Abstract
The kinetics of photolysis of ascorbic acid in cream formulations on UV irradiation has been studied using a specific spectrophotometric method with a reproducibility of ±5%. The apparent first-order rate constants (kobs) for the photolysis of ascorbic acid in creams have been determined. The photoproducts formed in the cream formulations include dehydroascorbic acid and 2,3-diketogulonic acid. The photolysis of ascorbic acid appears to be affected by the concentration of active ingredient, pH, and viscosity of the medium and formulation characteristics. The study indicates that the ionized state and redox potentials of ascorbic acid are important factors in the photostability of the vitamin in cream formulations. The viscosity of the humectant present in the creams appears to influence the photostability of ascorbic acid. The results show that the physical stability of the creams is an important factor in the stabilization of the vitamin. In the cream formulations stored in the dark, ascorbic acid undergoes aerobic oxidation and the degradation is affected by similar factors as indicated in the photolysis reactions. The rate of oxidative degradation in the dark is about seventy times slower than that observed in the presence of light.
KEY WORDS: ascorbic acid, cream formulations, kinetics, photostability, spectrophotometric method
INTRODUCTION
Ascorbic acid (vitamin C) is an essential micronutrient that performs important metabolic functions (1). It is sensitive to air and light (2,3) and is degraded by chemical (4) and photochemical oxidation (5–10). Ascorbic acid is an ingredient of anti-aging cosmetic products (11–15) and exerts several functions on the skin as collagen synthesis, depigmentation, and antioxidant activity (16). As an antioxidant it protects skin by neutralizing reactive oxygen species generated on exposure to sunlight (17). In biological systems it reduces both oxygen- and nitrogen-based free radicals (18) and thus delays the aging process. In view of the instability of ascorbic acid in skin care formulations (19), it is often used in combination with another redox partner such as alpha-tocopherol (vitamin E) to retard its oxidation (20). The methods of testing the photostability of dermal preparations have been described by Thoma and Spilgies (21). The present work has been undertaken to study the photolysis of ascorbic acid in cream formulations to evaluate the kinetics of the system under various conditions such as the concentration of active ingredient, pH, and viscosity of the medium and redox potentials of ascorbic acid. The study of the effect of formulation ingredients such as the emulsifiers and humectants on the photolysis of ascorbic acid may provide information to improve the stability of ascorbic acid in cream formulations on exposure to light.
MATERIALS AND METHODS
Ascorbic acid (AH2) and dehydroascorbic acid (DHA) were obtained from Sigma Chemical Co., St. Louis, MD. 2,3-Diketogulonic acid (DGA) was prepared by the method of Homann and Gaffron (22); Rf 0.065 (solvent system C mentioned under thin-layer chromatography (TLC)); UV (pH 7.0, 0.2 M phosphate buffer); λmax 290 nm. All the formulation ingredients, reagents, and solvents were of the purest form available from Merck and Co., Whitehouse Station, NJ.
Cream Formulations
On the basis of various skin care formulations reported in the literature (23–25), the following basic formula was used for the preparation of oil-in-water creams containing AH2:
| Oil phase | Percentage (w/w) | |
| Emulsifier | Myristic/palmitic/stearic acid | 12.0 |
| Cetyl alcohol | 3.0 | |
| Aqueous phase | ||
| Active ingredient | Ascorbic acid | 2.0 |
| Humectant | Ethylene glycol/propylene glycol/glycerin | 5.0 |
| Neutralizer | Potassium hydroxide | 1.0 |
| Continuous phase | Distilled water | Q.S. |
The details of the various cream formulations used in this study are given in Table I.
Table I.
Composition of Cream Formulations Containing Ascorbic Acid
| Cream number | pH | Ingredients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SA | PA | MA | CA | AH2 | GL | PG | EG | PH | DW | |||
| 1 | a | 4 | + | − | − | + | + | + | − | − | + | + |
| b | 5 | + | − | − | + | + | + | − | − | + | + | |
| c | 6 | + | − | − | + | + | + | − | − | + | + | |
| d | 7 | + | − | − | + | + | + | − | − | + | + | |
| 2 | a | 4 | − | + | − | + | + | + | − | − | + | + |
| b | 5 | − | + | − | + | + | + | − | − | + | + | |
| c | 6 | − | + | − | + | + | + | − | − | + | + | |
| d | 7 | − | + | − | + | + | + | − | − | + | + | |
| 3 | a | 4 | − | − | + | + | + | + | − | − | + | + |
| b | 5 | − | − | + | + | + | + | − | − | + | + | |
| c | 6 | − | − | + | + | + | + | − | − | + | + | |
| d | 7 | − | − | + | + | + | + | − | − | + | + | |
| 4 | a | 4 | + | − | − | + | + | − | + | − | + | + |
| b | 5 | + | − | − | + | + | − | + | − | + | + | |
| c | 6 | + | − | − | + | + | − | + | − | + | + | |
| d | 7 | + | − | − | + | + | − | + | − | + | + | |
| 5 | a | 4 | − | + | − | + | + | − | + | − | + | + |
| b | 5 | − | + | − | + | + | − | + | − | + | + | |
| c | 6 | − | + | − | + | + | − | + | − | + | + | |
| d | 7 | − | + | − | + | + | − | + | − | + | + | |
| 6 | a | 4 | − | − | + | + | + | − | + | − | + | + |
| b | 5 | − | − | + | + | + | − | + | − | + | + | |
| c | 6 | − | − | + | + | + | − | + | − | + | + | |
| d | 7 | − | − | + | + | + | − | + | − | + | + | |
| 7 | a | 4 | + | − | − | + | + | − | − | + | + | + |
| b | 5 | + | − | − | + | + | − | − | + | + | + | |
| c | 6 | + | − | − | + | + | − | − | + | + | + | |
| d | 7 | + | − | − | + | + | − | − | + | + | + | |
| 8 | a | 4 | − | + | − | + | + | − | − | + | + | + |
| b | 5 | − | + | − | + | + | − | − | + | + | + | |
| c | 6 | − | + | − | + | + | − | − | + | + | + | |
| d | 7 | − | + | − | + | + | − | − | + | + | + | |
| 9 | a | 4 | − | − | + | + | + | − | − | + | + | + |
| b | 5 | − | − | + | + | + | − | − | + | + | + | |
| c | 6 | − | − | + | + | + | − | − | + | + | + | |
| d | 7 | − | − | + | + | + | − | − | + | + | + | |
SA stearic acid, PA palmitic acid, MA myristic acid, CA cetyl alcohol, AH 2 ascorbic acid, GL glycerin, PG propylene glycol, EG ethylene glycol, PH potassium hydroxide, DW distilled water
Preparation of Creams
The emulsifiers were melted at 70–80°C in a glass jar immersed in a water bath. AH2 was separately dissolved in a small portion of distilled water. Potassium hydroxide and humectants were dissolved in the remaining portion of water and mixed with the oily phase with constant stirring until the formation of a thick white mass. It was cooled to ~40°C and the AH2 solution was added. The thick mass was mixed using a mechanical mixer with a glass stirrer at 1,000 rpm for 5 min. The pH of the cream was adjusted to the desired value and the contents again mixed for 10 min at 500 rpm. All the creams were prepared under uniform conditions to maintain their individual physical characteristics and stored at room temperature in airtight glass containers for a period of 3 months in the dark.
