Abstract
The aim of this study was to gain better insight into molecular changes which reflect disturbances in the balance between proliferation and apoptosis during progression of thyroid malignancy from papillary microcarcinoma (PMC) via clinically manifest papillary carcinoma (PTC) to anaplastic carcinoma (ATC). The apoptosis related molecules (Bcl-2, Bax) and proliferation related marker (PCNA) were analysed immunohistochemically in 120 archival cases comprising PMC (n=34), PTC (n=52) and ATC (n=34). In addition, in situ apoptotic cell death was analysed by the TUNEL method. The average Bcl-2 staining score did not differ between PMC and PTC (p>0.05), but was significantly lower in ATC (p<0.05).The Bax score was higher in PTCs and ATCs than in PMCs (p<0.05). Due to these changes, the Bcl-2/Bax ratio showed a marked decrease from PMC to ATC (p<0.05), while proliferation activity increased significantly from PTC to ATC (p<0.05). Despite high Bax expression, the rate of apoptotic cell death was low in the investigated carcinomas, especially in ATC, i.e. the increase in proliferative activity was not counterbalanced with appropriate cell death. Differences were found in the expression of apoptotic molecules (Bcl-2 and Bax), their ratio (Bcl-2 /Bax) and in the rate of apoptotic cell death and proliferative activity between PMC, PTC and ATC, indicating that disturbances in the balance between apoptosis and proliferation, in favour of the latter, occur gradually during the progression of malignancy in thyroid tumours.
Key words: thyroid, papillary microcarcinoma, papillary carcinoma, anaplastic carcinoma, Bcl-2, Bax, apoptosis, proliferation.
Thyroid cancer is not a common malignancy, but it presents with many intricate aspects concerning clinicopathological features and tumour biology. These tumours originate from either the follicular epithelial cells or the parafollicular cells. Carcinomas originating from follicular epithelium are generally classified into two groups: well-differentiated thyroid carcinomas, which are composed of papillary and follicular carcinomas, and undifferentiated/anaplastic thyroid carcinomas.
Papillary thyroid carcinoma (PTC) is the most common thyroid cancer and accounts for approximately 80% of all thyroid malignancies. Generally, PTC grows slowly and despite its high potential for regional nodal metastazation, it usually exhibits rather non-aggressive biological behavior and has a good prognosis. PTC can present in different histological variants, as well as in various sizes (Li Volsi, 1990; Rosai et al., 1992; Mazzaferri and Jhiang, 1994; Mizukami et al., 1995; Gillilland et al., 1997). PTCs that are not larger than 1 cm are classified as papillary microcarcinomas - PMCs (Hedinger et al., 1988) and they are the most common subtype of PTC. These lesions are frequently detected as incidental findings on autopsy or during examination of benign conditions. Most of them remain silent clinically and have an excellent prognosis. However, PMC may also represent the earliest form of a future large lesion, since all advanced carcinomas were microcarcinomas at some point in the past. Thus, the relationship between clinically evident PTC and PMC is not completely clear and many questions concerning the biological behaviour and, consequently, clinical management of PMC, still remain unanswered (Piersanti et al., 2003; Roti et al., 2006; Pelizzo et al., 2006; Baloch and Li Volsi, 2006).
Contrary to PTCs which are generally indolent slow-growing carcinomas, anaplastic thyroid carcinoma (ATC) is one of the most aggressive human malignancies known, characterized by an almost invariable rapid fatal outcome. Despite vigorous treatment, patients with ATC have a dismal prognosis, mainly due to the high rates of nodal and distant metastases and to local aggressiveness (Aldinger et al., 1978; Li Volsi, 1990; Guiffrida and Gharib, 2000). ATC is thought to arise from a background of differentiated (papillary or follicular) carcinoma, since many ATCs are accompanied by such cells.Thus, ATC represents a terminal dedifferentiation of preexisting differentiated carcinoma, i.e. the end stage of thyroid tumour progression (Spires et al., 1988; van der Laan et al., 1993).
Molecular and genetic changes underlying the progression of malignancy in thyroid tumours from PMC via PTC to ATC are still not clear and are under investigation. It is known that the biological behavior of each tumour depends on a number of factors, including the rate of growth. This in turn is related to the number of proliferating cells vs. those cells dying via necrosis or apoptosis, i.e. programmed cell death. Thus, the balance between proliferation and apoptosis may play a major role in determining the growth and aggressiveness of the tumour (Rubin et al., 2003).
