Abstract
Enterotoxigenic Escherichia coli (ETEC) infection is the most common type of porcine postweaning colibacillosis (PWC). Among fimbriae of porcine ETEC strains the best studied family of fimbriae are the members of F4 adhesins, existing in at least three variants: ab, ac, ad. Active immunization against porcine PWC is difficult due to: i) ETEC strains are only one of the essential predisposing factors, ii) the success of vaccinal antigen uptake depends on the presence of enterocyte receptors for F4 adhesins, iii) the intestinal immune system may react with tolerance or hypersensitivity to the same antigens depending on the dose and form of the vaccinal immunogen, and iv) kinetics of the specific immune responses may be different in the case of F4 (earlier) and the other ETEC adhesins, particularly F18 (later). The aim of this study was to test the effectiveness of a live attenuated F4ac+ non-ETEC vaccine against porcine PWC by analyzing quantitative differences in the small intestinal lymphoid and myeloid cell subsets of immunized (with or without levamisole given as an adjuvant) vs control non-immunized pigs. Four week-old pigs were intragastrically immunized with a vaccine candidate F4ac + non-ETEC strain 2407 at day 0, challenged 7 days later with a virulent F4ac+ strain ETEC 11-800/1/94, euthanatized at day 13 and sampled for immunohistology. Non-immunized pigs received saline at day 0 and were processed as the principals. Immunophenotypes of lymphoid and myeloid cell subsets were demonstrated within jejunal and ileal mucosa by immunohistochemical avidinbiotin complex method and corresponding morphometric data were analyzed using software program Lucia G for digital image analyses. Monoclonal antibodies reactive with surface molecules on porcine immune cells such as CD3, CD45RA, CD45RC, CD21 and SWC3 enabled clear insight into distribution patterns and amount of these cells within the gut-associated lymphoid tissues (GALT) examined. The numbers of jejunal and ileal cell subsets tested were significantly increased (at P<0.5 or lower) in both principal groups (vaccinated or levamisole primed-vaccinated) of pigs, compared to those recorded in the control non-vaccinated pigs. Based on the histomorphometric quantification of porcine intestinal immune cells from the GALT compartments tested, it is possible to differentiate the responses of pigs immunized by an experimental mucosal vaccine from those of non-immunized pigs.
Key words: adjuvanted/nonadjuvanted E. coli vaccine, intestinal immune cells, pigs.
Introduction
Intestinal mucosal surfaces represent the entry route of a wide range of harmless dietary antigens and harmful viral and bacterial pathogens. Certain enteric pathogens take advantage of host or other factors (such as diet or stress), which may alter or weaken gut immune system defences. For many of these, such as porcine enterotoxigenic Escherichia coli (ETEC) strains etiological agents of postweaning colibacillosis (PWC), no effective vaccine exists. Hence, it is important that prospective vaccines engender maximal immunity at these susceptible sites. Promising candidates that might be able to manage sufficient protection include live attenuated oral vaccine with F4ac+ non-ETEC strain. Porcine PWC is economically one of the most significant disease of swine, which encountered for the major productive losses in the swine industry due to morbidity, mortality or retarded growth. Commonoly induced by ETEC strains that mostly carry F4 or F18 fimbriae, it is etiologically complex disease triggered by numerous stressful events (i.e. weaning) and recently, an increase in incidence of outbreaks of severe E. coli-associated diarrhea has been observed worldwide.1 Lallès et al.2 discussed the influence of weaning and nutrition in the postweaning period on intestinal physiology and mucosal immunology. The ETEC strains isolated from pigs with PWC produce enterotoxins that may induce a severe watery diarrhea, starting 3–10 days after weaning. It is widely accepted that specific serotypes/patho-types of ETEC are responsible for the major part of PWC, but only a part although an essential one.3 It is also without dabate that weaning is a period during which there is a great change in the magnitude and variety of exposure to environmental antigens derived from food and potentially pathogenic microbes. As a consequence PWC commonoly occurs in pigs weaned abruptly at 3–5 weeks, and it is quite clearly associated with the event of weaning,2 accompanied with various other predisposing factors such as environmental, nutritional and physiological changes. Because parenterally administrated vaccines tend to stimulate systemic rather than local (intestinal) immune responses,4 novel strategies are aimed at designing of mucosal vaccines with attenuated non-ETEC strains producing F4 or F18 fimbrial antigens.1
The extensive research of Dacko et al.5 made possible classification of E. coli strains isolated from diarrheic suckling piglets into several groups: ETEC, vero- or Shigatoxigenic (VTEC or STEC), enteroaggregative (EAEC), enteropathogenic (EPEC) and necrotoxigenic (NTEC). The study demonstrated that the majority of the isolates (59.6%) were able to produce heat-labile (LTI) and/or heat-stable (STI) enterotoxins, and these were classified as typically ETEC strains. Zhang et al.6 have found the prevalence of F4 fimbrial genes in E. coli strains isolated from young pigs with PWC in the USA. Moreover, all toxin genes except the EAST1 toxin gene, were almost exclusively associated with F4+ or F18+ isolates, and most of these isolates carried multiple toxin genes.