pH Measurements
The pH measurements were carried out with an Elmetron LCD display pH meter (model–CP501, sensitivity ± 0.01 pH units, Poland) using a combination pH electrode. The pH of the cream formulations was maintained in the range of 4.0–7.0 with H3PO4/NaOH solution.
Photolysis
A 2-g quantity of the cream was uniformly spread on several rectangular glass plates (5 × 15 cm) covered with a 1-cm tape on each side to give a 1-mm-thick layer. The plates were irradiated in a dark chamber under constant temperature and humidity (25 ± 1°C/RH 60%) using a Philips 30 W TUV tube (100% emission at 254 nm, the wavelength absorbed by AH2 at pH 4–7), fixed horizontally at a distance of 30 cm from the center of the plates. Each plate was removed at appropriate interval and the cream was subjected to spectrophotometric assay and chromatographic examination.
Thin-Layer Chromatography
The photolysed creams containing AH2 and photoproducts were extracted with methanol and subjected to TLC using 250-μm silica gel GF254 plates (Merck) and the solvent systems: A, acetic acid–acetone–methanol–benzene (5:5:20:70, v/v) (26); B, ethanol–10% acetic acid (90:10, v/v) (27); and C, acetonitrile-butyl nitrile-water, (66:33:2, v/v) (28). The spots were detected under UV light (254 nm) (AH2) or by spraying with a 3% aqueous phenylhydrazine hydrochloride solution (DHA, DGA).
Spectral Measurements
All spectral measurements on methanolic extracts of freshly prepared/photolysed creams were carried out on a Shimadzu UV–1601 recording spectrophotometer using quartz cells of 10-mm-path length.
Light Intensity Measurements
The intensity of the Philips 30 W TUV tube was determined by potassium ferrioxalate actinometry (29) as 5.56 ± 0.12 × 1018 quanta s–1.
Assay Method
The photolysed cream was completely removed from the glass plate and transferred to a volumetric flask. The AH2 content was extracted with methanol (3 × 10 ml), the pH of combined methanolic solutions was adjusted to 2.0 (with H3PO4) and the volume made up to 100 ml. A 1-ml aliquot of the solution was diluted to 20 ml with acidified methanol (pH 2.0) and the absorbance was measured at 245 nm using an appropriate blank. A representative standard curve of absorbance versus concentration in the range 0.1–1.0 × 10–4 M resulted in the following linear least-squares regression equation: y = 0.9920x + 0.0012; r2 = 0.9996.
RESULTS AND DISCUSSION
Photoproducts of Ascorbic Acid in Creams
The formation of degradation products on the UV photolysis of AH2 in various creams (pH 4–7) has been studied by TLC and spectrophotometry. All the formulations showed the presence of DHA on detection by TLC along with AH2 using the solvent systems A, B, and C. However, DGA, a hydrolysis product of DHA (22) was only detected at pH 6 and 7. It appears that in the cream media at relatively acidic pH of 4 and 5, this compound is not formed. The identification of DHA was carried out by comparison of the Rf value and spot color with those of the authentic compound. The intensity of the spots of methanolic extracts of the photolysed creams shows that the amounts of DHA and DGA formed in various samples subjected to an equally irradiated time differ. This could be due to a difference in the rate of photolysis of AH2 in the creams depending on the nature of the formulation ingredients and factors such as pH and viscosity. It has been observed that the rate of formation of DHA and DGA is greater in the creams containing myristic acid as the emulsifier and ethylene glycol as the humectant compared to those containing stearic/palmitic acids and propylene glycol/glycerin. The reason for this pattern of photolysis is discussed under the effects of formulation characteristics and viscosity of the creams and carbon chain length of the emulsifying agents. The formation of DHA and DGA on photooxidation of AH2 solutions has previously been reported (22,30,31).
Spectral Characteristics of Photolysed Creams
The UV absorption spectra of the methanolic extracts of AH2 in photolysed creams show a gradual loss of absorbance around 245 nm, with time, as a result of the oxidation of the molecule to DHA (32,33) which does not absorb in this region due to the loss of conjugation. However, the magnitude of these changes varies with the change in the rate of photolysis of AH2 in a particular cream and appears to be a function of the polar character, pH, and viscosity of the creams. The absorbance loss at 245 nm is greater in the creams containing myristic acid and ethylene glycol.
Assay of Ascorbic Acid in Creams
The assay of AH2 in creams has been carried out in acidified methanol (pH 2.0) according to the UV spectrophotometric method of Zeng et al. (34). Aqueous solutions of AH2 (~pH 2) exhibit absorption maxima at 243 nm (2,35,36), 244 nm (37), and 245 nm (1,38). The absorption maxima of AH2 in methanol and phosphate buffer (pH 2.5) occur at 245 nm (34). Since dilute solutions of AH2 are highly susceptible to oxidation, the pH of the solutions were adjusted to 2.0 with phosphoric acid to maintain the molecule in the non-ionized form (99%) and to minimize degradation during the assay.
