]. Compared with the lowest quartile, the highest quartile of LDL cholesterol was associated with approximately a 3-fold increase in risk of dementia with stroke, after adjusting for vascular risk factors and demographic variables (relative risk, 3.1; 95% confidence interval [CI], 1.5-6.1) [115]. However, observational studies have not shown a decreased risk for VCI associated with statin or lipid-lowering therapy [114].
No RCTs have demonstrated that statins given in late life to individuals at risk of vascular disease prevents dementia [116]. In the Heart Protection Study, simvastatin treatment for 5 years did not prevent cognitive decline or dementia incidence among individuals with a history of coronary heart disease or risk factors for it, or CVD [117, 118]. Similarly, in the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER), pravastatin treatment did not affect cognitive decline during 3 years of follow-up among older adults at risk for vascular disease [119, 120]. Although statins may reduce the risk of VCI by preventing primary and recurrent stroke [117, 118, 121], whether treatment of elevated LDL cholesterol levels will reduce the risk of VCI independently of stroke needs to be determined.
Vascular Care
Comprehensive vascular care may represent 1 approach to the primary or secondary prevention of VCI. However, in a recent RCT, multi-component vascular care that combined pharmacological and nonpharmacological interventions did not slow functional or cognitive decline in 130 patients with AD and CVD [122]. A larger cluster, randomized trial with a 6-year follow-up in 3700 elderly subjects (70 to 78 years) will assess whether nurse-led intensive vascular care in primary care decreases the incidence of dementia, including VaD, and whether it reduces disability; secondary outcomes are mortality, incidence of vascular events, and cognitive functioning [123]. Given that recurrent stroke accelerates VCI, secondary stroke prevention is an important strategy to prevent cognitive declines in VCI [124].
Vitamins and Other Compounds
Some observational studies suggest a protective role of antioxidants in VCI. In the Canadian Study of Health and Aging, the history of anti-oxidant vitamin use (vitamin E and C supplements, and/or multivitamins) was associated with a lower incidence of VCI, but not dementia or AD for 5 years [125]. Cytidine 5′-diphosphocholine (CDP-choline or citicoline), a precursor needed for the synthesis of phosphatidylcholine, may attenuate ischemic injury by accelerating re-synthesis of phospholipids in brain cell membranes, by suppressing the release of free fatty acids, and perhaps by increasing noradrenaline and dopamine levels in the central nervous system [126]. Several small RCTs of chronic cerebrovascular patients (n ≤ 100) and 1 large (n = 536) unpublished RCT of VCI patients suggest improved cognition, particularly in the areas of attention and perceptual-motor performance with oral citicoline treatment [126]. However, a recent RCT (n = 30) showed no improvement in neuropsychological performance in VaD patients treated with citicoline for 12 months [127]. An unpublished study (n = 347) found that citicoline treatment for 6 months may prevent poststroke cognitive decline [128]. Although the drug seems to be safe and well tolerated, more study of its symptomatic and disease-modifying effects on VCI is needed. Despite some intriguing data, the efficacy of several compounds, including Hydergine [129], Ginkgo biloba [130], Huperzine A [131], and propentofylline [132] for VCI has not been established.
Anti-Depressants
Selective serotonin reuptake inhibitors (SSRIs) may improve cognitive function by effecting neural circuits that process cognitive information [133] and increase hippocampal neurogenesis [134]. In a retrospective case series of 35 nondepressed male veterans with VCI, open-label sertraline use was associated with improved executive function measured by the Executive Interview (EXIT25) perhaps through a dopaminergic mechanism [135]. In a post hoc subsample analysis (n = 129) of a recent multicenter trial of poststroke depression in patients treated within 3 months of recent hemorrhagic or ischemic stroke, escitalopram for 12 months improved scores in global cognition, and immediate and delayed memory, compared to placebo or problem solving therapy, even after controlling for depressive symptoms [133]. Future research is needed to study the effects of anti-depressants on cognition and neuropychiatric symptoms or behaviors linking mechanisms (e.g., serotonergic, noradrenergic, or dopaminergic) to cognitive or behavioral effects, and identifying subgroups or phenotypes of VCI with greater benefit.
Nonpharmacologic Therapies
Carotid Revascularization
Some data suggest that carotid artery stenosis (CAS) may be associated with cognitive dysfunction even in persons without stroke; however, whether revascularization influences the course of VCI is uncertain. In a recent observational study of 4,006 right-handed individuals aged 65 or older from the Cardiovascular Health Study, left-sided CAS ≥ 75% was associated with cognitive impairment at baseline and cognitive decline during a 5-year span [136]. Because the associations persisted after adjustment for right-sided CAS and vascular risk factors, the study authors postulated mechanisms independent of vascular risk factors or cardiovascular disease. A recent systematic review did not find consistent evidence that carotid revascularization affected cognition due to methodological differences and limitations among studies [137].