Members of the Bcl-2 family play an important role in the regulation of apoptosis (reviewed by Burlacu, 2003 and Kirkin et al., 2004). The representative member, Bcl-2, is a protein of the outer mitochondrial membrane with anti-apoptotic activity.The Bcl-2 homologue, Bax, a monomeric cytosolic protein, displays a pro-apoptotic function.The Bax gene is transcriptionally activated by p53, a tumour suppressor, now firmly established as a key inhibitory regulator of cell proliferation (Levine et al., 1991). If unsuccessful, p53 can also eliminate cells through the Bcl-2/Bax apoptotic pathway. The ratio between Bcl-2 and Bax appears to be important in deciding the life or death of a cell (Reed, 1994).
The aim of this study was to gain better insight into molecular changes that reflect disturbances in the balance between proliferation and apoptosis during progression of thyroid malignancy from PMC via PTC to ATC. For this purpose we examined the expression of apoptosis related molecules (Bcl-2, Bax) and of proliferation marker (PCNA) and furthermore analysed in situ apoptotic cell death in these tumours.
Materials and Methods
Tissue samples
Formalin-fixed paraffin-embedded tissues were obtained from the archival material of the Centre for Endocrine Surgery, Clinical Centre of Serbia, Belgrade. A total of 120 cases included: 34 cases taken from patients diagnosed as having PMC after undergoing surgery for benign or autoimmune conditions, 52 cases diagnosed as conventional PTC and 34 cases diagnosed as ATC. Histological slides from the thyroid tumor tissue stained by haematoxylin and eosin were reevaluated by the pathologist to confirm the diagnosis of papillary or anaplastic carcinoma, respectively, according to widely accepted cytohistological criteria (Li Volsi, 1990; Rosai et al., 1992; Hedinger et al., 1998).
Nuclear features (ground glass nuclei, grooved nuclei and nuclear pseudoinclusions, at least two of them), regardless of the growth pattern, were taken as the “gold standard” for confirming the diagnosis of papillary carcinoma. Selected cases included classical variant with papillary architecture and follicular variant (including areas with a solid growth pattern in some cases). Uncommon subtypes of papillary carcinoma (such as oncocytic, tall cell, columnar) were excluded. Selected cases were divided into two categories: papillary microcarcinoma (PMC) and clinically manifest papillary carcinoma (PTC).
Cytological features (large polygonal or spindle shape cells, frequently multinucleated and containing abnormal mitotic figures) confirmed the diagnosis of ATC.
The study protocol was approved by the Ethics Committee at the Centre for Endocrine Surgery, Clinical Centre of Serbia, Belgrade.
Immunohistochemistry
For immunohistochemical staining we used mouse monoclonal antibodies against human Bcl-2 (clone Bcl 2-100, Sigma, Germany) and PCNA (NCL-PCNA, Novocastra, UK) and rabbit polyclonal antibody against Bax (Dako, Carpinteria, California, USA).
Immunohistochemistry was performed using the avidin-biotin-peroxidase method, provided by a commercially available kit, according to the manufacturer’s recommendations (Vector Laboratories, Burlingame, California, USA).
For each specimen, 10% formalin-fixed, paraffin–embedded tissue block prepared previously was used. Tissue sections (4–6 µm thick) from each block were mounted on adhesive-coated slides, deparaffinized with xylene and rehydrated through a series of descending graded ethanol. Endogenous peroxidase activity was blocked with 0.3% H2O2/methanol for 30 min followed by incubation with non-immune horse serum for 20 min to block non-specific binding.Tissue sections were then incubated with primary antibody against Bcl-2 (1:200 dilution), primary antibody against Bax (1:25 dilution) or primary antibody against PCNA (1:100 dilution) at 4°C overnight.This was followed by incubation with biotinylated horse anti-mouse IgG (for Bcl-2 and PCNA) or with biotinylated goat-anti rabbit IgG (for Bax) for 30 min and thereafter with the avidin-biotin-peroxidase complex for 30 min. Between each step, sections were washed three times in phosphate buffered saline (PBS).The reaction was visualised using 3, 3′-diaminobenzidine tetra hydrochloride (DAB) solution as a chromogen.