The present study is aimed at evaluation of the immunogenicity of attenuated F4ac+ non-ETEC vaccine candidate strain against porcine PWC. We have tested the effectiveness of live oral vaccine by analyzing quantitative differences in lymphoid and myeloid cell subsets within the gut-associated lymphoid tissues (GALT) of weaned pigs specifically immunized (with or without levamisole applied as an adjuvant) vs control non-immunized and challenged pigs with homologous ETEC strain.
Materials and Methods
Bacterial strains
The recombinant avirulent F4ac+ non-ETEC vaccine candidate strain 2407 (serotype O9: K36: H19: F4ac: LT− STb− ) kindly donated by dr. sc. Thomas A. Casey from NADC, Ames, IA, USA, was used for the immunization.7 The authentic F4ac+ ETEC strain 11-800/1/94 (serotype O149: K91: F4ac: 987P: Hly+ LT+ STb+ ), isolated from diarrheic pigs aging between 3 and 4 weeks reared on swine farms in Croatia, was used for the challenge infection. Both strains were kept in the glycerin broth at −80°C until used.
Monoclonal antibodies
The mAbs reactive with porcine leukocyte surface molecules i.e. cluster of differentiation (CD) antigens that we have used to study in situ identification, distribution and quantification patterns of respective lymphoid and myeloid cell subsets are listed in Table 1.
Table 1. The mAbs specific for swine leukocyte CD/SWC antigens used in immunohistological demonstration of porcine intestinal lymphoid and myeloid cell subsets.
| Marker antigens | mAbs | Cells | Donor* |
|---|---|---|---|
| CD3a | BB23-8E6 | T cells | Pescovitz |
| CD3b | FY1H2 | T cells | Yang |
| CD45RA | MIL13 | Naïve T cells | Haverson |
| CD45RC | MIL5 | Memory T cells | Stokes |
| CD21 | BB6-11c9 | B cells | Pescovitz |
| SWC3 | 74-22-15 | Macrophages, monocytes, granulocytes | Lunney |
Kindly donated for research purposes and testings for the Swine CD Workshops held in Davis, CA, USA (1995), Ludhiana, India (1998), and Amsterdam, Netherlands (1999).
Study design
Fifteen conventionally reared crossbred pigs (Swedish Landrace × Yorkshire) from a large scale swine farm nearby Zagreb, Croatia were weaned at 4 weeks of age and purchased for this experiment. The pigs were housed in the animal facility at the Veterinary Faculty University of Zagreb and fed with a standard weaner diet. They were randomly divided into three groups comprising 5 animals each. After two days of accommodation pigs were treated as follows: control non-immunized pigs received 5 mL of saline intramuscularly (i.m.) at day 0, principal pigs were intragastrically (i.g.) immunized with 1010 CFU/mL of F4ac+ non-ETEC vaccine candidate strain 2407 in 60 mL of TSB at day 0 or i.m. primed with levamisole (Nilverm®; Pliva, Zagreb, Croatia) at the immunostimulatory dose of 2.5 mg/kg over three consecutive days (−2, −1, 0) and i.g. immunized with 1010 CFU/mL of F4ac+ non-ETEC vaccine candidate strain 2407 in 60 mL of TSB at day 0. Seven days later all pigs were challenged with 1010 CFU/mL of F4ac+ ETEC strain 11-800/1/94, and three out of each group were euthanatized at day 13 and sampled for immunohistology.
All treatments of pigs were conducted in accordance with the “Directive for the Protection of Vertebrate Animals used for Experimental and other Purposes” (86/609/EEC).
Clinical observations
Clinical observations for signs of colibacillosis, such as diarrhea, dehydration, weight loss, weakness and anorexia were recorded three times daily by the person blinded to given treatments. Pigs were weighed at the beginning of the trial (day -2), and 7 and 10 days after the immunization (at day 0) or 4 days after the challenge infection. The diarrhea developed by pigs was graded on a scale of intensity where scores (per pig per day of the experiment) were given based on stools consistency: +, soft feces = mild diarrhea; ++, fluid feces = moderate diarrhea; +++, watery feces = severe diarrhea. Pigs with normal firm feces were scored as diarrhea negative (−).
Isolation of vaccine and challenge strains from the feces
Beginning with day -2 before the treatments with levamisole, rectal swabs were taken from each pig and also at days 7 and 13 following immunization and challenge infection. Samples were diluted in serial dilutions up to 1010 in saline and 1 mL of each dilution was placed onto TSB with 5% sheep blood agar (Blood Agar Base, No.2, OXOID CM 271). After incubation at 37°C overnight, the numbers of colony-forming units (CFU) per mL were determined by counting on an automatic computer-assisted counter. Five E. coli colonies from each plate were serotyped by a slide agglutination test using rabbit OK antisera prepared from standard E. coli strains (Croatian Veterinary Institute, Zagreb, Croatia). Hemolytic isolates identified by plating on 5% sheep blood agar with esculine were further serologically checked for F4 fimbrial antigens. The presence or absence of heat stable and heat labile enterotoxins were confirmed using commercial test kits as afore mentioned. To detect natural infections with other E. coli strains, faecal samples were also plated onto plain agar.
