Abstract
Recent literature indicates that torsion of the left ventricle (LV) is a promising predictor for response to cardiac resynchronisation therapy (CRT). Among patients with severe heart failure, 45 to 75% of patients show rigid body rotation, where the base and apex rotate in the same direction, instead of normal, opposite rotation. The occurrence of this phenomenon seems to be a good indicator for response to CRT. From this review, it can be concluded that LV torsion might be a welcome addition to current selection criteria.
Keywords: Cardiac resynchronization therapy, LV torsion, MRI tagging, Speckle tracking, Heart failure
Introduction
Since the introduction of cardiac resynchronisation therapy (CRT) [1] as a treatment option for severe heart failure, response rates were found to be no higher than 50–70% [2] using standard selection criteria such as QRS width >120 ms and left ventricular (LV) ejection fraction <35% [3].
The search for better selection criteria for successful treatment with CRT remains an important topic since the PROSPECT trial [4] showed that conventional wall motion and velocity-based dyssynchrony measures are not better predictors than standard selection criteria.
Recent studies indicate that measures based on mechanical deformation patterns of myocardial tissue are better predictors for response to CRT [5, 6]. Strain parameters such as the circumferential uniformity ratio estimate (CURE) show promising results [7, 8]. In addition, recently LV torsion was assessed as a measure for prediction of CRT response. As this parameter is rather different from common indexes measuring dyssynchrony, and preliminary results are excellent, this review will discuss the potential role of LV torsion in ischaemic and/or dilated cardiomyopathy for selection of CRT candidates.
Imaging myocardial torsion
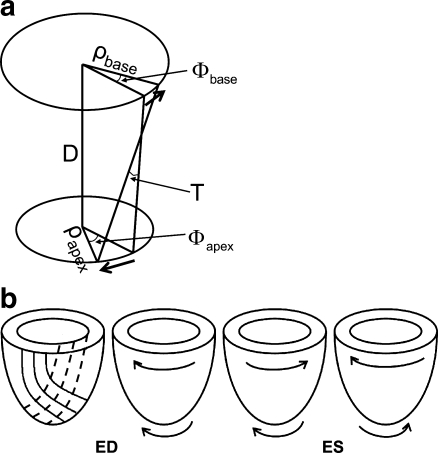
Myocardial torsion is the wringing motion or opposite rotation of the LV base and apex. Normally, the apex rotates anticlockwise (when viewed from the apex), and the base rotates clockwise. LV torsion is caused by oppositely oriented oblique fibre layers in the myocardial wall. Rotations are in the direction of the subepicardial layer, because of its longer lever arm [9] (Fig. 1).
Fig. 1.
Schematic drawing of LV torsion and its directions. a Torsion (T) defined as the CL shear angle, which normalises for the size of the heart (ρ: radius, ϕ : rotation, D: distance between slices). b Orientation of myofibre layers and normal rotational directions in the LV wall. The left-most image shows the myofibre directions. Dashed lines: endocardial fibre direction, solid lines: epicardial fibre direction. ED: end–diastole, ES: end–systole. Solid lines: epicardial region, dashed lines: endocardial regions. The right-most image shows untwisting
There is a direct relation between torsion and left ventricular ejection [10], as well as between untwisting and (early diastolic) suction [11] of blood. As a consequence, torsion is decreased in patients with impaired cardiac function. Changes in LV geometry, such as dilatation of the LV, will also affect torsion, due to changed myofibre direction.
LV torsion can be measured non-invasively by imaging with MRI tagging [12] or by ultrasound speckle tracking [13]. It is usually defined as the difference between basal and apical rotation. As rotation increases linearly over the length of the LV, it is important that measurements are normalised and/or taken at the same LV level [9] before they can be compared between subjects. The diameter as well as the length of the heart can differ between subjects and needs to be corrected for. When torsion is defined as the circumferential-longitudinal shear angle (Fig. 1), it is independent of LV size [14]. Regarding these physiological aspects and the definition of torsion, imaging should be best performed using a reference coordinate system. Hence, MRI can be considered the golden standard for measuring LV torsion. However, the studies described in this review do not always use the same description or definition of torsion. Therefore, numbers cannot always be compared in absolute terms. The focus will therefore be on relative differences and observed torsion patterns in heart failure patients with dilated or ischaemic cardiomyopathy.
