Abstract
Optimal treatment of oral mucositis (OM) due to graft versus host disease (GvHD) is currently not available. Platelet-derived growth factors (PDGFs) have high capability for tissue healing and may play a role in repairing the mucosal barrier. The aim of the present work was to develop a mucoadhesive formulation to administer platelet lysate to oral cavity prolonging contact time of platelet lysate with oral mucosa. The mucoadhesive formulation was characterized for in vitro properties (PDGF-AB concentration, mucoadhesive properties, cytotoxicity, fibroblast proliferation, wound healing). Moreover, a preliminary clinical study on seven GvHD patients with OM refractory to other therapies was conducted, to evaluate feasibility, safety, and efficacy. GVPL (mucoadhesive gel vehicle mixed with platelet lysate)showed good mucoadhesive properties; additionally, it was characterized by good biocompatibility in vitro on fibroblasts and it was able to enhance fibroblast proliferation and wound healing, maintaining the efficacy for up to 14 days following storage at 2–8°C. In vivo, clinical response was good-to-complete in five, fair in one, none in the remaining one. The in vitro results indicate that GVPL has optimal mucoadhesive and healing enhancer properties, maintained over time (up to 14 days); preliminary clinical results suggest that oral application of platelet lysate-loaded mucoadhesive formulation is feasible, safe, well tolerated, and effective. A larger controlled randomized study is needed.
KEY WORDS: in vitro proliferation and wound healing test, in vivo assessment in graft versus host disease, mucositis, platelet lysate growth factors
INTRODUCTION
Oral mucosa is a target of acute and chronic graft versus host disease (aGvHD, cGvHD) in a high proportion (around 70%) of patients after allogeneic stem-cell transplantation, where oral mucositis presents with ulcerations, bleeding, lichen planus, atrophy, and dryness. Recent data show a significant impact of aGvHD on severity and duration of oral mucositis (OM) and a correlation between OM severity and its duration (1). Mucosal barrier injury results in significant morbidity including non infectious (severe pain, poor feeding with consequent weight loss, need for artificial feeding, prolonged hospitalization, and increased consumption of health care resources) and infectious complications (mucosa-related bacteremia, mucosa-related invasive fungal disease, and typhlitis) (2). Pathobiology of mucositis in GvHD is complex, and therapeutic agents capable of accelerating the healing process or (even) preventing mucositis onset are highly desirable. A 2007 Cochrane systematic review reported the results of 277 studies on prevention or treatment of OM using very different approaches including Chinese medicine, prostaglandin, aloe vera, glutamine, granulocyte/macrophage colony stimulating factor (MG-CSF), recombinant keratinocyte growth factor (KGF-1) (3). Some of these interventions showed a benefit in preventing or reducing the severity of OM, although none was of definitive proven efficacy (4) and currently there are no universally accepted protocols. A shift from symptomatic and palliative treatments to reparative medicine is mandatory (5–7).
Platelets (PLT) are able to release a large number of biologically active substances from intracellular alpha granules, in response to activation (8–10). Among these, a very important category is represented by growth factors. They initiate and modulate tissue repair mechanisms such as cell proliferation, angiogenesis, chemotaxis, extracellular matrix depositing, and remodeling (11). A large number of platelet-derived growth factors (PDGFs) have been isolated, studied, and characterized so far, of which the most intensively investigated are PDGF (and its isoforms AB, AA, BB), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), and insulin growth factor (IGF) (11).
Some of these growth factors (GFs) are available in purified form, but it has been pointed out that tissue repair cannot be effectively mediated by a single agent, as multiple signals are required to complete the regeneration process (10). To better exploit the whole potential of the naturally occurring platelet GFs, it has been suggested the therapeutic employment of platelet-rich preparations, hemoderivatives from which platelets can release their complete pool of biologically active substances because their efficacy is bound to their synergistic action (10).
Ensuring a prolonged contact of PDGFs (preferably autologous to avoid the transfusion related risk) with the oral mucosa of GvHD patients could represent a new therapeutic chance; however, application of platelet lysate (PL), rich in PDGFs, to injured mucosal tissues arises particular formulation challenges that clearly differ from those that characterize the treatment of skin wounds or bone defects. Mucosal tissues are in fact exposed to the effect of physiological fluids that remove any active substances, thereby limiting their contact time. In the case of oral mucosa, also tongue movements play an important role; further, unpleasant taste can limit patient's compliance.