After counterstaining with haematoxylin, slides were dehydrated in ascending ethanol, cleared with xylene, mounted with coverslips using a permanent mounting medium and thereafter examined using a Reichart-Jung microscope supplied with a Photostar automatic camera system.
Sections of normal human lymph nodes served as a positive control for Bcl-2. In addition normal thyroid tissue adjacent to malignant cells served as an internal positive control for Bcl-2 and as a negative control for Bax and PCNA. Tissue samples receiving no primary antibody (anti-Bcl-2, anti-Bax or anti-PCNA), i.e. the antibody was replaced with PBS during staining procedure, exhibited no staining. and served as negative controls.
Scoring of immunohistochemical staining
Cytoplasmic staining for Bcl-2 and Bax was scored by two independent observers as follows: (−, 0) no staining; (+/−, 1) weak or focal staining; (+, 2) moderate staining in the majority of cells and (++, 3) strong staining in the majority of cells.
The Bcl-2/Bax ratio was estimated as follows: the average staining scores for Bcl-2 and for Bax were calculated (mean ± SD) from the individual staining score for each case (0–3) in the group and used to estimate Bcl-2/Bax ratios.
PCNA nuclear staining was scored as follows: (0) no staining, (1) up to 10% stained nuclei, (2) more than 10% and less than 25% stained nuclei and (3) more than 25% stained nuclei.
The average staining score for PCNA was calculated (mean ± SD) from the individual staining scores for each case in the group, and assigned as a Proliferative index (PI).
In situ detection of apoptotic cells
Apoptotic cells in tissue sections were detected by the terminal deoxynucleotidyl transferase-mediated deoxy uridine triphosphate nick-end labeling technique (TUNEL), using a commercial apoptosis kit (TACS TM TdT Kit, R&D Systems Europe, Abington,UK), according to the supplier’s instructions. In brief, tissue sections were deparaffinized with xylene and ethanol and rinsed with PBS. Sections were then treated with proteinase K in PBS, followed by quenching of endogenous peroxidase. Digoxigenin-dUTP was added to the 3′-OH ends of DNA by terminal deoxynucleotidyl transferase (TdT). After incubating with anti-digoxigen antibody conjugated with peroxidase, the sections were stained with diaminobenzidine (DAB) and counterstained with methyl green.
Experimental controls included during performing the protocol of the TUNEL method were:TACS-Nuclease-treated thyroid tissue sections as a positive control and the omission of TdT reaction step as a negative control, according to supplier’s instructions.
The percentage of apoptotic cells was determined by light microscope examination and the rate of apoptosis was graded as follows: (0) no detectable apoptotic cells, (1) up to 10% apoptotic cells, (2) more than 10% and less than 25% apoptotic cells and (3) more than 25% apoptotic cells.
The average apoptotic score was calculated (mean ± SD) from the individual staining scores for each case in the group, and assigned as an Apoptotic index (AI).
The ratio between proliferation and apoptosis (PI/AI ratio)
The ratio between proliferation and apoptosis was estimated as follows: the average staining scores for proliferative and for apoptotic cells were calculated (mean ± SD) from the individual staining score for each case in the group and used to estimate proliferation/apoptosis ratio (PI/AI ratio).
Statistical data
Statistical comparisons of data were made using Pearson's χ2 test and the Mann-Whitney U-test, as indicated in the Results section. A value p<0.05 was considered to be statistically significant.
Results
Immunohistochemical expression of Bcl-2 and Bax proteins was visualized as cytoplasmic staining and found in most of the carcinomas examined (Table 1).There was no statistically significant difference in Bcl-2 expression between PMC and PTC (94.12% positive cases and 92.31% positive cases, respectively), but the number of Bcl-2 positive cases was lower in the ATC group (76.47%, p<0.05).
