Abstract
Anionic polymer sodium carboxymethylcellulose (CELLOGEN® HP-HS and/or HP-12HS) was investigated for its ability to influence the release of three model drugs propranolol hydrochloride, theophylline and ibuprofen from polyethylene oxide (POLYOX™ WSR 1105 and/or Coagulant) hydrophilic matrices. For anionic ibuprofen and non-ionic theophylline, no unusual/unexpected release profiles were obtained from tablets containing a mixture of two polymers. However, for cationic propranolol HCl, a combination of polyethylene oxide (PEO) with sodium carboxymethylcellulose (NaCMC) produced a significantly slower drug release compared to the matrices with single polymers. The potential use of this synergistic interaction can be a design of new extended release pharmaceutical dosage forms with a more prolonged release (beyond 12 h) using lower polymer amount, which could be particularly beneficial for freely water-soluble drugs, preferably for once daily oral administration. In order to explain changes in the obtained drug release profiles, Fourier transform infrared absorption spectroscopy was performed. A possible explanation for the more prolonged propranolol HCl release from matrices based on both PEO and NaCMC may be due to a chemical bond (i.e. ionic/electrostatic intermolecular interaction) between amine group of the cationic drug and carboxyl group of the anionic polymer, leading to a formation of a new type/form of the active (i.e. salt) with sustained release pattern.
Key words: extended release, FT-IR, ibuprofen, matrix tablet, polyethylene oxide, polymer combination, propranolol hydrochloride, sodium carboxymethylcellulose, theophylline
INTRODUCTION
Matrices represent a popular and widely used approach for an oral extended release drug delivery due to economic, process development and scale-up reasons (1,2). Polyethylene oxide (3) and sodium carboxymethylcellulose (4) are popular matrix-forming polymers, with wide regulatory acceptance, availability in a range of viscosity grades and good swelling and erosion characteristics, which can be used to modulate the release of various drugs (3–11).
Many active substances have a relatively short plasma half-life, and therefore, patients are routinely asked to take the medicine in divided daily doses every 6 to 8 h (12,13). As a result, development of extended release (ER) formulations that can enable the drug to be given once daily is vital for improving patient compliance and therapeutic efficacy (13,14).
Due to the high cost of both natural polymers, synthesis of the new ones and testing their safety, a modern focus has been directed towards investigation of blends of pharmaceutically approved materials as matrix functional excipients to enhance single polymer performance by obtaining a variety of physical and chemical properties in a new formulation (15–19). It has been reported (4) that polymer blends can be used to prevent dose-dumping (‘burst release’), to increase the resistance to agitation (‘food effect’) and to lower microenvironmental pH within the matrix gel, which may be beneficial for improving solubility or stability of some basic active pharmaceutical ingredients (APIs).
Çaykara and Demirci (16) studied blends based on polyvinyl alcohol (PVA) and sodium alginate prepared by the solution casting method. The authors observed an improvement in the mechanical properties of the films made from blends compared to the films made from single polymers due to the hydrogen bonding interactions between the ether oxygen of PVA and the hydroxyl groups of alginate. Feely and Davis (20) investigated the effect of non-ionic polymers, polyethylene glycol (PEG) 6000 and ethyl cellulose (EC) on chlorpheniramine release from hypromellose matrices. The authors reported that PEG acted as a swellable polymer and EC appeared to have behaved as an inert diluent.
A number of studies (20,21) investigated the inclusion of anionic surfactants, polymers and ion exchange resins in hydroxypropyl methylcellulose (HPMC) ER formulations and claimed that the release of the active was dependent on drug-ionic excipient interaction.
There are also reports in the literature describing an interaction between basic cationic drug propranolol HCl and anionic polymers in the dissolution medium leading to the release rate retardation (22,23). A number of publications (22,24–26) described an existence of inter-polymer complexes that resulted in a near zero-order release from hydrophilic matrices achieved by using a combination of non-ionic (i.e. HPMC) with ionic polymers (i.e. NaCMC) for the following drugs: β-adrenergic blockers, oxprenolol hydrochloride and some bronchodilators.
However, a very limited number of publications regarding ER matrices based on a combination of PEO and an ionic polymer exists. Lu et al. (27) investigated electrospinning (simple and effective fabrication technique for producing nano- to microscale fibres) of sodium alginate from aqueous solution. The authors reported that sodium alginate alone did not electrospan and its processability was greatly improved by blending with PEO which enabled to produce smooth fibres, tensile strength and morphology of which were dependent on the polymer–polymer ratio. Authors claimed that this phenomenon was attributed to hydrogen bonding interaction between the etheric oxygen of PEO and hydroxyl groups of sodium alginate leading to a reduction of repulsive force among poly-anionic sodium alginate molecules.
Basavaraju et al. (28) demonstrated that the reduced viscosities for different compositions of PEO and xanthan gum could be attributed to the attraction of macromolecules in a solution with similar miscibility. They observed that the polymer blends were miscible only when the xanthan gum content was 40% (w/w) and above. Fourier transform infrared (FT-IR) spectrum confirmed the possibility of intermolecular H-bonding between PEO and xanthan gum. Similar observations were also made by Raviprakash and Rai (29) for poly(ethylene glycol) and sodium alginate combinations, by Basavaraju et al. (18) for poly(ethylene oxide) and hydroxypropyl methylcellulose blends and by Guru et al. (11) between poly(vinyl pyrrolidone) and xanthan gum, wherein all systems showed miscibility windows. In this study, the influence of anionic sodium carboxymethylcellulose on the release of three model drugs with different ionic nature and aqueous solubility from ER matrix tablets based on non-ionic polyethylene oxide was investigated.
MATERIALS AND METHODS
Formulation and Tablet Preparation
Model formulations (20 g blend) containing 49.75% (w/w) drug (see Table I for physicochemical properties of drugs); 49.75% (w/w) PEO, NaCMC or their 1:1 mixture used as a matrix former and 0.5% (w/w) of magnesium stearate (Peter Greven, UK) used as a lubricant were prepared. The choice of ER polymer viscosity grade was based on an aqueous solubility of API (4). PEO with relatively low molecular weight (Mw), POLYOX™ WSR 1105, was used in the ibuprofen and theophylline formulations and high Mw PEO (POLYOX™ WSR Coagulant) in the propranolol HCl formulations. Two grades of NaCMC with the same level of substitution (0.7), with relatively low Mw (CELLOGEN® HP-SH) and high Mw (CELLOGEN® HP-12HS), were investigated (Table II).
Table I.
Model Drugs Used in the Study
| Active | Manufacturer | Average particle size (μm) | Ionic nature | Molecular weight | Log P | pKa | Solubility in water (mg/mL) |
|---|---|---|---|---|---|---|---|
| Propranolol HCl | S.I.M.S., Italy | 10 | Cationic | 295.8 | 1.2 | 9.50 | 360.0 |
| Theophylline | Sigma-Aldrich, UK | 82 | Non-ionic | 180.2 | 0.0 | 8.60 | 7.4–8.0 |
| Ibuprofen | IMCD, UK | 29 | Anionic | 206.3 | 4.0 | 4.4; 5.20 | 0.02–0.06 |
Table II.
Matrix Formers Used in the Study
| Polymer | Manufacturer | Molecular weight (Da) | Viscosity (mPa s) | Average particle size (μm) | Ionic nature | Solubility in water (mg/mL) |
|---|---|---|---|---|---|---|
| PEO (POLYOXTM WSR 1105a or Coagulantb) | Dow, USA | 900,000 or 5,000,000 | 13,500 | 150 | Non-ionic | Soluble |
| 7,480 | ||||||
| NaCMC (CELLOGEN® HP-SHc) or NaCMC (CELLOGEN® HP-12HSd) | Dai-Ichi Kogyo Seiyaku, Japan | 150,000 or 350,000 | 465 | 177 | Anionic | Easily dispersed. Solubility depends on degree of substitution |
| 12,000 |
aViscosity 5% aqueous solution at 25°C; Brookfield RVF2, 20 rpm
bViscosity 1% aqueous solution at 25°C; Brookfield RVF2, 20 rpm
cViscosity 1% aqueous solution at 25°C; Brookfield LV2, 60 rpm
dViscosity 1% aqueous solution at 25°C; Brookfield LV4, 30 rpm
For all formulations, the ingredients with the exception of magnesium stearate were blended in a 1-L tumbler mixer (Turbula T2C, Willy A. Bachofen, Switzerland) at 64 rpm for 3 min. Then magnesium stearate was added to the mixtures, and samples were blended for an additional 1 min.