Torsion patterns and cardiac resynchronisation therapy
It has been known for over a decade that LV torsion is altered in heart failure; LV torsion decreases during ischaemic and dilated cardiomyopathy [15–17]. However, the relation between LV torsion and the response to CRT is a topic that has recently gained interest.
Rigid body rotation
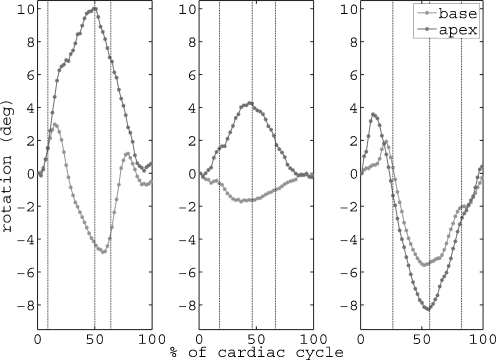
In 2003, Setser et al. [18] showed that in patients with end-stage heart failure, torsion was not only significantly reduced, but the basal and apical rotation sometimes followed the same direction of rotation (Fig. 2). This more or less results in absence of torsion or so-called ‘rigid body rotation’. In this study, 21 patients with dilated cardiomyopathy underwent an MRI scan with tagging. Patients were in NYHA functional class III-IV, and were eligible for partial LV ventriculectomy. Several rotation patterns were observed: Basal and apical rotation could be in the normal direction (base clockwise and apex anticlockwise when viewed from the apex), although significantly decreased; both basal and apical rotation could be clockwise; or both could be anticlockwise. This ‘rigid body rotation’ phenomenon appeared in the majority of the patients (Table 1).
Fig. 2.
Basal and apical rotation curves. Example of rotation curves for a healthy subject (left), a heart failure patient with normal rotation directions (middle) and a heart failure patient with rigid body rotation (right). Vertical black dotted lines indicate aortic valve opening, aortic valve closure and mitral valve opening, respectively
Table 1.
Prevalence of rigid body rotation in heart failure
| Study | Patients (#) | Rigid body rotation (#,% of patients) | Clockwise (#,% of rigid body rotation) | Anticlockwise (#,% of rigid body rotation) |
|---|---|---|---|---|
| Setser et al. [18] | 21 | 16, 76 | 13, 81 | 3, 19 |
| Kanzaki et al. [19] | 17 | Unclear | ||
| Popescu et al. [20] | 50 | 26, 52 | 22, 85 | 4, 15 |
| Van Dalen et al. [21] | 10 | 0, 0 | ||
| Sade et al. [22] | 33 | 15, 45 | 7, 47 | 8, 53 |
| Rüssel et al. [23] | 34 | 20, 59 | 18, 90 | 2, 10 |
A second study describing this LV rotation pattern was performed by Kanzaki et al. [19]. They included 17 patients with dilated cardiomyopathy, but without conduction delay, for MRI with tagging. All patients were non-ischaemic. Rigid body rotation was only observed in clockwise direction, and was worse with lower LV ejection fraction.
Popescu et al. [20] also found a relation between disease severity and rigid body rotation. In their study, in 50 patients with non-ischaemic dilated cardiomyopathy, rotation and torsion were analysed by ultrasound speckle tracking. About half of the patients showed rigid body rotation. In almost all patients with rigid body rotation, both basal and apical rotation were clockwise (Table 1). There was a significant relationship between rigid body rotation and more severe LV remodelling. Therefore, they speculated that increased sphericity of the LV might lead to a change in myofibre directions, which are responsible for rotation and torsion. Hence, disease progression (and the accompanying alteration of LV geometry) may lead to rigid body rotation.
A speckle tracking study in 10 patients with dilated cardiomyopathy and 10 patients with non-compaction cardiomyopathy by Van Dalen et al. [21] also reported about the occurrence of rigid body rotation. In both patient groups, 3 people had an electrical conduction delay (bundle branch block). Remarkably, all dilated cardiomyopathy patients were found to have normal rotation directions, whereas all patients with non-compaction cardiomyopathy showed rigid body rotation. In this group, 7 patients had clockwise rigid body rotation and 3 patients anticlockwise. Both groups had comparable LV volumes and ejection fraction. Following their results, the authors suggested rigid body rotation as a marker for non-compaction cardiomyopathy, and speculate that in other studies describing rigid body rotation this diagnosis might have been overlooked. However, in the study by Popescu et al. [20], non-compaction cardiomyopathy was an exclusion criterion. Likely, no rigid body rotation was found in the dilated cardiomyopathy groups because the number of patients was limited.