Table 1. Immunohistochemical expression of Bcl-2 and Bax in papillary microcarcinoma (PMC), papillary carcinoma (PTC) and anaplastic carcinoma (ATC) of the thyroid gland.
| Bcl-2 | Bax | |||||||
|---|---|---|---|---|---|---|---|---|
| − | +/− | + | ++ | − | +/− | + | ++ | |
| PMC (n = 34) | 2 | 6 | 17 | 9 | 9 | 4 | 9 | 12 |
| Bcl-2 positive: 32/34 (94.12%) | Bax positive: 25/34 (73.53%)c | |||||||
| PTC (n = 52) | 4 | 9 | 26 | 13 | 4 | 2 | 12 | 34 |
| Bcl-2 positive: 48/52 (92.31%)a | Bax positive: 48/52 (92.31%)d | |||||||
| ATC (n = 34) | 8 | 17 | 8 | 1 | 3 | 5 | 11 | 15 |
| Bcl-2 positive: 26/34 (76.47%)b | Bax positive: 31/34 (92.12%) | |||||||
Staining score: (−,0) no staining; (+/−,1) weak or focal staining; (+,2) moderate staining in the majority of cells; (++,3) strong staining in the majority of cells. Statistically significant difference (p<0.05) for a vs b and c vs d (Pearson's χ2 test).
Bax positivity was significantly higher in PTCs (92.31%) than in PMCs (73.53%, p<0.05).There was no difference in the number of Bax positive cases between PTCs and ATCs.
Since Bcl-2 and Bax positive carcinomas showed variability in immunostaining pattern, the average staining scores for both Bcl-2 and Bax were calculated (mean ± SD) from the individual staining score for each case (0–3) in the group. Thereafter, the Bcl-2/Bax ratio was estimated from those values (Table 2). The Bcl-2 score did not differ between PMC and PTC (1.971 and 1.923, respectively). ATC cases showed a significantly lower average Bcl-2 score than PMCs and PTCs (p<0.05). The average Bax score was markedly higher in PTCs (2.481) than in PMCs (1.706, p<0.05), but there was no significant difference between PTCs and ATCs. Due to the changes in the level of Bcl-2 and Bax expression during malignancy progression from PMC to ATC, the Bcl-2/Bax ratio decreased from 1.155 in PMC to 0.586 in ATC (p<0.05).
Table 2.
The average immunostaining score for Bcl-2 and Bax and the Bcl-2/Bax ratio* in papillary microcarcinoma (PMC), papillary carcinoma (PTC) and anaplastic carcinoma (ATC) of the thyroid gland.
| Bcl-2 score | Bax score | Bcl-2/Bax | |
|---|---|---|---|
| PMC (n = 34) | 1.971±0.834 | 1.706±1.219c | 1.155e |
| PTC (n = 52) | 1.923±0.860a | 2.481±0.897d | 0.775 |
| ATC (n = 34) | 1.241±0.776b | 2.118±0.977 | 0.586f |
Estimated as detailed in the Material and Methods section. Statistically significant differences (p<0.05) for a vs b, c vs d, e vs f (Mann-Whitney U-test).
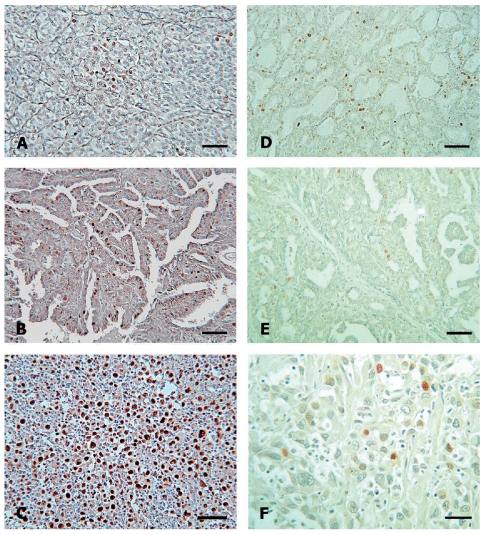
Immunohistochemical expression of PCNA revealed a significant enhancement from PMC to ATC (Figure 1 A, B, C).The relative number of high PCNA expressing cases (scores 2 and 3) was 35.39% for PMC, 53.85% for PTC and 82.35% for ATC (p<0.05). As shown in Table 3, the mean proliferation index (PI) for the analyzed carcinoma groups increased significantly (p<0.05) from PMC via PTC to ATC (PI=1.118, 1.615 and 2.412, respectively).
Figure 1.