The UV method of Zeng et al. (34) was originally used for the analysis of ascorbic acid in aqueous solution. It was, therefore, validated before its application to the assay of AH2 in photolysed creams. The reproducibility of the method was confirmed by the analysis of known amounts of AH2 in the concentration range likely to be found in the photolysed creams. The values of the recoveries of AH2 in creams by the UV spectrophotometric method are in the range of 90–96%. The values of RSD for the assays indicate the precision of the method within ±5% (Table II). The analytical data show gradually decreasing concentrations of AH2, with time, in photolysed creams indicating the accuracy of the assay method. This is also evident from the linearity of kinetic plots for the photolysis reactions.
Table II.
Recovery of Ascorbic Acid Added to Cream Formulations
| Cream formulationa | Added (mg%) | Found (mg%) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1a | 40.0 | 38.0 | 95.0 | 2.1 |
| 20.0 | 18.3 | 91.5 | 2.5 | |
| 2b | 40.0 | 37.1 | 92.8 | 1.5 |
| 20.0 | 18.5 | 92.5 | 2.5 | |
| 3c | 40.0 | 37.5 | 93.8 | 1.1 |
| 20.0 | 18.1 | 90.5 | 3.1 | |
| 4d | 40.0 | 38.4 | 96.0 | 1.3 |
| 20.0 | 18.9 | 94.5 | 2.1 | |
| 5b | 40.0 | 37.0 | 92.5 | 1.4 |
| 20.0 | 18.9 | 94.5 | 2.6 | |
| 6c | 40.0 | 36.9 | 92.3 | 1.0 |
| 20.0 | 19.0 | 95.0 | 2.2 | |
| 7d | 40.0 | 37.4 | 93.5 | 1.7 |
| 20.0 | 18.2 | 91.0 | 3.9 | |
| 8c | 40.0 | 38.0 | 95.0 | 1.5 |
| 20.0 | 18.8 | 94.0 | 3.3 | |
| 9d | 40.0 | 36.7 | 91.8 | 2.0 |
| 20.0 | 18.9 | 94.5 | 4.2 |
Values expressed as a mean of three to five determinations
aThe cream formulations represent combinations of each emulsifier (stearic acid, palmitic acid, myristic acid) with each humectant (glycerin, propylene glycol, ethylene glycol) to observe the efficiency of methanol to extract AH2 from different creams (Table I)
Kinetics of Photolysis
The photolysis of AH2 in various creams at pH 4–7 was found to follow first-order kinetics and the apparent first-order rate constants (kobs) are reported in Table III. The oxidative degradation of AH2 also occurs by first-order kinetics (3). The effects of formulation characteristics, concentration, carbon chain length of emulsifier, viscosity, and pH of the medium and redox potentials of AH2 on the kinetics of photolysis are discussed in the following sections.
Table III.
First-Order Rate Constants (k obs) for the Degradation of Ascorbic Acid in Cream Formulations in Light and Dark
| Cream formulation | Light, k obs × 103, min–1a,b,c, ±SD | Dark, k obs × 102, day–1a,b,c, ±SD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | 4.0 | 5.0 | 6.0 | 7.0 | 4.0 | 5.0 | 6.0 | 7.0 | |
| 1 | 0.44 ± 0.039 | 0.64 ± 0.062 | 1.00 ± 0.088 | 1.29 ± 0.120 | 1.28 ± 0.115 | 1.52 ± 0.099 | 1.91 ± 0.096 | 2.20 ± 0.132 | |
| 2 | 0.42 ± 0.036 | 0.60 ± 0.059 | 0.95 ± 0.090 | 1.20 ± 0.108 | 0.91 ± 0.055 | 1.10 ± 0.069 | 1.52 ± 0.097 | 1.82 ± 0.097 | |
| 3 | 0.47 ± 0.042 | 0.69 ± 0.066 | 1.07 ± 0.098 | 1.37 ± 0.106 | 1.48 ± 0.092 | 1.76 ± 0.097 | 2.20 ± 0.153 | 2.54 ± 0.152 | |
| 4 | 0.56 ± 0.050 | 0.72 ± 0.069 | 1.04 ± 0.097 | 1.31 ± 0.101 | 1.37 ± 0.099 | 1.61 ± 0.129 | 2.05 ± 0.123 | 2.36 ± 0.165 | |
| 5 | 0.50 ± 0.050 | 0.67 ± 0.064 | 0.97 ± 0.087 | 1.24 ± 0.111 | 1.21 ± 0.085 | 1.41 ± 0.080 | 1.75 ± 0.132 | 1.95 ± 0.105 | |
| 6 | 0.61 ± 0.059 | 0.79 ± 0.065 | 1.13 ± 0.105 | 1.40 ± 0.138 | 1.62 ± 0.105 | 1.94 ± 0.142 | 2.37 ± 0.190 | 2.65 ± 0.188 | |
| 7 | 0.60 ± 0.057 | 0.71 ± 0.067 | 1.08 ± 0.105 | 1.33 ± 0.099 | 1.64 ± 0.095 | 1.89 ± 0.132 | 2.22 ± 0.164 | 2.46 ± 0.145 | |
| 8 | 0.53 ± 0.048 | 0.62 ± 0.055 | 0.99 ± 0.076 | 1.26 ± 0.103 | 1.43 ± 0.099 | 1.67 ± 0.127 | 1.93 ± 0.149 | 2.12 ± 0.142 | |
| 9 | 0.65 ± 0.062 | 0.81 ± 0.080 | 1.17 ± 0.074 | 1.43 ± 0.112 | 1.84 ± 0.149 | 2.08 ± 0.162 | 2.51 ± 0.203 | 2.80 ± 0.178 | |
aThe rate constants at pH 4.0–7.0 represent the values for formulations a to d of each cream, respectively
bThe values of rate constants are relative and depend on specific experimental conditions including light intensity
c n = 3
Effect of Formulation Characteristics
The formulation characteristics play an important role in the stability of a drug in a product. It has been observed by various tests that palmitic acid as an emulsifier imparts better formulation characteristics such as consistency, uniformity, and compatibility (39) to enhance the stability of the product compared with the other emulsifiers. In such a medium, there is a greater possibility of achieving stabilization of the active ingredient. Therefore, AH2 has been found to be more stable in the presence of palmitic acid in the creams studied. The stabilization of proteins by palmitic acid has been reported (40,41). The creams containing myristic acid showed phase separation. It was observed visually and occurred to the extent of 4–5%. This could be due to the lower viscosity and shorter hydrocarbon chain length of the emulsifier compared with those of the other creams. The emulsifiers with relatively long hydrocarbon chains are reported to produce stable creams (42). The creams containing stearic acid became a little hard during storage. Stearic acid has been reported to possess the properties of a hardening agent and has shown evidence of drying out (43). This property of the emulsifier might have caused increase in the viscosity of creams resulting in hardening. This is specially true for creams containing glycerin as a humectant (43). However, no phase separation was observed in this case. The creams containing palmitic acid retained their original characteristics better than those containing the other emulsifiers. One of the reasons of greater stability of AH2 in the presence of palmitic acid is that it is compatible with reducing agents and thus prevents oxidation of AH2 whereas stearic acid is not compatible with reducing agents (43). The physical stability of a formulation is an important factor in the stabilization of an active ingredient (44).