LV torsion and the response to cardiac resynchronisation therapy
To our knowledge, the first study relating torsion to the response to CRT dates from 2008 [22]. In this study, Sade et al. assessed LV torsion using ultrasound speckle tracking in 33 patients receiving CRT. Both patients with short and long QRS duration were included. QRS duration was not related to torsion. In this group, 21 patients had ischaemic cardiomyopathy and 12 patients non-ischaemic. At baseline and after 8 months of CRT, there was no difference between these groups in terms of LV function or rotation/torsion. About a quarter of patients had rigid body rotation at baseline (Table 1), which normalised after CRT. CRT responders were defined by a decrease in end-systolic volume >10%. Torsion was significantly lower in responders at baseline and significantly improved after CRT (Table 2). Although not significant, torsion worsened in non-responders. From these results, it seems that the appearance of rigid body rotation is independent of the presence of ischaemia or QRS width.
Table 2.
Effect of CRT on torsion. All studies were speckle tracking studies in humans. Note that absolute numbers must not be compared between studies because of possible different descriptions of torsion and measurement methods [14]
| Responders | Non-responders | |||||
|---|---|---|---|---|---|---|
| Study | Baseline torsion (°) | Follow-up torsion (°) | p-value | Baseline torsion (°) | Follow-up torsion (°) | p-value |
| Sade et al. [22] (FU 8 months) | 1.5 ± 2.8 | 6.3 ± 3.6 | <0.0001 | 5.3 ± 3.1 (p = 0.01 vs baseline responders) | 2.0 ± 3.4 | NS |
| Bertini et al. [24] (FU 6 months) | 4.3 ± 2.4 | 8.5 ± 3.2 | <0.001 | 5.4 ± 2.9 (p = 0.07 vs baseline responders) | 3.3 ± 2.2 | <0.001 |
| Zhang et al. [25] (FU 3 months) | 6.8 ± 4.5 | 5.6 ± 5.6 | NS | 6.9 ± 4.1 (NS vs baseline responders) | 4.2 ± 3.7 | <0.05 |
*FU Follow-up
Previous own work could confirm the relation between rigid body rotation and response to CRT [23]. Rotation was measured by MRI with tagging, in 34 patients eligible for CRT. Patients were in NYHA class III-IV, QRS width >120 ms, and LV ejection fraction <35%. Eighteen patients had ischaemic cardiomyopathy, and 16 patients had non-ischaemic cardiomyopathy. It was found that the majority of patients showed a pattern of LV torsion, where the apical rotation was inverted (Table 1). In line with Sade’s study, no differences were found between the ischaemic and non-ischaemic group.
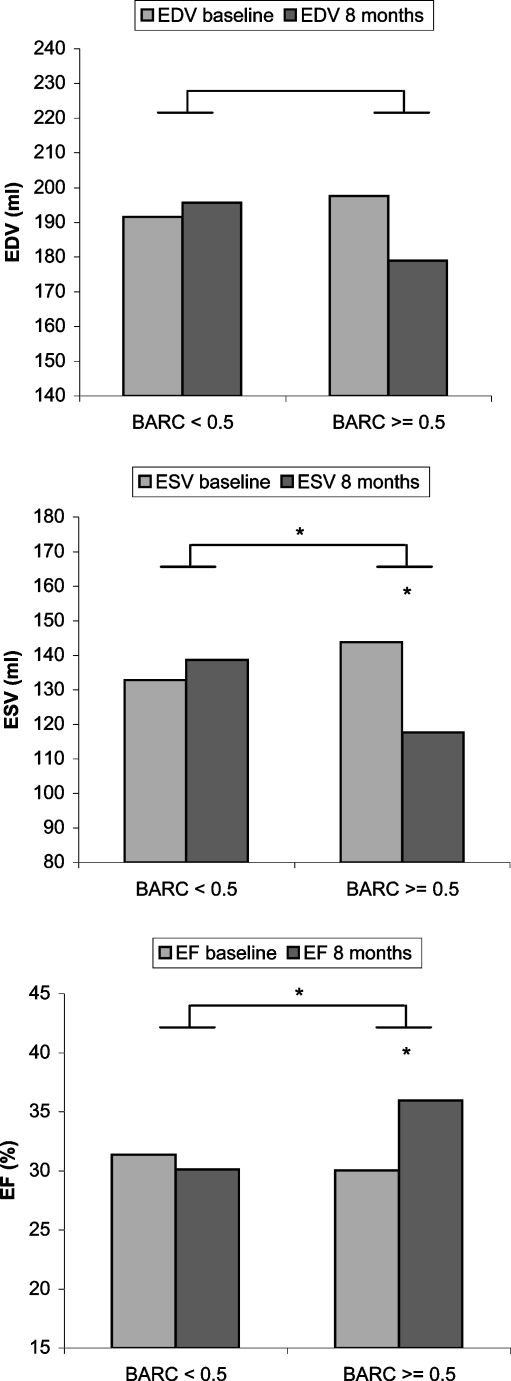
The degree of rigid body rotation was quantified by the correlation coefficient between the basal and apical rotation curve. Acute responders to CRT were classified as having an immediate increase in dP/dtmax of >10%. No difference in LV function was observed at baseline between responders and non-responders; hence there was no relation between LV function and rigid body rotation. This might be caused by the fact that only end-stage heart failure patients were included, in whom LV function was equally low. Patients with a high correlation coefficient, thus a high degree of rigid body rotation, responded best to CRT. Eight months after CRT, reversed remodelling was significantly better in the group with a high correlation coefficient (Fig. 3).
Fig. 3.