Proliferation and apoptosis in papillary microcarcinoma (PMC), clinically manifest papillary carcinoma (PTC) and anaplastic carcinoma (ATC) of the thyroid gland. Proliferative activity, assessed by PCNA immunohistochemical staining, in PMC (A, bar 30 µm), PTC (B, bar 30 µm) and ATC (C, bar 30 µm). Streptavidin-biotin immunoperoxidase technique, haematoxylin diaminobenzidine counterstaining. In situ apoptotic cell death in PMC (D, bar 30 µm), PTC (E, bar 20 µm) and ATC (F, bar 10 µm). TUNEL technique, methyl green counterstaining.
Table 3. Proliferation and apoptotic indices in papillary microcarcinoma (PMC), papillary thyroid carcinoma (PTC) and anaplastic thyroid carcinoma (ATC) as assessed by PCNA immunostaining and the TUNEL method.
| Proliferation index (PI)* | Apoptotic index (AI)* | PI/AI* | |
|---|---|---|---|
| PMC (n= 34) | 1.118±0.769a | 0.441±0.504 | 2.535 |
| PTC (n = 52) | 1.615±0.867b | 0.500±0.505d | 3.230f |
| ATC (n = 34) | 2.412±0.925c | 0.206±0.411e | 11.719g |
As detailed in the Material and Methods section. Statistically significant differences ( p<0.05) for a vs b, b vs c, d vs e, f vs g (Mann-Whitney U-test).
The number of apoptotic cells was low, with zero up to ten positive cells per 10 HPFs in most carcinomas studied (Figure 1 D, E, F). The apoptotic index (AI) for each carcinoma group is given in Table 3 and was similar in PMCs and PTCs (0.441 and 0.500, respectively), but significantly less in ATCs (0.206, p<0.05).The ratio between proliferation and apoptotic indices (PI/AI) increased significantly during progression from PMC (PI/AI = 2.535) to ATC (PI/AI = 11.719, p<0.05). There was a weak association between the decreased Bcl-2 expression and decreased apoptosis but no relation with Bax expression (statistically not significant).
Discussion
The thyroid gland represents an exceptional opportunity to study the regulation of proliferation and apoptosis during tumour initiation and progression from a precursor lesion (PMC) through a clinically manifest slow-growing tumour (PTC) to a fatal rapidly growing carcinoma (ATC).The biological behaviour of these subtypes of thyroid cancer is highly divergent, as reflected by differences in their prognoses (indices of morbidity and mortality), patterns of metastasis and “clinical aggressiveness”.
Besides other factors, tumour growth and aggressiveness depend on the ratio between anti-apoptotic and pro-apoptotic molecules and their balance with the proliferative activity of neoplastic cells. Therefore, in this study we analysed expression of the apoptosis related molecules, Bcl-2 and Bax, their ratio, in situ apoptotic cell death and proliferative activity during progression of thyroid malignancy from PMC via PTC to ATC.
Bcl-2 is an anti-apoptotic molecule that prevents apoptosis by preserving mitochondrial membrane integrity (Gross et al., 1999; Burlacu, 2003; Kirkin et al., 2004). In previous reports, Bcl-2 was found to be expressed in normal thyroid tissue and in well differentiated thyroid carcinomas (Moore et al., 1988; Pilotti et al., 1994; Pollina et al., 1996; Mitselou et al., 2004; Aksoy et al., 2005; Cvejic et al., 2008; Xu et al., 2007, 2008), but its expression was reported to decrease with dedifferentiation and aggressiveness (Moore et al., 1988; Pollina et al., 1996; Saltman et al., 2006).
The pro-apoptotic molecule Bax has been less investigated in thyroid carcinomas, with conflicting results, and its role in thyroid tumorigenesis and tumour biology is discussed controversially. While some authors could not detect Bax in well differentiated thyroid carcinomas (Manetto et al., 1997), others reported Bax expression in all or in the majority of PTCs investigated (Hermann et al., 2001; Letsas et al., 2005). In anaplastic thyroid carcinoma, Bax was found to be weakly expressed (Branet et al., 1996).
The results of this study showed significant changes in the expression of apoptosis–related molecules during progression of malignancy in thyroid tumours. Bcl-2 protein was present at similar high levels in PMC and PTC, but decreased significantly during progression to anaplastic carcinoma, which confirms previous results. On the contrary, the expression of Bax protein increased in PTC when compared to PMC, and thereafter was maintained at a similar level in ATC.