Effect of Concentration
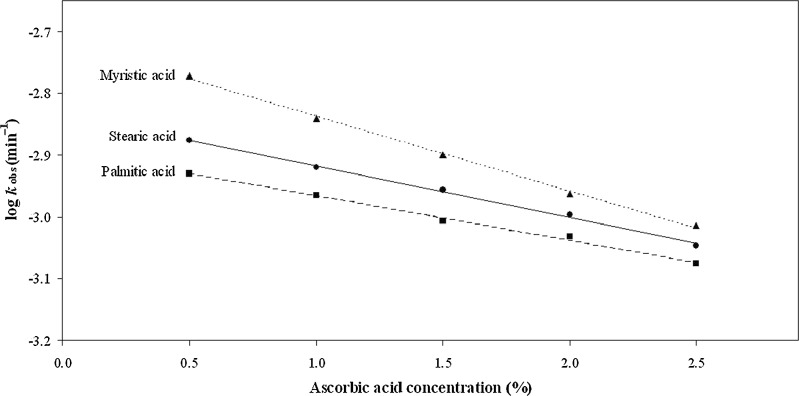
In order to observe the effect of concentration on the photolysis of AH2 in a cream containing different emulsifiers and glycerin as humectant, a plot of log kobs against percentage of concentration of AH2 was constructed which exhibited an apparent linear relationship between the two values (Fig. 1). Thus the rate of degradation of AH2 appears to be faster at a lower concentration on exposure to the same intensity of light. This may be due to a relatively greater number of photons available for excitation of the molecule at lower concentration compared to that at a higher concentration. The AH2 concentrations of creams used in this study are within the range (1–15%) reported by previous workers for topical applications to skin (13–15).
Fig. 1.
A plot of log k obs for photolysis against ascorbic acid concentrations in cream formulations containing myristic, stearic, and palmitic acids
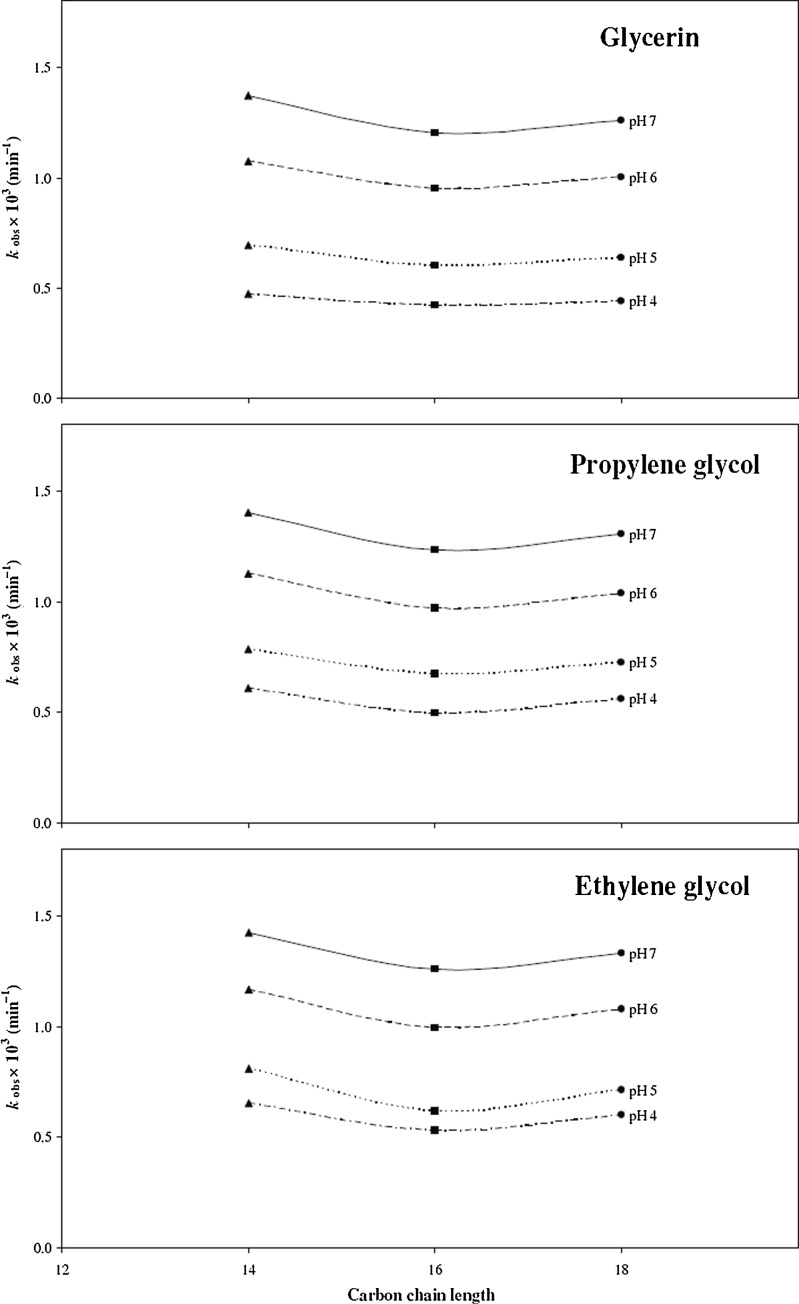
Effect of Hydrocarbon Chain Length of Emulsifiers
In order to observe the effect of hydrocarbon chain length of the emulsifiers on the photolysis of AH2 in various cream formulations, plots of kobs against the hydrocarbon chain length of emulsifiers were constructed (Fig. 2). It appeared that the photolysis of AH2 is affected by the emulsifiers in the order of:
myristic acid > stearic acid > palmitic acid.
