Abstract
Background
Atrial fibrillation (AF) is the most common sustained atrial arrhythmia and it is independently associated with an increased morbidity and mortality. As a result of the high prevalence of AF, the economic and clinical impact of the disease is substantial. This study describes the economic and clinical impact of AF in the Netherlands.
Methods
Epidemiological data on AF in the Netherlands were projected on population estimates of the Netherlands in 2009 and combined with data on the cost of AF and its interventions.
Results
Overall prevalence of AF in the Netherlands is 5.5% in the population over 55 years, corresponding to about 250,000 AF patients. The prevalence increases with age, and the mean age of AF patients is 69.3 years. Incidence of AF in the Netherlands varies with age, from 1188 new cases in the age group of 55 to 59 up to 7074 new cases in the age group 75 to 79. Total new cases amounts to 45,085 patients per year in the Netherlands. Total costs of AF in the Netherlands are € 583 million, of which the majority (70%) were accounted for by hospitalisations and in-hospital procedures. Pharmacotherapeutic management of AF totalled € 17 million in the Netherlands in 2009.
Discussion
AF is a serious disease with a high clinical and economic burden, especially due to hospitalisations as a result of cardiovascular events. The number of patients with AF in the Netherlands is considerable and will increase with the ageing population in the future.
Keywords: Atrial fibrillation, Burden of illness, Costs, Comorbidity
Introduction
Atrial fibrillation (AF) is the most common sustained atrial arrhythmia and it is independently associated with an increased morbidity and mortality [1–3]. In particular, it is a major cause of stroke, a highly lethal and disabling event frequently associated with prolonged hospitalisation [1–4]. AF is one of the typical diseases in the elderly, and the prevalence of AF is increasing with age [4]. However, it is suggested that the prevalence of AF is also increasing independent of the ageing of the general population [5]. As a consequence, around 1–2% of the general population suffers from AF [4].
Given the frequent comorbidities in AF, the economic burden for society is considerable and expected to grow in the near future [5, 6]. Several cost estimates have been published on the economic burden of AF, all reporting hospitalisation costs as the main cost driver [7–11]. Ringborg et al. described the cost of AF in five European countries, including the Netherlands, in the period 2003–2004. Mean annual costs per patient were reported to be €2328 for the Netherlands. Costs for interventional procedures and inpatient care were major cost drivers, while treatment costs and consultations accounted for a relatively small share of the total annual costs [11]. The aim of the current paper is to describe the clinical and economic burden of AF in the Netherlands.
Clinical spectrum and concomitant disease
AF can be subdivided into four different clinical types, based on the severity of the disease: first-detected AF, paroxysmal AF, persistent AF, and permanent AF. Patients who had their first episode of AF have first-detected AF. When a patient has had two or more episodes, AF is considered recurrent. If the patient converts back to sinus rhythm within 7 days it is called paroxysmal AF, while recurrent AF lasting longer than 7 days is called persistent AF, and the use of cardioversion therapy does not alter this designation. If conversion back to sinus rhythm failed or was not attempted, AF is defined as permanent [12]. About 60% to 90% of patients with AF experience symptoms [13, 14]. Most common symptoms include palpitations, shortness of breath, fatigue, chest pain, dizziness and syncope.
AF is associated with an up to two times increased risk for all-cause mortality, even in the absence of other cardiovascular mortality such as myocardial infarction, congestive heart failure, valvular heart disease, and stroke or transient ischaemic attack [1]. The risk for mortality appears to be higher in women than in men [1, 13]. Moreover, AF patients have a significantly increased risk of stroke which increases with age and in the presence of heart failure, hypertension, diabetes and especially a prior stroke or transient ischaemic attack [12, 15]. Stroke is the most debilitating complication of AF. Not only does AF increase the risk for stroke fivefold, [16, 17] it is also associated with more severe strokes and with a poorer prognosis compared with strokes not associated with AF. Following a stroke, AF patients had a significantly higher 30-day and 1-year mortality and a higher risk for stroke recurrence than non-AF patients suffering from a stroke [18–21].