Due to these changes, the Bcl-2/Bax ratio showed a significant decline during progression of malignancy from PMC to ATC. It is assumed that the ratio of Bcl-2 and Bax decides whether a cell will undergo apoptosis or not, i.e. Bax/Bcl-2 and Bax/Bax dimers are able to prolong cell life and induce cell death, respectively (Reed, 1994). In this study, In situ detection of apoptotic cells indicated a low apoptotic index in both papillary carcinoma types, as reported previously (Yoshida et al., 1999; Farid et al., 2001; Letsas et al., 2005).This is consistent with high levels of Bcl-2, the anti-apoptotic molecule. On the other hand, Bcl-2 prolongs cell life span and allows further mutations to accumulate, facilitating tumour progression from microscopic (subclinical) to clinically apparent carcinomas (PTCs), which are, as shown here, characterized by concomitant expression of Bcl-2 and Bax, increased proliferative activity and a low rate of apoptosis. Finally, the transition of the phenotype of well-differentiated papillary carcinoma to anaplastic carcinoma is a very rare event, requiring further sequential mutations in genes involved in growth signal transduction cascades, cell cycle regulation and apoptotic pathways due to genomic instability. Our results showed that this terminal phase of malignancy progression is characterized by permanent accelerated proliferative activity, a low level of Bcl-2 and a high level of Bax, but with a very low rate of apoptotic death.
However, an increase in apoptotic cell death in ATC compared to well-differentiated thyroid carcinoma was reported by Moore et al. (1988) for two cases analyzed, and also by Sreelekha et al. (2000), but the levels of Bax protein expression were not evaluated. It could be expected that the loss of Bcl-2 and an accumulation of Bax homodimers should lead to a higher rate of apoptosis. However, the reasons for inappropriate cell death in thyroid carcinomas, despite the presence of high levels of pro-apoptotic Bax protein are not yet understood.Thus, the complexity of genetic defects in the apoptotic pathway in thyroid carcinomas, for example defects in functions of Bax downstream effectors, such as caspases, should be investigated.
In contrast to the low level of apoptosis, proliferative activity in this study was shown to increase significantly during progression from PMC to ATC, and consequently the ratio between proliferation and apoptotic indices was shifted in favor of proliferation.
In conclusion, in this investigation we found differences in the expression of apoptotic molecules (Bcl-2 and Bax), their ratio (Bcl-2 /Bax), apoptotic cell death and proliferative activity between PMC, PTC and ATC, indicating that disturbances in the balance between apoptosis and proliferation, in favour of the latter, occur gradually during the progression of malignancy in thyroid tumours.
Acknowledgement
Supported by the Ministry of Science of the Republic of Serbia, project 143039: “Molecular mechanisms and biochemical basis of thyroid gland tumours”.
References
- Aksoy M, Giles Y, Kapran Y, Terzioglu T, Tezelman S. Expression of Bcl-2 in papillary thyroid cancers and its prognostic value. Acta Chir Belg. 2005;105:644–8. doi: 10.1080/00015458.2005.11679794. [DOI] [PubMed] [Google Scholar]
- Aldinger KA, Samaan NA, Ibanez M, Hill CS., Jr Anaplastic carcinoma of the thyroid: a review of 84 cases of spindle and giant cell carcinoma of the thyroid. Cancer. 1978;41:2267–75. doi: 10.1002/1097-0142(197806)41:6<2267::aid-cncr2820410627>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Baloch ZW, Li Volsi VA. Microcarcinoma of the thyroid. Adv Anat Pathol. 2006;13:69–75. doi: 10.1097/01.pap.0000213006.10362.17. [DOI] [PubMed] [Google Scholar]