Lifestyle Modification
Several observational studies suggest that physical activity may reduce the risk of VCI [44, 138]. Recently, a blinded, randomized pilot study of chronic stroke survivors showed that a brief 2-month aerobic exercise intervention improved cognitive performance and sensorimotor learning, including information processing speed [139]. In addition, smoking cessation was associated with improved cognition among normotensive patients with VaD or mixed dementia (n = 14) [95]. These preliminary data suggest avenues for future clinical and interventional studies of lifestyle modification for the primary and secondary prevention of VCI.
Cognitive Training and Rehabilitation
Cognitive training and cognitive rehabilitation represent possible nonpharmacological therapies for VCI. However, a Cochrane review found no evidence for the efficacy of cognitive training based on 9 RCTs for VCI, and insufficient evidence to evaluate individualized cognitive rehabilitation [140].
Future Directions
Key gaps remain in our understanding of the risk factors, causal pathways, and pathophysiology of VCI. Potential biological, pathological, environmental, and genetic mechanisms of VCI warrant further investigation. In addition, how vascular risk factors and their treatments affect VCI independent of primary or recurrent stroke needs to be determined. Clinical studies are contributing to our understanding of vascular risk factor treatments in cognition among middle-aged and older adults with or at risk for VCI [141–143]. Despite limited efficacy and frequent adverse effects, drugs for dementia are prescribed off-label to a substantial proportion of VCI patients, with a prescription rate of 35 to 45% for cholinesterase inhibitors [144]. [47]Clinical and effectiveness studies are needed to determine whether there are subgroups of patients with VCI who may tolerate and receive benefit from symptomatic treatments. Research and clinical approaches to VCI will likely need to advance beyond primary and secondary stroke prevention and the use of AD therapies. Moreover, these research and clinical approaches will need to consider the pathological and clinical heterogeneity of VCI. Given its substantial health, social, and economic burden in the growing population of older adults, the prevention and treatment of VCI are critical priorities for clinical care and research.
Electronic supplementary materials
(PDF 510 kb)
Acknowledgments
This work was supported by research from the National Institutes of Health (NIH) (P30 AG024824-07 subproject PI) (D.A.L.), and research from the NIH (R01 AG030155) (K.M.L.). There is no real or perceived conflict of interest.
Full conflict of interest disclosure is available in the electronic supplementary material for this article.
References
- 1.Lobo A, Launer LJ, Fratiglioni L, et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54:S4–9. [PubMed] [Google Scholar]
- 2.Wahlund LET, Gauthier S. Vascular Cognitive Impairment in Clinical Practice. Cambridge, UK: Cambridge University Press; 2009. [Google Scholar]
- 3.He W, Sengupta M, Velkoff VA, DeBarros KA. U.S. Census Bureau, Current Population Reports, P23-209, 65+ in the United States: 2005, U.S. Government Printing Office,Washington, DC, 2005.
- 4.Ukraintseva S, Sloan F, Arbeev K, Yashin A. Increasing rates of dementia at time of declining mortality from stroke. Stroke. 2006;37:1155–1159. doi: 10.1161/01.STR.0000217971.88034.e9. [DOI] [PubMed] [Google Scholar]
- 5.Hachinski VC, Bowler JV. Vascular dementia. Neurology. 1993;43:2159–2160. doi: 10.1212/wnl.43.10.2159-a. [DOI] [PubMed] [Google Scholar]
- 6.Bowler JV, Steenhuis R, Hachinski V. Conceptual background to vascular cognitive impairment. Alzheimer Dis Assoc Disord. 1999;13(Suppl 3):S30–37. [PubMed] [Google Scholar]
- 7.O'Brien JT, Erkinjuntti T, Reisberg B, et al. Vascular cognitive impairment. Lancet Neurol. 2003;2:89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 8.Moorhouse P, Rockwood K. Vascular cognitive impairment: current concepts and clinical developments. Lancet Neurol. 2008;7:246–255. doi: 10.1016/S1474-4422(08)70040-1. [DOI] [PubMed] [Google Scholar]
- 9.Rockwood K, Brown M, Merry H, Sketris I, Fisk J. Societal costs of vascular cognitive impairment in older adults. Stroke. 2002;33:1605–1609. doi: 10.1161/01.str.0000017878.85274.44. [DOI] [PubMed] [Google Scholar]
