Abstract
The objective of the present study was to formulate and evaluate microemulsion systems for topical delivery of clotrimazole (CTM). The solubility of CTM in various oils was determined to select the oil phase of the microemulsion systems. Pseudoternary phase diagrams were constructed to identify the area of microemulsion existence. Five CTM microemulsion formulations (M1–M5) were prepared and evaluated for their thermodynamic stability, pH, refractive index, droplet size, viscosity, and in vitro release across cellulose membrane. Among the prepared microemulsion formulations, M3 (lemon oil/Tween 80/n-butanol/water) and M4 (isopropyl myristate/Tween 80/n-butanol/water) microemulsion systems were found to be promising according to their physical properties and CTM cumulative percentage release. Gel form of M3 and M4 were prepared using 1% Carbopol 940 as the hydrogel matrix. Both formulations were evaluated in the liquid and gel forms for drug retention in the skin in comparison to the marketed CTM topical cream and their stability examined after storage at 40°C for 6 months. Microemulsion formulations achieved significantly higher skin retention for CTM over the CTM cream. Stability studies showed that M4 preparations were more stable than M3. The in vitro anti-fungal activity of M4 against Candida albicans was higher than that of the conventional cream. Moreover, clinical evaluation proved the efficacy and tolerability of this preparation in the treatment of various topical fungal infections.
Key words: clotrimazole, microemulsion, skin retention, topical cream, topical gel
INTRODUCTION
Clotrimazole (CTM) is a broad spectrum antimycotic agent effective against pathogenic dermatophytes, yeasts, and several species of Candida, Tricophyton, Microsporum, Epidermophyton, and Malassezia. CTM is known to be very effective locally and presents no major side effects (1). CTM is available in several conventional dosage forms such as creams, gels, pessaries, and ovules. However, poor water solubility of CTM (0.49 μg/ml) (2) presents a hindrance for its local availability and limits its effective antifungal therapy.
Many formulation approaches have been attempted to improve the solubility of clotrimazole such as mucoadhesive thermosensitive gels (3), nanospheres (4), solid lipid nanoparticles (5), liposomal/niosomal delivery systems (6), bioadhesive liposomal gels (7), and inclusion complexes with β-cyclodextrin (8).
Microemulsions are clear, stable, isotropic mixtures of oil, water, and surfactant, frequently in combination with a cosurfactant (9–11). Microemulsions have several advantages such as enhanced drug solubility, good thermodynamic stability, and higher transdermal permeability over conventional formulations (11,12). Many studies reported the use of microemulsions as topical drug delivery vehicles showed their ability to improve transdermal and dermal delivery properties (12–15). Microemulsions have several permeation enhancement mechanisms such as increase in concentration gradient and thermodynamic activity toward the skin (16).
In the present study, different formulations of CTM microemulsion were prepared in liquid and gel forms for topical application and evaluated for their physicochemical properties and ability to deliver CTM inside the skin. The in vitro antifungal activities as well as the clinical efficacy of these formulations in treating different skin fungal infections were also examined.
MATERIALS AND METHODS
Materials
CTM was kindly donated as a gift by Al-Arabia Company (Egypt). Polysorbate 80 (Tween 80), n-butanol, isopropyl myristate (IPM), oleic acid, benzyl alcohol, polyoxy-35-castor oil (Cremophor® EL), and triethanolamine were purchased from Sigma Aldrich (Germany). Lemon oil was purchased from Morgan chemicals industry company (Egypt). Carbopol 940 was from Goodrich Chemical Co. (London, England). Sabroud dextrose agar was purchased from Britania laboratories (Argentina). Canesten® skin cream (Clotrimazole 1% w/w, manufactured by Memphis Co. For Pharm. & Chem. Ind., Cairo, Egypt under license of Bayer Leverkusen, Germany) was purchased from a local pharmacy store. All other materials were of analytical grade.
Methods
Determination of Saturation Solubility of CTM in Different Oils
The solubility of CTM in various oils was determined by adding excess amount of drug to 4 g of oil in capped glass test tubes. The tubes were equilibrated at 37°C for 72 h in a thermostatically controlled water bath shaker. The suspensions were centrifuged at 6,000 rpm for 15 min followed by filtration of the obtained supernatant through 0.45 μm Millipore membrane filters. One gram of the filtrate was diluted appropriately with methanol and used for the determination of CTM concentration spectrophotometrically at 260 nm using oil in methanol at the same dilution as blank.