The finding of a suitable mucoadhesive vehicle that prolongs the contact time of PDGFs with the damaged oral mucosa without interfering with their biological characteristics and capable of masking the unpleasant taste of plasma is highly desirable. Mucoadhesive polymers are able to interact with the mucous layer of the mucosa giving efficient, though reversible, bonds, through hydrogen-forming groups, chains interpenetration, or electrostatic interactions. Carbomers are among the better-known mucoadhesive polymers, and produce gels with good mechanical strength (which is important to maintain the biomaterial-PL formulation at the oral mucosa longer). Of paramount importance is also compatibility of biomaterial with the active substance, whose efficacy and stability must not be impaired.
The aim of the present work was to develop a mucoadhesive formulation to administer platelet lysate to oral cavity and to develop in vitro test for a fast evaluation of platelet lysate activity. The preliminary results of a pilot study on seven GvHD patients with OM are reported. This study was aimed at assessing feasibility, safety, and in vitro and in vivo efficacy of the developed mucoadhesive vehicle mixed with platelet lysate (GVPL) specifically designed to prolong its contact time with the oral mucosa.
EXPERIMENTAL PART
Materials
Platelet Lysate
A 40 mL anticoagulated (ACD-A) peripheral blood sample (autologous) was taken from four cGvHD patients with a platelet PLT count ≥150,000/μL (i.e. minimum accepted normal PLT value in our laboratory). The sample was centrifuged at 900 rpm for 10 min and the platelet-rich plasma (PRP) was collected and freezed at −80°C (thermal shock), and subsequently thawed at 37°C to induce lysis and PDGFs release.
For three additional patients, one with aGvHD and two with cGvHD, with platelet count <150,000/μL (30,000, 70,000, and 90,000/μL), an aliquot of 30 mL was taken from a single donor PLT apheresis collection. All apheresis products were qualified according to the Italian legislation.
Both autologous blood samples and apheresis products were diluted with a saline solution at a final concentration of 1:1 (one volume of PL and one volume of saline solution).
All manipulations were performed under sterile conditions.
Polymer
Carbopol 974P-NF (polyacrylic acid (PAA)) (Batch # CC61NAB896, Lubrizol, Brussel, Belgium) was used (viscosity 29400–34900 cP). This is the grade with less content of solvents in particular no benzene (EP class I solvent) and cyclohexane (EP class II solvent) residues are present. In fact, this grade is polymerized in ethyl acetate (EP class III solvent) less critical. Carbopol 974P NF has a medium cross-linking and medium–high viscosity and it is recommended for high viscosity gel.
METHODS
Preparation of Vehicle and Formulation
Gel vehicle (GV) was prepared in saline solution (0.9% w/v NaCl) using PAA at 5% w/w. Sodium saccharine (Ph. Eur. Compliant; Polichimica s.r.l., Bologna, Italy) at 0.2% w/w and flavor (mint-liquorice batch # 261016/1 1 × 1,000 or strawberry batch #260308/2 1 × 1,000, DKS aromatic, Corsico, Italy) at 0.2% w/w were added. After the complete dispersion of PAA the vehicle was buffered at pH 7.0 using NaOH (Ph. Eur. Grade; Riedel de Haen, Milano, Italy) solution 4 N.
GV was sterilized using steam sterilization at 121°C for 15 min (Alpha Junior, PBI International, Milan, Italy) under nitrogen atmosphere.
The final formulation (GVPL) for clinical use was prepared by mixing 1:1 (w/w) vehicle with PL. GVPL contained a final concentration of PAA equal to 2.5% w/w. Finally, GVPL was aliquoted in sterile disposables, 4-mL volume each. Disposables were maintained at 4°C for a maximum of 14 days.
For the in vitro characterization, GVPL was prepared by mixing 1:1 (w/w) GV with PL. GVPL contained a final concentration of PAA equal to 2.5% w/w and PL concentration was one fourth of that in the apheresis samples.
Quality Controls
A sample from PL and from GV preparation was taken for microbial cultures to detect aerobic–anaerobic, bacterial, and fungal contamination. Besides, a sample from GVPL (immediately after preparation and after 14 days of storage at 4°C) was obtained in order to detect potential contaminations due to manipulation. Eight milliliters for each sample were inoculated in the culture medium (Bact/Alert FA and BacT/Alert FN, Organon Teknika Corp., Durham, NC) and incubated for 10 days at 37°C.
