Abstract
Hepatocyte growth factor (HGF) exerts proliferative activities in thyrocytes upon binding to its tyrosine kinase receptor c-met and is also expressed in benign thyroid nodules as well as in Hashimoto's thyroiditis (HT).
The simultaneous expression of HGF/c-met and three trasducers of tyrosine kinase receptors (STAT3, PI3K, RHO) in both the nodular and extranodular tissues were studied by immunohistochemistry in 50 benign thyroid nodules (NGs), 25 of which associated with HT. The ligand/tyrosine kinase receptor pair HGF/c-met and the two trasducers PI3K and RHO were expressed in NGs, regardless of association with HT, with a higher positive cases percentage in HT-associated NGs compared to not HT-associated NGs (25/25 or 100% vs 7/25 or 28%; P<0.001). HGF, PI3K and RHO expression was only stromal (fibroblasts and endothelial cells), in all 32 reactive NGs, while c-met localization was consistently epithelial (thyrocyes). Immunoreactions for HGF, c-met, PI3K and RHO were also apparent in the extra-nodular tissue of HT specimens, where HGF and PI3K were expressed not only in stromal cells but also in thyrocyes along with the c-met. Finally, a positive correlation was observed between the proportion of HGF, c-met, PI3K follicular cells and the grade of lymphoid aggregates in HT. In conclusion, HGF, c-met, PI3K are much more frequently and highly expressed in HT compared to NGs, and among all NGs in those present in the context of HT. A paracrine effect of HFG/c-met on nodule development, based on the prevalent stromal expression, may be suggested along with a major role of HGF/c-met and PI3K in HT. Finally, the expression of such molecules in HT may be regulated by lymphoid infiltrate.
Key words: HGF/c-met signaling, PI3K, RHO, Hashimoto's thyroiditis, thyroid nodules.
Introduction
Hashimoto's thyroditis (HT) is the most prevalent autoimmune thyroid disease worldwide and is characterized by variable clinical presentation with respect to proliferation of the follicular cells.1,2 Thyrocyte proliferation may be very intense in HT, thus leading to multiple nodular lesions.3–6 Increased prevalence of HT patients with associated nodular goiter remains high in moderately iodine-deficient areas such as southern Italy.5
Growth factors other than thyrotropin (TSH), and cytokines favor the development of diffuse and/or nodular goiter.7–9 However, only few studies have evaluated the role of different growth factors in the nodular variant of HT.10,11
Recently, we reported the immunohistochemical expression of the hepatocyte growth factor (HGF) in HT-associated nodular goiter specimens and demonstrated that it was more frequent and intense than what observed in non-HT goiters.4 Upon binding to its specific tyrosine-kinase receptor (HGF-R or c-met), HGF exerts mitogenic and anti-apoptotic activities in various cell types, including follicular thyroid cells.12–14 Previous studies demonstrated that HGF and c-met are expressed in hyperplastic nodules (but non in normal thyroid tissue) and are over-expressed in papillary thyroid carcinomas (PTC).15–18
Similarly to activation of the other ligand/ tyrosine kinase receptors, activation of the HGF/c-met signaling system recruits several intracellular effectors, including phosphatidylinositol 3-kinase (PI3K), Ras, adaptators GRB2 and SHC, the docking protein Gab1, the member of the signal transducers and activators of transcription family STAT3, β catenin and RHO.19–23 Such effectors are ubiquitous, as they are expressed in all human tissues, and trigger distinct biological events, i.e. growth, scattering and morphogenesis, in epithelial cells.21–29 Concerning thyroid oncology, expression of these ubiquitous effectors (for instance, STAT3, PI3K) has been investigated mainly in malignant thyroid tumors arising from the follicular epithelium,16,30–33 but rarely together with expression of HGF/c-met.16,30 The few data available on the expression of such molecules in thyroid nodules of HT patients are mainly focused on the possible association between HT and thyroid cancer.34 Larson and coworkers reported that the PI3K pathway components p-Akt, Akt1, and Akt2 were highly expressed in HT and HT-associated PTC, as well as in non-PTC, but not in the normal follicular epithelium. On this basis, they suggest that the PI3K/Akt pathway activation might represent a common molecular mechanism between the chronic autoimmune inflammation of thyroid and PTC.34
Moreover, two recent studies related PI3K expression/activation with the mechanisms of immunity response.35,36 It is well known that autoimmune thyroide diseases is related with the balance between T helper types 1 and 2 (Th1 and Th2) responses, by an involvement of Toll-like receptors (TLR).1,2,37 The subunit p85 of PI3K participates, in this framework, in the ligation with TLR controlling the Th1/Th2 balance for an advantage of Th1 cytokines.35 Moreover, PI3K regulatory subunits p85 regulates the motility of T and B lymphocytes.36
Here we evaluate the immunohistochemical expression of ligand/tyrosine kinase receptor pair HGF/c-met and three trasducers of activated tyrosine kinase receptors (STAT3, PI3K, RHO) in benign thyroid nodules, half of which developed in the context of background HT. To the best of our knowledge, the expression of these molecules has not been previously examined in HT.