Construction of Microemulsion Phase Diagrams
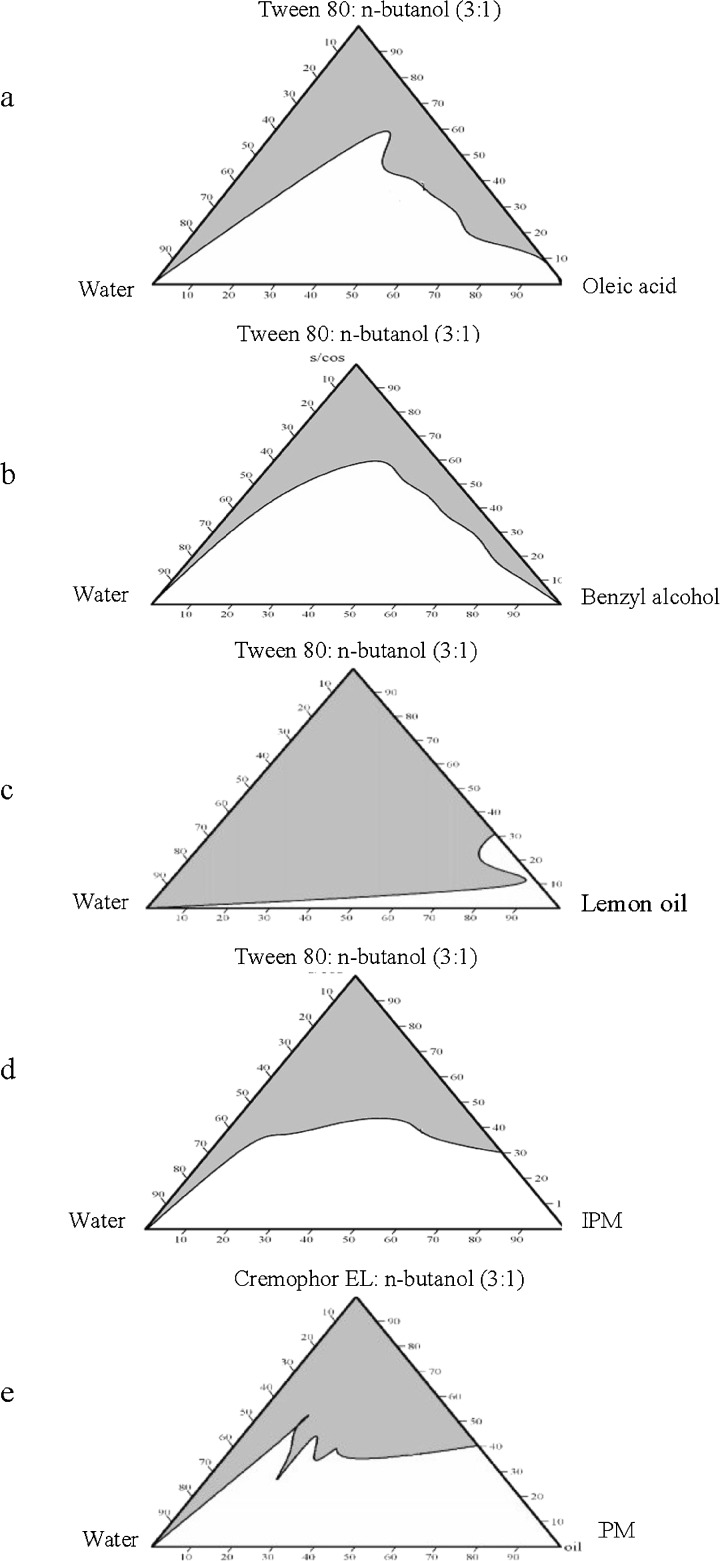
The oil phase studied included lemon oil, oleic acid, benzyl alcohol, or isopropyl myristate. Tween 80 and Cremophor EL were selected as surfactants (S) while n-butanol was used as the cosurfactant (CoS) in an S/CoS weight ratios of 1:1, 1:2, and 1:3. The composition of the prepared systems is presented in Table I. The pseudoternary-phase diagrams of each oil in combination with the different S/CoS weight ratios were constructed using water titration method at 25°C (17). Samples were titrated with water in a drop-wise manner and mixed thoroughly by vortexing until clear and transparent microemulsion phase regions could be identified. The clear areas corresponding to microemulsion were constructed inside the triangular phase diagram using Chemix School 3.5 software.
Table I.
Composition of the Prepared Systems
| System | Oil | S | CoS |
|---|---|---|---|
| M1 | Oleic acid | Tween 80 | n-butanol |
| M2 | Benzyl alcohol | Tween 80 | n-butanol |
| M3 | Lemon oil | Tween 80 | n-butanol |
| M4 | IPM | Tween 80 | n-butanol |
| M5 | IPM | Cremophor®EL | n-butanol |
Preparation of CTM Microemulsion
The appropriate oils and S to CoS weight ratio used in the microemulsions were chosen from the constructed phase diagrams (Fig. 1). The selected percent composition for all the microemulsion systems was 5.5% w/w oil, 49.5% w/w water, 45% w/w S/CoS at a ratio of 3:1. The selection of this ratio was based on being a common system in all the phase diagrams constructed with the different oils used. Also, this ratio was expected to give an O/W microemulsion system due to the relatively low weight ratio of oil to water. This aspect is important because CTM is lipophilic drug thus, preferably incorporated into the internal phase of the microemulsion. The drug was accurately weighted to represent 1% of the total weight of the formulation and added to the oily phase, composed of the chosen oil and CoS, and vortexed together until the drug was completely dissolved. The surfactant was then added to the mixture, vortexed, and water added dropwise with continuous mixing.
Fig. 1.
Pseudoternary phase diagrams of various oils—S/CoS (3:1 weight ratio)—water at 25°C (the gray area represents microemulsion region)
Assessment of Thermodynamic Stability of CTM Microemulsions
To assess the thermodynamic stability of the prepared microemulsions, two tests were carried out:
Centrifuge Stress Test
Microemulsions were centrifuged at 6,000 rpm for 30 min and then examined for phase separation (18).
Freeze–Thaw Cycle Stress Test
Microemulsions were subjected to a total of three complete freeze–thaw cycles, each cycle consisting of 24 h at 25°C followed by 24 h at −5°C (19).
Characterization of CTM Microemulsion Systems
pH Measurements
The pH of the systems was measured by direct immersion of the electrode of the pH meter (Mettler Toledo MP 220, Switzerland) in the system at room temperature.
Refractive Index
Refractive indices of the systems were determined at 25°C using refractometer M46.17/63707 (Higler and Walts Ltd., England, UK).
Morphology Scanning of CTM Microemulsions
Morphology of the microemulsion droplets was examined using a transmission electron microscopy (Jeol Jem Dos electron microscopy, Japan). A drop of the microemulsion preparation was placed on a microscope slide and stained with one drop of 3% phosphotungestic acid and the stained microemulsion droplets were imaged.
Droplet Size Determination
The size of droplets in the different CTM microemulsion systems was determined using Laser diffraction Mastersizer X Ver.2.15 (Malvern instruments Ltd. Malvern, UK) at 25 ± 0.5°C.
