Abstract
Background
The Norwood procedure consists of three palliative operations, performed in neonates with hypoplastic left heart syndrome. Especially the first stage (Norwood I) is associated with the highest mortality rates in paediatric cardiac surgery (up to 25%). During surgery, the aorta is reconstructed and a systemic-to-pulmonary shunt is applied. Originally the modified Blalock-Taussig shunt was used, but recently the right-ventricle-to-pulmonary-artery shunt is increasingly being employed. We reviewed the results of our operative strategy, where an individualised choice of shunt is made. Furthermore, attempts to reduce interstage mortality (between Norwood I and II) were assessed.
Methods
All neonates who underwent Norwood stage I palliation from August 2004 until November 2010 were included in this retrospective analysis. Mortality rates and management strategies were compared.
Results
Thirty-six patients were available for analysis. Overall 30-day mortality was 5.6% (2 patients) and interstage mortality after discharge was 14% (5 patients). In 2006, a novel clinical protocol was introduced, aimed at reduction of mortality during the interstage period. This resulted in reduction of interstage mortality from 23% to 9% (3 of 13 infants, versus 2 of 23), with a cumulative survival of 82% (maximum follow-up 4 years).
Conclusion
Early surgical results following the Norwood procedure using an individualised shunt choice are favourable.
Keywords: Congenital heart disease, Hypoplastic left heart syndrome, Survival, Mortality
Introduction
The Norwood procedure is the standard treatment for hypoplastic left heart syndrome (HLHS). In this complex congenital heart defect, occurring in 1 in 5000 live births, a restricted foramen ovale during foetal life results in a hypoplastic left ventricle, stenotic or atretic mitral and aortic valves, and consequent hypoplasia of the ascending aortic arch [1]. The diagnosis is usually made antenatally, and treatment options include termination of the pregnancy, compassionate care, or surgical palliation.
The Norwood trajectory is the most common surgical procedure, and consists of three stages of repair, ultimately resulting in a Fontan circulation. The first stage (Norwood I) is performed at neonatal age and results in a univentricular circulation. The stage II and III repairs are performed at 4–6 months and 2 years, respectively, in which first a partial, and later a total cavo-pulmonary connection are constructed.
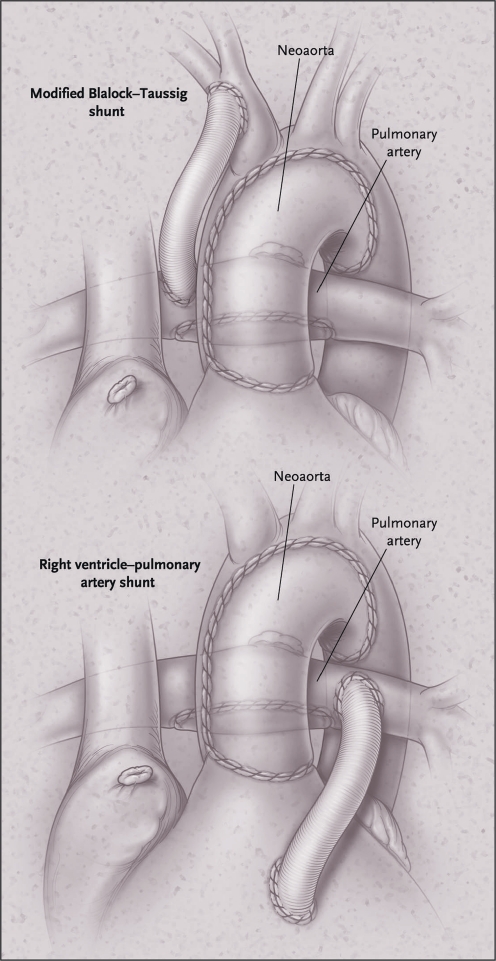
The first stage of the Norwood procedure is associated with the highest mortality rates within the field of congenital heart surgery, with reported early mortality rates varying between 6 and 25% [2–6]. The procedure results in the right ventricle providing systemic circulation through a newly constructed ‘neo-aorta’. Pulmonary circulation is warranted by the placement of a systemic-to-pulmonary shunt. The original Norwood procedure includes the modified Blalock-Taussig shunt (BT shunt): a tube graft connecting the brachiocephalic artery to the right pulmonary artery (Fig. 1). Perfusion through the shunt mainly takes place during diastole, which entails the risk of a lower perfusion pressure of the coronary arteries [7]. Therefore, recently the right-ventricle-to-pulmonary-artery (RV-PA or Sano) shunt has gained popularity [2]. This shunt type, however, may negatively impact cardiac function in the long term, due to the development of fibrotic tissue of the right ventricle [6, 8]. In our centre the modified BT shunt is used preferentially. However, in selected infants with specific characteristics (low weight, very small ascending aorta or severe tricuspid insufficiency) the RV-PA shunt is employed.
Fig. 1.