- 10.Sicras A, Rejas J, Arco S, et al. Prevalence, resource utilization and costs of vascular dementia compared to Alzheimer's dementia in a population setting. Dement Geriatr Cogn Disord. 2005;19:305–315. doi: 10.1159/000084556. [DOI] [PubMed] [Google Scholar]
- 11.Hill J, Fillit H, Shah SN, Valle MC, Futterman R. Patterns of healthcare utilization and costs for vascular dementia in a community-dwelling population. J Alzheimers Dis. 2005;8:43–50. doi: 10.3233/jad-2005-8105. [DOI] [PubMed] [Google Scholar]
- 12.Boyle PA, Cahn-Weiner D. Assessment and prediction of functional impairment in vascular dementia. Expert Rev Neurother. 2004;4:109–114. doi: 10.1586/14737175.4.1.109. [DOI] [PubMed] [Google Scholar]
- 13.Nys GM, Zandvoort MJ, Worp HB, et al. Early cognitive impairment predicts long-term depressive symptoms and quality of life after stroke. J Neurol Sci. 2006;247:149–156. doi: 10.1016/j.jns.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Fillit H, Hill J. The costs of vascular dementia: a comparison with Alzheimer's disease. J Neurol Sci. 2002;203–204:35–39. doi: 10.1016/s0022-510x(02)00257-5. [DOI] [PubMed] [Google Scholar]
- 15.Knopman DS, Rocca WA, Cha RH, Edland SD, Kokmen E. Survival study of vascular dementia in Rochester, Minnesota. Arch Neurol. 2003;60:85–90. doi: 10.1001/archneur.60.1.85. [DOI] [PubMed] [Google Scholar]
- 16.Fitzpatrick AL, Kuller LH, Lopez OL, Kawas CH, Jagust W. Survival following dementia onset: Alzheimer's disease and vascular dementia. J Neurol Sci. 2005;229–230:43–49. doi: 10.1016/j.jns.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 17.Langa KM, Foster NL, Larson EB. Mixed dementia: emerging concepts and therapeutic implications. JAMA. 2004;292:2901–2908. doi: 10.1001/jama.292.23.2901. [DOI] [PubMed] [Google Scholar]
- 18.Jin YP, Legge S, Ostbye T, Feightner JW, Hachinski V. The reciprocal risks of stroke and cognitive impairment in an elderly population. Alzheimers Dement. 2006;2:171–178. doi: 10.1016/j.jalz.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Knopman DS, Roberts RO, Geda YE, et al. Association of prior stroke with cognitive function and cognitive impairment: a population-based study. Arch Neurol. 2009;66:614–619. doi: 10.1001/archneurol.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tatemichi TK, Desmond DW, Paik M, et al. Clinical determinants of dementia related to stroke. Ann Neurol. 1993;33:568–575. doi: 10.1002/ana.410330603. [DOI] [PubMed] [Google Scholar]
- 21.Desmond DW, Moroney JT, Sano M, Stern Y. Incidence of dementia after ischemic stroke: results of a longitudinal study. Stroke. 2002;33:2254–2260. doi: 10.1161/01.str.0000028235.91778.95. [DOI] [PubMed] [Google Scholar]
- 22.Desmond DW, Moroney JT, Paik MC, et al. Frequency and clinical determinants of dementia after ischemic stroke. Neurology. 2000;54:1124–1131. doi: 10.1212/wnl.54.5.1124. [DOI] [PubMed] [Google Scholar]
- 23.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 24.Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer's disease. Lancet. 1999;354:919–920. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]
- 25.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 26.Skoog I, Nilsson L, Palmertz B, Andreasson LA, Svanborg A. A population-based study of dementia in 85-year-olds. N Engl J Med. 1993;328:153–158. doi: 10.1056/NEJM199301213280301. [DOI] [PubMed] [Google Scholar]
- 27.Ahtiluoto S, Polvikoski T, Peltonen M, et al. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology. 2010;75:1195–1202. doi: 10.1212/WNL.0b013e3181f4d7f8. [DOI] [PubMed] [Google Scholar]
- 28.Hachinski V. The 2005 Thomas Willis Lecture: stroke and vascular cognitive impairment: a transdisciplinary, translational and transactional approach. Stroke. 2007;38:1396. doi: 10.1161/01.STR.0000260101.08944.e9. [DOI] [PubMed] [Google Scholar]
- 29.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's Disease Diagnostic and Treatment Centers. Neurology. 1992;42:473–480. doi: 10.1212/wnl.42.3.473. [DOI] [PubMed] [Google Scholar]
- 30.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 31.Diagnostic and statistical manual of mental disorders. 4. Washington, DC: APA Press; 1994. [Google Scholar]
- 32.The ICD-10 classification of mental and behavioral disorders Clinical descriptions and diagnostic guidelines. Switzerland: WHO; 1992. [Google Scholar]
- 33.Sachdev P. Is it time to retire the term "dementia"? J Neuropsychiatry Clin Neurosci. 2000;12:276–279. doi: 10.1176/jnp.12.2.276. [DOI] [PubMed] [Google Scholar]