Rheological Study
The formed CTM microemulsion systems were studied for their rheological behavior at 25 ± 1°C using Brookfield viscometer model DV-III equipped with spindle number 40 (Engineering Laboratories, Inc., Middleboro, MA, USA). All the studied microemulsion systems were subjected to continuous variation of shear rate from 75 s−1 to 938 s−1 and the resulting shear stress was measured. The rheological behavior of the microemulsion systems was evaluated by constructing rheograms where the shear stress (dyne/cm2) versus shear rate (s−1) was plotted.
In Vitro Release of CTM from Microemulsions
Prior to testing, a piece of cellulose membrane (Molecular weight cut off 12,000–14,000 Da, Spectra/Pro, Spectrum Laboratories, Inc., USA) of suitable dimensions was soaked in sufficient amount of distilled water for about 24 h. Later, this membrane was fixed in position to cover one end of a top-cut plastic syringe used to represent a dialyzing tube of 1.9 cm internal diameter, thus, providing an effective release area of approximately 2.84 cm2. The membrane was made water tight by rubber band and an accurately weighed 2 g of the test preparation was placed in the designed release assembly. The tube enclosing the test sample was then attached to the shaft of a dissolution apparatus I (Hanson Research, California, USA) instead of its basket. The dialyzing tube was carefully lowered and adjusted so that the membrane just touched the surface of the release medium. A volume of 50 ml Sorenson’s phosphate buffer (pH 7.4) containing 1% sodium lauryl sulfate (SLS) maintained at 37°C and stirred at a speed of 100 rpm was used as the release medium (20). Aliquots (2 ml) of the medium were withdrawn at 1, 2, 3, 6, and 8 h, and replaced with equal volume of the fresh medium to maintain the volume of the release medium constant throughout the duration of the experiment. The samples were filtered through 0.45 μm Millipore membrane filters and analyzed for CTM content using high-performance liquid chromatography (HPLC).
HPLC Assay of CTM
Samples were analyzed for clortimazole concentration by using a validated HPLC assay (21) with slight modifications. The HPLC system consisted of a Shimadzu (Tokyo, Japan) chromatographic system equipped with Shimadzu LC-10 AD vp pump, Shimadzu SPD-10A vp UV/Visible detector, DGU-12A degasser and SCL-10A vp system controller. Samples were injected using a manual injector at injection volume of 20 μl and 1.5 ml/ min flow rate. The used column was a C18 column (Shim-pack vp-ODS, 4.6 × 150 mm). A mobile phase consisting of a mixture of methanol/dipotassium hydrogen phosphate (0.025 M) at a ratio of 75:25 v/v was used in an analysis that was conducted in an isocratic elution mode. Prior to use, the mobile phase was sonicated and filtered through 0.45 μm cellulose membrane under vacuum. Data acquisition and integration were carried out using Shimadzu Class vp software (version 6.12 SP4). The detection wavelength was 254 nm. All operations were carried out at ambient temperature.
Preparation of CTM Microemulsion Gel
In general, most microemulsions possess a very low viscosity, which may restrict their clinical application, due to the inconvenience of application (11). In order to overcome this disadvantage, some gelling agents have been used to increase viscosity and form microemulsion-based hydrogels, which are more suitable for topical application when compared with microemulsion as a vehicle for drug delivery. In this study, Carbopol 940 was selected, based on previous reports, as the hydrogel matrix (22,23). An amount of drug representing 1% w/w of the formulation was added to an oily phase, consisting of the chosen oil and CoS which was vortexed until the drug was completely dissolved. The surfactant was then added to the resulting oily phase. An aqueous phase of Carbopol 940 was prepared by dispersing an amount of the gelling agent, equivalent to 1% w/w of the formulation in water. After complete swelling of the Carbopol in water, the pH of the aqueous phase was adjusted by adding triethanolamine and the microemulsion gel obtained by adding the oily phase to the water-swelled gel with vigorous mixing.
Skin Retention Study
Approval to carry skin retention study was obtained from the Animal Ethics Committee of Faculty of Pharmacy, Helwan University. Guidelines of the ethics committee were followed for the study. Skin was obtained from the abdomen of female mice weighing 21 ± 2 g. The full-thickness skin was excised after hair was removed with a depilatory. Subcutaneous fat and other extraneous tissues were trimmed; the skin washed with physiological saline followed by phosphate-buffered saline (pH 7.4) and then visually inspected for integrity to ensure the absence of holes or other imperfections. The excised mice skins were stored at −21°C and used within 1 week of skin harvest. Prior to testing, the skin was brought to room temperature then fixed in position to top-cut plastic syringe representing a dialyzing tube of 1.9 cm internal diameter. The skin was placed with its stratum corneum facing upward (inside of the tube) and dermal side downward (to face the medium). The position-fixed skin was made water tight by a rubber band. One gram of the tested microemulsion (liquids and gels) and the conventional CTM cream was accurately weighed in the plastic tube, which was then attached through its other end to the shaft of the dissolution tester. The whole assembly was adjusted in the same manner as previously mentioned under the in vitro release study. However, in this study, the skin was touching the surface of a medium composed of only 20 ml Sorenson’s phosphate buffer (pH 7.4) containing 1% SLS at 37°C and stirred at 50 rpm. After 24 h, the effective diffusion area of the skin was separated, washed several times with distilled water, to remove formulation excess, and then cut into small piece. The segments obtained were weighed in capped glass vials, vortexed with 3 ml methanol then left soaking for 24 h, to ensure effective extraction of the retained drug from the skin, before being subjected to three sonication cycles, 30 min each, in an ultrasound bath. The resulting mixture was then filtered using 0.45 μm syringe filter and 1 ml from the filtrate was diluted with 1 ml Sorenson’s phosphate buffer (pH 7.4) containing 1% SLS then refiltered and CTM quantified using HPLC.