Round flat-faced 10-mm-diameter tablets with a target weight of 320 mg (n = 3 for each formulation) were produced by direct compression using a semi-automated hand-press (Atlas T8, Specac, UK) at 20 kN compression force. Additionally, the effect of PEO-to-NaCMC (HP-12HS) ratio (1:5, 1:4, 1:2.3, 1:1.5, 1.5:1, 2.3:1, 4:1 and 5:1) on propranolol hydrochloride release was investigated.
Physical Properties of the Tablets
The diameter and thickness of each tablet were measured to ±0.001 mm using a 25-mm digital micrometer (Model 293-766-30, Mitutoyo, Japan). Tablet porosity (ε, percent) was calculated using Eq. 1 (30).
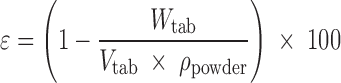 |
1 |
where Wtab is the tablet weight (grams), Vtab represents the apparent volume of the tablet (cubic centimetres) and ρpowder is the true density of powder (grams per cubic centimetre).
The true density of powders was determined using ultrapychnometer (Quantachrome Instruments, UK). The surface area (SA, square millimetres), apparent volume (Vtab, cubic millimetre) and SA/Vtab ratio for each tablet were determined using the following three equations, Eqs. 2–4, suitable for round flat-faced compacts (31):
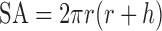 |
2 |
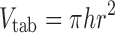 |
3 |
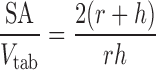 |
4 |
where h and r are tablet thickness (millimetres) and radius (square millimetres), respectively.
It has been shown that the drug release from matrix tablets is affected by several variables/parameters, such as tablet shape, size, surface area and ratio of surface area to volume (SA/Vtab) (32). The tablets with larger (SA/Vtab) ratio typically have faster release profiles, in spite of the dose or size and when the above ratio is held constant the drug release rates are usually similar, regardless of the tablet shape (round or oval) (33). This is the main reason for calculating SA/Vtab parameter in the present study. Apparent tablet density was calculated according to the following equation (Eq. 5):
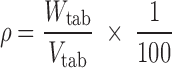 |
5 |
where ρ is apparent tablet density and Wtab is tablet weight.
Drug Release Analysis
In vitro drug release was obtained in a USP compliant dissolution bath (AT7 Smart, Sotax, Switzerland) using Apparatus II (paddles) with 15 × 31-mm sinkers (Sotax), operated at 100 rpm in 900 mL of purified water at 37.0 ± 0.5°C. Measurements at each time point were performed in triplicates, and mean and standard deviation values were calculated. Sink conditions (34) for ibuprofen were only achieved in pH 7.2 phosphate buffer.
Absorbance was measured using a UV–Vis spectrophotometer (Lambda 25, Perkin Elmer, US) at 222, 272 and 319 nm for ibuprofen, theophylline and propranolol HCl, respectively. The produced dissolution profiles were compared by model-independent approach using a similarity f2 factor. The similarity factor is a logarithmic reciprocal square root transformation of the sum of squared error and is a measurement of the similarity in the percent dissolution between the two curves (Eq. 6).
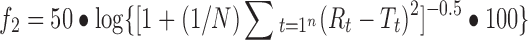 |
6 |
where N is a number of time points, Rt is a dissolution value of the reference (pre-change) batch at time t and Tt is a dissolution value of the test (post-change) batch at time t. For curves to be considered similar, f2 values should be close to 100. Generally, f2 values greater than 50 (50–100) ensure sameness or equivalence of the two dissolution profiles (5).
The dissolution profiles were described by the power law firstly proposed by Korsemeyer and Peppas and known as Peppas model, where log cumulative percentage of drug release is plotted against a log time (35,36). In this approach, so-called diffusion model, it is assumed that the drug is initially uniformly distributed through a polymeric matrix. In order to investigate the release kinetics from the studied matrices, the drug release data between 5% and 60% was fitted to Eq. 7 proposed by Ritger and Peppas (37):
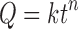 |
7 |
where Q is the percentage of drug released at time t, k is the kinetic constant representing structural and geometric characteristic of the tablet and n is the release exponent indicative of the drug release mechanism (37).
According to Ford et al. (38) and Efentakis et al. (39) for matrix (cylindrical) tablets, an n value equal or less than 0.45 indicates Fickian mechanism (or case I) mainly controlled by diffusion. For n ≥ 0.89 (i.e. 0.89 < n < 1.00), a super case II transport takes place, when dissolution process is controlled mainly by erosion and the release rate is independent of time (‘zero-order’ kinetics). Intermediate values (i.e. 0.45 ≤ n ≤ 0.89) represent a non-Fickian or anomalous transport and suggest that erosion (polymer matrix relaxation) and drug diffusion both contribute to the overall drug release mechanisms (37,39). A very high k value may be an indication of a burst release from the matrix.
Dissolution efficiency (DE) is a quantitative approach to assess in vitro drug release profile (40). According to the same author, the higher the DE, the better the release efficiency of the active ingredient from the matrix form. The DE of a pharmaceutical dosage form is defined as the area under the dissolution curve up to the time, t, expressed as the percentage of the area of the rectangle (Eq. 8):
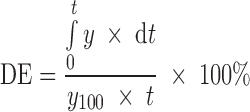 |
8 |
where y is the percent of drug dissolved at time t (12 h in this study).
An alternative parameter that describes the dissolution rate is the mean dissolution time (MDT), the most likely time for a molecule to be dissolved from a solid dosage form. Therefore, MDT is the mean time for the drug to dissolve under in vitro dissolution conditions and was calculated using the Eq. 9:
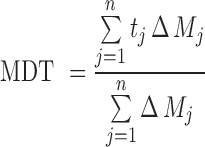 |
9 |
where j is the sample number, tj is the midpoint of the jth time period (calculated as (t + t − 1)/2) and ΔMj is the additional amount of drug dissolved between tj and t − 1.
The mean dissolution rate (MDR) was calculated according to the following equation (Eq. 10):
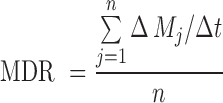 |
10 |
where n is the number of dissolution sample times, Δt is the time at midpoint between t and t − 1 (calculated as (t + t − 1)/2) and ΔMj is the additional amount of drug dissolved between tj and t − 1.
The release rates (percent per square root hour) were calculated using Higuchi equation, as in Eq. 11 (41,42):
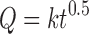 |
11 |
where the release rate (k) values were extracted from the slope of percentage drug release (Q) versus square root of time t.
Preparation of Propranolol Hydrochloride–Anionic Polymer Complex
The method was adapted from Takka (23) where drug (0.1–0.2 g) was dissolved in purified water (900 mL) and after 1 h a single polymer (0.1–0.2 g) or polymer combination (1:1 ratio) was slowly added to the API (propranolol HCl) solution and left constantly stirred at 37°C for 2 h. The formed precipitate (∼0.1 g) was later dried in an oven (Heraeus UT6, Thermo Electron Corporation, UK) at 40°C for 3 days under the reduced pressure, triturated and further analysed using FT-IR.
Fourier Transform Infrared Absorption Spectroscopy
In order to determine the presence and nature of interaction between drug and polymer, the samples of physical mixtures (powders) and complexes of propranolol HCl with NaCMC (in triplicates) were analysed using FT-IR spectrophotometer (Nicolet 380, Thermo Electron Corporation, USA). All samples (1 to 2 mg) were placed in the middle of the sample holder, and force was applied by the top of the arm of the sample stage. Each sample was scanned 32 times from 4,000 to 400 cm−1 at 1 cm−1 resolution.
RESULTS AND DISCUSSION
Effect of NaCMC on Physicomechanical Properties of PEO ER Matrices
Robust, mechanically strong (10–14 kp; 1.9–2.7 MPa) matrices were produced for all studied formulations. Table III shows the physicomechanical properties of the produced compacts. For all formulations, tablets with relatively similar weight (320.0–338.0 mg), diameter (10.0–10.1 mm), thickness (3.0–3.9 mm), volume (0.2–0.3 cm3), density (1.0–1.4 g/cm3) and SA/Vtab ratio (0.9–1.1) were produced. In this study (SA/Vtab) ratio for all model drugs was similar (0.91 to 1.06 mm2/mm3) indicating that the difference in drug release seen from various formulations was not due to the above parameter but most likely due to other factors, such as drug–polymer and/or polymer–polymer interaction which is discussed below.
Table III.