PDGF-AB Assay
Due to the high number of PDGFs (more than 300), it is quite impossible to test every single PDGFs. Among all PDGFs, we focused on the concentration of platelet-derived growth factor isoform AB only (in both platelet lysate and formulation) due to its wide range of activity on cell proliferation, cell migration, and angiogenesis
The concentration of PDGF isoform AB in the PL and the formulation was assayed by means of ELISA test (Human PDGF-AB Quantikine PharmPak, R&D systems, Minneapolis, MN, USA; assay range: 31.2–2,000 pg/mL). A calibration curve was performed adding to each standard concentration a prefixed concentration of PAA (0.125% w/w, same concentration of formulation diluted 1/20) in saline to assess the potential interference of polymer on kit response. The concentration of PDGF-AB in the formulation was compared to the concentration of PDGF-AB in the PL.
In Vitro Characterization
Mucoadhesion Properties
Mucoadhesion measurements were performed using TA.XT plus (Texture analyser, ENCO, Spinea, Italy) equipped by load cell of 1 kg and cylinder probe of 1 cm and the measuring system A/MUC (mucoadhesion test ring) (12). The measuring system A/MUC holds the biological substrate (100 μl of mucin dispersion: 8% w/w in phosphate buffer pH 6.4, mucin type: type II crude, Sigma Aldrich, Milano, Italy). Twenty milligrams of each sample were applied on the cylinder probe of the apparatus TA.XT plus. Sample and biologic substrate were put in contact with a preload of 6,000 mN for 3 min. Cylinder probe was moved upward at a prefixed speed of 2.5 mm/min up to the complete separation of the mucoadhesive interface (mucin sample).
The force of detachment as a function of probe displacement was recorded and the parameter work of adhesion (millinewtons per millimeter (mN.mm)) (AUC) was calculated as area under the curve force vs displacement.
Cytotoxicity and Proliferation on Fibroblasts
Normal Human Dermal Fibroblasts from juvenile foreskin (PromoCell GmbH, Heidelberg, Germany) were used. Cells between the second and fifth passage were used for all the experiments. Fibroblasts were grown in presence of Dulbecco's modified Eagle medium (DMEM) (Sigma) and supplemented with 10% fetal calf serum (FCS, Sigma, Milano, Italy) with 200 IU/mL penicillin, and with 0.2 mg/mL streptomycin and kept at 37°C in a 5% CO2 atmosphere with 95% of relative humidity.
For the cytotoxicity study fibroblasts were seeded in each well of 96-well plates (area of 0.34 cm2) at density of 105 cells/cm2. Cells were grown for 24 h to obtain sub-confluence. After 24 h, cells were washed with saline solution and 200 μl of PAA solutions (having concentration ranging from 0.5% w/w (2.5% w/w of PAA diluted 1:5) to 0.125% w/w (2.5% w/w of PAA diluted 1:20) in HBSS pH 7.4) were put in contact with cell substrates. The complete medium was used as control. Cells were maintained in contact with PAA samples for 3 h and subsequently the Neutral Red test was performed (Tox Kit 4, Sigma Aldrich, Milano, Italy).
For the proliferation experiments, fibroblasts were seeded in 96-well plates with area of 0.34 cm2 at the concentrations of 7,500 cells/well. The cells were suspended in the following media: complete growth medium; minimal medium (mM; not supplemented with fetal calf serum) for the control; mM containing PL diluted 1:20 and 1:40; mM containing the formulation diluted to PL concentration 1:20 and 1:40; 200 μl of each sample were added to each well and the well plate kept at 37°C in an atmosphere of 95% air and 5% CO2 and 95% of relative humidity for 24 h. After 24 h, the cells were sub-confluent and attached to the well bottom and neutral red test was performed. Each well was washed with saline phosphate buffer (PBS) and 200 μl of neutral red solution (0.33 mg/mL in mM) were put in each well for 2 h of contact time. Cell substrates were then washed with PBS to remove neutral red not entrapped in the cells and the fixing medium (1% w/v CaCl2 and 0.5% w/v formaldehyde aqueous solution) was added to fix the substrate. After removal of the fixing solution, a solubilizing solution (1% v/v of acetic acid in ethanol) was added to the cell substrates to cause cell disruption and to release neutral red captured by viable cells. Neutral red released after cell lysis was assayed by means of ELISA plate reader at 490 nm with reference of 655 nm. Cell proliferation was calculated as% ratio between the absorbance of each sample and the absorbance of complete growth medium.
In Vitro Wound Healing Test
In vitro wound healing test is based on the employment of μ-Dish (Ibidi, Giardini, Milan, Italy) device that is a Petri dish in which an insert is enclosed. The insert consists of two chambers with a growth area of 0.22 cm² divided by a septum with a width of cell free gap of 500 ± 50 μm.