Stability Study on the Selected CTM Microemulsion Liquid and Gel
Based on results of previous studies, liquid and gel formulations of M3 and M4 were stored in well-stoppered dark brown glass containers for 6 months at 40°C and 75% relative humidity. The stored samples were checked for optical clarity and phase separation by visual inspection and measurement of pH, refractive index, mean droplet size, and CTM content at 0-, 3-, and 6-month storage.
Antifungal Activity of CTM in the Selected Microemulsion Liquid and Gel Forms
The antifungal activity study of M4 liquid and gel in comparison with the CTM conventional cream against Candida albicans was determined by agar diffusion method. The plates were first sterilized in hot air oven at 160°C for 60 min. C. albicans suspension (100 μl) was introduced into each plate and 40 ml of sterile Sabroud dextrose agar was poured into each plate. The plates were agitated carefully to allow for both an even distribution of the agar in the plates and a homogenous mixing of the agar with the test organism. The plates were left on a flat solid surface and allowed to harden. In each plate, three cups, each 10 mm in diameter were bored in the medium with cork borer. The disks of agar were removed by sterilized dissecting needle while being careful not to damage the cups. In each plate, 0.1 g of M4 microemulsion, gel or CTM cream was placed in one of the cups and the plates incubated at 37°C for 24 h. The entire operation was carried out under aseptic conditions and the mean inhibition zone from six plates was calculated.
Clinical Evaluation of CTM Microemulsion Gel
After approval by local ethical committee, a clinical trial was conducted to evaluate the clinical efficacy of the prepared 1% CTM M4 gel. All procedures followed were in accordance with the Declaration of Helsinki (1975, amended 2000) on experimentation on human subjects. Twenty patients with different skin fungal infections were included in this study. Their ages ranged from 18 to 50 years (mean 34 years). Informed consent had been obtained from each patient prior to treatment. All patients were attending the outpatient clinic of the Department of Dermatology, Helwan public hospital in Helwan area, Cairo, Egypt. To be eligible for participation in the study, patients had to meet the following inclusion criteria: clinically diagnosed patients suffering from tinea corporis, tinea circinata, and tinea pedis with skin lesion area of less than 10% of the total body surface area. Diabetic patients, patients taking chemotherapy, pregnant, and lactating women were excluded. Before initiation of the study, for each patient, the severity of various symptoms such as redness, itching, and infection degree was recorded on a scale from 0 (no symptoms) to 3 (severe symptoms) by a dermatologist. The patients were instructed to use the M4 gel as a thin film over the diseased area three times daily. After 1 and 2 weeks of treatment, the scores for the symptoms of fungal infection were recorded by a dermatologist. No other systemic or local antifungal medications were allowed to be taken.
RESULTS AND DISCUSSION
Determination of Saturation Solubility of CTM in Different Oils
The drug was freely soluble in benzyl alcohol and showed a reasonably high solubility in oleic acid and lemon oil (187 and 64 mg/g, respectively) compared to the other oils examined (Table II). Based on the high drug solubility, which was important to achieve, benzyl alcohol, oleic acid, and lemon oil were selected for preparation of the CTM microemulsions (24). Although the drug had a relative low solubility in IPM compared to other oils, it was also selected for the preparation of microemulsions due to its well-known permeation-enhancing property (25) and biocompatibility (26).
Table II.
Saturation Solubility of CTM in Various Oils (n = 3)
| Oil | Solubility (mg/g oil) |
|---|---|
| Olive oil | 18.50 ± 0.94 |
| Soybean oil | 6.37 ± 2.67 |
| Sesame oil | 14.35 ± 1.53 |
| Light liquid paraffin | 0.31 ± 0.22 |
| IPM | 10.11 ± 0.16 |
| Oleic acid | 187.14 ± 7.45 |
| Lemon oil | 63.9 ± 16.45 |
| Benzyl alcohol | Freely soluble |
Values are mean ± SD of three replicates
Construction of Microemulsion Phase Diagrams
It was observed that percentage area of microemulsion region in the majority of phase diagrams was largest at S/CoS weight ratio of 3:1 compared to 1:1 and 2:1 ratios (data not shown). This can be explained on the basis of the enhancement in micelle formation with the increase in S/CoS ratio, which consequently increases the solubilizing capacity of the microemulsion (27). It was also observed that lemon oil formed the largest microemulsion area (88.80%) while oleic formed the smallest microemulsion area (32.29%) at the same S/CoS weight ratio of 3:1 (Fig. 1).
Thermodynamic Stability of CTM Microemulsions
Visual examination showed that all the CTM microemulsion systems were stable after being subjected to centrifugation or freeze–thaw cycles.
Characterization of CTM Microemulsion Systems
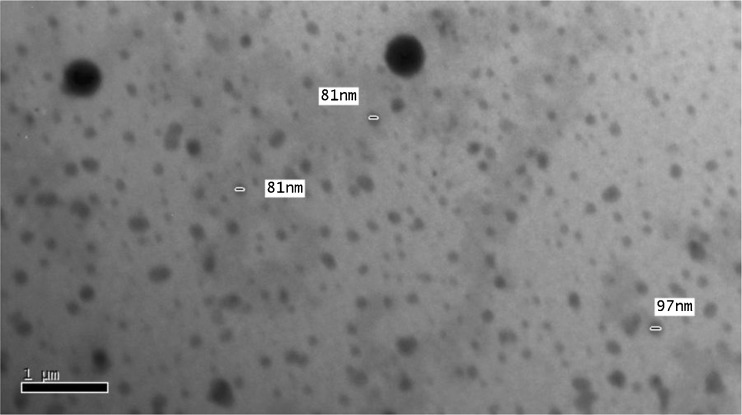
The results of pH measurements, refractive indices, droplet diameters, and viscosity measurements are shown in Table III. It is evident from Table III that all the systems are within the required physiologic pH range accepted for dermal preparations (4.0–7.0 pH units) and that the refractive index measurements for all tested microemulsions were in the range expected for transparent isotropic systems (28). The mean droplet size of the prepared microemulsions ranged between 197.50 ± 18.32 and 296.25 ± 77.45 nm, which was slightly higher than the usual microemulsion droplet size range of 20–200 nm (29). Transmission electron microscope photographs (Fig. 2) showed spherical microemulsion droplets with some large droplet sizes, which might be due to the dynamic property of microemulsion where the interface is continuously and spontaneously fluctuating (30). The higher droplet radius can be explained as a temporal, apparent, and aggregated radius (13). Rheological study showed that all the systems prepared followed the Newtonian behavior expected for microemulsions (data not shown) (31,32). Consequently, the viscosity of microemulsions was calculated from the slope of shear stress vs. shear rate plots (Table III). The highest viscosity value of M5 containing Cremophor EL relative to those containing Tween 80 (M1–M4) might be because Cremophor EL is semisolid while Tween 80 is liquid at room temperature (33).