Physicomechanical Properties of the Studied Dry Compacts
| API | Polymer | Weight (mg) | Diameter (mm) | Thickness (mm) | Volume (cm3) | Density (g/cm3) | Porosity (%) | SA/V tab (mm2/mm3) |
|---|---|---|---|---|---|---|---|---|
| Ibuprofen | PEO | 320.00 ± 0.01 | 10.03 ± 0.00 | 3.91 ± 0.10 | 0.31 ± 0.00 | 1.04 ± 0.00 | 16.30 ± 0.01 | 0.91 ± 0.00 |
| NaCMC | 332.00 ± 0.01 | 10.07 ± 0.00 | 3.45 ± 0.06 | 0.28 ± 0.00 | 1.19 ± 0.00 | 18.23 ± 0.00 | 0.97 ± 0.00 | |
| PEO/NaCMC | 329.00 ± 0.01 | 10.09 ± 0.01 | 3.75 ± 0.09 | 0.30 ± 0.01 | 1.10 ± 0.00 | 23.37 ± 0.00 | 0.93 ± 0.01 | |
| Theophylline | PEO | 338.00 ± 0.00 | 10.01 ± 0.00 | 3.38 ± 0.01 | 0.27 ± 0.00 | 1.27 ± 0.01 | 13.38 ± 0.01 | 0.99 ± 0.01 |
| NaCMC | 332.00 ± 0.00 | 10.05 ± 0.01 | 3.04 ± 0.05 | 0.24 ± 0.00 | 1.38 ± 0.00 | 17.11 ± 0.00 | 1.06 ± 0.00 | |
| PEO/NaCMC | 334.00 ± 0.01 | 10.03 ± 0.00 | 3.20 ± 0.08 | 0.25 ± 0.01 | 1.32 ± 0.00 | 17.73 ± 0.00 | 1.02 ± 0.00 | |
| Propranolol HCl | PEO | 327.00 ± 0.00 | 10.02 ± 0.00 | 3.57 ± 0.00 | 0.28 ± 0.00 | 1.16 ± 0.00 | 14.76 ± 0.01 | 0.96 ± 0.00 |
| NaCMC | 336.00 ± 0.00 | 10.06 ± 0.00 | 3.33 ± 0.02 | 0.26 ± 0.00 | 1.27 ± 0.00 | 9.62 ± 0.00 | 1.00 ± 0.00 | |
| PEO/NaCMC | 330.00 ± 0.01 | 10.03 ± 0.00 | 3.41 ± 0.07 | 0.27 ± 0.01 | 1.22 ± 0.01 | 10.38 ± 0.00 | 0.98 ± 0.01 |
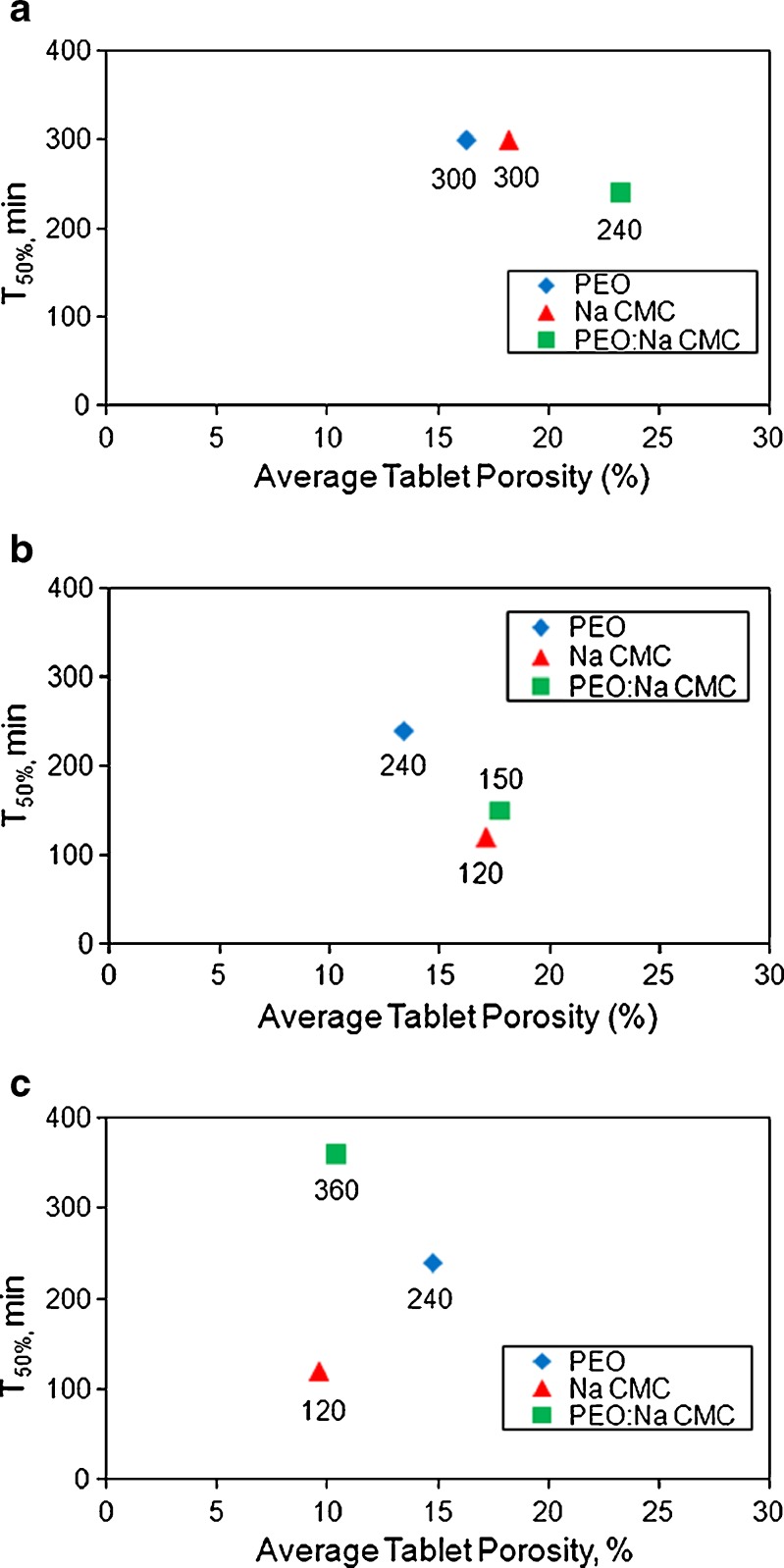
The porosity values for all tablets produced in this study fall within wide (9.6–23.4%) range. No influence of tablet porosity on API release from PEO, Na CMC and PEO/Na CMC matrices was observed (Fig. 1a–c). These results were in agreement with other studies (43–45) that identified no significant effect of porosity on drug release from matrices manufactured with the same amount of ER polymer, once a critical tablet mechanical strength was achieved.
Fig. 1.
The influence of tablet porosity on a ibuprofen, b theophylline and c propranolol HCl release from PEO, NaCMC and PEO/Na CMC formulations
Effect of NaCMC on Drug Release from PEO ER Matrices
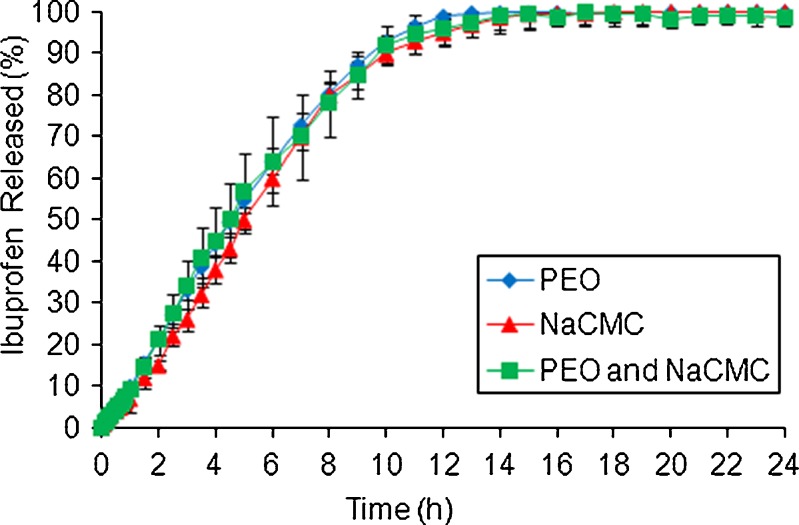
Figures 2, 3 and 4 show the influence of NaCMC on ibuprofen, theophylline and propranolol HCl release from PEO ER matrices, respectively. For ibuprofen, drug release was not dependant on the choice of the polymer as a matrix former (Fig. 2).