Fibroblasts were seeded in each chamber at 105 cells/cm2 and grown at confluence at 37°C in a 5% CO2 atmosphere with 95% of relative humidity. After 24 h, cells reached confluence and the insert was removed displaying two areas of cell substrates divided from the prefixed gap. Cell substrates were put in contact with 200 μl of PL at 1:20 concentration and formulation diluted to have the same concentration of PL and mM. At prefixed times (0, 72, 96 h), microphotographs were taken to evaluate the invasion and cell growth in the gap.
Patients Characteristics, GVPL Administration, and Preliminary Evaluation
Prior to study start, approval from the Ethical Committee of Fondazione IRCCS S. Matteo was obtained. Seven patients with OM, of whom one with grade III aGvHD and grade IV mucositis and six with extensive cGvHD and grade III mucositis, were enrolled after obtaining their informed consent.
The patients had been shown to be refractory to standard immunosuppressive therapy, extracorporeal photopheresis (13), and conventional local therapy (oral rinse with lydocaine, prednisone, sucralfate, chlorexidine). All patients were under systemic analgesia with opioid drugs (morphine).
GVPL was applied on the oral mucosa three times per day for 30 days. All were treated as out-patients. Patients or their relatives received training for correctly maintaining the storage and performing the application procedure.
GVPL was applied on oral mucosa by means of sterile and disposable spatula. After the application the patients avoided eating and drinking for 1 h.
Use of analgesics, OM area, local or systemic infections, and percentage of body weight increase were evaluated every 2 days to the end of treatment. Any side effects were recorded.
Statistical Methods
Mean and standard deviation are presented for normally distributed variables, and median and interquartile range (IQR) for non-normally distributed variables, proportions for categorical variables. Student t test (rank sum test or Mann–Whitney test for non-normal distributions) was used to compare quantitative variables, and Pearson's χ2 test (Fisher exact test where appropriate) for categorical variables. Tests for paired data were used wherever appropriate.
Overall response was evaluated at the end of treatment and defined as following:
No response (NR): size of lesions unchanged/worsened, no change in analgesic intake, feeding with liquid food;
Twenty-five percent response (25% R): reduction in the size of one lesion, mild reduction of oral pain, no change in analgesic intake, feeding with liquid food;
Fifty percent response (50% R): reduction in the size of two or more lesions, important reduction of oral pain, reduction in analgesics intake more or equal to 50%, no difficulty in feeding with solid food;
One hundred percent response (100% R): disappearance of all lesions, no analgesic intake, regular feeding with any type of food.
RESULTS
PDGF-AB Assay
PAA demonstrated to not interfere with ELISA test, as assessed by means of a calibration curve performed using PAA in the diluent that had very close parameters (slope and intercept) with respect to the curve obtained in the kit standard diluent (data not shown).
The concentration of PDGF-AB was equal to 44.7 ng/mL (±1.0) for PL and 49.7 ng/mL (±0.7) for GVPL: no significant decrease in PDGF-AB concentration could be evidenced after loading PL in GV.
Mucoadhesion Properties
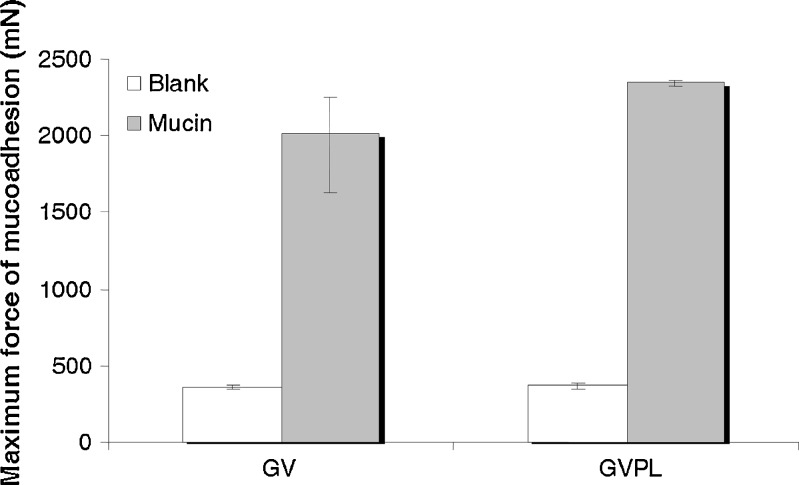
Figure 1 shows the mucoadhesion properties of the vehicle and of the formulation. The increase of the force necessary to detach the sample from mucin with respect to the blank was statistically significant both for the vehicle and for the formulation.