- 34.Sachdev PS, Brodaty H, Valenzuela MJ, et al. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology. 2004;62:912–919. doi: 10.1212/01.wnl.0000115108.65264.4b. [DOI] [PubMed] [Google Scholar]
- 35.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 36.Roman GC. Vascular dementia revisited: diagnosis, pathogenesis, treatment, and prevention. Med Clin North Am. 2002;86:477–499. doi: 10.1016/s0025-7125(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 37.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5:735–741. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 38.O'Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 39.Yoshitake T, Kiyohara Y, Kato I, et al. Incidence and risk factors of vascular dementia and Alzheimer's disease in a defined elderly Japanese population: the Hisayama Study. Neurology. 1995;45:1161–1168. doi: 10.1212/wnl.45.6.1161. [DOI] [PubMed] [Google Scholar]
- 40.Kuller LH, Lopez OL, Jagust WJ, et al. Determinants of vascular dementia in the Cardiovascular Health Cognition Study. Neurology. 2005;64:1548–1552. doi: 10.1212/01.WNL.0000160115.55756.DE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer's and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28:75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer's disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 43.Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4:103–109. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 44.Ravaglia G, Forti P, Lucicesare A, et al. Physical activity and dementia risk in the elderly: findings from a prospective Italian study. Neurology. 2008;70:1786–1794. doi: 10.1212/01.wnl.0000296276.50595.86. [DOI] [PubMed] [Google Scholar]
- 45.Casserly I, Topol E. Convergence of atherosclerosis and Alzheimer's disease: inflammation, cholesterol, and misfolded proteins. Lancet. 2004;363:1139–1146. doi: 10.1016/S0140-6736(04)15900-X. [DOI] [PubMed] [Google Scholar]
- 46.Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke. 2009;40:S40–44. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iadecola C, Gorelick PB. Converging pathogenic mechanisms in vascular and neurodegenerative dementia. Stroke. 2003;34:335–337. doi: 10.1161/01.str.0000054050.51530.76. [DOI] [PubMed] [Google Scholar]
- 48.Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210–1215. doi: 10.1001/archneurol.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66:300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34:806–812. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- 51.Murray AM. The brain and the kidney connection: A model of accelerated vascular cognitive impairment. Neurology. 2009;73:916–917. doi: 10.1212/WNL.0b013e3181b99a2e. [DOI] [PubMed] [Google Scholar]
- 52.Honig LS, Tang MX, Albert S, et al. Stroke and the risk of Alzheimer disease. Arch Neurol. 2003;60:1707–1712. doi: 10.1001/archneur.60.12.1707. [DOI] [PubMed] [Google Scholar]
- 53.Puccio D, Novo G, Baiamonte V, et al. Atrial fibrillation and mild cognitive impairment: what correlation? Minerva Cardioangiol. 2009;57:143–150. [PubMed] [Google Scholar]
- 54.Purandare N, Voshaar RC, Morris J, et al. Asymptomatic spontaneous cerebral emboli predict cognitive and functional decline in dementia. Biol Psychiatry. 2007;62:339–344. doi: 10.1016/j.biopsych.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 55.Oijen M, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Fibrinogen is associated with an increased risk of Alzheimer disease and vascular dementia. Stroke. 2005;36:2637–2641. doi: 10.1161/01.STR.0000189721.31432.26. [DOI] [PubMed] [Google Scholar]
- 56.Gallacher J, Bayer A, Lowe G, et al. Is sticky blood bad for the brain?: Hemostatic and inflammatory systems and dementia in the Caerphilly Prospective Study. Arterioscler Thromb Vasc Biol. 2010;30:599–604. doi: 10.1161/ATVBAHA.109.197368. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto H, Watanabe T, Miyazaki A, et al. High prevalence of Chlamydia pneumoniae antibodies and increased high-sensitive C-reactive protein in patients with vascular dementia. J Am Geriatr Soc. 2005;53:583–589. doi: 10.1111/j.1532-5415.2005.53204.x. [DOI] [PubMed] [Google Scholar]
- 58.Zuliani G, Guerra G, Ranzini M, et al. High interleukin-6 plasma levels are associated with functional impairment in older patients with vascular dementia. Int J Geriatr Psychiatry. 2007;22:305–311. doi: 10.1002/gps.1674. [DOI] [PubMed] [Google Scholar]