In addition, AF frequently coexists with heart failure and a reciprocal relation exists between both diseases [22]. Wang et al. reported that in 1470 subjects in the Framingham study developing AF and/or congestive heart failure, 26% developed both conditions. In 38% of these subjects, the diagnosis of AF was first established and for 41% the diagnosis of heart failure was first, while for 21% both diagnoses were established on the same day [23]. The relative risk of developing AF from having heart failure, 6.1 to 17.5, is significant compared with that for hypertension and becomes larger with increasing severity of heart failure [2]. Moreover, Wang reported a worsening of the prognosis and increased mortality with either combination of AF and heart failure as compared with having only one of these conditions [23]. In the Euro Heart Survey, prevalence of heart failure was 26% in patients with first-detected AF, 23% in patients with paroxysmal AF, 35% in patients with persistent AF and 49% in patients with permanent AF, and in the overall group heart failure was a major risk factor for mortality and morbidity during 1 year follow-up [6, 24]. In return, AF may worsen the prognosis of heart failure, [22] indicating a strong interaction between the two diseases.
Moreover, hypertension has been demonstrated to be an important risk factor for the development of AF [25]. Within the Euro Heart Survey, mean prevalence of hypertension in AF patients was 64%, which did not differ significantly between AF subtypes [6]. The relative risk of developing AF for hypertensive patients is reported to be 1.4 to 2.1 compared with normotensive patients [26]. Given the high prevalence of hypertension in the Netherlands, its contribution to the burden of AF is considerable. For example, in the Rotterdam study, 21.4% of the general population over 55 years of age suffered from hypertension [27]. Hypertension has become the most dominant risk factor for AF worldwide [25, 26].
The impact of AF on quality of life (QoL) is considerable [28]. Especially patients with more frequent symptoms and those experiencing cardiovascular comorbidity, such as stroke and heart failure, have a poorer quality of life with regard to physical and mental health [29]. However, this may not apply to the elderly population of AF patients, as older patients generally have a lower QoL and in addition may experience less symptoms caused by AF [30]. QoL is reported to depend on comorbidity and women consistently report a lower QoL than men [31]. In patients who can maintain sinus rhythm, quality of life is better compared with those remaining in AF. Of note, the latter is independent of the treatment strategy chosen. Indeed, a study by Reynolds suggests that treatment, either rate control or antiarrhythmic, may improve quality of life scores in AF patients up to normal population scores [31].
Epidemiology
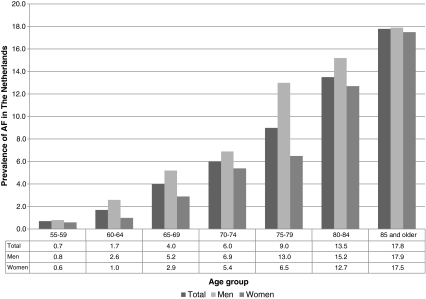
Prevalence estimates of AF in the Netherlands have been reported by Heeringa et al. [27]. General prevalence in the Netherlands was 5.5% in the population over 55 years of age in 1990 [27]. Mean age of that population was 69.3 years and the prevalence was higher for men (6.0%) than for women (5.1%). AF prevalence increases with older age. Heeringa et al. report an age-stratified prevalence ranging from 0.7% for ages 55 to 59 to 17.8% for 85 years and older, as is graphically depicted in Fig. 1 [27]. Literature reports several estimates of the prevalence of AF in other countries. Go et al. report an overall prevalence of 0.95% in the adult (age ≥20 years) population that received care within a large health maintenance organisation in Northern California [17]. The diagnosis was established based on the reported diagnosis in automated clinical databases. The mean age of their patient population was 71.2 years. The disease was more prevalent in men (6.0%) than in women (5.1%). In that study, prevalence increases from 0.1% in the population younger than 55 years, to 9.0% in persons aged 80 years and older [17]. In addition, Furberg et al. reported a prevalence of 4.8% for women and 6.2% for men in a randomly selected population aged ≥65 years from the US [32]. As the selection of AF patients by Heeringa et al. was based on both their population study and general practitioner files, a higher, probably more reliable, prevalence estimate was found for the Netherlands [27].
Fig. 1.