Fig. 1.
Mucoadhesive properties of the vehicle (GV) and of the formulation (GVPL): maximum force of mucoadhesion (millinewton) (median and IQR; n = 6). Comparisons: among GV, blank vs mucin p < 0.001; among GVPL, blank vs mucin p < 0.001; mucin GV vs mucin GVPL, p = 0.37
Carbopol 974P-NF is a high-molecular-weight polymer readily swell in water, providing a large adhesive surface for maximum contact with the mucin (the glycoprotein predominant in the mucous layer). Carbopol polymer chains are able to interpenetrate between mucin chains of the mucus layer: this causes a marked increase in the polymer-mucus interface viscosity. This phenomena combined with the formation of hydrogen bonding consolidates and strengthens the mucoadhesive joint (14).
Cytotoxicity and Proliferation on Fibroblasts
Figure 2 shows the percentage of viability of fibroblasts in contact with Carbopol 974P samples having different concentrations (cytotoxicity test). No decrease in viability was seen when the Carbopol 974P concentration was increased up to 0.25% w/w. A lower than 100% viability (even if not statistically significant) occurred only at 0.5% w/w Carbopol 974P concentration.
Fig. 2.
Percent viability of fibroblasts in contact with Carbopol 974P samples having different concentrations (median and IQR; n = 8)
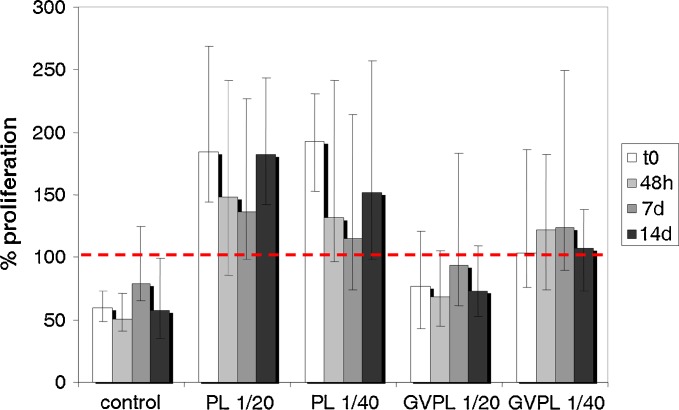
Figure 3 shows the values of 24-h cellular proliferation of fibroblasts in presence of PL or of formulation that had been maintained at 4°C for different times (time zero T0, 2 days, 7 days, 14 days). The proliferation obtained in complete culture medium (with fetal calf serum, FCS) is assumed as reference for each experiment performed at the same day; it is considered 100% and is indicated by the dotted line. “Control” refers to the percentage of proliferation obtained with mM. At each storage time up to 14 days for both the PL samples (diluted 1:20 and 1:40) and for the PL diluted 1:40 in the formulation (form 1:40), the difference in proliferation versus the corresponding control was statistically significant (Mann–Whitney). Globally, non-statistically significant differences in proliferation have been found among PL or GVPL as a function of dilution (Mann–Whitney). However the percentage of proliferation of GVPL diluted 1:20 were lower than those of GVPL diluted 1:40—this could be due to higher viscosity of 1:20 sample that can delay cell growth.
Fig. 3.