- 59.Ivan CS, Seshadri S, Beiser A, et al. Dementia after stroke: the Framingham Study. Stroke. 2004;35:1264–1268. doi: 10.1161/01.STR.0000127810.92616.78. [DOI] [PubMed] [Google Scholar]
- 60.Hebert R, Lindsay J, Verreault R, Rockwood K, Hill G, Dubois MF. Vascular dementia : incidence and risk factors in the Canadian study of health and aging. Stroke. 2000;31:1487–1493. doi: 10.1161/01.str.31.7.1487. [DOI] [PubMed] [Google Scholar]
- 61.Baum L, Lam LC, Kwok T, et al. Apolipoprotein E epsilon4 allele is associated with vascular dementia. Dement Geriatr Cogn Disord. 2006;22:301–305. doi: 10.1159/000095246. [DOI] [PubMed] [Google Scholar]
- 62.Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 63.Barba R, Martinez-Espinosa S, Rodriguez-Garcia E, Pondal M, Vivancos J, Ser T. Poststroke dementia: clinical features and risk factors. Stroke. 2000;31:1494–1501. doi: 10.1161/01.str.31.7.1494. [DOI] [PubMed] [Google Scholar]
- 64.Peters N, Opherk C, Bergmann T, Castro M, Herzog J, Dichgans M. Spectrum of mutations in biopsy-proven CADASIL: implications for diagnostic strategies. Arch Neurol. 2005;62:1091–1094. doi: 10.1001/archneur.62.7.1091. [DOI] [PubMed] [Google Scholar]
- 65.Dotti MT, Federico A, Mazzei R, et al. The spectrum of Notch3 mutations in 28 Italian CADASIL families. J Neurol Neurosurg Psychiatry. 2005;76:736–738. doi: 10.1136/jnnp.2004.048207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang SC, Lee MJ, Jeng JS, Yip PK. Arg332Cys mutation of NOTCH3 gene in the first known Taiwanese family with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J Neurol Sci. 2005;228:125–128. doi: 10.1016/j.jns.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 67.Leblanc GG, Meschia JF, Stuss DT, Hachinski V. Genetics of vascular cognitive impairment: the opportunity and the challenges. Stroke. 2006;37:248–255. doi: 10.1161/01.STR.0000195177.61184.49. [DOI] [PubMed] [Google Scholar]
- 68.Division of Neuropharmacologic Drug Products, US Food and Drug Administration (FDA). Issues paper on vascular dementia. Peripheral and Central Nervous System Advisory Committee meeting, March 14, 2001. Available at: http://www.fda.gov/ohrms/dockets/ac/01/briefing/3724b2_01_VasDementia.pdf. Accessed on November 29, 2010.
- 69.Roman GC, Kalaria RN. Vascular determinants of cholinergic deficits in Alzheimer disease and vascular dementia. Neurobiol Aging. 2006;27:1769–1785. doi: 10.1016/j.neurobiolaging.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 70.Black S, Roman GC, Geldmacher DS, et al. Efficacy and tolerability of donepezil in vascular dementia: positive results of a 24-week, multicenter, international, randomized, placebo-controlled clinical trial. Stroke. 2003;34:2323–2330. doi: 10.1161/01.STR.0000091396.95360.E1. [DOI] [PubMed] [Google Scholar]
- 71.Wilkinson D, Doody R, Helme R, et al. Donepezil in vascular dementia: a randomized, placebo-controlled study. Neurology. 2003;61:479–486. doi: 10.1212/01.wnl.0000078943.50032.fc. [DOI] [PubMed] [Google Scholar]
- 72.Roman GC, Salloway S, Black SE, et al. Randomized, placebo-controlled, clinical trial of donepezil in vascular dementia: differential effects by hippocampal size. Stroke. 2010;41:1213–1221. doi: 10.1161/STROKEAHA.109.570077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malouf R, Birks J. Donepezil for vascular cognitive impairment. Cochrane Database Syst Rev 2004:CD004395. [DOI] [PMC free article] [PubMed]
- 74.Kavirajan H, Schneider LS. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a meta-analysis of randomised controlled trials. Lancet Neurol. 2007;6:782–792. doi: 10.1016/S1474-4422(07)70195-3. [DOI] [PubMed] [Google Scholar]
- 75.Dichgans M, Markus HS, Salloway S, et al. Donepezil in patients with subcortical vascular cognitive impairment: a randomised double-blind trial in CADASIL. Lancet Neurol. 2008;7:310–318. doi: 10.1016/S1474-4422(08)70046-2. [DOI] [PubMed] [Google Scholar]
- 76.Bores GM, Huger FP, Petko W, et al. Pharmacological evaluation of novel Alzheimer's disease therapeutics: acetylcholinesterase inhibitors related to galanthamine. J Pharmacol Exp Ther. 1996;277:728–738. [PubMed] [Google Scholar]
- 77.Maelicke A, Albuquerque EX. Allosteric modulation of nicotinic acetylcholine receptors as a treatment strategy for Alzheimer's disease. Eur J Pharmacol. 2000;393:165–170. doi: 10.1016/s0014-2999(00)00093-5. [DOI] [PubMed] [Google Scholar]
- 78.Erkinjuntti T, Kurz A, Gauthier S, Bullock R, Lilienfeld S, Damaraju CV. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: a randomised trial. Lancet. 2002;359:1283–1290. doi: 10.1016/S0140-6736(02)08267-3. [DOI] [PubMed] [Google Scholar]