Prevalence of AF in the Netherlands, by age group and gender [27]
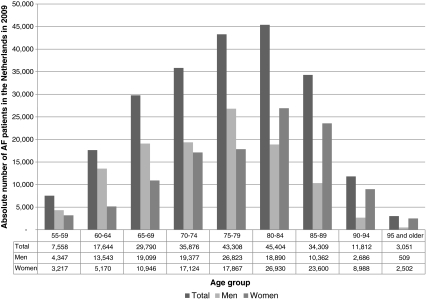
If projected on the Netherlands population in 2009, the prevalence figures by Heeringa et al. add up to 250,470 AF patients, 55 years of age and older, accounting for 1.5% of the total Netherlands population in 2009. Figure 2 shows the projected number of patients with AF in the Netherlands in 2009, distributed by age class and gender, based on the prevalence estimates by Heeringa et al. [27]. As these figures are already 20 years old and the age- and gender-adjusted prevalence of AF is reported to be increasing [5], the actual prevalence of AF in the Netherlands in 2009 may be higher.
Fig. 2.
Number of AF patients in the Netherlands in 2009, by age group and gender [27]
The incidence of AF in the Netherlands was reported to be 9.9/1000 person-years in the population over 55 years of age, increasing from 1.1/1000 person-years at ages 55–60 to 18.2/1000 patient-years at ages 85 years of age and higher [27]. Across all age groups, the incidence was higher in men than in women [27]. Projected on the Netherlands population, these incidence figures account for 45,085 new cases of AF in 2009, ranging from 1188 in the age group of 55 to 59, to 7074 new cases in the age group of 75 to 79 years.
Rate and rhythm management of AF
Treatment guidelines for the management of AF are available from the Netherlands Association of General Practitioners (NHG) for first-line management, and from the European Society of Cardiology for second-line management [12, 33]. Diagnosis is usually based on both clinical examination and the patient’s history, and confirmed by ECG recording [12]. Newly diagnosed AF patients start with rate-control therapy in combination with appropriate antithrombotic treatment, usually acetylsalicylic acid or oral anticoagulants. Beta-blockers are most commonly used for rate control, while second choices are calcium channel blockers or digoxin in the event of heart failure. When AF persists, rate-control therapy may be continued in combination with antithrombotic treatment. However, sinus rhythm may also be restored by cardioversion [12, 33].
In patients with paroxysmal AF, the initial choice is rate control in combination with antithrombotic treatment. When troublesome symptoms persist, antiarrhythmic therapy can be initiated. Although achieving sinus rhythm was associated with a considerable reduction in the risk of death in comparison with not achieving sinus rhythm [34], several trials have implied that currently available antiarrhythmics have no beneficial effect with regard to the risk for stroke or mortality [35, 36]. Thus, beneficial effects of antiarrhythmic drugs with regard to mortality may be offset by their adverse effects, as is suggested by Corley et al. [34]. AF ablation techniques may offer an alternative and potential curative strategy for patients with paroxysmal, persistent or permanent AF [37]. However, ablation is not suitable for every AF patient and it remains uncertain whether ablation will become available for the majority of AF patients [38]. Therefore, there is still a need for an effective method to maintain sinus rhythm with less severe side effects [34]. Of note, the current AF guideline strongly suggests that when deciding on treatment strategy, safety rather than efficacy should guide the choice of rate- or rhythm-control drugs and ablation therapy [12].
Costs and economic burden of disease
Based on the mean annual costs of €2328 per patient for AF, as calculated by Ringborg et al., the total cost of AF in the Netherlands is estimated to be € 583,093,264, of which 3.7% for drug therapy (including vitamin K antagonists, other antithrombotic treatment, and antiarrhythmic rate-control treatment) [11, 27]. The total costs accounted for 1.3% of the healthcare budget of the Netherlands in 2008 [39]. It is likely that the new types of anticoagulant therapy may provide an increase in the costs of medicinal treatment of AF, but this cannot be concluded from our study. Table 1 displays the estimated total costs of AF in the Netherlands distributed by age group [6, 11]. Due to the increasing prevalence with age, costs also increase with age. However, the mean costs provided by Ringborg et al. are mean costs over all age groups [11]. As the risk for cardiovascular events increases with age, costs related to these events will also increase with age. It can thus be expected that the true costs per age group are even more skewed to the patient groups with higher ages.