Percentage of fibroblast proliferation in presence of two dilutions (1:20 or 1:40) of PL or of PL in GVPL after different times storage (T0 time zero, 48 h 2 days, 7 d 7 days, 10 d 10 days, 14 d 14 days) (median and IQR; n = 32 at each time point). t = 0: control vs PL 1:20, p < 0.001; control vs PL 1/40, <0.001; control vs GVPL 1/20, p = 0.202; control vs GVPL 1/40, p < 0.001; PL 1/20 vs PL 1/40, p = 0.392; GVPL 1/20 vs GVPL 1/40, p = 0.012; PL 1/20 vs GVPL 1/20, p ≤ 0.001; PL 1/40 vs GVPL 1/40, p ≤ 0.001. t = 48 h: control vs PL 1/20, p < 0.001; control vs PL 1/40, p < 0.001; control vs GVPL 1/20, p = 0.113; control vs GVPL 1/40, p < 0.001; PL 1/20 vs PL 1/40, p = 0.491; GVPL 1/20 vs GVPL 1/40, p = 0.002; PL 1/20 vs GVPL 1/20, p ≤ 0.001; PL 1/40 vs GVPL 1/40, p = 0.101. t = 7 d: control vs PL 1/20, p < 0.001; control vs PL 1/40, p = 0.051; control vs GVPL 1/20, p = 0.312; control vs GVPL 1/40, p = 0.008; PL 1/20 vs PL 1/40, p = 0.130; GVPL 1/20 vs GVPL 1/40, p = 0.065; PL 1/20 vs GVPL 1/20, p = 0.021; PL 1/40 vs GVPL 1/40, p = 0.258. t = 14 d: control vs PL 1/20, p < 0.001; control vs PL 1/40, p < 0.001; control vs GVPL 1/20, p = 0.197; control vs GVPL 1/40, p = 0.001; PL 1/20 vs PL 1/40, p = 0.075; GVPL 1/20 vs GVPL 1/40, p = 0.014; PL 1/20 vs GVPL 1/20, p ≤ 0.001; PL 1/40 vs GVPL 1/40, p = 0.048
In vitro Wound Healing Test
In Figure 4, pictures of fibroblast proliferation in the in vitro wound healing test at different times are shown. In the case of PL, the growth of the fibroblasts was quite fast, and after 72 h a complete and confluent invasion of the gap occurred. In the case of the formulation, cell growth within the gap occurred more slowly, and after 72 h, sub-confluent growing of the cells in the gap was observed. Even if the cell growth was slower in presence of Carbopol 974P with respect to PL alone (as demonstrated in proliferation test for the same concentrations—1:20), no evidence of cell death was observed and no cell detached from Petri bottom and round shaped in suspension were present.
Fig. 4.
Microphotographs of fibroblasts at time 0 (t = 0) and after exposure to a PL 1/20 and b PL 1/20 in GVPL at 72 and 96 h
Quality Controls
No microbial and fungal contaminations in samples analyzed were detected.
Response to Treatment
The patient with grade IV mucositis, treated with allogeneic GVPL showed NR. Out of the six patients with grade III mucositis, two showed a 100% R, one had 25% R, and three had 50% R. Response to treatment (overall response, weight percentage increase, use of analgesics) and complications (oral and systemic infections) are detailed in Table I.
Table I.
Patients' Characteristics and Results after 30 days of GVPL Application on Oral Mucosa in Seven GVHD Patients with Oral Mucositis
| Patient's number | Age | GvHD | Mucositis (grade) | PLT lysate | Weight (% increase) | Response | Mucositis after treatment (grade) | Use of analgesics | Oral infection |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 13 | Acute (grade III) | IV | Allogeneic | 0 | NR | IV | Unchanged | No |
| 2 | 51 | Chronic extensive | III | Autologous | 10 | 100% R | 0 | Withdrawal | No |
| 3 | 34 | Chronic extensive | III | Allogeneic | 7 | 50% R | I | Reduction | No |
| 4 | 33 | Chronic extensive | III | Autologous | 2 | 25% R | II | Unchanged | No |
| 5 | 17 | Chronic extensive | III | Allogeneic | 10 | 100% R | 0 | Withdrawal | No |
| 6 | 47 | Chronic extensive | III | Autologous | 4 | 50% R | I | Reduction | No |
| 7 | 54 | Chronic extensive | III | Autologous | 6 | 50% R | I | Reduction | No |
DISCUSSION
Oral mucosa is an important target of GvHD post allogeneic stem-cell transplantation. OM results in a significant morbidity (painful ulcers, need for opioids, malnutrition, weight loss), impaired quality of life, and an increased risk for bacteremia and sepsis (15,16). Optimal therapy for OM has not yet been established; it ranges from “homemade remedies” (local ice chips, chamomile, honey etc.) to very expensive new molecules (KGF-1 Palifermin, KGF-2 Repifermin, FGF-20 Velafermin). Even if some approaches showed a benefit, no definitive solution was found (4–7). OM in GvHD is a peculiar condition sustained by a continuous immunologic aggression that makes particularly difficult a definitive tissue repair. Hence, we tested a platelet lysate mucohadesive formulation acting like a drug delivery system able to continuously release in situ and for an extended time PDGFs with the aim to induce a definitive tissue healing of oral mucosa.
No decrease in PDGF-AB concentration after loading PL in GV was observed, demonstrating that PDGF-AB was compatible with PAA and conceivably no degradation or inactivation of PDGFs occurred after the contact with the polymer. Additionally, our results confirm that the well known mucoadhesion properties that characterize Carbopol 974P are not affected by the presence of the PL and by the dilution.