- 79.Auchus AP, Brashear HR, Salloway S, Korczyn AD, Deyn PP, Gassmann-Mayer C. Galantamine treatment of vascular dementia: a randomized trial. Neurology. 2007;69:448–458. doi: 10.1212/01.wnl.0000266625.31615.f6. [DOI] [PubMed] [Google Scholar]
- 80.Craig D, Birks J. Galantamine for vascular cognitive impairment. Cochrane Database Syst Rev 2006:CD004746. [DOI] [PubMed]
- 81.Moretti R, Torre P, Antonello RM, Cazzato G, Bava A. Rivastigmine in subcortical vascular dementia: an open 22-month study. J Neurol Sci. 2002;203–204:141–146. doi: 10.1016/s0022-510x(02)00280-0. [DOI] [PubMed] [Google Scholar]
- 82.Ballard C, Sauter M, Scheltens P, et al. Efficacy, safety and tolerability of rivastigmine capsules in patients with probable vascular dementia: the VantagE study. Curr Med Res Opin. 2008;24:2561–2574. doi: 10.1185/03007990802328142. [DOI] [PubMed] [Google Scholar]
- 83.Craig D, Birks J. Rivastigmine for vascular cognitive impairment. Cochrane Database Syst Rev 2005:CD004744. [DOI] [PubMed]
- 84.Gardoni F, Luca M. New targets for pharmacological intervention in the glutamatergic synapse. Eur J Pharmacol. 2006;545:2–10. doi: 10.1016/j.ejphar.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 85.Kornhuber J, Weller M, Schoppmeyer K, Riederer P. Amantadine and memantine are NMDA receptor antagonists with neuroprotective properties. J Neural Transm Suppl. 1994;43:91–104. [PubMed] [Google Scholar]
- 86.Orgogozo JM, Rigaud AS, Stoffler A, Mobius HJ, Forette F. Efficacy and safety of memantine in patients with mild to moderate vascular dementia: a randomized, placebo-controlled trial (MMM 300) Stroke. 2002;33:1834–1839. doi: 10.1161/01.str.0000020094.08790.49. [DOI] [PubMed] [Google Scholar]
- 87.Wilcock G, Mobius HJ, Stoffler A. A double-blind, placebo-controlled multicentre study of memantine in mild to moderate vascular dementia (MMM500) Int Clin Psychopharmacol. 2002;17:297–305. doi: 10.1097/00004850-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 88.McShane R, Areosa Sastre A, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev 2006:CD003154. [DOI] [PubMed]
- 89.Qaseem A, Snow V, Cross JT, Jr, et al. Current pharmacologic treatment of dementia: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2008;148:370–378. doi: 10.7326/0003-4819-148-5-200803040-00008. [DOI] [PubMed] [Google Scholar]
- 90.O'Brien JT, Burns A. Clinical practice with anti-dementia drugs: a revised (second) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol published online 18 November 2010. doi:10.1177/0269881110387547. The online version of this article can be found at: http://jop.sagepub.com/content/early/2010/11/17/0269881110387547. [DOI] [PubMed]
- 91.Wong CL, Bansback N, Lee PE, Anis AH. Cost-effectiveness: cholinesterase inhibitors and memantine in vascular dementia. Can J Neurol Sci. 2009;36:735–739. doi: 10.1017/s0317167100008350. [DOI] [PubMed] [Google Scholar]
- 92.Yamada M, Kasagi F, Sasaki H, Masunari N, Mimori Y, Suzuki G. Association between dementia and midlife risk factors: the Radiation Effects Research Foundation Adult Health Study. J Am Geriatr Soc. 2003;51:410–414. doi: 10.1046/j.1532-5415.2003.51117.x. [DOI] [PubMed] [Google Scholar]
- 93.in’t Veld BA, Ruitenberg A, Hofman A, Stricker BH, Breteler MM. Antihypertensive drugs and incidence of dementia: the Rotterdam Study. Neurobiol Aging. 2001;22:407–412. doi: 10.1016/s0197-4580(00)00241-4. [DOI] [PubMed] [Google Scholar]
- 94.Peila R, White LR, Masaki K, Petrovitch H, Launer LJ. Reducing the risk of dementia: efficacy of long-term treatment of hypertension. Stroke. 2006;37:1165–1170. doi: 10.1161/01.STR.0000217653.01615.93. [DOI] [PubMed] [Google Scholar]
- 95.Meyer JS, Judd BW, Tawaklna T, Rogers RL, Mortel KF. Improved cognition after control of risk factors for multi-infarct dementia. JAMA. 1986;256:2203–2209. [PubMed] [Google Scholar]
- 96.Forette F, Seux ML, Staessen JA, et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352:1347–1351. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- 97.Forette F, Seux ML, Staessen JA, et al. The prevention of dementia with antihypertensive treatment: new evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Arch Intern Med. 2002;162:2046–2052. doi: 10.1001/archinte.162.18.2046. [DOI] [PubMed] [Google Scholar]
- 98.Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033–1041. [DOI] [PubMed]