Fig. 2.
Plots of k obs for photolysis of ascorbic acid in creams (1–9) against carbon chain length of the emulsifier. Stearic acid (black circle); palmitic acid (black square); myristic acid (black triangle). Humectant used: glycerin (1–3); propylene glycol (4–6); ethylene glycol (7–9)
The kinetic results indicate that AH2 exhibits greater stability in the presence of palmitic acid than that observed in the presence of other emulsifiers. However, there is little difference in the values of kobs in formulations 1 and 2 at pH 4.0 (0.44 and 0.42 × 10–3 min–1) and at pH 5.0 (0.64 and 0.60 × 10–3 min–1), respectively. Thus the hydrocarbon chain length effect is not very prominent in these cases and other factors may be involved in stabilization as discussed under the effect ofhumectant. A consideration of kobs obtained for the degradation of AH2 in the dark indicates a significant difference in formulations 1 and 2 at pH 4.0 (1.28 and 0.91 × 10–2 day–1) and at pH 5.0 (1.52 and 1.10 × 10–2 day–1), respectively. These data provide a better indication of the overall greater stability of AH2 in the presence of palmitic acid compared with the other emulsifiers.
Effect of Humectants
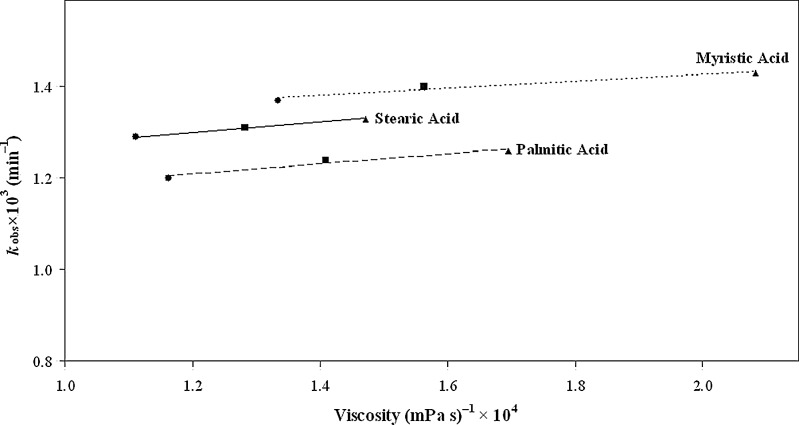
The rate of a chemical reaction may be affected by the viscosity of the medium and this can greatly influence the stability of oxidisable substances (45,46). Plots of kobs for the photolysis of AH2versus inverse of the viscosity of creams containing different humectants (viscosity, mPa s: ethylene glycol, 17.4; propylene glycol, 56.1; 85% glycerine, 109.0) (47) in combination with the individual emulsifier have been found to be linear (Fig. 3). Thus an increase in the cream viscosity leads to a decrease in the rate of photolysis of AH2. The plots indicate that for each combination the rates are affected by the magnitude of the viscosity. The highest rates are observed with myristic acid (lowest viscosity range), followed by those in the presence of stearic acid (highest viscosity range). A combination of humectants with palmitic acid shows the lowest rates of photolysis. A similar effect of palmitic acid on rates in the presence of different humectants has been observed (Fig. 2) and discussed under the effect of hydrocarbon chain length of emulsifiers.
Fig. 3.
Plots of k obs versus inverse of viscosity in creams containing: glycerin (black circle); propylene glycol (black square); and ethylene glycol (black triangle) as humectants with different emulsifiers
The viscosity of the medium affects the rate at which molecules can diffuse through the solution. This, in turn, may affect the rate at which a compound can suffer oxidation at the liquid surface. This applies to AH2 and an increase in the viscosity of the medium makes access to air at the surface more difficult to prevent oxidation (45). The stabilizing effect of viscosity imparting substances on AH2 solutions has been reported (48).
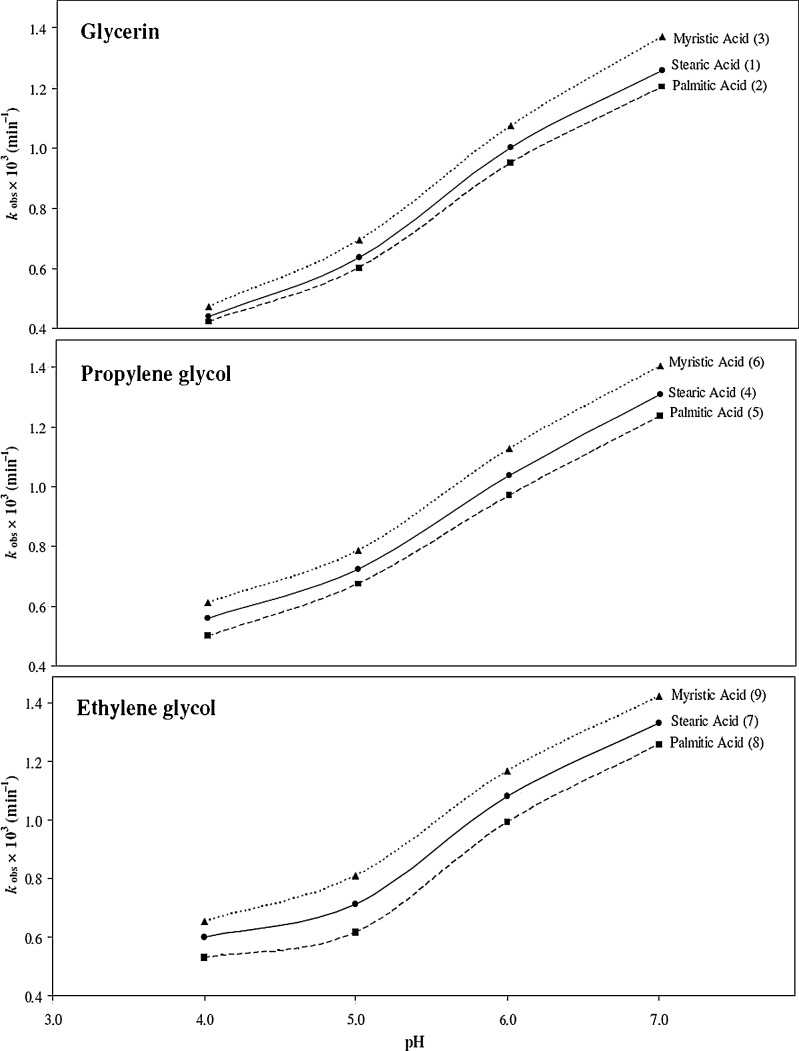
Effect of pH and Redox Potentials
The pH effect on the rate of photolysis of AH2 in some typical creams (4–6) at pH 4–7 (Fig. 4) represents a sigmoid type curve indicating the oxidation of the ionized form (AH−) of AH2 (pKa, 4.1) (35) with pH. The AH− species appears to be more susceptible to photooxidation than the non-ionized form (AH2). The behavior of AH2, on photooxidation in the pH range 4–7, is similar to that observed for the chemical oxidation of AH2 by molecular oxygen (3) and involves the interaction of AH2 with singlet oxygen on UV irradiation (5). The AH− species (predominant in the pH range 4.2–7.0, 55.7–99.9%) is more reactive towards singlet oxygen than its protonated form, the AH2 molecule (49) and, therefore, the rate of photooxidation is higher in the pH range above 4.1 corresponding to the pKa1 of AH2. The major goal of a rate–pH profile is to determine the optimum pH range for a particular formulation. Several workers have studied the rate–pH profiles of the chemical oxidation of AH2 in the pH range 2–7 (3,50); however, the kinetics of photooxidation of AH2 in cream formulations at different pH values has not been reported.