The two variants of the Norwood I procedure; with modified BT shunt (top) and RV-PA shunt (bottom). Re-used with permission from Ohye et al. [8]
Beyond survival of the Norwood I, the period between the first and second stages is associated with high risk for mortality. To reduce this interstage mortality at our centre, from 2006 onwards, a new clinical protocol was introduced. This consisted of the prolonged admission of high-risk infants between Norwood stages I and II, and electively performing the stage II repair at a younger age.
In this study, we reviewed the early surgical results of the Norwood procedure at our centre and the impact of the altered strategy during the interstage period.
Methods
Retrospectively, all consecutive infants who underwent the Norwood procedure from August 2004 until November 2010 were reviewed. Demographic data were recorded, and outcome data included mortality and unplanned re-interventions. The ethical committee of our hospital waived the need for parental consent for this study.
All patients underwent the procedure by the same surgical team. Standard cardiopulmonary bypass techniques were used in all patients, including cooling to deep hypothermia. A modified BT shunt was placed routinely, except when the following patient characteristics were present: an ascending aorta of <2 mm diameter, weight <2.5 kg at surgery or severe tricuspid insufficiency; the RV-PA shunt was employed in these cases.
During postoperative management on the paediatric intensive care, a multidisciplinary team of paediatric cardiothoracic surgeons, paediatric cardiac anaesthesiologists, paediatric cardiologists and paediatric intensivists was involved. Extracorporeal membrane oxygenation (ECMO) apparatus was not available during the study period.
Before November 2006, stage II repair was electively planned at an age of 6 months, and patients awaited the procedure at home. After this date, clinical practice during the interstage was altered. Selected infants that did not prove to be completely haemodynamically stable (i.e. recurrent dips in oxygen saturation or a mean oxygen saturation of <70% without additional oxygen) remained admitted in hospital during this interstage period. Furthermore, in all patients efforts were made to routinely perform the stage II repair at a younger age (from 2 to 4 months onwards).
Stage III repair was subsequently planned at approximately 2 years of age.
Statistics
Group characteristics are displayed as medians and (interquartile) ranges as appropriate. Actuarial survival analysis was performed using Kaplan-Meier estimates, and a log-rank test was used to compare survival before and after 2006. Analyses were performed with SPSS Version 15.0 (SPSS Inc, Chicago, Ill, USA).
Results
A total of 36 infants underwent the Norwood procedure, with a median age of 7 (range 4–55) days and median weight of 3.5 kg (range 1.8–4.6) at surgery. Twenty-four neonates were diagnosed with classical HLHS, and the remaining 12 had HLHS-associated anomalies.
Using the above-described considerations for shunt choice, the modified BT shunt was used in 29 cases (81%), as opposed to the RV-PA shunt in 7 cases (19%). The proportion of either shunt type did not change during the study period.
Two patients died within 30 days after the Norwood I procedure (5.6%), both due to low cardiac output syndrome. A modified BT shunt was used in one, RV-PA shunt in the other. Unplanned re-interventions (surgical or by interventional catheterisation) before stage II repair were necessary in 6 infants (16.7%), of which half were due to shunt obstructions.
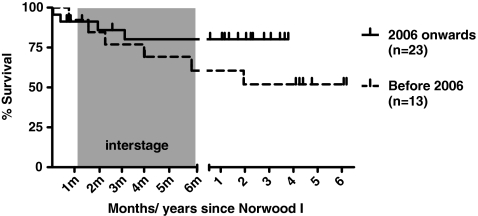
For analysis of the effect of the altered management protocol in the interstage period, neonates undergoing surgery before and after 2006 were compared, resulting in 13 neonates in the early period versus 23 in the later period. The change in protocol resulted in a younger age at stage II repair (median 4.5 versus 6 months). Stage III repair was performed at a median age of 2 years in both groups.
In the early period, interstage mortality (occurring after discharge following the Norwood procedure and before stage II repair) occurred in 3 neonates (23%). In the later period, 2 of 23 infants died (9%). Later mortality was incidental, with one patient within 30 days after stage III repair due to a failing Fontan circulation.
Figure 2 shows the survival of infants before and after 2006 (p = 0.19 between the two periods). In the current era, survival to date is currently 82%, with a maximal follow-up of 4 years.
Fig. 2.
Kaplan-Meier survival curve
Discussion
In the current surgical era, the Norwood procedure still carries among the highest mortality rates within paediatric cardiac surgery. In this study, we reviewed the results of our recent surgical and management protocols in this high-risk group, and found a favourable early mortality of 5.6%. Overall mortality is largely dependent on patients who died while awaiting their stage II repair, but the current management protocols seem to have improved the overall results markedly.