Table III.
Characterization of CTM Microemulsion Systems
| System | pH | Refractive index | Droplet diameter (nm) | Viscosity (cps) |
|---|---|---|---|---|
| M1 | 5.10 | 1.39 | 197.50 ± 18.32 | 92.22 ± 10.72 |
| M2 | 5.74 | 1.39 | 225.00 ± 28.28 | 42.32 ± 4.46 |
| M3 | 5.94 | 1.39 | 207.50 ± 23.15 | 60.59 ± 7.27 |
| M4 | 5.99 | 1.39 | 220.00 ± 66.55 | 86.70 ± 7.54 |
| M5 | 6.88 | 1.40 | 296.25 ± 77.45 | 146.80 ± 1.68 |
Values are mean ± SD of three replicates
Fig. 2.
Transmission electron microphotograph of CTM microemulsion droplets of formulation M4 stained with 3% phosphotungestic acid
In Vitro Release of CTM from Microemulsions
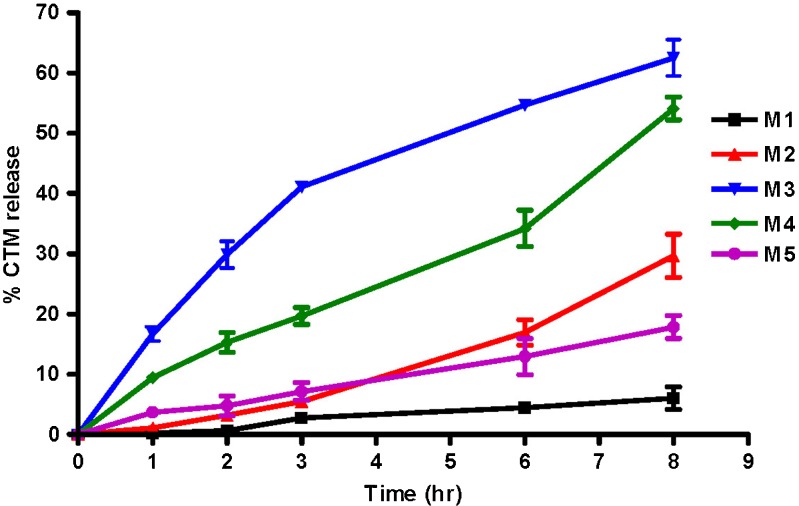
As illustrated in Fig. 3, the cumulative percent CTM released after 8 h was 6.05%, 29.69%, 62.50%, 54.11%, 17.86% for M1, M2, M3, M4, M5, respectively. The in vitro release of CTM from all the microemulsion systems followed zero-order kinetics except for M3 where the Higuchi diffusion model showed a better fit (data not shown). The lower cumulative percent release observed for M5 than M4 might be due to the larger droplet size and higher viscosity created by Cremophor EL, the surfactant used in M5, compared to Tween 80 in M4 (Table III). The solubility of CTM in lemon oil and IPM (64 and 10 mg/g oil, respectively) was lower than in oleic acid and benzyl alcohol (187 mg/g and freely soluble, respectively; Table II). The weaker interaction, indicated by the lower solubility, between the drug and the oil for the former oils might have been the driving force for a faster drug release from their respective microemulsions (M3 and M4) compared with those containing the latter oils (M1 and M2). The lower viscosity of M3 than M4, reflecting the higher fluidity for lemon oil compared to IPM, provided better mobility for the drug molecules, and consequently a faster release with a diffusion-controlled release mechanism (Table III). Based on these results, M3 and M4 were prepared in gel form and subjected to further skin retention and stability studies.
Fig. 3.
Release profiles of CTM from different microemulsion systems
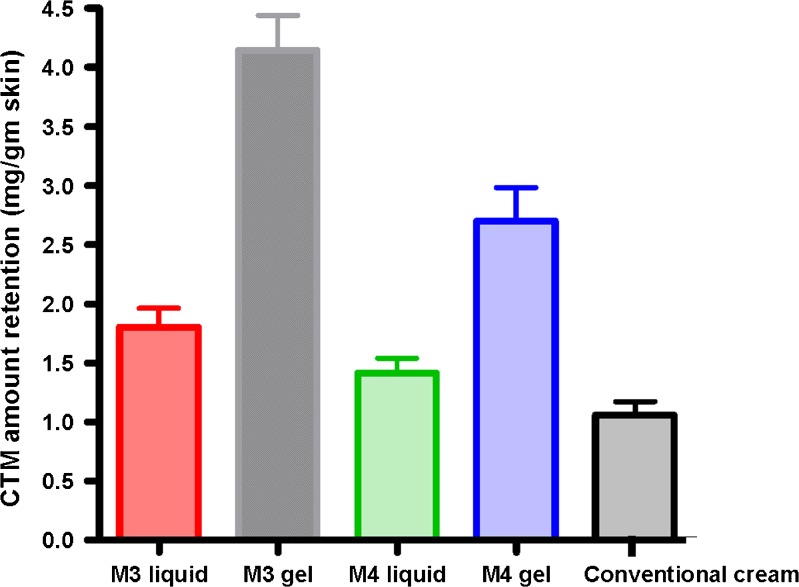
Skin Retention Study
A significantly higher retention of drug in the skin was obtained for the tested microemulsion (liquids and gels) than the conventional CTM cream (Fig. 4). Moreover, the gels provided two- to threefold increase in skin retention compared to their corresponding liquid microemulsion. This might be due to the tighter contact with the skin provided by the adhesive property of the gel. More drug retention in the skin was observed for M3 formulations (liquid and gel) compared to their M4 counterparts. This might be due to either the faster release observed for the drug from M3 compared to M4 or the complex composition of lemon oil with the various terpinenes and components that might be aiding in the penetration and retention of the drug in the skin.