- Branet F, Brousset P, Krajewski S, Schlaifer D, Selves J, Reed JC, et al. Expression of the cell death-inducing gene Bax in carcinomas developed from the follicular cells of the thyroid gland. J Clin Endocrinol Metab. 1996;81:2726–30. doi: 10.1210/jcem.81.7.8675602. [DOI] [PubMed] [Google Scholar]
- Burlacu A. Regulation of apoptosis by Bcl-2 family proteins. J Cell Mol Med. 2003;7:249–57. doi: 10.1111/j.1582-4934.2003.tb00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvejic D, Selemetjev S, Savin S, Paunovic I, Petrovic I, Tatic S. Apoptosis and proliferation related molecules (Bcl-2, Bax, p53, PCNA) in papillary microcarcinoma versus papillary carcinoma of the thyroid. Pathology. 2008;40:475–80. doi: 10.1080/00313020802026989. [DOI] [PubMed] [Google Scholar]
- Farid P, Gomb SZ, Peter I, Szende B. Bcl-2, p53 and bax in thyroid tumors and their relation to apoptosis. Neoplasma. 2001;48:299–301. [PubMed] [Google Scholar]
- Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from Surveillance, Epidemiology and END Results (SEER) program 1973–1991. Cancer. 1997;79:564–73. doi: 10.1002/(sici)1097-0142(19970201)79:3<564::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Gross A, McDonell JM, Korsmeyer SJ. Bcl-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Guiffrida D, Gharib H. Anaplastic thyroid carcinoma: Current diagnosis and treatment. Annals of Oncology. 2000;11:1083–9. doi: 10.1023/a:1008322002520. [DOI] [PubMed] [Google Scholar]
- Hedinger C, Williams ED, Sobin LH. International Histological Classification of Tumours. 2nd edn. World Health Organization; Geneva: Springer-Verlag: 1988. Histological typing of thyroid tumours; pp. 1–66. [Google Scholar]
- Hermann S, Sturm I, Mrozek I, Klosterhalfen B, Hauptmann S, Dörken B, Daniel PT. Bax expression in benign and malignant thyroid tumours: dysregulation of wild type p53 is associated with a high Bax and p21 expression in thyroid carcinoma. Int J Cancer. 2001;92:805–811. doi: 10.1002/ijc.1284. [DOI] [PubMed] [Google Scholar]
- Kirkin V, Joos S, Zörnig M. The role of Bcl-2 family members in tumorigenesis. Biochim Biophys Acta. 2004;1644:229–49. doi: 10.1016/j.bbamcr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Letsas KP, Frongou-Lazaridis M, Skyrlas A, Tsatsoulis A, Malamou-Mitsi V. Transcription factor-mediated proliferation and apoptosis in benign and malignant thyroid lesions. Pathology International. 2005;55:694–702. doi: 10.1111/j.1440-1827.2005.01899.x. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Momand J, Finlay CA. The p53 tumor suppressor gene. Nature. 1991;351:453–6. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- LiVolsi VA. Surgical pathology of the thyroid. Philadelphia: WB Saunders. 1990:136–72. [Google Scholar]
- LiVolsi VA. Surgical pathology of the thyroid. Vol. 253. Philadelphia: WB Saunders; 1990. Undifferentiated or anaplastic carcinoma of the thyroid; 74 pp. [Google Scholar]
- Manetto V, Lorenzini R, Cordon-Cardo C, Krajewski S, Rosai J, Reed JC, et al. Bcl-2 and Bax expression in thyroid tumours. An immunohistochemical and western blot analysis. Virchow Arch. 1997;430:125–30. doi: 10.1007/BF01008033. [DOI] [PubMed] [Google Scholar]
- Mazzaferri EL, Jhiang S. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–28. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- Mitselou A, Peschos D, Dallas P, Charalabopoulos K, Agnantis N J, Vougiouklakis T. Immunohistochemical analysis of expression of Bcl-2 protein in papillary carcinomas and papillary microcarcinomas of the thyroid gland. Exp Oncol. 2004;26:282–286. [PubMed] [Google Scholar]
- Mizukami Y, Michigishi T, Nonomura A, Noguchi M, Nakamura T. Thyroid carcinoma: clinical, pathologic correlations. Crit Rev Oncol/Hematol. 1995;18:67–102. doi: 10.1016/1040-8428(94)00121-9. [DOI] [PubMed] [Google Scholar]