LV volume and ejection fraction changes at 8 months follow-up. LV volume and EF changes between baseline and follow-up are shown for patients with a BARC (basal-apical rotation correlation) below and above the cut-off value of 0.5. *p< = 0.05
A study by Bertini et al. [24] investigated the effect of CRT on LV torsion, using 2D speckle tracking analysis. They also related this change in torsion to LV reverse remodelling after 6 months of CRT. Seventy-one CRT candidates were included, with QRS width >120 ms, NYHA III-IV and LV ejection fraction <35%. Patients either had ischaemic or non-ischaemic dilated cardiomyopathy. Immediately after CRT, torsion significantly increased and the change in torsion was strongly related to the change in LV ejection fraction. At six-month follow-up, 40 patients (56%) were responding well to CRT (defined as a decrease in end-systolic volume ≥15%). Baseline LV torsion was lower in the responder group, although not significant (p = 0.07). In the responder group, torsion significantly increased, whereas in the non-responder group, there was a significant decrease in LV torsion, which confirms the study by Sade et al. (Table 2). It was not reported if and in how many of the patients rigid body rotation could be observed.
The increase in torsion after six months of CRT was largest in those patients with the LV lead placed in a posterolateral position. The more apical, the better. This is in line with our own study, where the best response to CRT was also obtained with the LV lead in the posterolateral position. There are several hypotheses for this finding. First, the myocardial wall is thinner at the apex. Therefore, the Purkinje network might be more easily reached with pacing in this region. Second, normal activation is from apex to base. Pacing in a more basal region might therefore disturb the torsion pattern. Furthermore, since torsion is generated by oppositely oriented fibre layers in the myocardial wall, and torsion is normally in the direction of the epicardial layer, it might be that pacing in the posterolateral/apical region gives best access to activate this epicardial layer, and torsion direction can be restored.
In contrast to the above, other studies did not find an effect of CRT on LV torsion. Zhang et al. [25] studied LV torsion with speckle tracking in 39 patients who received CRT at baseline and after 3 months of CRT. At baseline, LV torsion was not different between responders (21 patients with reduction in end-systolic volume ≥15%), and non-responders. Furthermore, torsion did not improve in responders but worsened in non-responders (Table 2). However, myocardial strain did improve in responders. Possibly, there were not many patients with rigid body rotation among the group. Furthermore, a follow-up time of only 3 months might be too short for torsion to recover. In a study by Ashikaga et al. [26], seven dogs with left bundle branch block and pacing-induced heart failure were included. LV rotation and torsion mechanics were measured with MRI tagging. Immediately after CRT, haemodynamic parameters improved, but not torsion. From CRT literature, it should be evident that torsion is not the only parameter affecting CRT response; however, recent studies indicate that it may play an important role.