Tissue sampling and immunohisto-chemical staining
Samples of middle part of jejunum and ileum were collected immediately after euthanasia and fixed (as 10×10×5 mm blocks) in fresh 10% neutral buffered formaldehyde (pH 7.0–7.6) for 24 hours. Tissue blocks were then dehydrated in 75%, 80%, 95%, 100% ethanol and embedded in paraplast embedding media (Sigma, Deisenhofen, Germany).
Paraplast-embedded sections were cut at 5 µm and floated on a water bath containing distilled water, heated at approximately 42°C. The selected sections are picked up with the precoated slides and dried horizontally on a 37°C warming tray overnight. Then, the slides were dewaxed in xylene three times for 5 minutes each, hydrated in 100%, 95%, 80%, 75% ethanol for 3 minutes, washed in phosphate buffered saline (PBS) pH 7.2 twice for 3 minutes each and immersed into distilled water for 5 minutes.
Immunohistochemical staining was performed by the avidin biotin complex (ABC) method.8,9 For blocking of endogenous peroxidase activity, the slides were immersed into 3% hydrogen peroxide solution for 30 minutes, then washed in PBS for 2×3 minutes, drained and wiped. Blocking of background staining was performed by incubating the intestinal tissue sections with 5% rabbit serum and 5% pig serum diluted in PBS, for 30 minutes at room temperature in a humid chamber. The blocking serum was tapped off and the excess was wiped away without rinse. The primary antibody (mouse anti-swine), diluted 1:2 in PBS with 1% swine serum and 1% rabbit serum were added to cover the sections and incubated for 1 hour at room temperature. Also, negative control sections either without primary or secondary antibody were prepared. Slides were gently rinsed in PBS bath for 3×5 minutes and wiped. The secondary antibody (biotinylated rabbit anti-mouse IgG) (Sigma, St. Louis, USA) diluted 1:500 in PBS was applied to cover the tissue sections and incubated at room temperature for 1 hour. Slides were rinsed three times for 5 minutes each in PBS, wiped and the tertiary antibody (streptavidin- peroxidase complex) (ICN, ImmunoBiologicals, USA) diluted 1:1000 in PBS was applied for 1 hour at room temperature. After rinsing three times for 5 minutes each in PBS, the reaction was visualized with a 0.05% solution of 3,3-diaminobenzidine tetrachloride (DAB) in 0.05 M Tris-HCl (pH 7.6) containing 0.01% H2O2. The sections were dehydrated in raising concentrations of ethanol, mounted in canada balsam and coverslip.
Morphometric analyses
Immunophenotypes of lymphoid and myeloid cell subsets within jejunal and ileal mucosa demonstrated by immunohistochemical ABC method were analyzed using software program Lucia G for digital image analyses (DIA). Morphometric analyses were performed by counting of the immune cells in 12 randomly selected tissue section fields at ×200 on screen magnification including the areas of lamina propria and adjacent epithelium, the areas of villus and crypts and also the Peyer’s patches. The results are expressed as the mean values and standard deviations of the number of jejunal and ileal immune cells per µm2 of an average tissue section field of 672387.5 µm2. Mean values of stained cell numbers for each group were compared using statistical analysis.
Statistical analysis
Data were analyzed by a non-parametric Mann-Whitney U test and differences between number of cells recorded in principal and control groups were considered as significant at P<0.05 and lower values.
Results
Clinical examinations
The mean weight (kg ±SD) per group of pigs at day -2 were as follows: control = 7.43±1.44 vaccinated = 6.25±0.7 and levamisole-primed vaccinated = 6.68±0.8. All these body weights were statistically similar. Seven days following the treatments, vaccinated pigs had much lower (P<0.05) body weight (4.75±0.6) as compared to that in control pigs (7.75±1.0). Weight gain in levamisole-primed vaccinated pigs was also lower (5.93±0.5), but not significantly. Ten days after the treatments, vaccinated pigs still gain weight slower (5.5±1.0) although not significantly. In the control pigs (8.68±1.3) as well as in levamisole-primed vaccinated pigs (7.75±0.5) body weight was increased 10 days after the treatments. However, such increases were not significantly different between days 2 and 10. All pigs gained body weight 4 days following challenge inoculation, but the values were not significantly different between days 2 and 13 of the experiment.
Fecal shedding of the vaccine and challege strain
The isolated E. coli strains and their numbers were assessed from the rectal swabs before and after the treatments as shown in Table 3. The vaccine candidate strain (O9:K36:H19:F4ac) was isolated from none of controls, 2/5 vaccinated (at Day 7) and 5/5 levamisole primed-vaccinated pigs (2.5×106 CFU/mL) at day 13 of the experiment. Thus, the vaccine candidate strain (applied in the concentration of 1010 CFU/mL at day 0) could be cultured in a minor quantity of 0.25% 14 days after the specific immunization. This strain could not be recovered from neither fecal material nor intestinal content in F4ac+ non-ETEC strain treated pigs. We were able to isolate challenge strain (O149:K91:F4ac) from 4/5 and 5/5 control and vaccinated pigs (1.0×106 and 5.5×106 CFU/mL), respectively, at day 13 of the experiment. However, the challenge strain cold not be recovered from levamisole primed-vaccinated pigs. We speculate that this occurs due to the prolonged presence (from day -2 to day 13) of homologous farm strain (O8:K87:F4ac:Hly− ) in the gastrointestinal tract of these pigs, which resulted in its more rapid passage through the intestines following challenge infection. Although in minor quantities, we were able to isolate both vaccinal (0.25%) and challenge strain (0.10–0.55%) 14 or 7 days following the inoculation, respectively. It seems that challenge strain was passing through the intestines almost three times slower than the vaccinal strain. Accordingly, it would be more appropriate to vaccinate pigs on at least three consecutive days, starting at weaning with much higher doses of vaccine candidate strain.