No cytotoxicity due to Carbopol 974P was evidenced towards fibroblasts, although at 0.5% w/w Carbopol 974P concentration a lower viability was observed. This might be due to an in vitro interference of the polymer on cell growth, reasonably due to the viscosity of the polymeric solution that could impair the oxygen supply and the diffusion of nutrients to the cell substrate.
The results of in vitro proliferation demonstrate that the repairing capacity of PL is maintained also in presence of the PAA based vehicle up to 14 days of storage at 2–8°C. This allows patients to use the formulation for 2 weeks before returning for additional blood drawing to obtain another preparation.
In the in vitro wound healing study, the cell growth in presence of Carbopol confirms the results of the proliferation test. The in vitro proliferation and wound healing tests represent a valuable instrument to increase the confidence in quality and consistency of the active substance that is in this case PL. Both proliferation and wound healing have the advantage among other possible in vitro test, such as ELISA, of estimating the effect of the whole network of PDGFs.
Though the cell growth was slower in presence of Carbopol 974P with respect to PL alone, no evidence of cell death was observed (cells detached from Petri bottom and round shaped in suspension). The slower growth may be attributed to the viscosity of Carbopol 974: even if the concentration of Carbopol used is biocompatible with this cell substrate as evidenced by cytotoxicity study, the slight increase of viscosity of the medium might have produced a moderate interference of cell growth in vitro on fibroblast culture.
In vitro properties of PL are not affected by the combination with PAA, which in turn is expected to increase in vitro the efficacy of the formulation increasing contact time of platelet lysate on oral mucosa.
The preliminary in vivo experience was also encouraging: the compliance of GVPL was excellent for all patients. Out of seven patients with OM refractory to any therapy, a response in six patients with grade III mucositis was obtained while no response was obtained in only one patient with grade IV mucositis. The latter patient suffered from a severe autoimmune disease (ulcerative colitis) that, along with the employment of allogeneic PLT, may have impaired the response. Of note, out of the six responders, two achieved complete “restitutio ad integrum” of oral mucosa and the remaining patients showed an important improvement that is reduction of at least 25% OM and, in all patients, except one, reduction of analgesics. The possibility of a significant tapering of analgesics (withdrawn in two patients, reduced in three patients) and the contemporary improvement of feeding and therefore nutritional status (weight gain) was remarkable for its impact in the quality of life. The absence of bacterial and fungal contamination in GVPL and the lack of oral infections during the entire lapse of GVPL application allow us to consider our approach safe. It is important to note that the significant reduction of oral lesions may also reduce the risk of disseminated infections.
CONCLUSIONS
The in vitro proliferation test demonstrated to be a suitable cell culture model to evaluate proliferation performance of platelet lysate in easy and fast way. This will be useful to develop and screen innovative formulations intended for the delivery of platelet lysate. In particular, it will be possible to put in evidence the compatibility of the platelet lysate growth factors with possible excipients and also to compare the stability data of platelet lysate action in a polymeric vehicle. In addition the in vitro wound healing test demonstrated to be a proof of concept evaluation.
The mucoadhesive formulation was characterized in vitro by good technological properties (consistency and mucoadhesion) and good in vitro proliferation and wound healing properties. The in vivo preliminary results suggest that oral application of GVPL is feasible, safe, well tolerated, and effective in GvHD patients with OM, even if these results need to be confirmed in a larger clinical study, possibly controlled and randomized. Also, other categories of patients might benefit from this approach, for instance all patients with OM after high-dose chemo/radiotherapy with the potential to open a simple and economic possibility to cure mucosal lesions otherwise very difficult to treat.
Acknowledgments
The authors wish to thank Cariplo Foundation for the financial support (Project title: “Platelet gel bioadhesive systems for mucositis in chemio/radio therapy and stem cell trasplantation” call 2006).