Fig. 4.
Skin retention of 1% CTM microemulsion preparations and conventional cream
Stability Studies
Stability studies were carried out to detect any changes in pH, refractive index, droplet size, and drug content of the preparations through 6 months of storage at 40°C and 75% relative humidity. All preparations were physically stable retaining homogeneity with no phase separation after 3 and 6 months (Table IV).
Table IV.
Stability Evaluation of Microemulsion M3 and M4 Liquids and Gel Formulation
| Parameters | Storage periods in months at 40 ± 2°C | ||
|---|---|---|---|
| 0 | 3 | 6 | |
| pH values | |||
| M3 liquid | 5.94 ± 0.02 | 5.44 ± 0.03 | 5.11 ± 0.04 |
| M3 gel | 6.12 ± 0.10 | 5.57 ± 0.05 | 5.31 ± 0.01 |
| M4 liquid | 5.99 ± 0.06 | 5.71 ± 0.01 | 5.63 ± 0.02 |
| M4 gel | 6.21 ± 0.04 | 6.15 ± 0.08 | 5.99 ± 0.03 |
| Refractive index | |||
| M3 liquid | 1.395 ± 0.001 | 1.392 ± 0.010 | 1.391 ± 0.002 |
| M3 gel | 1.389 ± 0.003 | 1.396 ± 0.006 | 1.392 ± 0.005 |
| M4 liquid | 1.395 ± 0.003 | 1.400 ± 0.001 | 1.425 ± 0.001 |
| M4 gel | 1.393 ± 0.004 | 1.399 ± 0.003 | 1.411 ± 0.001 |
| Droplet size (nm) | |||
| M3 | 207.50 ± 23.15 | 237.15 ± 11.44 | 286.25 ± 55.61 |
| M3 gel | 200.49 ± 10.00 | 232.33 ± 20.11 | 256.79 ± 32.43 |
| M4 | 220.00 ± 66.55 | 262.55 ± 35.46 | 311.50 ± 26.59 |
| M4 gel | 237.97 ± 48.32 | 250.65 ± 20.43 | 266.89 ± 11.76 |
| CTM content (%) | |||
| M3 liquid | 100.00 | 82.60 ± 5.87 | 63.47 ± 7.40 |
| M3 gel | 100.00 | 85.88 ± 5.45 | 68.99 ± 12.80 |
| M4 liquid | 100.00 | 97.15 ± 2.11 | 83.63 ± 1.32 |
| M4 gel | 100.00 | 95.39 ± 4.90 | 90.22 ± 7.14 |
The clarity and isotropy of all the preparations were preserved as indicated by the refractive index measurements throughout the 6-month storage period. However, there was a gradual growth in the mean droplet size with the time of storage. Moreover, drug degradation was found to increase with time of storage and reach 36%, 31%, 16%, and 10% after 6 months for M3 liquid and gel, and M4 liquid and gel, respectively. The higher degradation rate of CTM in both formulations correlated with the reduction in the pH readings after 6-month storage (Table IV). This might be attributed to the acidic pH catalyzed degradation of CTM. Clotrimazole degradation has been reported to be acid catalyzed (34). The identified products of this degradation are imidazole, an aromatic weak basic compound with a pKa of 6.9, and (2-chlorophenyl) diphenylmethanol, a relatively more acid compound (35). It is the formation of the latter that is believed to have caused the drop in pH with time. It might be possible that one or some of the aldehydic components (such as gernial and neral) and alcohols (such as linalool, geraniol, and nerol) present in lemon oil interacted with CTM thus, forming intermediates that facilitated drug degradation from M3 to a greater extent than M4 (36). Therefore as a result of this higher stability of M4, its liquid and gel preparations were further subjected to in vitro antifungal activity evaluation.
Antifungal Activity of CTM in Liquid and Gel Preparations of M4
The results of in vitro antifungal activity of M4 liquid and gel against C. albicans showed that the mean diameter of the inhibition zone due to M4 preparations was highly significant than that of conventional CTM cream. These results reflect the higher antifungal activity of M4 liquid and gel preparations in comparison to conventional CTM cream (P < 0.05). The higher in vitro antifungal activity of M4 preparations could be attributed to the smaller globule size of the microemulsion with its larger surface area compared to the ordinary emulsion present in the cream. This might have allowed for a greater drug release per unit time from the droplets resulting in a higher concentration gradient across the fungal cell wall and more of drug getting into the cell to efficiently inhibit ergosterol synthesis (34).