Table 3. Isolation and quantification of E. coli strains from either rectal swabs or intestinal contents of weaned pigs before and after the treatments; numerical data are expressed as either average values of CFU/mL or percent values of hemolytic colonies in the isolates for each group of pigs.
| Treatment of pigsa | Day of experiment | No. of E. coli positive pigs/total no. of pigs | E. coli isolate | CFU/mL | Hemolytic isolates (%) |
|---|---|---|---|---|---|
| None | −2 | 3/5 | O8:K87:F4 acc | - | - |
| 7 | 5/5 | E. colid | - | - | |
| 10 | 1/5 | E. coli | - | - | |
| 13 | 4/5 | O138:K81, | 3.0×107 | 10 | |
| O149:K91:F4ac | 1.0×106 | 20 | |||
| F4ac+ non-ETEC | −2 | 1/5 | E. coli | - | - |
| 7 | 2/5 | O8:K87:F4ac : | - | 75 | |
| O9:K36:H19:F4ac | |||||
| 10 | 4/5 | O149:K91:F4ac | - | - | |
| 13 | 2/5 | O149:K91:F4ac | 5.5×106 | 22.5 | |
| Levamisole + F4ac+ non-ETEC | −2 | 1/5 | O8:K87:F4ac | - | - |
| 7 | 3/5 | E. coli | - | - | |
| 10 | 1/5 | E. coli | - | - | |
| 13 | 5/5 | O8:K87:F4ac | 3.5×106 | ||
| O9:K36:H19:F4ac | 2.5×106 | 15.0 |
Groups comprised five 4-weeks-old pigs each.bControl pigs received saline as a placebo.
“Farm strain”.
Non-pathogenic strain.
Immunohistochemical findings
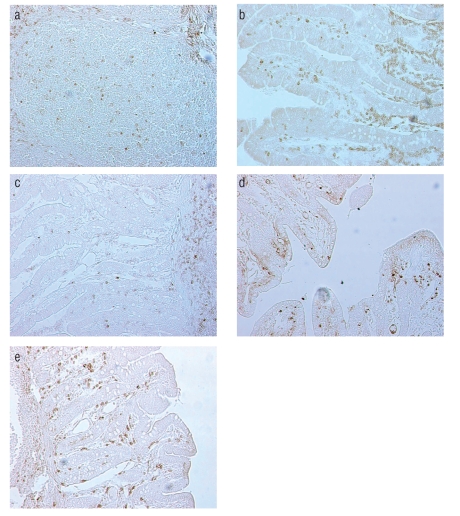
The in situ localization and distribution patterns of lymphoid and myeloid cells within jejunal and ileal mucosa are shown in Figure 1 a–e. According to location numerous CD3+ cells are among intraepithelial lymphocytes. CD3+ T cells were uniformly distributed between crypts and villi. The cells expressing CD3 antigen were also found in the mucosal lamina propria and inside the follicles of Peyer's patches. We have found numerous clusters of CD3+ T lymphocytes in the interfollicular area. CD45RA+ naive T cells were more abundant in the crypts area whereas CD45RC+ memory T cells were mostly found in the villi and interfollicular areas. Predominance of CD45RA+ naïve T cells was found in Peyer’s patches and these cells were also numerous in the extrafollicular areas. The CD45RC+ memory T cells were abundant in the mucosal lamina propria and in the interfollicular areas, but quite scarce in Peyer’ patches. Numerous CD21+ B cells were observed in the villi: these cells were also frequent in the mucosal lamina propria and inside the follicles of Peyer's patches. SWC3+ myeloid cells (marcophages/granulocytes) were particularly frequent in the areas directly below the epithelium of the villi, in the mucosal lamina propria and between the crypts. Small amount of these cells was scattered through ileal Peyer's patches.
Figure 1.
Immunohistochemical localization of CD3+ T cells in the interfollicular area of ileum (a), CD45RA+ naïve T cells in the lamina propria and between crypts of jejunum (b), CD45RC+ memory T cells in ileal lamina propria and Peyer’s patches (c), CD21+ B cells (d) and SWC3+ myeloid cells (e) in the lamina propria of ileum from 4-week-old pig as demonstrated by ABC method; original magnification 200×.
Histomorphometric findings
Following oral administration of vaccine candidate F4ac+ non-ETEC strain (with or withou levamisole applied as an adjuvant) and challenge infection with the homologous ETEC strain, we have evaluated the intestinal immune responses of immunized pigs by quantification of numbers of their CD3+, CD45RA+, CD45RC+ and CD21+ lymphoid and SWC3+ myeloid cells in the sections of jejunum (Table 4) and ileum (Table 5) using a computer-assisted histomorphometry.