Fig. 4.
Plots of k obs versus pH for the photolysis of ascorbic acid in creams (1–9)
The photooxidation of AH2 is influenced by its redox potential which varies with pH. The greater photostability of AH2 at pH 5–6 compared to that at pH 7 and above is due to its lower rate of oxidation–reduction in this range (E0 pH 5.0 = +0.127 V) (35). The increase in the rate of photooxidation, with pH, is due to a corresponding increase in the redox potential (E0 pH 7.0 = +0.058 V) (51) of AH2 and is similar to the photolysis behavior of riboflavin at pH 5–6 (E0 pH 5.0 = −0.117 V) (52) compared to that at pH 7.0 (E0 pH 7.0 = −0.207 V) (52,53). Since the ionization as well as the redox potentials of AH2 is a function of pH, the rate of photooxidation depends upon the specific species present and its redox behavior at a particular pH. The photolysis of AH2 in creams may involve a polar semiquinone intermediate (1) which, depending on the polar character of the medium, undergoes oxidation with varying rates. This is similar to the behavior of riboflavin analogs on photolysis in various media (54).
Degradation of AH2 in the Dark
In view of the photolysis of AH2 in creams, a comparative study has been carried out to observe its degradation in the dark. The apparent first-order rate constants for the degradation of AH2 in the dark are reported in Table III. The values of these rate constants indicate that the degradation of AH2 in the dark is about 70 times slower than those of the creams exposed to UV irradiation (Table III). The degradation of AH2 in creams in the dark is due to chemical oxidation (3,4) and occurs in the order of emulsifier:
myristic acid > stearic acid > palmitic acid.
It appears that palmitic acid exerts a stabilizing effect against chemical oxidation of AH2 in the dark. This could result from its compatibility with reducing agents as discussed in the section on “Effect of Formulation Characteristics”.
The rate of degradation of AH2 also appears to be affected by the viscosity of the cream in the order of humectant:
ethylene glycol > propylene glycol > glycerin.
Thus the presence of glycerin in creams imparts the most stabilizing effect on the degradation of AH2. This is the same order as observed in the case of the photolysis of AH2 in creams as discussed under “Effect of Humectants”. The airtight containers used for the storage of creams make the access of air to the creams difficult to cause chemical oxidation of AH2. However, in the presence of some air, it has been observed that the degradation of AH2 is highest in the upper layer of the creams compared to that of the middle and the bottom layers.
The effect of pH on the degradation of AH2 in the creams shows that the degradation increases with an increase in pH as observed in the case of the photolysis of AH2 in creams. This is due to an increase in the ionization and redox potentials of AH2, with pH, causing greater oxidation of the molecule. However, the difference between the rate of degradation at pH 4 and 7 is less than that observed in the presence of light (Table III). This could be due to the effect of photooxidation of AH2 compared to the aerobic oxidation as evident from the magnitude of the rate constants under the light and dark reactions.
CONCLUSION
The present work shows that the rate of photolysis of ascorbic acid in cream formulations on UV irradiation is affected by concentration, viscosity of the medium, pH, and redox potentials of ascorbic acid as well as the formulation characteristics of creams. An increase in the rate of photolysis, with pH as well as the redox potentials, in cream formulations is due to the gradual ionization of ascorbic acid. Ascorbic acid exhibits maximum photostability in the creams at pH 4 in all the formulations. The main species involved as an intermediate in the photolysis of ascorbic acid at pH 4 and above is the monohydrogen ascorbate anion (AH−) which is oxidized at a higher rate with an increase in the pH of the medium. The photoproducts of ascorbic acid detected in the cream formulations are dehydroascorbic acid and 2,3-diketogulonic acid. The degradation of ascorbic acid in the dark is also affected by the factors mentioned above and is much slower than that observed in the presence of light. Palmitic acid has been observed to exert a stabilizing effect against degradation of the vitamin in creams.
REFERENCES
- 1.Johnston CS, Steinberg FM, Rucker RB. Ascorbic acid. In: Zempleni J, Rucker RB, McCormick DB, Suttie JW, editors. Handbook of Vitamins. 4. Boca Raton: CRC Press; 2007. [Google Scholar]
- 2.British Pharmacopoeia. London: Her Majesty's Stationary Office; 2009. Electronic version.