Fig. 2.
Effect of NaCMC on ibuprofen release from PEO ER matrices in pH 7.2 phosphate buffer
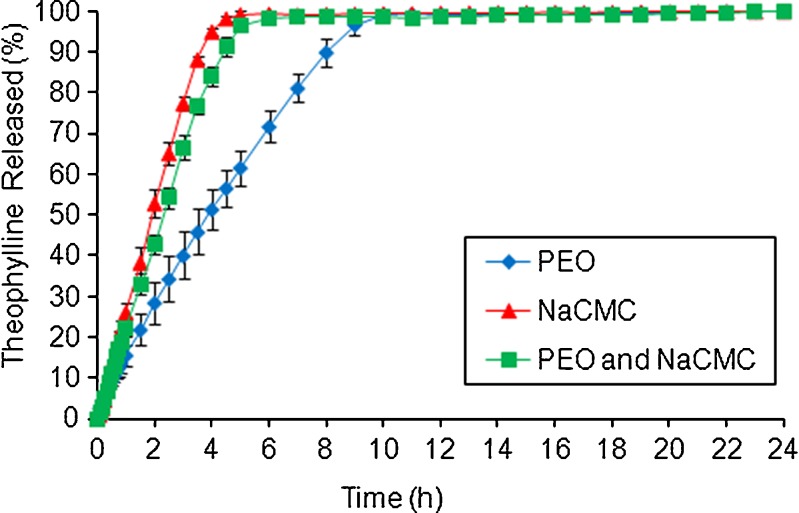
Fig. 3.
Effect of NaCMC on theophylline release from PEO ER matrices in water
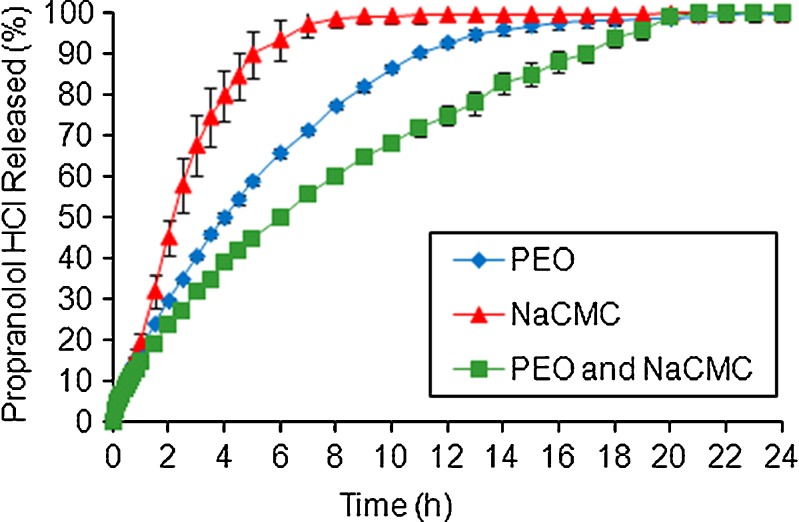
Fig. 4.
Effect of NaCMC on propranolol HCl release from PEO ER matrices in water
For theophylline, a relatively fast release (100% in 4 h) was obtained from matrices containing NaCMC (Fig. 3). The rapid drug dissolution/diffusion and relatively fast erosion (n > 0.8, Table IV) of those matrices may be explained by the high aqueous solubility and hygroscopic nature of NaCMC due to the presence of ionized carboxylic acid groups within the polymer structure. The latter may possibly lead to an increase in rate and extent of water uptake due to ion-pair repulsion, stretch in the gel network and break of the bonds responsible for the gel structure (46–48). The slowest theophylline release was obtained from PEO tablets. A combination of PEO and NaCMC resulted in theophylline dissolution profile similar to the one obtained from the matrices based on NaCMC (Fig. 3).
Table IV.
Values of the Kinetic Constant (k), Diffusion Exponent (n) and Correlation Coefficient (R 2) for the Matrices Containing Various APIs and Polymers
| API | Polymer | k | n | R 2 |
|---|---|---|---|---|
| Ibuprofen | PEO | 10.204 | 1.055 | 0.996 |
| NaCMC | 6.841 | 1.190 | 0.998 | |
| PEO/NaCMC | 9.412 | 1.170 | 1.000 | |
| Theophylline | PEO | 16.279 | 0.812 | 0.999 |
| NaCMC | 25.800 | 1.100 | 0.994 | |
| PEO/NaCMC | 21.879 | 1.013 | 0.998 | |
| Propranolol HCl | PEO | 17.673 | 0.750 | 1.000 |
| NaCMC | 21.929 | 0.832 | 0.958 | |
| PEO/NaCMC | 14.068 | 0.697 | 0.999 |
The most interesting results were produced for propranolol HCl (Fig. 4). For this cationic API, the slowest release profile was obtained when both PEO and NaCMC were used in the formulation. As a result, 100% of the drug dissolution occurred after 20 h. This indicated a potential synergistic interaction (chemical or/and physical) between drug–polymer and/or polymer–polymer, the mechanism of which is currently under investigation.
For example, one explanation may be that carboxylic (COOH) group of NaCMC reacts with the amine (HNR2) group of strong basic drug (pKa = 9.5) propranolol hydrochloride due to a lone pair of electrons on nitrogen of the latter group pulling/attracting positively charged carbon ions of NaCMC towards it, leading to a formation of some type of the chemical reversible bonding between drug and polymer. During this reaction, the drug may form a new salt type with the CMC polymer, i.e. propranolol H+(CMC−), which retards the release. Similar results were previously reported for NaCMC and HPMC combinations with propranolol hydrochloride as a model API (23,47,48). The authors claimed an ionic interaction by hydrogen bonding between the amine group of propranolol HCl and carboxyl group of NaCMC resulted in a formation of a propranolol–NaCMC complex leading to a retardation of the drug release rate from HPMC ER matrices.
Similarity factor (f2) values between formulations containing single NaCMC polymer and PEO/NaCMC blend were calculated and equal to 68, 61 and 33 for ibuprofen, theophylline and propranolol HCl, respectively. These results indicated that there was no influence of NaCMC on anionic ibuprofen or non-ionic theophylline release and a significant effect on the release of cationic propranolol HCl from PEO ER matrices. In addition, the Kruskal–Wallis nonparametric test (GraphPad Software Inc., San Diego, CA, USA) followed by the Dunn post hoc multiple comparison test was used to investigate the differences between the three propranolol HCl formulations. All dissolution profiles were found to differ significantly (P < 0.0001) indicating a strong effect of the NaCMC on drug release.
The dissolution data parameters [kinetic constant (k), the release exponent (n) and correlation coefficient (R2)] were determined from the drug release data and summarized in Table IV. Good correlation (R2 > 0.96) was achieved for all studied formulations. The poorly water-soluble ibuprofen and the slightly water-soluble theophylline were released mainly by erosion (n = 0.8–1.2), whereas freely water-soluble propranolol HCl was mainly released by a combination of diffusion and erosion processes (n = 0.70–0.83) (49).
All matrices based on NaCMC produced higher (×1.20-fold) n values compared to other formulations and, in most cases, had a relatively higher k values. That could be explained by a more rapid dissolution of the drug in the first few hours due to faster erosion of NaCMC matrices. No initial fast release was observed for ibuprofen–NaCMC formulation due to poor solubility of this drug in water as compared to highly water-soluble propranolol HCl and slightly water-soluble theophylline.
In order to facilitate comparison between drug release from tablets made using various model drugs and polymer combinations, additional independent dissolution parameters such as AUC, DE, MDT, MDR and release rates were calculated (Table V). For ibuprofen and theophylline formulations with the polymer blend, no unexpected dissolution data parameters were obtained. In contrary, propranolol HCl formulations containing a mixture of PEO and NaCMC appeared to be a more complex process. For examples, Table IV shows that propranolol HCl binary formulations produced lower n values compared to each single polymers (approximately by 1.2-fold), indicating a higher input into drug release by diffusion. Table V shows an increase (approximately by 1.7-fold) in MDT values together with 1.3–1.7- and 1.3–1.4-fold reduction in AUC and MDR values (all parameters calculated at 12 h dissolution) from binary propranolol HCl mixtures, compared to PEO and NaCMC only formulations, respectively. Table V shows that release rate constants (percent per half hour) and dissolution efficiency (percent) for propranolol HCl formulations containing binary mixtures of polymers were also significantly lower in comparison to the same single polymer formulation, i.e. release rate values reduction by 1.4- and 1.7-fold for PEO and NaCMC only formulations and DE12 h of 60%, 79% and 45% for PEO, NaCMC and PEO/NaCMC formulations, respectively.