Table 4. Morphometric data of lymphoid and myeloid cell subsets in jejunum of pigs immunized with either vaccine candidate F4ac+ non-ETEC strain or with combination of levamisole and F4ac+non-ETEC. The results are expressed as the mean values and standard deviations of the number of cells per µm2 of tissue section field; in every sample the cells were counted in 12 randomly chosen fields with the average area of 672387.5 µm2.
| Treatment of pigsa | Mean±SD(×10−5 ) number of lymphoid and myeloid cells residing jejunal mucosa | ||||
|---|---|---|---|---|---|
| CD3 | CD45RA | CD45RC | CD21 | SWC3 | |
| Noneb | 1.54±0.61 | 6.84±2.06 | 2.25±0.50 | 1.7±1.19 | 1.39±0.09 |
| F4ac+ non ETEC | 20.3±3.84** | 60.4±7.44** | 13.8±4.32* | 46.0±4.42** | 7.96±2.15* |
| Levamisole+ F4ac+ non-ETEC | 15.9±5.44* | 79.1±13.8** | 18.5±4.55* | 27.5±8.29* | 7.62±1.71* |
Groups comprised five 4-weeks-old pigs each.
Control pigs received saline as a placebo. Significantly different at:
P<0,01 or
<0.001 than in the control nontreated pigs.
Table 5. Morphometric data of lymphoid and myeloid cell subsets in ileum of pigs immunized with either vaccine candidate F4ac+non-ETEC strain or with combination of levamisole and F4ac+non-ETEC. The results are expressed as the mean values and standard deviations of the number of cells per µm2 of tissue section field; in every sample the cells were counted in 12 randomly chosen fields with the average area of 672387.5 µm2.
| Treatment Of pigsa | Mean±SD(×10−5 ) number of lymphoid and myeloid cells residing ileal mucosa | ||||
|---|---|---|---|---|---|
| CD3 | CD45RA | CD45RC | CD21 | SWC3 | |
| Noneb | 3.14±0.47 | 12.2±4.17 | 2.98±0.7 | 4.93±1.67 | 1.42±0.86 |
| F4ac+ non ETEC | 21.6±9.47* | 113.0±21.8*** | 15.2±9.97 | 84.2±16.8*** | 9.26±1.25*** |
| Levamisole +F4ac+ non-ETEC | 18.6±3.39*** | 155.0±65.3* | 19.5±6.85* | 88.4±23.5** | 10.6±3.13** |
Groups comprised five 4-weeks-old pigs each.
Control pigs received saline as a placebo. Significantly different at:
P<0.05,
<0,01 or
<0.001 than in the control nontreated pigs.
Quantitative immunophenotypic analyses showed that pigs immunzied with F4ac+ non-ETEC vaccine candidate strain had highly increased numbers of jejunal and ileal CD3+ T cells (P<0.001 and P<0.05, respectively) as compared to those recorded in non-treated control pigs (Table 4 and Table 5). In the pigs immunized with levamisole adjuvanted vaccine these cells were also highly elevated in the mucosal sites of jejunum (P<0.01) and ileum (P<0.001). Naive and memory T cells (expressing isoforms of CD45RA+ and CD45RC+surface antigens) were also significantly increased in the jejunum (P<0.001 and P<0.01, respectively) of boths principal groups of pigs. While CD45RA+ cells were also increased in the ileum of both principal groups (P<0.001 and P<0.05), CD45RC+ were higher than the control values (P< 0.05) in the ileum of pigs that had been immunized with nonadjuvanted and levamisole-adjuvanted vaccine, but not in the ileum of pigs immunized with nonadjuvanted vaccine. Both principal groups of pigs had increased numbers of CD21+ B cells within both jejunum (P<0.001 and P<0.05) and ileum (P<0.001 and P<0.01). Similar finding was observed for SWC3+ marcophages/ granulocytes (Table 4 and Table 5). The effect of levamisole when given as an ajuvant with the experimental vaccine was calculated by comparison to nonadjuvanted vaccine. A significant difference was obtained only for the number of CD21+ B cells (at P<0.5) which was slightly higher in the pigs treated with vaccine candidate strain than in the pigs treated with levamisole plus vaccinal strain (Table 4).