Conclusion
Recent studies indicate that besides current selection criteria for CRT, such as QRS width >120 ms and ejection fraction <35%, measures of torsion and/or rigid body rotation are very promising in the prediction of response to CRT. Evaluation of LV torsion appears to reveal important new insights into the disease process. The general observation seems to be that in about 45–75% of dilated cardiomyopathy patients, depending on the spread of LV function in the group, rigid body rotation occurs. This might be related to increased sphericity of the LV, or altered electrical conduction. However, the occurrence of rigid body rotation seems to be independent of QRS width. Also, the presence of ischaemia seems unrelated.
The majority of rigid body rotation is in the clockwise direction, meaning that the apex follows the rotation of the base. Specific causes for the direction in which rigid body rotation occurs, as well as the influence of the direction of rigid body rotation on the response to CRT, remain unclear.
Besides current selection criteria for CRT such as QRS width >120 ms and ejection fraction <35%, evaluation of LV torsion appears to reveal important information about response. However, although not discussed in this review, the evaluation of dyssynchrony measures (either based on myocardial strain or wall motion/velocity) is probably also of added value. Mainly the relationships between these measures and LV torsion patters are of interest for a more thorough understanding of disease progression and the mode of operation of CRT.
Measurements of myocardial function such as strain and torsion have been mainly evaluated in a research setting using MRI as golden standard. With the upcoming quality and availability of ultrasound speckle tracking, the clinical perspective is that in the near future, it will be possible to add such measures to current CRT screening procedures.
Contributor Information
I. K. Rüssel, Phone: +31-70-2102736, Email: i.russel@hagaziekenhuis.nl
M. J. W. Götte, Phone: +31-70-2102811
References
- 1.Cleland JGF, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization therapy on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–49. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 2.Bleeker GB, Bax JJ, Fung JWH, et al. Clinical versus echocardiographic parameters to assess response to cardiac resynchronization therapy. Am J Cardiol. 2006;97:260–3. doi: 10.1016/j.amjcard.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 3.Strickberger SA, Conti J, Daoud EG, et al. Patient selection for cardiac resynchronization therapy. Circ. 2005;111:2760–7. doi: 10.1161/01.CIR.0000161276.09685.4A. [DOI] [PubMed] [Google Scholar]
- 4.Chung ES, Leon AR, Tavazzi L, et al. Results of the predictors of response to CRT (PROSPECT) trial. Circ. 2008;117:2608–16. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 5.Rüssel IK, Zwanenburg JJM, Germans T, et al. Mechanical dyssynchrony or myocardial shortening as MRI predictor of response to biventricular pacing? J Magn Res Imaging. 2007;26:1452–60. doi: 10.1002/jmri.21133. [DOI] [PubMed] [Google Scholar]
- 6.Carasso S, Rakowski H, Witte KK, et al. Left Ventricular strain patterns in dilated cardiomyopathy predict response to cardiac resynchronization therapy: timing is not everything. J Am Soc Echocardiogr. 2009;22:242–50. doi: 10.1016/j.echo.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Helm RH, Leclercq C, Faris OP, et al. Cardiac dyssynchrony analysis using circumferential versus longitudinal strain: implications for assessing cardiac resynchronization. Circ. 2005;111:2760–7. doi: 10.1161/CIRCULATIONAHA.104.508457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilchick KC, Dimaano V, Wu KC, et al. Cardiac magnetic resonance assessment of dyssynchrony and myocardial scar predicts function class improvement following cardiac resynchronization therapy. JACC Cardiovasc Imaging. 2008;1:561–8. doi: 10.1016/j.jcmg.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorenz CH, Pastorek JS, Bundy JM. Delineation of normal human left ventricular twist throughout systole by tagged magnetic resonance imaging. J Cardiovasc Magn Reson. 2000;2:97–108. doi: 10.3109/10976640009148678. [DOI] [PubMed] [Google Scholar]