Table V.
Dissolution Parameters for the Matrices Containing Various APIs and Polymers
| API | Polymer | AUC12 h (% h) | DE12 h (%) | MDT12 h (h) | MDR12 h (% h−1) | Release rate (% h−1/2) |
|---|---|---|---|---|---|---|
| Ibuprofen | PEO | 706.79 | 58.90 | 4.85 | 8.72 | 33.97 ± 3.10 |
| NaCMC | 629.79 | 52.48 | 5.27 | 7.48 | 32.96 ± 3.28 | |
| PEO/NaCMC | 739.04 | 61.59 | 4.30 | 8.66 | 33.54 ± 10.68 | |
| Theophylline | PEO | 785.77 | 65.48 | 4.08 | 10.43 | 34.91 ± 5.81 |
| NaCMC | 996.63 | 83.05 | 1.99 | 15.33 | 38.44 ± 3.60 | |
| PEO/NaCMC | 952.69 | 79.39 | 2.33 | 13.91 | 38.27 ± 2.95 | |
| Propranolol HCl | PEO | 717.52 | 59.79 | 4.26 | 10.72 | 30.44 ± 1.03 |
| NaCMC | 941.56 | 78.46 | 2.55 | 11.30 | 37.56 ± 6.54 | |
| PEO/NaCMC | 539.81 | 44.98 | 4.42 | 8.11 | 22.58 ± 2.73 |
The time necessary for 20%, 50% and 80% drug release to occur for ibuprofen and theophylline were as expected (i.e. the release rate values from polymer blends fall in between formulations containing single polymers). However, for propranolol HCl, T20–80% was significantly higher for the polymer blend formulation in comparison to tablets containing only either PEO or NaCMC, i.e. 240, 120 and 360 min for 50% drug release for PEO, NaCMC and PEO/NaCMC formulations, respectively.
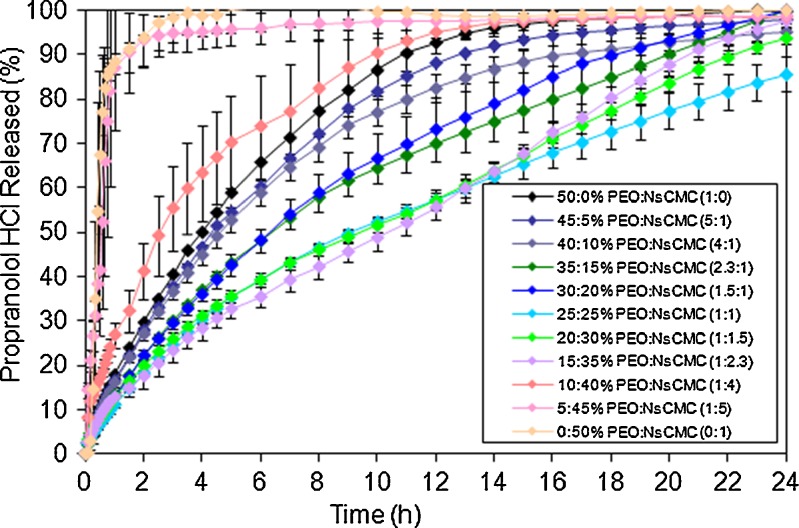
Effect of PEO/NaCMC Ratio on Propranolol HCl Release
Figure 5 and Tables VI and VII show a significant effect of PEO/NaCMC ratio on propranolol HCl release. On a subsequent addition of NaCMC to PEO matrices, the drug release rate and dissolution efficiency values decreased for most of the binary formulations compared to single polymer tablets. For example a 2-fold decrease was observed in the release rate from 30.4 down to 16.8% h−1/2 for the PEO (50%, w/w) and PEO/NaCMC (15:35%, w/w), respectively. The threshold point in PEO-to-NaCMC concentration was found to be 15% to 35%, where the release rate was the slowest, i.e. reduction by 1.6- and 1.8-fold in MDR and AUC values, respectively, followed by an increase of 1.1-fold in MDT value for the above ratio compared to the formulation without NaCMC. However, a further reduction in PEO concentration leads to a significant increase of drug release rate, i.e. from 33.4 up to 275.4% h−1/2 for the PEO/NaCMC (10:40%, w/w) and NaCMC (50%, w/w), respectively (Fig. 5; Table VII). Therefore, a minimum of 15% (w/w) PEO amount out of a total 50% polymer concentration in ER matrices was necessary in order to maintain the integrity of the matrix and to produce an extended drug release.
Fig. 5.
Effect of PEO/NaCMC ratio on propranolol HCl release
Table VI.
Values of the Kinetic Constant (k), Diffusion Exponent (n) and Correlation Coefficient (R 2) for Propranolol HCl Matrices Containing Various PEO/NaCMC Ratios, Where All Formulations Contained 50% (w/w) Total Polymer Concentration
| Polymer concentrations (%, w/w) and their ratio | k | n | R 2 |
|---|---|---|---|
| 50% PEO, 0% NaCMC (1:0) | 17.673 | 0.750 | 1.000 |
| 45% PEO, 5% NaCMC (5:1) | 16.348 | 0.760 | 0.999 |
| 40% PEO, 10% NaCMC (4:1) | 16.027 | 0.742 | 0.999 |
| 35% PEO, 15% NaCMC (2.3:1) | 13.128 | 0.732 | 0.999 |
| 30% PEO, 20% NaCMC (1.5:1) | 12.827 | 0.616 | 0.999 |
| 25% PEO, 25% NaCMC (1:1) | 11.154 | 0.684 | 0.998 |
| 20% PEO, 30% NaCMC (1:1.5) | 12.827 | 0.616 | 0.999 |
| 15% PEO, 35% NaCMC (1:2.3) | 12.555 | 0.588 | 0.998 |
| 10% PEO, 40% NaCMC (1:4) | 27.547 | 0.543 | 0.979 |
| 5% PEO, 45% NaCMC (1:5) | 66.389 | 0.634 | 0.983 |
| 0% PEO, 50% NaCMC (0:1) | 117.290 | 0.974 | 0.951 |
Table VII.
Dissolution Parameters for the Propranolol HCl Matrices Containing Various Ratios of PEO to NaCMC
| Polymer concentrations (%, w/w) and their ratio | AUC12 h (% h) | DE12 h (%) | MDT12 h (h) | MDR12 h (% h−1) | Release rate (% h−1/2) |
|---|---|---|---|---|---|
| 50% PEO, 0% NaCMC (1:0) | 717.52 | 59.79 | 4.26 | 10.72 | 30.42 ± 1.03 |
| 45% PEO, 5% NaCMC (5:1) | 672.36 | 56.03 | 4.37 | 10.22 | 28.45 ± 2.40 |
| 40% PEO, 10% NaCMC (4:1) | 643.88 | 53.66 | 4.20 | 9.59 | 27.66 ± 3.16 |
| 35% PEO, 15% NaCMC (2.3:1) | 533.23 | 44.44 | 4.37 | 7.94 | 23.30 ± 1.79 |
| 30% PEO, 20% NaCMC (1.5:1) | 541.00 | 45.08 | 4.06 | 7.73 | 23.49 ± 10.77 |
| 25% PEO, 25% NaCMC (1:1) | 434.94 | 36.25 | 4.40 | 6.32 | 18.66 ± 4.08 |
| 20% PEO, 30% NaCMC (1:1.5) | 438.41 | 36.53 | 4.33 | 6.58 | 17.76 ± 2.08 |
| 15% PEO, 35% NaCMC (1:2.3) | 407.11 | 33.93 | 4.68 | 6.82 | 16.76 ± 3.41 |
| 10% PEO, 40% NaCMC (1:4) | 811.28 | 67.61 | 3.46 | 12.00 | 33.35 ± 0.79 |
| 5% PEO, 45% NaCMC (1:5) | 1,099.94 | 91.66 | 0.71 | 32.74 | 74.78 ± 25.76 |
| 0% PEO, 50% NaCMC (0:1) | 1,123.12 | 93.59 | 0.62 | 36.45 | 275.40 ± 6.68 |
The dissolution parameters [kinetic constant (k), the release exponent (n) and correlation coefficient (R2)] determined from the drug release data were calculated for formulations containing different polymer–polymer ratio and summarized in Table VI, where all tablets contained 50% (w/w) total polymer amount. Good correlation (R2 > 0.95) was achieved for all studied formulations.