Table 1.
Total economic burden of AF in the Netherlands by age group
| Age group (years) | Prevalence (%) [27] | Number of patients in the Netherlands in 2009 | Total costs |
|---|---|---|---|
| 55–59 | 0.7 | 7558 | € 17,593,862 |
| 60–64 | 1.7 | 17,644 | € 41,075,258 |
| 65–69 | 4.0 | 29,790 | € 69,351,027 |
| 70–74 | 6.0 | 35,876 | € 83,520,120 |
| 75–79 | 9.0 | 43,308 | € 100,821,443 |
| 80–84 | 13.5 | 45,404 | € 105,701,478 |
| 85–89 | 17.8 | 34,309 | € 79,870,444 |
| 90–94 | 17.8 | 11,812 | € 27,499,351 |
| 95 and older | 17.8 | 3051 | € 7,102,956 |
| 55 and older | 5.5 | 250,470 | € 583,093,264 |
Based on a population of 16,430,473 for the Netherlands in 2009
An estimation of the costs for pharmacological treatment of AF can be derived from a projection of the use of antiarrhythmics and rate-control therapy as reported in the Euro Heart Survey, combined with data from the Netherlands Healthcare Insurance Board [11, 40]. Costs for the 125,232 patients using rate-control agents in the Netherlands in 2009 were € 11,330,579, based on the costs of the most frequently administered medicinal products. In addition to that, costs for 50,314 sotalol users were € 826,979 and costs for 27,502 amiodarone users were € 2,511,237 in 2009. The use of other antiarrhythmics, including flecainide and propafenone, amounted to € 2,565,907 in 2009. In total, annual pharmacological treatment costs totalled € 17,234,702 in the Netherlands in 2009 [11].
In addition, AF patients contribute to a significant part of the healthcare resource use especially because of hospital admissions. Ringborg et al. calculated that patients with AF in the Netherlands annually generate an average cost of €798 (34%) for the relatively few number of hospital interventions such as coronary artery bypass grafting, catheter ablation, and pacemaker implantation; and €834 (36%) for inpatient care (share of total annual costs per patient). Here, it should be noted that these interventions are also related to comorbidity in AF patients.
In addition, €160 (7%) was annually spent on diagnostic procedures such as transthoracic echocardiography, chest X-rays, Holter monitoring, thyroid function tests, exercise tests and transoesophageal echocardiography [11]. Consequently, the total costs for hospitalisations and in-hospital procedures for all AF patients in the Netherlands add up to €456,356,340.
The higher risk for cardiovascular morbidity causes a significant economic burden not only due to hospitalisation, but also due to loss of work. Although the majority of patients with AF are older than 65 and have stopped working, the costs of productivity losses are still considerable. Ringborg et al. calculated that Netherlands AF patients incurred an annual mean cost of €391. These costs were the highest of all costs due to work loss of all the countries in the study by Ringborg [11]. Of note these costs were averaged over all patients, whereas indirect costs are only incurred by the employed population. Thus, the average indirect costs per employed patients are even higher.
Conclusion
This paper has described the clinical and economic burden of AF in the Netherlands. AF is a seriously debilitating disease with a high risk of mortality and cardiovascular events such as stroke. The severity of the disease is growing further, and the prognosis worsens with increasing age. In addition, the number of patients with AF in the Netherlands is considerable, and this number is likely to increase as a result of an ageing population.
The total costs of AF in the Netherlands are estimated to be € 583 million in 2009, which would account for 1.3% of the Netherlands healthcare expenditure. The majority of these costs are incurred for hospitalisations and in-hospital procedures, while drug therapy only accounts for 3.7% of these costs. The total costs are likely to be an underestimation of the true costs of AF in the Netherlands. Many patients have concomitant cardiovascular diseases that result in a further increase in the costs of the management of these patients. Moreover, costs will increase with age as a result of the higher prevalence and increasing risk for cardiovascular events that occur with older age. Consonant with the increasing prevalence of AF, this will result in an increasing economic burden of AF in the near future. Consequently, better medicinal treatment of the risk factors for AF, AF itself, or of stroke in patients with AF, will lead to a minor increase in the costs of treatment, but at the same time to a significant reduction in serious events and related hospitalisations, which carry the bulk of the costs for patients with AF.