Discussion
As the preventive use of antibiotics in veterinary medicine became prohibited in the EU there is a rapidly increasing need for alternative approaches in the area of swine colibacillosis as well. Other approaches to control this disease include, beside attenuated E. coli vaccines, supplementation of feed with egg yolk antibodies from the chickens immunized with F4 or F18 fimbriae, breeding of F18– and F4-resistant swine, supplementation of diets with zinc, medium-chain fatty acids and/or spraydried plasma, dietary acidification, phage therapy or the use of probiotics.1,3,11 However, it shoud be taken into consideration that immunization methods and routes for inducing systemic immunity often delayed or prevented induction of mucosal immunity and vice versa.12 Namely, parenterally administrated E. coli vaccines tend to stimulate systemic rather than the mucosal immune system.4 Alexa et al.13 suggested the efficiency of combined parenteral and oral immunization against ETEC diarrhea in weaned piglets. Felder et al.14 examined the feasibility of peroral immunization with microencapsulated E. coli and detached fimbriae to prevent ETEC infections in pigs. In 2002, Cox et al.15 have reported that oral administration of F4 fimbrial antigens to the F4R+ pigs resulted in an intestinal mucosal immune response that completely protected the pigs against a challenge infection, while in F4R- pigs such response did not occur. Snoeck et al.16 studied the feasibility of oral vaccination of suckling piglets against F4+ ETEC infection with F4 fimbriae, but despite the induction of an immune response, the colonization of the small intestine by F4+ ETEC upon oral challenge could not be prevented. Application of F4 or F18 fimbriae in microspheres has also not proven useful.17 The protective effects of DNA vaccines (using whole plasmids containing specific virulence genes of ETEC) are still far to be seen.18 In 2004, Verdonck et al.19 reported that oral immunization of piglets with recombinant F4 fimbrial adhesin FaeG monomers induced both mucosal and systemic F4-specific immune responses. Such live attenuated oral vaccines have been suggested to be relatively effective in preventing ETEC-induced PWC in pigs, but the mechanisms responsible for protection have not been elucidated. Priming by levamisole of the vaccinated pigs has a tendency to trigger the mucosal rather than systemic immune system and abrogates the inefficacy of vaccination against porcine PWC induced by the vaccine alone.20 Božić et al.21 suggested that enhanced recruitment and activation of T cells in ileal Peyer's patches was a consequence of an interaction between resident T and B cells of levamisole primed vaccinated pigs. Snoeck et al.22 have shown that jejunal Peyer’s patches are the major inductive sites for the F4-specific intestinal antibody responses. Božić et al.23 observed that priming by levamisole of weaned pigs vaccinated with experimental F4ac+ E. coli oral vaccine stimulated their gut immune system, including the immune cell subsets within jejunal lamina propria and ileal Peyer's patches, upon virulent challenge. Božić et al.24 implied that levamisole exerts its potentiating activity in the mesenteric lymph node by augmenting both recruitment and activation of cells that participate in the cell-mediated immunity. More recent study,25 suggested that synergistic action of the drug and vaccine enhanced the T cell-mediated immunity in ileal Peyer’s patches. The results of our current study showed that vaccine candidate F4ac+ non-ETEC strain stimulated increase in the numbers of CD3+ T cells, CD45RA+ naive and CD45RC+ memory T cells in the lamina propria and Peyer’s patches of jejunum and ileum of weaned pigs. It is likely that increased numbers of CD3+, CD45RA+ and CD45RC+ T cells in jejunal and ileal lamina propria and ileal Peyer's patches are reflecting the initiation of specific responses induced by vaccination (with or without levamisole applied as an adjuvant), which may result in the induction of the protective immunity at mucosal surfaces of the gut. Our quantitative data on intestinal B cell response recorded by changes in the number of CD21+ cells following nonajuvanted and levamisole-adjuvanted vaccination revealed that later combination had less effect on proliferation of jejunal B cells, indicating that levamisole did not affect these cell subset as it did with T cell subsets tested. This is in agreement with earlier findings that levamisole has ability to stimulate T cells and macrophages, but not B cells26 or that its effect on these cells is questionable.27 Indeed, the immunostimulatory effect of levamisole (when given in combination with the vaccine) was more pronounced for T cells, particularly CD45RA+ naïve and CD45RC+ memory T cells, than for CD21+B cells. Increased numbers of SWC3+ myeloid cells (macrophages/granulocytes) in jejunum and ileum, and their distribution in the areas below epithelium of villi and inside Peyer’s patches, indicated their role in presenting antigens of vaccine candidate non-ETEC strain to T cells, and thus, commencing induction of specific immune response within small intestinal mucosa. Since we have found increased number of SWC3+ cells in the small intestinal mucosa of both principal groups of pigs, it seems that levamisole given as an adjuvant was not prerequisite for recruitment of jejunal and ileal macrophages/granulocytes as suggested by others.26
The succes of vaccination against PWC in our model system depends largely upon quantitative parameters of intestinal cellular immunity, but it also relies on data regarding mortality, incidence/severity of diarrhea, growth performance and shedding of virulent strains of E. coli. Although all 5 pigs developed diarrhea in both principal groups of pigs and only 3 of 5 in control group of pigs, it is very likely that they were infected prior to start of the experiment. Namely, they became diarrheic at day 1 and died at day 9 (in nonajuvanted-vaccinated group) and at day 3 (in levamisole adjuvanted-vaccinated group) of the experiment.
According to diarrhea scoring, it is obvious that pigs from group treated with vaccine candidate strain had moderate to severe diarrhea of 4–8 days of duration. Control pigs and those that received levamisole adjuvanted vaccine had mild to moderate diarrhea of either 3–5 or 2–6 days of duration, respectively.
However, control pigs had highest CFU number (4.0×107 ) as compared to both principal groups (5.5–6.0×106 ). As we have observed diarrhea in each group of pigs (Table 2; although of different intensity/duration, and in different no. of pigs per group) we have selected for euthanasia and sampling 3 pigs per group based on the following criteria: i) presence or absence of diarrhea, ii) intensity of diarrhea, and iii) duration of diarrhea. In order to obtain as much as possible uniform samples of jejunum and ileum for immunohistology, we have euthanatized pigs that were closely uniform regarding diarrhea status.