For most of the freely water-soluble propranolol HCl matrices (with exception of 50% (w/w) NaCMC, n = 0.97, representing erosion), the mechanism of release was mainly controlled by a combination of diffusion and erosion (n = 0.54–0.76) (49). Most of the polymer blend matrices (>15% (w/w) PEO) produced lower n and k values compared to the formulation where single PEO was used (approximately by ×1.3–1.4-folds), indicating a higher input of diffusion into drug release.
Further reduction in the concentration of matrix-forming polymer PEO leads to an increase in both n and k values resulting in higher tablet erosion and faster disintegration. The time taken for 20%, 50% and 80% of the drug to be released was higher for most of the polymer binary formulations in comparison to tablets where only one polymer was used. However, this was observed for PEO concentrations greater than 15% (w/w), i.e. T50% was increased from 240 up to 600 min for PEO (50%, w/w) and PEO/NaCMC (15:35%, w/w). Matrices containing 10% (w/w) PEO produced ER. However, very high (up to 19.6) standard deviation values were recorded, suggesting that the tablets were not as robust due to insufficient amount of matrix-forming polymer (i.e. PEO) to maintain matrix integrity and to provide quick gel formation. Tablets with less than 10% PEO disintegrated within first 25–35 min of the dissolution test. Therefore, in order to achieve ER, at least 15% of PEO was required in the formulation.
FT-IR Spectroscopy
The decrease in the propranolol HCl release rate from PEO and NaCMC matrices could be related to either polymer–polymer or drug–polymer interaction. According to Çaykara and Demirci (16), blending of various polymers could result in a polymer–polymer intermolecular interaction strength of which would be depended on the compatibility or miscibility between the two mixed materials at a molecular level.
In other studies where NaCMC formulations with hydrophilic polymers (HPMC) and propranolol HCl were investigated, it was suggested that dissolution was mainly controlled by an interaction between the cationic API and anionic polymer (23,50,51). Other researchers (26,52) reported a formation of a propranolol–NaCMC precipitate leading to a modification of the drug release rate.
It is known that a polymer molecule containing ionisable subunits acts as a polyelectrolyte or ionomer. Sodium carboxymethylcellulose, although neutral in nature, can dissociate into sodium ion and polymeric anion (CMC−), becoming negatively charged and so-called anionic polymer. Propranolol hydrochloride exists in non-ionic state as a salt, formed with inorganic and organic acids. It has an ability to lose and exchange its chloride atom and attract carboxylate anion. As a result of this reaction, formation of no-covalently attached H+ protons is increased, and drug eventually becomes positively charged and therefore so-called cationic (50). Due to the oppositely charge molecule interactions between counter ions (23,51), a complex formation between propranolol hydrochloride and NaCMC was anticipated.
However, the decrease in the propranolol HCl release rate from PEO and NaCMC matrices could be related to either polymer–polymer or drug–polymer interaction. According to Çaykara and Demirci (16), blending of various polymers could result in a polymer–polymer intermolecular interaction strength of which would be depended on the compatibility or miscibility between the two mixed materials at a molecular level.
In another study, of NaCMC formulations with hydrophilic polymers (HPMC) and propranolol hydrochloride, it was suggested that dissolution was mainly controlled by interaction between the cationic API and anionic polymer (23,51). Other researchers (26,52) reported a formation of a propranolol–NaCMC precipitate leading to a modification of the drug release rate.
Another possible explanation of the observed release patterns could be attributed to the increased viscosity of a formed gel. According to Walker and Wells (53), the addition of NaCMC to a non-ionic polymer (i.e. HPMC) may have increased gel viscosity and thus lead to lower drug diffusion from the matrix. The authors claimed that this phenomenon could possibly be attributed to the strong hydrogen bonding between the carboxyl groups in NaCMC and the hydroxyl groups of HPMC, leading to a strong cross-linking between the two polymers. In order to confirm an existence (if any) and nature of chemical or physical bond in polymer combinations containing propranolol HCl, FT-IR analysis was carried.
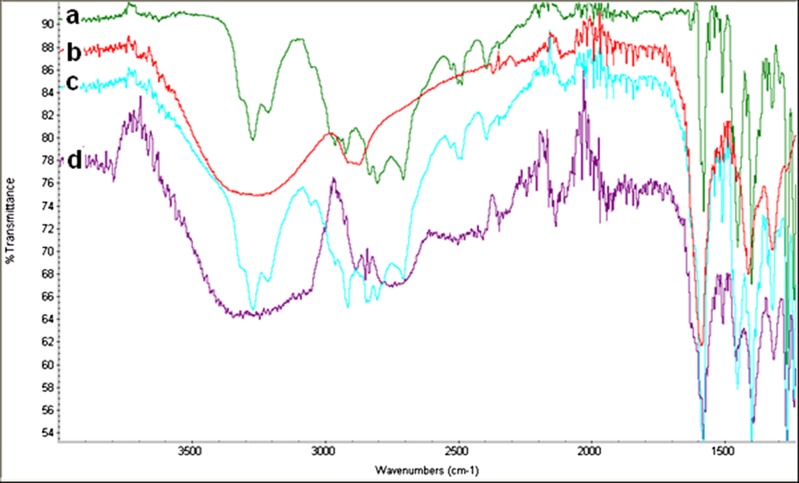
The FT-IR spectra in the absorbance mode for the pure propranolol HCl, single polymer and the polymer blends (powder and complex) are shown in Fig. 6. The results of this study suggested a presence of an intermolecular interaction with appearances of new peaks between amine group of propranolol HCl and carboxylic groups in the structure of NaCMC, enabling a chemical bonds (i.e. ionic bond formed through an electrostatic attraction between two oppositely charged ions) leading to a formation of a new less water-soluble form of the drug, i.e. propranolol H+(CMC−). Table VIII shows characteristic bands of carboxylic acid groups vibrations at 1,488.81 cm−1 (possible –C = C– and –C = O vibration/stretch and –NH deformation) and 1,829.00 cm−1 (carbonyl COOH stretching, C = O stretching) which may be the result of salt formation. The absorption bands characterizing intermolecular bonding in the complex due to –C–H and –OH or –NH were observed at 2,850.38 and 3,330.58 cm−1, respectively.
Fig. 6.
FT-IR scans of powder samples: a propranolol HCl, b NaCMC, c dry powder mixture of propranolol HCl and NaCMC and d propranolol HCl and NaCMC complex
Table VIII.
FT-IR Spectra for Dry Physical Mixture (Powder) and Complex of Propranolol HCl and NaCMC
| Propranolol HCl/NaCMC (powder) | Propranolol HCl/NaCMC (complex) | Comments |
|---|---|---|
| 689.59 | New peak | |
| 706.98 | New peak | |
| 736.68 | 733.56 | |
| 769.46 | 770.19 | |
| 796.76 | 793.87 | |
| 900.25 | 900.09 | |
| 990.30 | New peak | |
| 1,029.92 | 1,026.53 | |
| 1,038.15 | New peak | |
| 1,105.06 | 1,101.25 | Band width change |
| 1,141.66 | ||
| 1,156.11 | Change in S = O symmetric stretching | |
| 1,240.46 | 1,240.17 | |
| 1,266.67 | 1,268.03 | Band width change, C–O Stretch |
| 1,321.89 | 1,314.39 | Band width change, C–O Stretch |
| 1,400.15 | 1,392.10 | Band width change |
| 1,452.14 | 1,456.80 | Band width change |
| 1,488.81 | New peak: –C = C– and –C = O vibration/stretch and –NH deformation | |
| 1,509.64 | 1,507.65 | |
| 1,521.15 | 1,521.14 | |
| 1,580.14 | 1,580.13 | |
| 1,845.13 | 1,829.99 | Carbonyl COOH stretching, C = O stretch; salt formation |
| 1,918.79 | 1,918.53 | |
| 1,923.96 | New peak | |
| 1,943.99 | 1,943.59 | |
| 1,968.54 | 1,969.12 | |
| 2,134.36 | New peak | |
| 2,177.05 | 2,164.05 | Stretch |
| 2,347.08 | New peak | |
| 2,732.59 | New peak | |
| 2,748.55 | New peak | |
| 2,850.38 | New peak, –C–H | |
| 3,249.04 | New peak | |
| 3,271.55 | ||
| 3,330.58 | New peak, –NH and –OH stretching |
Similar results were reported for propranolol HCl matrices containing Eudragit (S100 and L100-55) and NaCMC by Takka (23). The author claimed that when ionization occurred, the resonance between the two C–O bands together with formation of the COO− groups was possible and stated that conversion of carboxylic acid into a salt was carried out by the addition of an amine group to the former solution, confirmed by DSC and FT-IR analysis. Similarly, Sriwongjanya and Bodmeier (54) claimed that for the propranolol HCl and anionic exchange resin (Amberlite® IRP 69) in HPMC (various grades of MethocelTM) tablets retarded release was mainly due to a drug–resin complex formation in situ within the matrix gel regions.