- 99.Tzourio C, Anderson C, Chapman N, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069–1075. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- 100.Bosch J, Yusuf S, Pogue J, et al. Use of ramipril in preventing stroke: double blind randomised trial. BMJ. 2002;324:699–702. doi: 10.1136/bmj.324.7339.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008;7:683–689. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- 102.Lithell H, Hansson L, Skoog I, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21:875–886. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 103.Diener HC, Sacco RL, Yusuf S, et al. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study. Lancet Neurol. 2008;7:875–884. doi: 10.1016/S1474-4422(08)70198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McGuinness B, Todd S, Passmore P, Bullock R. Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane Database Syst Rev 2009:CD004034. [DOI] [PMC free article] [PubMed]
- 105.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 106.Wang LY, Larson EB, Sonnen JA, et al. Blood Pressure and Brain Injury in Older Adults: Findings from a Community-Based Autopsy Study. J Am Geriatr Soc. 2009;57:1975–1981. doi: 10.1111/j.1532-5415.2009.02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anderson C, Teo K, Gao P, et al. Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurol. 2011;10:43–53. doi: 10.1016/S1474-4422(10)70250-7. [DOI] [PubMed] [Google Scholar]
- 108.Lopez-Arrieta JM, Birks J. Nimodipine for primary degenerative, mixed and vascular dementia. Cochrane Database Syst Rev 2002:CD000147. [DOI] [PubMed]
- 109.Moretti R, Torre P, Antonello RM, Cazzato G, Pizzolato G. Different responses to rivastigmine in subcortical vascular dementia and multi-infarct dementia. Am J Alzheimers Dis Other Demen. 2008;23:167–176. doi: 10.1177/1533317507312558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Broe GA, Grayson DA, Creasey HM, et al. Anti-inflammatory drugs protect against Alzheimer disease at low doses. Arch Neurol. 2000;57:1586–1591. doi: 10.1001/archneur.57.11.1586. [DOI] [PubMed] [Google Scholar]
- 111.Meyer JS, Rogers RL, McClintic K, Mortel KF, Lotfi J. Randomized clinical trial of daily aspirin therapy in multi-infarct dementia. A pilot study. J Am Geriatr Soc. 1989;37:549–555. doi: 10.1111/j.1532-5415.1989.tb05688.x. [DOI] [PubMed] [Google Scholar]
- 112.Williams PS, Spector A, Orrell M, Rands G. Aspirin for vascular dementia. Cochrane Database Syst Rev 2000:CD001296. [DOI] [PubMed]
- 113.Dufouil C, Richard F, Fievet N, et al. APOE genotype, cholesterol level, lipid-lowering treatment, and dementia: the Three-City Study. Neurology. 2005;64:1531–1538. doi: 10.1212/01.WNL.0000160114.42643.31. [DOI] [PubMed] [Google Scholar]
- 114.Reitz C, Tang MX, Luchsinger J, Mayeux R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol. 2004;61:705–714. doi: 10.1001/archneur.61.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moroney JT, Tang MX, Berglund L, et al. Low-density lipoprotein cholesterol and the risk of dementia with stroke. JAMA. 1999;282:254–260. doi: 10.1001/jama.282.3.254. [DOI] [PubMed] [Google Scholar]
- 116.McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. Cochrane Database Syst Rev 2009:CD003160. [DOI] [PubMed]
- 117.Collins R, Armitage J, Parish S, Sleight P, Peto R. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet. 2004;363:757–767. doi: 10.1016/S0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- 118.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 119.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 120.Trompet S, Vliet P, Craen AJ, et al. Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J Neurol. 2010;257:85–90. doi: 10.1007/s00415-009-5271-7. [DOI] [PubMed] [Google Scholar]
- 121.Amarenco P, Bogousslavsky J, Callahan A, 3rd, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 122.Richard E, Kuiper R, Dijkgraaf MG, Gool WA. Vascular care in patients with Alzheimer's disease with cerebrovascular lesions-a randomized clinical trial. J Am Geriatr Soc. 2009;57:797–805. doi: 10.1111/j.1532-5415.2009.02217.x. [DOI] [PubMed] [Google Scholar]
- 123.Richard E, Heuvel E. Moll van Charante EP, et al. Prevention of dementia by intensive vascular care (PreDIVA): a cluster-randomized trial in progress. Alzheimer Dis Assoc Disord. 2009;23:198–204. doi: 10.1097/WAD.0b013e31819783a4. [DOI] [PubMed] [Google Scholar]