Table 2. Extent of diarrhea intensity expressed as scores based on stools consistency: −, firm feces = no diarrhea; +, soft feces = mild diarrhea; ++, fluid feces = moderate diarrhea; +++, watery feces = severe diarrhea.
| Treatment of pigsa | Pig no. | Day of experiment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9–13 | ||
| Noneb | 1 | |||||||||
| 2 | ++ | ++ | +++ | ++ | ++ | |||||
| 3 | ++ | + | + | |||||||
| 4 | ++ | + | + | |||||||
| 5 | ||||||||||
| F4ac+ non-ETEC | 1 | + | + | ++ | ++ | |||||
| 2 | ++ | ++ | ++ | ++ | ||||||
| 3 | ++ | +++ | +++ | ++ | ++ | ++ | ++ | |||
| 4 | ++ | +++ | ++ | ++ | ++ | ++ | ++ | +++ | c | |
| 5 | + | + | ++ | ++ | ||||||
| Levamisole +F4ac+ non-ETEC | 1 | ++ | + | + | + | + | ||||
| 2 | + | + | ||||||||
| 3 | ++ | ++ | +++ | ++ | ++ | ++ | ||||
| 4 | +++ | +++ | c | |||||||
| 5 | + | + | ||||||||
Groups comprised five 4-weeks-old pigs each.
Control pigs received saline as a placebo. cDied at either Day 9 or Day 3 due to diarrheal disease.
Moreover, analyzing quantitative data obtained by histomorphometry (Tables 4 and 5), it is obvious that the presence of diarrhea in sampled pigs from all three groups did not influence neither the expression of CD/SWC antigens on lymphoid and myeloid cell subsets tested nor their quantitative and distribution patterns. Thus, we assume that such sampling may objectively reflect the effects of immunizing agents applied on development of the protective immunity as established by highly elevated proliferation of immune cell subsets tested in both groups of immunized pigs. Our assumption is based on the fact that both principal groups of pigs had significantly increased numbers of all cell subsets tested at day 13 following either specific or non-specific/specific immunization and challenge infection. Also, based on DIA analyses we may undoubtedly differentiate the intestinal immune response of pigs induced by an experimental mucosal (ajuvanted or nonajuvanted) vaccine from that observed in the respective sites of the GALT of non-vaccinated pigs.
Finally, our results confirmed that F4ac+ non-ETEC strain stimulated proliferation of several immune cell subsets in the jejunal and ileal mucosa of weaned pigs, and thus it should be further studied as a vaccine candidate strain for the prevention and control of PWC. However, present study has failed to confirm levamisole, when given with F4ac+ non-ETEC strain, as an efficient adjuvant for mucosal vaccines. Although our recent approach has been at least partially successful, such as those in the past, developing of a safe and effective vaccine against PWC that will provide solid and sustained immune responses remains a formidable challenge.
Acknowledgments:
this study was supported by the grant nos. 053-0532265-2255, 053-0532265-2242 and 053-0532265-2248 from the Ministry of Science, Education and Sport of Croatia. The authors thank Mrs. Zrinka Benčina for excellent technical assistance and Gordana Gregorović, B.S., M.S. for image analyses support.
Supported by the Ministry of Science, Education and Sport of Croatia (Grant Nos. 053-0532265-2255, 053-0532265-2242 and 053-0532265-2248).
References
- 1.Fairbrother JM, Nadeau E, Gyles CL. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim Health Res Rev. 2005;6:17–39. doi: 10.1079/ahr2005105. [DOI] [PubMed] [Google Scholar]
- 2.Lalles JP, Bosi P, Smidt H, et al. A challenge to gut physiologists. Liv Sci. 2007;108:82–93. [Google Scholar]
- 3.Nagy B, Fekete PZs. Enterotoxigenic Escherichia coli (ETEC) in farm animals. Vet Res. 1999;30:259–84. [PubMed] [Google Scholar]
- 4.Moon HW, Bunn TO. Vaccines for preventing enterotoxigenic Escherichia coli infections in farm animals. Vaccine. 1993;11:213–20. doi: 10.1016/0264-410X(93)90020-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dacko J, Weiner M, Osek J. Classifying Escherichia coli strains isolated from piglets diagnosed with diarrhea through amplifying virulence markers with PCR. Med Wet. 2004;60:952–7. [Google Scholar]