CONCLUSIONS
In this study, mechanically robust ER matrices of three model APIs were produced for PEO/NaCMC formulations. Drug release was dependent on the nature of the drugs used. For the anionic ibuprofen and the non-ionic theophylline, no unusual/unexpected release profiles were obtained from matrices containing a mixture of two polymers. However, for the cationic salt propranolol HCl, a combination of PEO with NaCMC produced a significantly slower drug release (beyond 12 h), compared to the matrices with single polymers. A possible explanation for this phenomenon may be an intermolecular interaction (ionic/electrostatic) due to bonding between amine group of cationic API with carboxyl group of the anionic polymer NaCMC (confirmed by FT-IR), leading to the formation of a new form of a less water-soluble salt, with a more prolonged release compared to the original active substance.
The PEO-to-NaCMC ratio showed a significant effect on propranolol HCl release. An ER was observed at PEO concentration greater than 15% (w/w) where the slowest drug release was observed for formulations containing 15% PEO and 35% NaCMC. This synergistic drug–polymer and/or polymer–polymer interaction can be used to design new oral ER pharmaceutical dosage forms with more prolonged and/or ‘zero-order’ release using lower polymer amounts, which could be beneficial for freely/very water-soluble drugs, particularly when formulation of high doses is required and once daily administration is preferred. The mechanism of a possible drug–polymer and/or polymer–polymer interaction is currently under investigation and will be the subject of a future publication.
Acknowledgements
The authors are grateful to Dai-Ichi Kogyo Seiyaku and IMCD for generous supply of Na CMC and ibuprofen, Charlotte Penny (Sekisui Diagnostics) for assistance in interpretation of potential chemical interaction and Colorcon for providing Ph.D. scholarship.
References
- 1.Vazquez MJ, Perez-Marcos B, Gomez-Amoza JL, Martinez-Pacheco R, Souto C, Concheiro A. Influence of technological variables on release of drug from hydrophilic matrices. Drug Dev Ind Pharm. 1992;20:2519–2526. [Google Scholar]
- 2.Juarez H, Rico G, Villafuerte L. Influence of admixed carboxymethylcellulose on release of 4-aminopyridine from hydroxypropyl methylcellulose matrix tablets. Int J Pharm. 2001;216:115–125. doi: 10.1016/S0378-5173(01)00583-X. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Hardy RJ, Gu X. Effect of drug solubility on polymer hydration and drug dissolution from polyethylene oxide (PEO) matrix tablets. AAPS PharmSciTech. 2008;9:437–443. doi: 10.1208/s12249-008-9060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiwari SB, Rajabi-Siahboomi AR. Extended-release oral drug delivery technologies: monolithic matrix systems, from methods in molecular biology, Chapter 11. In: Jain KK, editor. Drug delivery systems, 437. Totowa: Humana; 2008. pp. 217–243. [DOI] [PubMed] [Google Scholar]
- 5.Moore JW, Flanner HH. Mathematical comparison of curves with an emphasis on in-vitro dissolution profiles. Pharm Tech. 1996;20:64–74. [Google Scholar]
- 6.Velasco MW, Ford JL, Rowe P, Rajabi-Siahboomi AR. Influence of drug: hydroxypropylmethylcellulose ratio, drug and polymer particle size and compression force on the release of diclofenac sodium from HPMC tablets. J Contr Rel. 1999;57:75–85. doi: 10.1016/S0168-3659(98)00110-2. [DOI] [PubMed] [Google Scholar]
- 7.Choi SU, Lee J, Choi YW. Development of a directly compressible poly(ethylene oxide) matrix for the sustained-release of dihydrocodeine bitartrate. Drug Dev Ind Pharm. 2003;29:1045–1052. doi: 10.1081/DDC-120025863. [DOI] [PubMed] [Google Scholar]
- 8.Levina M, Rajabi-Siahboomi AR. The influence of excipients on drug release from hydroxypropyl methylcellulose matrices. J Pharm Sci. 2004;93:2746–2754. doi: 10.1002/jps.20181. [DOI] [PubMed] [Google Scholar]
- 9.Levina M, Gothoskar A, Rajabi-Siahboomi AR. Application of a modelling system in the formulation of extended release hydrophilic matrices. Pharm Tech Eur. 2006;18:20–26. [Google Scholar]
- 10.Palmer D, Levina M, Rajabi-Siahboomi AR. The influence of in vitro dissolution method on the release of a highly water soluble drug from polyethylene oxide and hypromellose hydrophilic extended release matrices. AAPS annual meeting and exposition. 2008; Atlanta, USA
- 11.Guru GS, Prasad P, Shivakumar HR, Rai SK. Miscibility studies of polysaccharide xanthan gum/PVP blend. J Polym Environ. 2010;18:135–140. doi: 10.1007/s10924-010-0191-2. [DOI] [Google Scholar]
- 12.Cid E, Mella F, Lucchini L, Carcamo M, Monasterio J. Plasma concentrations and bioavailability of propranolol by oral, rectal and intravenous administration in man. Biopharm Drug Dispos. 1986;7:559–566. doi: 10.1002/bdd.2510070605. [DOI] [PubMed] [Google Scholar]
- 13.Taylan B, Capan Y, Guven O, Kes S, Hincall AA. Design and evaluation of sustained release and buccal adhesive propranolol hydrochloride tablets. J Control Release. 1996;37:11–20. doi: 10.1016/0168-3659(95)00094-1. [DOI] [Google Scholar]
- 14.Serlin MJ, Orme ML, Maciver M, Green GJ, Sibeon RG, Beckenridge AM. The pharmacodynamics and pharmacokinetics of conventional and long acting propranolol in patients with moderate hypertension. Brit J Clin Pharmacol. 1983;15:519–527. doi: 10.1111/j.1365-2125.1983.tb02085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebube NK, Jones AB. Sustained release of acetaminophen from heterogeneous mixture of two hydrophilic non-ionic cellulose ether polymers. Int J Pharm. 2004;272:19–27. doi: 10.1016/j.ijpharm.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 16.Çaykara T, Demirci S. Preparation and characterization of blend films of poly(vinyl alcohol) and sodium alginate. J Macromol Sci. 2006;43:1113–1121. doi: 10.1080/10601320600740389. [DOI] [Google Scholar]
- 17.Conti S, Maggi L, Segale L, Ochoa Machiste E, Conte U, Grenier P, Vergnault G. Matrices containing Na CMC and HPMC. 1. Dissolution performance characterization. Int J Pharm. 2007;333:136–142. doi: 10.1016/j.ijpharm.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 18.Basavaraju KC, Damappa T, Rai SK. Miscibility studies of hydroxypropyl methyl cellulose and poly(ethylene oxide) by viscometry, ultrasonic, and refractometric methods. J Macromol Sci. 2008;47:417–425. doi: 10.1080/00222340801954712. [DOI] [Google Scholar]
- 19.Rakkappan C, Anbalagan S. Ultrasonic and FTIR studies on aqueous biodegradable polymer blend solutions. Am Eurasia J Sci Res. 2009;4:281–284. [Google Scholar]
- 20.Feely LC, Davis SS. The influence of polymeric excipients on drug release from hydroxypropylmethylcellulose matrices. In J Pharm. 1988;44:131–139. [Google Scholar]
- 21.Nokhodchi A, Norouzi-Sani S, Siahi-Shadbad MR, Lotfipoor F, Saeedi M. The effect of various surfactants on the release rate of propranolol hydrochloride from hydroxypropylmethylcellulose (HPMC)-Eudragit matrices. Eur J Pharm Biopharm. 2002;54:349–356. doi: 10.1016/S0939-6411(02)00120-0. [DOI] [PubMed] [Google Scholar]
- 22.Baveja SK, Ranga Rao KV, Padmaltha Devi K. Zero-order release hydrophilic matrix tablets of β-adrenergic blockers. Int J Pharm. 1987;39:39–45. doi: 10.1016/0378-5173(87)90196-7. [DOI] [Google Scholar]