References
- 1.Vokurka S, Steinerova K, Karas M, Koza V. Characteristics and risk factors of oral mucositis after allogeneic stem cell transplantation with FLU/MEL conditioning regimen in context with BU/CY2. Bone Marrow Transplant. 2009;44:601–605. doi: 10.1038/bmt.2009.66. [DOI] [PubMed] [Google Scholar]
- 2.Blijlevens NM. Implications of treatment-induced mucosal barrier injury. Curr Opin Oncol. 200;17:605–610. [PubMed] [Google Scholar]
- 3.Worthington HV, Clarkson JE, Eden OB. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Syst Rev. 2007;4:CD000978. doi: 10.1002/14651858.CD000978.pub3. [DOI] [PubMed] [Google Scholar]
- 4.van der Velden WJ, Herbers AH, Blijlevens NM. Palifermin in allogeneic HSCT: many questions remain. Bone Marrow Transplant. 2009;43:85–86. doi: 10.1038/bmt.2008.269. [DOI] [PubMed] [Google Scholar]
- 5.Langner S, Staber P, Schub N, Gramatzki M, Grothe W, Behre G, Rabitsch W, Urban C, Linkesch W, Neumeister P. Palifermin reduces incidence and severity of oral mucositis in allogeneic stem-cell transplant recipients. Bone Marrow Transplant. 2008;42:275–279. doi: 10.1038/bmt.2008.157. [DOI] [PubMed] [Google Scholar]
- 6.Gori E, Arpinati M, Bonifazi F, Errico A, Mega A, Alberani F, Sabbi V, Costazza G, Leanza S, Borrelli C, Berni M, Feraut C, Polato E, Altieri MC, Pirola E, Loddo MC, Banfi M, Barzetti L, Calza S, Brignoli C, Bandini G, De Vivo A, Bosi A, Baccarani AM. Cryotherapy in the prevention of oral mucositis in patients receiving low-dose methotrexate following myeloablative allogeneic stem cell transplantation: a prospective randomized study of the Gruppo Italiano Trapianto di Midollo Osseo nurses group. Bone Marrow Transplant. 2007;39:347–352. doi: 10.1038/sj.bmt.1705590. [DOI] [PubMed] [Google Scholar]
- 7.Crowther M, Avenell A, Culligan DJ. Systematic review and meta-analyses of studies of glutamine supplementation in haematopoietic stem cell transplantation. Bone Marrow Transplant. 2009;44:413–425. doi: 10.1038/bmt.2009.41. [DOI] [PubMed] [Google Scholar]
- 8.Mazzucco L, Medici D, Serra M, Panizza R, Rivara G, Orecchia S, Libener R, Cattana E, Levis A, Betta PG, Borzini P. The use of autologous platelet gel to treat difficult-to-heal wounds: a pilot study. Transfusion. 2004;44:1013–1018. doi: 10.1111/j.1537-2995.2004.03366.x. [DOI] [PubMed] [Google Scholar]
- 9.Rajkumar VS, Shiwen X, Bostrom M, Leoni P, Muddle J, Ivarsson M, Gerdin B, Denton CP, Bou-Gharios G, Black CM, Abraham DJ. Platelet-derived growth factor-beta receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am J Pathol. 2006;169:2254–2265. doi: 10.2353/ajpath.2006.060196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anitua E, Sanchez M, Nurden AT, Nurden P, Orive G, Andia I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006;24:227–234. doi: 10.1016/j.tibtech.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Borzini P, Mazzucco L. Platelet gels and releasates. Curr Opin Hematol. 2005;12:473–479. doi: 10.1097/01.moh.0000177831.70657.e8. [DOI] [PubMed] [Google Scholar]
- 12.Bonferoni MC, Sandri G, Rossi S, Ferrari F, Gibin S, Caramella C. Chitosan citrate as multifunctional polymer for vaginal delivery. Evaluation of penetration enhancement and peptidase inhibition properties. Eur J Pharm Sci. 2008;5(33):166–176. doi: 10.1016/j.ejps.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Perotti C, Del Fante C, Tinelli C, Viarengo G, Scudeller L, Zecca M, Locatelli F, Salvaneschi L. Extracorporeal photochemotherapy in graft-versus-host disease: a longitudinal study on factors influencing the response and survival in pediatric patients. Transfusion. 2010;50:1359–1369. doi: 10.1111/j.1537-2995.2009.02577.x. [DOI] [PubMed] [Google Scholar]
- 14.Lubrizol Pharmaceutical Bulletin 23: Bioadhesion, http://www.lubrizol.com/Pharmaceutical/Literature/Bulletins.html
- 15.Picardi A, Lanti A, Cudillo L, Cerretti R, Dentamaro T, De Angelis G, Ferraro A, Di Veroli A, Adorno G, Arcese W. Platelet gel for treatment of mucocutaneous lesions related to graft-versus-host disease after allogeneic hematopoietic stem cell transplant. Transfusion. 2009;50:501–506. doi: 10.1111/j.1537-2995.2009.02439.x. [DOI] [PubMed] [Google Scholar]
- 16.Imanguli MM, Pavletic SZ, Guadagnini JP, Brahim JS, Atkinson JC. Chronic graft versus host disease of oral mucosa: review of available therapies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:175–183. doi: 10.1016/j.tripleo.2005.08.028. [DOI] [PubMed] [Google Scholar]