In recent literature, mortality rates for the first stage of the Norwood procedure vary between 6 and 25% [2–6]. Choice of shunt type (modified BT versus RV-PA shunt) has received much attention with controversial results [2, 4, 5, 9]. A large recently completed randomised controlled multicentre trial comparing the two shunt types showed higher survival in infants who had received the RV-PA shunt at 1 year of age [8]. However, the RV-PA shunt was associated with more unexpected re-interventions, mainly due to shunt obstructions. At our centre, the choice for a modified BT or RV-PA shunt is based on specific patient characteristics. As the RV-PA shunt has the important disadvantage of an incision in the systemic ventricle, which may negatively impact ventricular function or lead to ventricular arrhythmias, there is a preference for the modified BT shunt. At our centre the RV-PA shunt is reserved for low birth weight infants, where the modified BT shunt may cause pulmonary overperfusion, and for patients with a very small ascending aorta, as a lower coronary perfusion pressure, often associated with use of the modified BT shunt, may lead to myocardial ischaemia in these patients. Finally, if severe tricuspid insufficiency is present, the RV-PA shunt is employed due to the theoretical advantage of reducing volume load of the right ventricle. In our opinion this individualised approach is the main reason for the low early mortality seen in our cohort.
Following discharge after the Norwood hospitalisation, the interstage period is known to be associated with a continued risk for mortality. Shunts may become obstructed, or changes in systemic or pulmonary vascular resistance may induce alterations in the ratio of systemic and pulmonary perfusion. The prolonged admission of selected neonates during this period, and the decision to routinely perform the stage II repair at a younger age in all patients, resulted in a marked reduction of this interstage mortality. This did not reach statistic significance, presumably due to the relatively small sample size. However, we cannot exclude that the reduced mortality over the years also reflects improvements in other (perioperative) management strategies that we are not aware of at this time.
The cumulative effects of all these efforts have resulted in the survival of 82%, with a maximal follow-up of 4 years. Recently published survival rates for 1 or 2 years of age vary between 64 and 81%, with most mortality taking place shortly after the Norwood procedure or during the interstage period [2, 4, 5, 8]. Over the years, survival after the Norwood procedure has improved drastically since it was first performed in the 1980s [10]. However, unfortunate side effects of these complex cardiac procedures are becoming increasingly apparent. Although the severity varies widely, up to 60% of children surviving the entire trajectory suffer from a wide range of neurocognitive deficits [2, 11]. The challenge is now to unravel the mechanisms leading to cerebral injury.
In conclusion, when reviewing the recent results of the Norwood procedure at our centre, a favourable surgical outcome is observed. The individualised choice for shunt type is regarded as an important factor. Beyond the first stage, prolonged admittance of high-risk patients and early timing of the stage II repair have likely contributed to improved survival in this group.
Acknowledgments
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Hazekamp MG, Rijlaarsdam ME, Schoof PH, et al. Favourable results with surgical treatment in 43 children with hypoplastic left-heart syndrome or similar disorders, 1999–2005. Ned Tijdschr Geneeskd. 2006;150:1930–5. [PubMed] [Google Scholar]
- 2.Atallah J, Dinu IA, Joffe AR, et al. Two-year survival and mental and psychomotor outcomes after the Norwood procedure: an analysis of the modified Blalock-Taussig shunt and right ventricle-to-pulmonary artery shunt surgical eras. Circulation. 2008;118:1410–8. doi: 10.1161/CIRCULATIONAHA.107.741579. [DOI] [PubMed] [Google Scholar]
- 3.European Association for Cardio-Thoracic Surgery: Mortality versus procedure, EACTS congenital database 2005. http://www.ctsnet.org/. Accessed 10 February 2011.
- 4.Ghanayem NS, Hoffman GM, Mussatto KA, et al. Perioperative monitoring in high-risk infants after stage 1 palliation of univentricular congenital heart disease. J Thorac Cardiovasc Surg. 2010;140:857–63. doi: 10.1016/j.jtcvs.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Graham EM, Zyblewski SC, Phillips JW, et al. Comparison of Norwood shunt types: do the outcomes differ 6 years later? Ann Thorac Surg. 2010;90:31–5. doi: 10.1016/j.athoracsur.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 6.Sano S, Ishino K, Kado H, et al. Outcome of right ventricle-to-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome: a multi-institutional study. Ann Thorac Surg. 2004;78:1951–7. doi: 10.1016/j.athoracsur.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 7.Ohye RG, Ludomirsky A, Devaney EJ, et al. Comparison of right ventricle to pulmonary artery conduit and modified Blalock-Taussig shunt hemodynamics after the Norwood operation. Ann Thorac Surg. 2004;78:1090–3. doi: 10.1016/S0003-4975(03)01386-9. [DOI] [PubMed] [Google Scholar]
- 8.Ohye RG, Sleeper LA, Mahony L, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362:1980–92. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizarro C, Mroczek T, Malec E, et al. Right ventricle to pulmonary artery conduit reduces interim mortality after stage 1 Norwood for hypoplastic left heart syndrome. Ann Thorac Surg. 2004;78:1959–63. doi: 10.1016/j.athoracsur.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Murdison KA, Baffa JM, Farrell PE., Jr et al. Hypoplastic left heart syndrome. Outcome after initial reconstruction and before modified Fontan procedure. Circulation. 1990;82:IV199–IV207. [PubMed] [Google Scholar]
- 11.Sarajuuri A, Jokinen E, Puosi R, et al. Neurodevelopment in children with hypoplastic left heart syndrome. J Pediatr. 2010;157:414–20. doi: 10.1016/j.jpeds.2010.04.027. [DOI] [PubMed] [Google Scholar]