- 6.Zhang W, Zhaoa M, Ruescha L, et al. Prevalence of virulence genes in Escherichia coli strains recently isolated from young pigs with diarrhea in the US. Vet Microbiol. 2007;123:145–52. doi: 10.1016/j.vetmic.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Casey TA, Moon HW. Genetic characterization and virulence of enterotoxigenic Escherichia coli mutants which have lost virulence genes in vivo. Infec Immun. 1990;58:4156–8. doi: 10.1128/iai.58.12.4156-4158.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guesdon JL, Ternynck T, Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979;27:1131–9. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- 9.Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–80. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 10.Nagy B, Fekete PZs. Enterotoxigenic Escherichia coli in veterinary medicine. Int J Med Microbiol. 2005;295:443–54. doi: 10.1016/j.ijmm.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Skrivanova E, Marounek M, Benda V, Brezina P. Susceptibility of Escherichia coli, Salmonella sp and Clostridium perfringens to organic acids and monolaurin. Vet Med. 2006;51:81–8. [Google Scholar]
- 12.MacDonald TT. Enhancement and suppression of Peyer's patch immune response by systemic priming. Clin Exp Immunol. 1982;49:441–441. [PMC free article] [PubMed] [Google Scholar]
- 13.Alexa P, Salajka E, Salajková Z, Máchová A. Combined parenteral and oral immunization against enterotoxigenic Escherichia coli diarrhea in weaned piglets. Vet Med. 1995;40:365–70. [PubMed] [Google Scholar]
- 14.Felder CB, Vorlaender N, Gander B, et al. Microencapsulated entero-toxigenic Escherichia coli and detached fimbriae for peroral vaccination of pigs. Vaccine. 2000;19:706–15. doi: 10.1016/s0264-410x(00)00264-4. [DOI] [PubMed] [Google Scholar]
- 15.Cox E, Van der Stede Y, Verdonck F, et al. Oral immunization of pigs with fimbrial antigens of enterotoxigenic E. coli: an interesting model to study mucosal immune mechanisms. Vet Immunol Immunopathol. 2002;87:287–90. doi: 10.1016/s0165-2427(02)00054-5. [DOI] [PubMed] [Google Scholar]
- 16.Snoeck V, Verdonck F, Cox E, Goddeeris BM. Inhibition of adhesion of F18+ Escherichia coli to piglet intestinal villous enterocytes by monoclonal antibody against blood group H-2 antigen. Vet Microbiol. 2004;100:241–6. doi: 10.1016/j.vetmic.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Snoeck V, Verfaillie T, Verdonck F, et al. The jejunal Peyer’s patches are the major inductive sites of the F4-specific immune response following intestinal immunization of pigs with F4 (K88) fimbriae. Vaccine. 2006;24:3812–20. doi: 10.1016/j.vaccine.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 18.Verfaillie T, Melkebeek V, Snoeck V, et al. Priming of piglets against ente-rotoxigenic Escherichia coli F4 fimbriae by immunisation with FAEG DNA. Vaccine. 2004;22:1640–7. doi: 10.1016/j.vaccine.2003.09.045. [DOI] [PubMed] [Google Scholar]
- 19.Verdonck F, Cox E, Van der Stede Y, Goddeeris BM. Oral immunization of piglets with recombinant F4 fimbrial adhesin FaeG monomers induces a mucosal and systemic F4-specific immune response. Vaccine. 2004;22:4291–9. doi: 10.1016/j.vaccine.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Božić F, Bilić V, Valpotić I. Modulation by levamisole of CD45RA and CD45RC isoforms expression in the gut of weaned pigs vaccinated against colibacillosis. J Vet Pharmacol Therap. 2002;25:69–72. doi: 10.1046/j.1365-2885.2002.00379.x. [DOI] [PubMed] [Google Scholar]
- 21.Božić F, Lacković G, Prevendar-Crnić A, et al. Levamisole stimulates intestinal T-cell-mediated immune responses of weaned pigs to vaccination against colibacillosis. J Vet Pharmacol Therap. 2003;26:282–307. [Google Scholar]
- 22.Snoeck V, Huyghebaert N, Cox E, et al. Enteric-coated pellets of F4 fimbriae for oral vaccination of suckling piglets against enterotoxigenic Escherichia coli infections. Vet Immunol Immunopathol. 2003;96:219–27. doi: 10.1016/j.vetimm.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Božić F, Šver L, Valpotić I. CD45RA and CD45RC isoforms expression in weaned pigs vaccinated with non-enterotoxigenic F4ac+ Escherichia coli strain against colibacillosis. Vet Med. 2002;47:5–11. [Google Scholar]
- 24.Božić F, Bilić V, Valpotić I. Levamisole mucosal adjuvant activity for a live attenuated Escherichia coli oral vaccine in weaned pigs. J Vet Pharmacol Therap. 2003;26:225–31. doi: 10.1046/j.1365-2885.2003.00458.x. [DOI] [PubMed] [Google Scholar]
- 25.Božić F, Lacković G, Kovšca-Janjatović A, et al. Levamisole synergizes experimental F4ac+ Escherichia coli oral vaccine in stimulating ileal Peyer’s patch T cells in weaned pigs. J Vet Pharmacol Therap. 2006;29:199–204. doi: 10.1111/j.1365-2885.2006.00731.x. [DOI] [PubMed] [Google Scholar]
- 26.Symoens J, Rosenthal M. Levamisole in the modulation of the immune response: the current experimental and clinical state. J Reticul Soc. 1977;21:175–221. [PubMed] [Google Scholar]
- 27.Van Wauwe J, Janssen PAJ. On the biochemical mode of action of levamisole: an update. Int J Immunopharm. 1991;13:3–9. doi: 10.1016/0192-0561(91)90019-4. [DOI] [PubMed] [Google Scholar]