Clinical Evaluation of 1% CTM M4 Gel
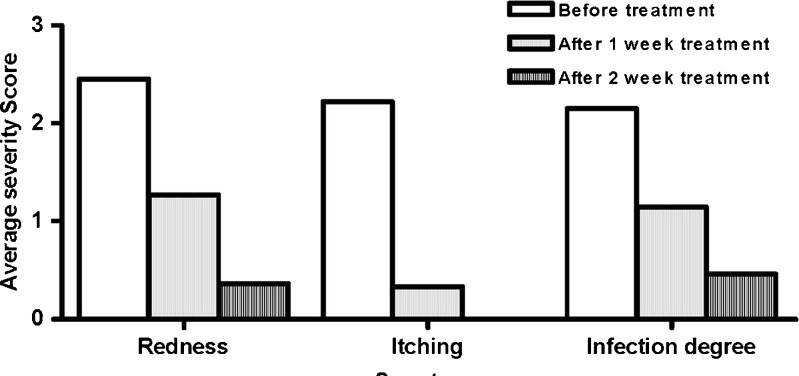
Thirteen patients (ten females and three males) completed the treatment course whereas seven patients did not and their data was excluded from the study. After 1- and 2-week treatment, 1% CMT M4 gel showed a significant reduction in the scores of symptoms of the evaluated skin fungal infections (tinea corporis, tinea circinata, and tinea pedis; Fig. 5). The overall evaluation of the clinical efficacy of M4 gel was good to excellent in 92.31% of the patients who completed the study. Moreover, the preparation was well tolerated by all patients with no discontinuation of treatment due to any side effects. Thus, the results proved the effectiveness of the selected formula in treatment of topical fungal diseases.
Fig. 5.
Average severity score of symptoms of fungal infections before and after 1- and 2-week treatment (n = 11 for redness, n = 9 for itching, n = 13 for infection degree)
CONCLUSION
A microemulsion gel formulation comprising of isopropyl myristate, Tween 80, n-butanol, and water was deemed promising as a successful topical delivery system of CTM for the treatment of skin fungal infections. Although, the gel preparation was more effective in vitro as an antifungal agent than the conventional skin cream, more comparative clinical studies are needed to confirm the benefit it provides over the available marketed products.
REFERENCES
- 1.Ritter W, Patzschke K, Krause U, Stettendorf S. Pharmacokinetic fundamentals of vaginal treatment with clotrimazole. Chemotherapy. 1982;28:37–42. doi: 10.1159/000238150. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen M, Bjerregaard S, Jacobsen J, Sørensen AM. Agenuine clotrimazole γ-cyclodextrin inclusion complex—isolation, antimycotic activity, toxicity and an unusual dissolution rate. Int J Pharm. 1998;176:121–31. doi: 10.1016/S0378-5173(98)00310-X. [DOI] [Google Scholar]
- 3.Chang JY, Oh YK, Kong HS, Kim EJ, Jang DD, Nam KT, Kim CK. Prolonged antifungal effects of clotrimazole-containing mucoadhesive thermosensitive gels on vaginitis. J Control Release. 2002;82:39–50. doi: 10.1016/S0168-3659(02)00086-X. [DOI] [PubMed] [Google Scholar]
- 4.Memişoğlu E, Bochot A, Ozalp M, Sen M, Duchêne D, Hincal AA. Direct formation of nanospheres from amphiphilic beta-cyclodextrin inclusion complexes. Pharm Res. 2003;20(1):117–25. doi: 10.1023/A:1022263111961. [DOI] [PubMed] [Google Scholar]
- 5.Souto EB, Wissing SA, Barbosa CM, Müller RH. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm. 2004;278:71–7. doi: 10.1016/j.ijpharm.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 6.Ning M, Gu Z, Pan H, Yu H, Xiao K. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antifungal drug clotrimazole. Indian J Exp Biol. 2005;43:150–7. [PubMed] [Google Scholar]
- 7.Pavelic Z, Skalko-Basnet N, Jalsenjak I. Characterization and in vitro evaluation of bioadhesive liposome gels for local therapy of vaginitis. Int J Pharm. 2005;301:140–8. doi: 10.1016/j.ijpharm.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Parbagar B, Yoo BK, Woo JS, Kim JA, Rhee JD, Piao MG, Choi HG, Yong CS. Enhanced bioavailability of poorly water-soluble clotrimazole by inclusion with β cyclodextrin. Arch Pharm Res. 2007;30(2):249–54. doi: 10.1007/BF02977701. [DOI] [PubMed] [Google Scholar]
- 9.Comelles F, Pascual A. Microemulsions with butyl lactate as cosurfactant. J Dispers Sci Tech. 1997;18:161–75. doi: 10.1080/01932699708943725. [DOI] [Google Scholar]
- 10.Park KM, Kim CH. Preparation and evaluation of flurbiprofen-loaded microemulsion for parenteral delivery. Int J Pharm. 1999;181:173–9. doi: 10.1016/S0378-5173(99)00029-0. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Del Rev. 2000;45:89–121. doi: 10.1016/S0169-409X(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 12.Osborne DW, Ward AJI, O’Neil KJ. Microemulsions as topical drug delivery vehicles: in vitro transdermal studies of a model hydrophilic drug. J Pharm Pharmacol. 1991;43:451–4. doi: 10.1111/j.2042-7158.1991.tb03511.x. [DOI] [PubMed] [Google Scholar]
- 13.Baroli B, López-Quintela MA, Delgado-Charro MB, Fadda AM, Blanco-Méndez J. Microemulsions for topical delivery of 8-methoxsalen. J Control Release. 2000;69:209–18. doi: 10.1016/S0168-3659(00)00309-6. [DOI] [PubMed] [Google Scholar]
- 14.Sintov AC, Shapiro L. New microemulsion vehicle facilitates percutaneous penetration in vitro and cutaneous drug bioavailability in vivo. J Control Release. 2004;95:173–83. doi: 10.1016/j.jconrel.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Delgado-Charro MB, Iglesias-Vilas G, Blanco-Mendez J, López-Quintela MA, Marty JP, Guy RH. Delivery of a hydrophilic solute through the skin from novel microemulsion systems. Eur J Pharm Biopharm. 1997;43:37–42. doi: 10.1016/S0939-6411(96)00016-1. [DOI] [Google Scholar]