- Moore D, Ohene-Fianko D, Garcia B, Chakrabarti S. Apoptosis in thyroid neoplasms: relationship with p53 and Bcl-2 expression. Histopathology. 1988;32:35–42. doi: 10.1046/j.1365-2559.1998.00319.x. [DOI] [PubMed] [Google Scholar]
- Pelizzo MR, Boschin IM, Toniato A, Piotto A, Bernante P, Pagetta C, et al. Papillary thyroid microcarcinoma (PTMC): Prognostic factors, management and outcome in 403 patients. Eur J Surg Oncol. 2006;32:1144–8. doi: 10.1016/j.ejso.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Piersanti M, Ezzat S, Asa SL. Controversies in papillary microcarcinoma of the thyroid. Endocrine Pathol. 2003;14:183–92. doi: 10.1007/s12022-003-0011-5. [DOI] [PubMed] [Google Scholar]
- Pilotti S, Collini P, Rilke F, Cattoreti G, Del Bo R, Pierotti MA. Bcl-2 protein expression in carcinomas originating from the follicular epithelium of the thyroid gland. J Pathol. 1994;172:337–42. doi: 10.1002/path.1711720408. [DOI] [PubMed] [Google Scholar]
- Pollina L, Pacini F, Fontanini G, Vignati S, Bevilacqua G, Basolo F. Bcl-2, p53 and proliferating cell nuclear antigen expression are related to the degree of differentiation in thyroid carcinomas. Br J Cancer. 1996;73:139–43. doi: 10.1038/bjc.1996.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosai J, Carcangiu ML, De Lellis RA. Atlas of Tumor Pathology, Fascicle 5, 3rd Series. Washington DC: Armed Forces Institute of Pathology; 1992. Tumors of the thyroid gland; pp. 161–82. [Google Scholar]
- Roti E, Rossi R, Transforini G, Bertelli F, Ambrosio MR, Busutti, et al. Clinical and histological characteristics of papillary thyroid microcarcinoma: results of a retrospective study in 243 patients. J Clin Endocrinol Metab. 2006;91:2171–8. doi: 10.1210/jc.2005-2372. [DOI] [PubMed] [Google Scholar]
- Rubin LI, Philpott Ki, Brooks SF. The cell cycle and cell death. Curr Biol. 2003;3:391–4. doi: 10.1016/0960-9822(93)90211-6. [DOI] [PubMed] [Google Scholar]
- Saltman B, Simgh B, Hedvat CV, Wreesmann VB, Ghossein R. Patterns of expression of cycle/apoptosis genes along the spectrum of thyroid carcinoma progression. Surgery. 2006;140:899–905. doi: 10.1016/j.surg.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Spires JR, Schwartz MR, Miller RH. Anaplastic thyroid carcinoma. Association with differentiated thyroid carcinoma. Arch Otolaryngol Head Neck Surgery. 1988;114:40–4. doi: 10.1001/archotol.1988.01860130044012. [DOI] [PubMed] [Google Scholar]
- Sreelekha TT, Pradeep VM, Vijayalakshmi K, Belthazar A, Chellam VG, Nair MB, et al. In situ apoptosis in the thyroid. Thyroid. 2000;10:117–22. doi: 10.1089/thy.2000.10.117. [DOI] [PubMed] [Google Scholar]
- Van der Laan B, Freeman JL, Tsang RW, Asa SL. The association of well-differentiated thyroid carcinoma with insular or anaplastic thyroid carcinoma: Evidence for dedifferentiation in tumor progression. Endocr Pathol. 1993;4:215–21. doi: 10.1007/BF02915464. [DOI] [PubMed] [Google Scholar]
- Xu WC, Chen SR, Huang JX, Zheng ZC, Chen LX, Lin JJ, et al. Expression and distribution of S-100 protein, CD83 and apoptosis-related proteins (Fas, FasL and Bcl-2) in thyroid tissues of autoimmune thyroid diseases. Eur J Histochem. 2007;51:291–300. doi: 10.4081/1154. [DOI] [PubMed] [Google Scholar]
- Xu W, Li X, Chen S, Huang J, Lin S, Lin J, Li Y, Tan X. Expression and distribution of S-100, CD83 and apoptosis-related proteins (Fas, FasL and Bcl-2) in tissues of thyroid carcinoma. Eur J Histochem. 2008;52:153–62. doi: 10.4081/1206. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Nakamura Y, Imada T, Asaga T, Shimizu A, Harada M. Apoptosis and proliferative activity in thyroid tumors. Surg Today. 1999;29:204–20. doi: 10.1007/BF02483007. [DOI] [PubMed] [Google Scholar]