Acknowledgments
Funding This study was funded by Sanofi-Aventis. The authors were independent from Sanofi-Aventis in the conduct and the reporting of this study.
References
- 1.Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271(11):840–844. doi: 10.1001/jama.1994.03510350050036. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82(8A):2N–9N. doi: 10.1016/S0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 3.Stewart S, Hart CL, Hole DJ, et al. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113(5):359–364. doi: 10.1016/S0002-9343(02)01236-6. [DOI] [PubMed] [Google Scholar]
- 4.Kirchhof P, Bax J, Blomstrom-Lundquist C, et al. Early and comprehensive management of atrial fibrillation: proceedings from the 2nd AFNET/EHRA consensus conference on atrial fibrillation entitled ‘research perspectives in atrial fibrillation’. Europace. 2009;11(7):860–885. doi: 10.1093/europace/eup124. [DOI] [PubMed] [Google Scholar]
- 5.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 6.Nieuwlaat R, Capucci A, Camm AJ, et al. Atrial fibrillation management: a prospective survey in ESC member countries: the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2005;26(22):2422–2434. doi: 10.1093/eurheartj/ehi505. [DOI] [PubMed] [Google Scholar]
- 7.McBride D, Mattenklotz AM, Willich SN, et al. The costs of care in atrial fibrillation and the effect of treatment modalities in Germany. Value Health. 2008;12(2):293–301. doi: 10.1111/j.1524-4733.2008.00416.x. [DOI] [PubMed] [Google Scholar]
- 8.Moeremans K, Aliot E, Chillou C, et al. Second line pharmacological management of paroxysmal and persistent atrial fibrillation in France: a cost analysis. Value Health. 2000;3(6):407–416. doi: 10.1046/j.1524-4733.2000.36001.x. [DOI] [PubMed] [Google Scholar]
- 9.Stewart S, Murphy NF, Walker A, et al. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart. 2004;90(3):286–292. doi: 10.1136/hrt.2002.008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reynolds MR, Essebag V, Zimetbaum P, et al. Healthcare resource utilization and costs associated with recurrent episodes of atrial fibrillation: the FRACTAL registry. J Cardiovasc Electrophysiol. 2007;18(6):628–633. doi: 10.1111/j.1540-8167.2007.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ringborg A, Nieuwlaat R, Lindgren P, et al. Costs of atrial fibrillation in five European countries: results from the Euro Heart Survey on atrial fibrillation. Europace. 2008;10(4):403–411. doi: 10.1093/europace/eun048. [DOI] [PubMed] [Google Scholar]
- 12.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery. Eur Heart J. 2010;31(19):2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 13.Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 14.Rho RW, Page RL. Asymptomatic atrial fibrillation. Prog Cardiovasc Dis. 2005;48(2):79–87. doi: 10.1016/j.pcad.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 16.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988. doi: 10.1161/01.STR.22.8.983. [DOI] [PubMed] [Google Scholar]
- 17.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 18.Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27(10):1760–1764. doi: 10.1161/01.STR.27.10.1760. [DOI] [PubMed] [Google Scholar]
- 19.Sandercock P, Bamford J, Dennis M, et al. Atrial fibrillation and stroke: prevalence in different types of stroke and influence on early and long term prognosis (Oxfordshire community stroke project) BMJ. 1992;305(6867):1460–1465. doi: 10.1136/bmj.305.6867.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marini C, Santis F, Sacco S, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke. 2005;36(6):1115–1119. doi: 10.1161/01.STR.0000166053.83476.4a. [DOI] [PubMed] [Google Scholar]
- 21.Kaarisalo MM, Immonen-Räihä P, Marttila RJ, et al. Atrial fibrillation and stroke. Mortality and causes of death after the first acute ischemic stroke. Stroke. 1997;28(2):311–315. doi: 10.1161/01.STR.28.2.311. [DOI] [PubMed] [Google Scholar]