- 10.Arts T, Reneman RS, Veenstra PC. A model of the mechanics of the left ventricle. Ann Biomed Eng. 1979;7:299–318. doi: 10.1007/BF02364118. [DOI] [PubMed] [Google Scholar]
- 11.Notomi Y, Popovic ZB, Yamada H, et al. Ventricular untwisting: a temporal link between left ventricular relaxation and suction. Am J Physiol Heart Circ Physiol. 2008;294:H505–13. doi: 10.1152/ajpheart.00975.2007. [DOI] [PubMed] [Google Scholar]
- 12.Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology. 1989;171:841–5. doi: 10.1148/radiology.171.3.2717762. [DOI] [PubMed] [Google Scholar]
- 13.Helle-Valle T, Crosby J, Edvardsen T, et al. New noninvasive method for assessment of left ventricular rotation: speckle tracking echocardiography. Circ. 2005;112:3149–456. doi: 10.1161/CIRCULATIONAHA.104.531558. [DOI] [PubMed] [Google Scholar]
- 14.Rüssel IK, Götte MJW, Bronzwaer JG, et al. Left ventricular torsion: an expanding role in the analysis of myocardial function. JACC Cardiovasc Imaging. 2009;2:648–55. doi: 10.1016/j.jcmg.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Hansen DE, Daughters GT, Alderman EL, et al. Effect of acute cardiac allograft rejection on left ventricular systolic torsion and diastolic recoil measured by intramyocardial markers. Circ. 1987;76:988–1008. doi: 10.1161/01.CIR.76.5.998. [DOI] [PubMed] [Google Scholar]
- 16.Young AA, Dokos S, Powell KA, et al. Regional heterogeneity of function in nonischemic dilated cardiomyopathy. Cardiovasc Res. 2001;49:308–18. doi: 10.1016/S0008-6363(00)00248-0. [DOI] [PubMed] [Google Scholar]
- 17.Nagel E, Stuber M, Lakatos M, et al. Cardiac rotation and relaxation after anterolateral myocardial infarction. Coron Artery Dis. 2000;11:261–7. doi: 10.1097/00019501-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Setser RM, Kasper JM, Lieber ML, et al. Persistent abnormal left ventricular systolic torsion in dilated cardiomyopathy after partial left ventriculectomy. J Thorac Cardiovasc Surg. 2003;126:48–55. doi: 10.1016/S0022-5223(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 19.Kanzaki H, Nakatani S, Yamada N, et al. Impaired systolic torsion in dilated cardiomyopathy: reversal of apical rotation at mid-systole characterized with magnetic resonance tagging method. Basic Res Cardiol. 2006;101:465–70. doi: 10.1007/s00395-006-0603-6. [DOI] [PubMed] [Google Scholar]
- 20.Popescu BA, Beladan CC, Calin A, et al. Left ventricular remodeling and torsional dynamics in dilated cardiomyopathy: reversed apical rotation as a marker of disease severity. Eur J Heart Fail. 2009;11:945–51. doi: 10.1093/eurjhf/hfp124. [DOI] [PubMed] [Google Scholar]
- 21.Dalen BM, Caliskan K, Soliman OII, et al. Left ventricular solid body rotation in non-compaction cardiomyopathy: a potential new objective and quantitative functional diagnostic criterion? Eur J Heart Fail. 2008;10:1088–93. doi: 10.1016/j.ejheart.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Sade LE, Demir O, Atar I, et al. Effect of mechanical dyssynchrony and cardiac resynchronization therapy on left ventricular rotational mechanics. Am J Cardiol. 2008;101:1163–9. doi: 10.1016/j.amjcard.2007.11.069. [DOI] [PubMed] [Google Scholar]
- 23.Rüssel IK, Götte MJW, Roest GJ, et al. Loss of opposite left ventricular basal and apical rotation predicts acute response to cardiac resynchronization therapy and is associated with long-term reversed remodeling. J Card Fail. 2009;15:717–25. doi: 10.1016/j.cardfail.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Bertini M, Marsan A, Delgado V, et al. Effects of cardiac resynchronization therapy on left ventricular twist. J Am Coll Cardiol. 2009;54:1317–25. doi: 10.1016/j.jacc.2009.05.063. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Fung JW, Yip GW, et al. Improvement of left ventricular myocardial short-axis, but not long-axis function or torsion after cardiac resynchronization therapy: an assessment by two-dimensional speckle tracking. Heart. 2008;94:1464–71. doi: 10.1136/hrt.2007.127498. [DOI] [PubMed] [Google Scholar]
- 26.Ashikaga H, Leclercq C, Wang J, Kass DA, McVeigh ER. Hemodynamic improvement in cardiac resynchronization does not require improvement in left ventricular rotation mechanics: three-dimensional tagged MRI analysis. Circ Cardiovasc Imaging. 2010, May 17 (epub ahead of print). [DOI] [PMC free article] [PubMed]