- 3.Blaugh SM, Hajratwala B. Kinetics of aerobic oxidation of ascorbic acid. J Pharm Sci. 1972;61:556–62. doi: 10.1002/jps.2600610412. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y, Zhan X, Ma L, Lin B, Li L, Li C, He N. Compressed oxygen in drug stability experiments. Chem Pharm Bull. 2007;55:87–91. doi: 10.1248/cpb.55.87. [DOI] [PubMed] [Google Scholar]
- 5.Silva E, Quina FH. Photoinduced processes in the eye lens: do flavins really play a role. In: Silva E, Edwards AM, editors. Flavins photochemistry and photobiology. Cambridge: The Royal Society of Chemistry; 2006. [Google Scholar]
- 6.Vaid FHM, Shaikh RH, Ansari IA, Ahmad I. Spectral study of the photolysis of aqueous thiamine hydrochloride and ascorbic acid solution in the presence and absence of riboflavin. J Chem Soc Pak. 2005;27:227–32. [Google Scholar]
- 7.Vaid FHM, Shaikh RH, Ansari IA, Ahmad I. Chromatographic study of the photolysis of aqueous thiamine hydrochloride and ascorbic acid solutions in the presence and absence of riboflavin. J Chem Soc Pak. 2006;28:464–81. [Google Scholar]
- 8.Ahmad I, Sheraz MA, Shaikh RH, Ahmed S, Vaid FHM. Photostability of ascorbic acid in aqueous and organic solvents. J Pharm Res. 2010;3:1237–9. [Google Scholar]
- 9.Sheraz MA, Ahmed S, Ahmad I, Qadeer K, Vaid FHM. Photodegradation and photostabilization of ascorbic acid in pharmaceutical preparations. Int J Chem Anal Sci. 2010;1:68–70. [Google Scholar]
- 10.Sheraz MA, Ahmed S, Ahmad I, Vaid FHM, Iqbal K. Formulation and stability of ascorbic acid in topical preparations. System Rev Pharm. 2011 (in press).
- 11.Gallarate M, Carlotti ME, Trotta M, Bovo S. On the stability of ascorbic acid in emulsified systems for topical and cosmetic use. Int J Pharm. 1999;188:233–41. doi: 10.1016/S0378-5173(99)00228-8. [DOI] [PubMed] [Google Scholar]
- 12.Raschke T, Koop U, Düsing HJ, Filbry A, Sauermann K, Jaspers S, Wenck H, Wittern KP. Topical activity of ascorbic acid: from in vitro optimization to in vivo efficacy. Skin Pharmacol Physiol. 2004;17:200–6. doi: 10.1159/000078824. [DOI] [PubMed] [Google Scholar]
- 13.Placzek M, Gaube S, Kerkmann U, Gilbertz KP, Herzinger T, Haen E, Przybilla B. Ultraviolet B-induced DNA damage in human epidermis is modified by the antioxidants ascorbic acid and D-alpha-tocopherol. J Invest Dermatol. 2005;124:304–7. doi: 10.1111/j.0022-202X.2004.23560.x. [DOI] [PubMed] [Google Scholar]
- 14.Lin FH, Lin JY, Gupta RD, Tournas JA, Burch JA, Selim MA, Monteiro-Riviere NA, Grichnik JM, Zielinski J, Pinnell SR. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J Invest Dermatol. 2005;125:826–32. doi: 10.1111/j.0022-202X.2005.23768.x. [DOI] [PubMed] [Google Scholar]
- 15.Heber GK, Markovic B, Hayes A. An immunohistological study of anhydrous topical ascorbic acid compositions on ex vivo human skin. J Cosmet Dermatol. 2006;5:150–6. doi: 10.1111/j.1473-2165.2006.00242.x. [DOI] [PubMed] [Google Scholar]
- 16.Nusgens BV, Humbert P, Rougier A, Colige AC, Haftek M, Lambert CA, Richard A, Creidi P, Lapière CM. Topically applied vitamin C enhances the mRNA level of collagens I and III their processing enzymes and tissue inhibitor of matrix metalloproteinase 1 in the human dermis. J Invest Dermatol. 2001;116:853–9. doi: 10.1046/j.0022-202x.2001.01362.x. [DOI] [PubMed] [Google Scholar]
- 17.Shindo Y, Witt E, Han D, Packer L. Dose-response effect of acute ultraviolet irradiation on antioxidants and molecular markers of oxidation in murine epidermis and dermis. J Invest Dermatol. 1994;102:470–5. doi: 10.1111/1523-1747.ep12373027. [DOI] [PubMed] [Google Scholar]
- 18.Higdon JV, Frei B. The antioxidant vitamin C and E. Arlington: AOAC Press; 2002. pp. 1–16. [Google Scholar]
- 19.Bissett DL. Anti-aging skin care formulations. In: Draelos ZD, Thaman LA, editors. Cosmetic Formulation of Skin Care Products. New York: Taylor & Francis Group; 2006. [Google Scholar]
- 20.Wille JJ. Thixogel-novel topical delivery systems for hydrophobic plant actives. In: Rosen MR, editor. Delivery system handbook for personal care and cosmetic products-technology, applications and formulations. Norwich: William Andrew Inc; 2005. [Google Scholar]
- 21.Thoma K, Spilgies H. Photostabilization of solid and semisolid dosage forms. In: Piechocki JT, Thoma K, editors. Pharmaceutical photostability and stabilization technology. New York: Informa Healthcare; 2007. [Google Scholar]
- 22.Homann P, Gaffron H. Photochemistry and metal catalyzes: studies in a flavin sensitized oxidation of ascorbic acid. Photochem Photobiol. 1964;3:499–515. doi: 10.1111/j.1751-1097.1964.tb08170.x. [DOI] [Google Scholar]
- 23.Betageri G, Prabhu S. Semisolid preparations. In: Swarbrick J, Boylan JC, editors. Encyclopedia of pharmaceutical technology. 2. New York: Marcel Dekker; 2002. pp. 2436–57. [Google Scholar]
- 24.Flynn GL. Cutaneous and transdermal delivery-processes and systems of delivery. In: Banker GS, Rhodes CT, editors. Modern pharmaceutics. New York: Marcel Dekker; 2002. [Google Scholar]
- 25.Lu GW, Flynn GL. Cutaneous and transdermal delivery-processes and systems of delivery. In: Florence AT, Siepmann J, editors. Modern pharmaceutics-applications and advances. Vol. 2. 5. New York: Informa Healthcare Inc; 2009. [Google Scholar]