- 16.Peltola S, Saarinen-Savolainen P, Kiesvaara J, Suhonen TM, Urtti A. Microemulsions for topical delivery of estradiol. Int J Pharm. 2003;254:99–107. doi: 10.1016/S0378-5173(02)00632-4. [DOI] [PubMed] [Google Scholar]
- 17.Boonme P, Krauel K, Graf A, Rades T, Junyaprasert VB. Characterization of microemulsion structure in the pseudoternary phase diagram of isopropyl palmitate/water/Brij 97:1-butanol. AAPS PharmSciTech. 2006;7(2):Article 45. doi: 10.1208/pt070245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiwari NG, Bajaj AN. Formulation development of eucalyptus oil microemulsion for intranasal delivery. Indian J Pharm Sci. 2007;69:731–3. doi: 10.4103/0250-474X.33148. [DOI] [Google Scholar]
- 19.Brime B, Moreno MA, Frutos G, Ballesteros MA, Frutos P. Amphotericin B in oil–water lecithin-based microemulsions: formulation and toxicity evaluation. J Pharm Sci. 2002;91(4):1178–85. doi: 10.1002/jps.10065. [DOI] [PubMed] [Google Scholar]
- 20.Alam MA, Ahmad FJ, Khan ZI, Khar RK, Ali M. Development and evaluation of acid-buffering bioadhesive vaginal tablet for mixed vaginal infections. AAPS PharmSciTech. 2007;8(4):E109. doi: 10.1208/pt0804109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoogerheide JG, Strusiak SH, Taddei CR, Townley ER, Wyka BE. High performance liquid chromatographic determination of clotrimazole in pharmaceutical formulations. J Assoc Off Anal Chem. 1981;64(4):864–9. [PubMed] [Google Scholar]
- 22.Chen H, Mou D, Du D, Chang X, Zhu D, Liu J, Xu H, Yang X. Hydrogel-thickened microemulsion for topical administration of drug molecule at an extremely low concentration. Int J Pharm. 2007;341:78–84. doi: 10.1016/j.ijpharm.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 23.Mou D, Chen H, Du D, Mao C, Wan J, Xu H, Yang X. Hydrogel-thickened nanoemulsion system for topical delivery of lipophilic drugs. Int J Pharm. 2008;353:270–6. doi: 10.1016/j.ijpharm.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 24.Baboota S, Shakeel F, Ahuj A, Shafiq S. Design, development and evaluation of novel nanoemulsion formulations for transdermal potential of celecoxib. Acta Pharm. 2007;57:315–32. doi: 10.2478/v10007-007-0025-5. [DOI] [PubMed] [Google Scholar]
- 25.Panigrahi L, Ghosal SK, Snigdha P, Maharana L, Barik BB. Effect of permeation enhancers on the release and permeation kinetics of lincomycin hydrochloride gel formulations through mouse skin. Indian J pharm Sci. 2006;68:205–11. doi: 10.4103/0250-474X.25716. [DOI] [Google Scholar]
- 26.Gupta S, Moulik SP. Biocompatible microemulsions and their prospective uses in drug delivery. J Pharm Sci. 2008;97:22–45. doi: 10.1002/jps.21177. [DOI] [PubMed] [Google Scholar]
- 27.Kawakami K, Yoshikawa T, Hayashi T, Nishihara Y, Masuda K. Microemulsion formulation for enhanced absorption of poorly soluble drugs. II. In vivo study. . J Control Release. 2002;81:75–82. doi: 10.1016/S0168-3659(02)00050-0. [DOI] [PubMed] [Google Scholar]
- 28.Junyaprasert VB, Boonme P, Songkro S, Krauel K, Rades T. Transdermal delivery of hydrophobic and hydrophilic local anesthetics from o/w and w/o Brij 97-based microemulsions. J Pharm Pharm Sci. 2007;10(3):288–98. [PubMed] [Google Scholar]
- 29.Talegaonkar S, Azeem A, Ahmad FJ, Khar RK, Pathan SA, Khan ZI. Microemulsions: a novel approach to enhanced drug delivery. Recent Pat Drug Deliv Formul. 2008;2:238–57. doi: 10.2174/187221108786241679. [DOI] [PubMed] [Google Scholar]
- 30.Lam AC, Schechter RS. The theory of diffusion in microemulsion. J Colloid Interface Sci. 1987;120:56–63. doi: 10.1016/0021-9797(87)90322-5. [DOI] [Google Scholar]
- 31.Alany RG, Tucker IG, Davies NM, Rades T. Characterizing colloidal structures of pseudoternary phase diagrams formed by oil/water/amphiphile systems. Drug Dev Ind Pharm. 2001;27:31–8. doi: 10.1081/DDC-100000125. [DOI] [PubMed] [Google Scholar]
- 32.Djordjevic L, Primorac M, Stupar M. In vitro release of diclofenac diethylamine from caprylocaproyl macrogolglycerides based microemulsions. Int J Pharm. 2005;296:73–9. doi: 10.1016/j.ijpharm.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Ammar H, Salama A, Ghorab M, Mahmoud A. Nanoemulsion as a potential ophthalmic delivery system for dorzolamide hydrochloride. AAPS PharmSciTech. 2009;10(3):808–19. doi: 10.1208/s12249-009-9268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bachhav YG, Patravale VB. Microemulsion-based vaginal gel of clotrimazole: formulation, in vitro evaluation, and stability studies. AAPS PharmSciTech. 2009;10(2):476–81. doi: 10.1208/s12249-009-9233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hajkova R, Sklenarova H, Matysova L, Svecova P, Solich P. Development and validation of HPLC method for determination of clotrimazole and its two degradation products in spray formulation. Talanta. 2007;73:483–9. doi: 10.1016/j.talanta.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 36.Kirbaslar FG, Tavman A, Dulger B, Turker G. Antimicrobial activity of Turkish citrus peel oils. Pak J Bot. 2009;41(6):3207–12. [Google Scholar]