- 22.Efremidis M, Pappas L, Sideris A, et al. Management of atrial fibrillation in patients with heart failure. J Card Fail. 2008;14(3):232–237. doi: 10.1016/j.cardfail.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 23.Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 24.Nieuwlaat R, Prins MH, Heuzey JY, et al. Prognosis, disease progression, and treatment of atrial fibrillation patients during 1 year: follow-up of the Euro Heart Survey on atrial fibrillation. Eur Heart J. 2008;29(9):1181–1189. doi: 10.1093/eurheartj/ehn139. [DOI] [PubMed] [Google Scholar]
- 25.Conen D, Osswald S, Albert CM. Epidemiology of atrial fibrillation. Swiss Med Wkly. 2009;139(25–26):346–352. doi: 10.4414/smw.2009.12500. [DOI] [PubMed] [Google Scholar]
- 26.Healey JS, Connolly SJ. Atrial fibrillation: hypertension as a causative agent, risk factor for complications, and potential therapeutic target. Am J Cardiol. 2003;91(10A):9G–14G. doi: 10.1016/S0002-9149(03)00227-3. [DOI] [PubMed] [Google Scholar]
- 27.Heeringa J, Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27(8):949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds MR, Ellis E, Zimetbaum P. Quality of life in atrial fibrillation: measurement tools and impact of interventions. J Cardiovasc Electrophysiol. 2008;19(7):762–768. doi: 10.1111/j.1540-8167.2007.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang Y. Relation of atrial arrhythmia-related symptoms to health-related quality of life in patients with newly diagnosed atrial fibrillation: a community hospital-based cohort. Heart Lung. 2006;35(3):170–177. doi: 10.1016/j.hrtlng.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Howes CJ, Reid MC, Brandt C, et al. Exercise tolerance and quality of life in elderly patients with chronic atrial fibrillation. J Cardiovasc Pharmacol Ther. 2001;6(1):23–29. doi: 10.1177/107424840100600103. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds MR, Lavelle T, Essebag V, et al. Influence of age, sex, and atrial fibrillation recurrence on quality of life outcomes in a population of patients with new-onset atrial fibrillation: the Fibrillation Registry Assessing Costs, Therapies, Adverse events and Lifestyle (FRACTAL) study. Am Heart J. 2006;152(6):1097–1103. doi: 10.1016/j.ahj.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furberg CD, Psaty BM, Manolio TA, et al. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study) Am J Cardiol. 1994;74(3):236–241. doi: 10.1016/0002-9149(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 33.Nederlands Huisartsen Genootschap. NHG-standaard atriumfibrilleren M79. 2009. Available from: http://nhg.artsennet.nl/kenniscentrum/k_richtlijnen/k_nhgstandaarden/Samenvattingskaartje-NHGStandaard/M79_svk.htm. Accessed 23 Dec 2009.
- 34.Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109(12):1509–1513. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 35.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 36.Burkhardt JD, Natale A. New technologies in atrial fibrillation ablation. Circulation. 2009;120(15):1533–1541. doi: 10.1161/CIRCULATIONAHA.109.858233. [DOI] [PubMed] [Google Scholar]
- 37.Fisher JD, Spinelli MA, Mookherjee D, et al. Atrial fibrillation ablation: reaching the mainstream. Pacing Clin Electrophysiol. 2006;29(5):523–537. doi: 10.1111/j.1540-8159.2006.00388.x. [DOI] [PubMed] [Google Scholar]
- 38.Ehrlich JR, Nattel S. Novel approaches for pharmacological management of atrial fibrillation. Drugs. 2009;69(7):757–774. doi: 10.2165/00003495-200969070-00001. [DOI] [PubMed] [Google Scholar]
- 39.Gezondheid en zorg in cijfers 2009. Centraal Bureau voor de Statistiek. Dec 2009. Available from: http://www.cbs.nl/nl-NL/menu/themas/gezondheid-welzijn/publicaties/publicaties/archief/2009/2009-c156-pub.htm. Accessed 5 Jan 2010.
- 40.College voor Zorgverzekeringen. www.medicijnkosten.nl. Accessed 5 Jan 2010.