- 26.Ganshirt H, Malzacher A. Separation of several vitamins of the B group and C by chromatography. Naturwiss. 1960;47:279–80. doi: 10.1007/BF01210760. [DOI] [Google Scholar]
- 27.Bolliger HR, Konig A. Water-soluble vitamins. In: Stahl E, editor. Thin-layer chromatography. Berlin: Springer; 1969. pp. 304–6. [Google Scholar]
- 28.Saari JC, Baker EM, Sauberlich HE. Thin-layer chromatographic separation of the oxidative degradation products of ascorbic acid. Anal Biochem. 1967;18:173–7. doi: 10.1016/0003-2697(67)90069-3. [DOI] [Google Scholar]
- 29.Hatchard CG, Parker CA. A new sensitive chemical actinometer II Potassium ferrioxalate as a standard chemical actinometer. Proc R Soc London Ser A. 1956;A235:518–36. [Google Scholar]
- 30.Heelis PF, Parsons BJ, Phillips GO, McKellar JF. The flavin sensitized photooxidation of ascorbic acid: a continuous and flash photolysis study. Photochem Photobiol. 1981;37:7–13. doi: 10.1111/j.1751-1097.1981.tb04289.x. [DOI] [Google Scholar]
- 31.Lavoie JC, Chessex P, Rouleau T, Migneault D, Comte B. Light-induced byproducts of vitamin C in multivitamin solutions. Clin Chem. 2004;50:135–40. doi: 10.1373/clinchem.2003.025338. [DOI] [PubMed] [Google Scholar]
- 32.Davies MB, Austin J, Partridge DA. Vitamin C: its chemistry and biochemistry. Cambridge: The Royal Society of Chemistry; 1991. [Google Scholar]
- 33.Rumsey SC, Levine M. Vitamin C. In: Song WO, Beecher GR, Eitenmiller RR, editors. Modern analytical methodologies in fat- and water-soluble vitamins. New York: Wiley; 2000. [Google Scholar]
- 34.Zeng W, Martinuzzi F, MacGregor A. Development and application of a novel UV method for the analysis of ascorbic acid. J Pharm Biomed Anal. 2005;36:1107–11. doi: 10.1016/j.jpba.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 35.O'Neil MJ, editor. The Merck Index. 13th ed. Rahway NJ, USA: Merck & Co Inc.; 2001; Electronic version.
- 36.Moffat AC, Osselton MD, Widdop B. Clarke's analysis of drugs and poisons. 3. London: Pharmaceutical Press; 2004. pp. 649–50. [Google Scholar]
- 37.Ogata Y, Kosugi Y. Solvent effect on the autooxidation of L-ascorbic acid. Tetrahedron. 1969;25:1055–62. doi: 10.1016/S0040-4020(01)82678-8. [DOI] [PubMed] [Google Scholar]
- 38.Verma KK, Jain A, Verma A, Chaurasia A. Spectrophotometric determination of ascorbic acid in pharmaceuticals by background correction and flow injection. Analyst. 1991;116:641–5. doi: 10.1039/an9911600641. [DOI] [PubMed] [Google Scholar]
- 39.Billany M. Suspensions and emulsions. In: Aulton ME, editor. Pharmaceutics: the science of dosage form design. 2. Philadelphia: Churchill Livingstone; 2002. [Google Scholar]
- 40.Saso L, Valentini G, Mattei E, Panzironi C, Casini ML, Grippa E, Silvestrini B. Stabilization of rat serum proteins following oral administration of fish oil. Arch Pharm Res. 1999;22:485–90. doi: 10.1007/BF02979157. [DOI] [PubMed] [Google Scholar]
- 41.Aucamp JP, Cosme AM, Lye GJ, Dalby PA. High-throughput measurement of protein stability in microtiter plates. Biotechnol Bioeng. 2005;89:599–607. doi: 10.1002/bit.20397. [DOI] [PubMed] [Google Scholar]
- 42.Kim CJ. Surface chemistry and colloids. In: Advanced pharmaceutics, physicochemical principles. CRC Press: Boca Raton; 2004. Chap. 4.
- 43.Rowe RC, Sheskey PJ, Quinn ME. Handbook of pharmaceutical excipients. 6. London: Pharmaceutical Press; 2009. [Google Scholar]
- 44.Im-Esap W, Siepmann J. Disperse systems. In: Banker GS, Rhodes CT, editors. Modern pharmaceutics. New York: Marcel Dekker; 2002. [Google Scholar]
- 45.Wallwork SC, Grant DJW. Physical chemistry for students of pharmacy and biology. 3. New York: Longmann; 1977. pp. 502–5. [Google Scholar]
- 46.Laidler KJ. Chemical kinetics. 3. New York: Harper and Row; 1987. pp. 183–5. [Google Scholar]
- 47.Flick EW. Industrial solvents handbook. 5. New Jersey: Noyes Data Corporation; 1998. pp. 359–74. [Google Scholar]
- 48.Kassem MA, Kassem AA, Ammar HO. Studies on the stability of injectable L-ascorbic acid solutions I Effect of pH solvent light and container. Pharm Acta Helv. 1969;44:611–23. [PubMed] [Google Scholar]
- 49.Bisby RH, Morgan CG, Hamblett I, Gorman AA. Quenching of singlet oxygen by trolox C ascorbate and amino acids: effect of pH and temperature. J Phys Chem. 1999;103:7454–9. [Google Scholar]
- 50.Moura T, Gaudy D, Jacob M, Cassanas G. pH influence on the stability of ascorbic acid spray-drying solutions. Pharm Acta Helv. 1994;69:77–80. [Google Scholar]
- 51.Fasman GD, editor. CRC handbook of biochemistry and molecular biology. 3 rd ed. Physical Chemical Data, Vol. 1, Ohio: CRC Press Cleveland; 1976. pp. 122–30.
- 52.Sinko PJ. Chemical kinetics and stability. Martin's physical pharmacy and pharmaceutical sciences. 5th ed. Baltimore: Lippincott Williams & Wilkins; 2006. Chap 8, 15, 18.
- 53.Ahmad I, Fasihullah Q, Noor A, Ansari IA, Ali QNM. Photolysis of riboflavin in aqueous solution: a kinetic study. Int J Pharm. 2004;280:199–208. doi: 10.1016/j.ijpharm.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 54.Ahmad I, Tollin G. Solvent effect on flavin electron transfer reactions. Biochemistry. 1981;20:5925–8. doi: 10.1021/bi00523a042. [DOI] [PubMed] [Google Scholar]