- 23.Takka S. Propranolol hydrochloride–anionic polymer binding interaction. II Farmaco. 2003;58:1051–1056. doi: 10.1016/S0014-827X(03)00181-2. [DOI] [PubMed] [Google Scholar]
- 24.Baveja SK, Ranga Rao KV, Singh A, Gombar VK. Release characteristics of some bronchodilators from compressed hydrophilic polymeric matrices and their correlation with molecular geometry. Int J Pharm. 1988;41:55–62. doi: 10.1016/0378-5173(88)90135-4. [DOI] [Google Scholar]
- 25.Baveja SK, Ranga Rao KV, Padmaltha Devi K. Relationship between gum content and half-life of soluble β-blockers from hydrophilic matrix tablets. Int J Pharm. 1988;47:133–139. doi: 10.1016/0378-5173(88)90224-4. [DOI] [Google Scholar]
- 26.Ranga Rao KV, Padmalatha Devi K, Buri P. Influence of molecular size and water solubility of the solute on its release from swelling and erosion controlled polymeric matrices. J Control Release. 1990;12:133–141. doi: 10.1016/0168-3659(90)90089-C. [DOI] [Google Scholar]
- 27.Lu JW, Zhu YL, Gu ZX, Hu P, Yu J. Electrospinning of sodium alginate with poly(ethylene oxide) Polymer. 2006;47:8026–8031. doi: 10.1016/j.polymer.2006.09.027. [DOI] [Google Scholar]
- 28.Basavaraju TM, Rai SK. Miscibility studies of polysaccharide xanthan gum and PEO (polyethylene oxide) in dilute solution. Carbohyd Polym. 2007;69:462–466. doi: 10.1016/j.carbpol.2007.01.004. [DOI] [Google Scholar]
- 29.Raviprakash SD, Rai KS. Miscibility studies of sodium alginate/poly(vinyl glycol) blend in water by viscosity, ultrasonic and refractive index methods. Int J Plast Tech. 2004;8:334. [Google Scholar]
- 30.Mu X, Tobyn MJ, Staniforth JN. Influence of physiological variables on the in-vitro drug-release behaviour of a polysaccharide matrix controlled release system. Drug Dev Ind Pharm. 2003;29:19–29. doi: 10.1081/DDC-120016680. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds TD, Mitchell SA, Balwinski KM. Investigation of the effect of tablet surface area/volume on drug release from hydroxypropyl methylcellulose controlled-release matrix tablets. Drug Dev Ind Pharm. 2002;28:457–466. doi: 10.1081/DDC-120003007. [DOI] [PubMed] [Google Scholar]
- 32.Colombo P, Catellani PL, Peppas NA, et al. Swelling characteristics of hydrophilic matrices for controlled release: new dimensionless number to describe the swelling and release behavior. Int J Pharm. 1992;88:99–109. doi: 10.1016/0378-5173(92)90307-N. [DOI] [Google Scholar]
- 33.Reynolds TD, Mitchell SA, Balwinski KM. Investigation of the effect of tablet surface area/volume on drug release from hydroxypropylmethylcellulose controlled-release matrix tablets. Drug Dev. Ind. Pharm. 2004;28(4):457–466. doi: 10.1081/DDC-120003007. [DOI] [PubMed] [Google Scholar]
- 34.Gibaldi M, Feldman S. Establishment of sink conditions in dissolution rate determinations—theoretical considerations and applications to non-disintegrating dosage forms. J Pharm Sci. 1967;56:1238–1242. doi: 10.1002/jps.2600561005. [DOI] [PubMed] [Google Scholar]
- 35.Korsemeyer RW, Peppas NA. Macromolecular and modelling aspects of swelling-controlled systems. In: Mansdorf SZ, Roseman TJ, editors. Controlled release delivery systems. New York: Marcel Dekker; 1983. p. 77. [Google Scholar]
- 36.Siepmann J, Kranz H, Bodmeier R, Peppas NA. HPMC-matrices for controlled drug delivery: a new model combining diffusion, swelling, and dissolution mechanisms and predicting the release kinetics. Pharm Res. 1999;16:1748–1756. doi: 10.1023/A:1018914301328. [DOI] [PubMed] [Google Scholar]
- 37.Ritger PL, Peppas NA. A simple equation for description of solute release. I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J Cont Rel. 1987;5:23–36. doi: 10.1016/0168-3659(87)90034-4. [DOI] [PubMed] [Google Scholar]
- 38.Ford JL, Mitchell K, Rowe PH, Armstrong DJ, Elliott PNC, Rostron C, Hogan JE. Mathematical modelling of drug release from HPMC matrices: effect of temperature. Int J Pharm. 1991;71:95–104. doi: 10.1016/0378-5173(91)90071-U. [DOI] [Google Scholar]
- 39.Efentakis M, Koutlis A, Vlachou M. Development and evaluation of oral multiple-unit and single-unit hydrophilic controlled-release systems. AAPS PharmSciTech. 2000;1:1–9. doi: 10.1208/pt010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan KA. The concept of dissolution efficiency. J Pharm Pharmacol. 1975;27:48–49. doi: 10.1111/j.2042-7158.1975.tb09378.x. [DOI] [PubMed] [Google Scholar]
- 41.Higuchi T. Rate of release of medicaments from ointment bases containing drugs in suspensions. J Pharm Sci. 1961;50:874–875. doi: 10.1002/jps.2600501018. [DOI] [PubMed] [Google Scholar]
- 42.Higuchi T. Mechanism of sustained action medication. Theoretical analysis of rate release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 43.Timmin P, Delargy A, Minchom C, Howard J. Influence of some process variables on product properties for a hydrophilic matrix controlled release tablet. Eur J Pharm Biopharm. 1992;38:113–118. [Google Scholar]
- 44.Nockhodchi A, Ford JL, Rowe P, Rubinstein MH. The effects of compression rate and force on the compaction properties of different viscosity grades of hydroxypropyl methylcellulose 2208. Int J Pharm. 1996;129:21–31. doi: 10.1016/0378-5173(95)04236-9. [DOI] [Google Scholar]
- 45.Liew CV, Chan LW, Ching AL, Heng PWS. Evaluation of sodium alginate as drug release modifier in matrix tablets. Int J Pharm. 2006;309:25–37. doi: 10.1016/j.ijpharm.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 46.Shah NH, Lazarus JH, Jarwoski CL. Carboxymethylcellulose: effect of degree of polymerization and substitution on tablet disintegration and dissolution. J Pharm Sci. 1981;70:611–613. doi: 10.1002/jps.2600700609. [DOI] [PubMed] [Google Scholar]
- 47.Hussain AS, Johnson RD, Shivanand P, Zoglio MA. Effects of blending a non-ionic and an anionic cellulose ether polymer on drug release from hydrophilic matrix capsules. Drug Dev Ind Pharm. 1994;20:2645–2657. doi: 10.3109/03639049409042668. [DOI] [Google Scholar]
- 48.Dabbagh MA, Ford JL, Rubinstein MH, Hogan JE, Rajabi-Siahboomi AR. Release for propranolol hydrochloride from matrix tablets containing sodium carboxymethylcellulose and hydropropylmethylcellulose. Pharm Dev Technol. 1999;4:313–324. doi: 10.1081/PDT-100101367. [DOI] [PubMed] [Google Scholar]
- 49.Oren PL, Seidler WMK. Sustained release matrix. 1990; US patent no. 4,968,508.
- 50.Rogers V, Dor PJM, Fix JA, Kojima H, Sako K. Soluble drug extended release system, 2004; United States patent, US 2004/0091518 A1, 1–14.
- 51.Takka S, Rajbhandari S, Sakr A. Effect of anionic polymers on the release of propranolol hydrochloride from matrix tablets. Eur J Pharm Biopharm. 2001;52:75–82. doi: 10.1016/S0939-6411(01)00147-3. [DOI] [PubMed] [Google Scholar]
- 52.Tucker IG, Ahmed GH, Stewart PJ. The binding of propranolol hydrochloride to sodium carboxymethylcellulose. Aust J Hosp Pharm. 1988;18:196–199. [Google Scholar]
- 53.Walker CV, Wells JI. Rheological synergism between ionic and non-ionic cellulose gums. Int J Pharm. 1982;11:309–322. doi: 10.1016/0378-5173(82)90081-3. [DOI] [Google Scholar]
- 54.Sriwongjanya M, Bodmeier R. Effect of ion exchange resins on the drug release from matrix tablets. Euro J Pharm Biopharm. 1998;46:321–327. doi: 10.1016/S0939-6411(98)00056-3. [DOI] [PubMed] [Google Scholar]