- 124.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 125.Maxwell CJ, Hicks MS, Hogan DB, Basran J, Ebly EM. Supplemental use of antioxidant vitamins and subsequent risk of cognitive decline and dementia. Dement Geriatr Cogn Disord. 2005;20:45–51. doi: 10.1159/000085074. [DOI] [PubMed] [Google Scholar]
- 126.Fioravanti M, Yanagi M. Cytidinediphosphocholine (CDP-choline) for cognitive and behavioural disturbances associated with chronic cerebral disorders in the elderly. Cochrane Database Syst Rev 2005:CD000269. [DOI] [PMC free article] [PubMed]
- 127.Cohen RA, Browndyke JN, Moser DJ, Paul RH, Gordon N, Sweet L. Long-term citicoline (cytidine diphosphate choline) use in patients with vascular dementia: neuroimaging and neuropsychological outcomes. Cerebrovasc Dis. 2003;16:199–204. doi: 10.1159/000071116. [DOI] [PubMed] [Google Scholar]
- 128.Alvarez-Sabin J, Roman GC. Citicoline in vascular cognitive impairment and vascular dementia after stroke. Stroke. 2011;42:S40–43. doi: 10.1161/STROKEAHA.110.606509. [DOI] [PubMed] [Google Scholar]
- 129.Olin J, Schneider L, Novit A, Luczak S. Hydergine for dementia. Cochrane Database Syst Rev 2001:CD000359. [DOI] [PMC free article] [PubMed]
- 130.Napryeyenko O, Borzenko I. Ginkgo biloba special extract in dementia with neuropsychiatric features. A randomised, placebo-controlled, double-blind clinical trial. Arzneimittelforschung. 2007;57:4–11. doi: 10.1055/s-0031-1296579. [DOI] [PubMed] [Google Scholar]
- 131.Hao Z, Liu M, Liu Z, Lv D. Huperzine A for vascular dementia. Cochrane Database Syst Rev 2009:CD007365. [DOI] [PMC free article] [PubMed]
- 132.Frampton M, Harvey RJ, Kirchner V. Propentofylline for dementia. Cochrane Database Syst Rev 2003:CD002853. [DOI] [PubMed]
- 133.Jorge RE, Acion L, Moser D, Adams HP, Jr, Robinson RG. Escitalopram and enhancement of cognitive recovery following stroke. Arch Gen Psychiatry. 2010;67:187–196. doi: 10.1001/archgenpsychiatry.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29:450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- 135.Royall DR, Cordes JA, Roman G, et al. Sertraline improves executive function in patients with vascular cognitive impairment. J Neuropsychiatry Clin Neurosci. 2009;21:445–454. doi: 10.1176/jnp.2009.21.4.445. [DOI] [PubMed] [Google Scholar]
- 136.Johnston SC, O'Meara ES, Manolio TA, et al. Cognitive impairment and decline are associated with carotid artery disease in patients without clinically evident cerebrovascular disease. Ann Intern Med. 2004;140:237–247. doi: 10.7326/0003-4819-140-4-200402170-00005. [DOI] [PubMed] [Google Scholar]
- 137.Rango P, Caso V, Leys D, Paciaroni M, Lenti M, Cao P. The role of carotid artery stenting and carotid endarterectomy in cognitive performance: a systematic review. Stroke. 2008;39:3116–3127. doi: 10.1161/STROKEAHA.108.518357. [DOI] [PubMed] [Google Scholar]
- 138.Rockwood K, Middleton L. Physical activity and the maintenance of cognitive function. Alzheimers Dement. 2007;3:S38–44. doi: 10.1016/j.jalz.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 139.Quaney BM, Boyd LA, McDowd JM, et al. Aerobic exercise improves cognition and motor function poststroke. Neurorehabil Neural Repair. 2009;23:879–885. doi: 10.1177/1545968309338193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Clare L, Woods RT, Moniz Cook ED, Orrell M, Spector A. Cognitive rehabilitation and cognitive training for early-stage Alzheimer's disease and vascular dementia. Cochrane Database Syst Rev 2003:CD003260. [DOI] [PubMed]
- 141.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 142.Hajjar I, Hart M, Milberg W, Novak V, Lipsitz L. The rationale and design of the antihypertensives and vascular, endothelial, and cognitive function (AVEC) trial in elderly hypertensives with early cognitive impairment: role of the renin angiotensin system inhibition. BMC Geriatr. 2009;9:48. doi: 10.1186/1471-2318-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Williamson JD, Miller ME, Bryan RN, et al. The Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Study (ACCORD-MIND): rationale, design, and methods. Am J Cardiol. 2007;99:112i–122i. doi: 10.1016/j.amjcard.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 144.Frisoni GB, Canu E, Geroldi C, et al. Prescription patterns and efficacy of drugs for patients with dementia: physicians' perspective in Italy. Aging Clin Exp Res. 2007;19:349–355. doi: 10.1007/BF03324714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 510 kb)