Materials and Methods
Tissues collection
Fifty surgical thyroid specimens were retrieved from the files of our Pathology Department, while 5 normal thyroids (NT) were harvested at autopsy. All specimens were 4% formalin-fixed and paraffin-embedded. The 50 surgical specimens were from patients diagnosed and followed up at our Endocrine Unit and included benign nodular goiters (NG), of which 25 were associated with HT. In such patients, the diagnosis of HT was based on the currently accepted clinical, laboratory (circulating thyroid antibodies) and ultrasonographic criteria. All nodular lesions were studied along with the non-nodular tissue from the contralateral thyroid lobe (Table 1).
Table 1. Expression of HGF, c-met, PI3K and RHO on epithelial and stromal cells in a series of 25 colloid nodular goiters (NGs) and of 25 nodular Hashimoto's thyroditis (HT) examined along with the corresponding extra-nodular thyroid tissue.
| Thyroid lesions Cases | Positive Cases | Reactive* Cells* | Percentage of positive cells (mean ±SD)° | |||
|---|---|---|---|---|---|---|
| HGF | c-met | PI3K | RHO | |||
| Colloid nodular goiters (NGs, n = 25) | 7 (28%) | Epithelial (DN-EC) | 0 | 4±2 | 0 | 0 |
| stromal | 9±2 | 0 | 7±3 | 8±3 | ||
| NGs associated with HT (n = 25) | 25 (100%) | Epithelial (DN-EC) | 0 | 4±2 | 0 | 0 |
| stromal | 12±8 | 0 | 8 ± 4 | 7±3 | ||
| Extra-nodular thyroid tissue of HT (n = 25) | 25 (100%) | Epithelial | ||||
| DN-EC | 58±20 | 19±5 | 25 ± 8 | 0 | ||
| DN-OC | 24±25 | 9±8 | 0 | 0 | ||
| CN-EC | 21±13 | 8±5 | 0 | 0 | ||
| stromal | 11±8 | 0 | 8±4 | 8±3 | ||
Thyroid lesions were catalogued by cytological morphology of thyrocytes based on nuclear and cytoplasm features at light microscopy,4,39 as detailed in Material and Methods. Based on these morphological features, the cells of each lesion were categorized as: i) dark nucleus and eosinophilic cytoplasm (DN-EC); ii) dark nucleus and oncocytic cytoplasm (DN-OC); iii) clear nucleus and eosinophilic cytoplasm (CN-EC).
The count of the reactive epithelial and stromal cells was based on evaluation of 1000 cells/case, using a 50X magnification. Values pertaining to stromal cells are typed in italics.
Hematoxylin-eosin-stained (H&E) sections of each specimen were re-evaluated by the pathologists in order to confirm the HT diagnosis, evaluate the intra-glandular inflammatory lymphoid aggregates and catalogue the lesions by cytological morphology. HT was diagnosed using histopathologic criteria of Li Volsi.38
The lymphoid aggregates were categorized as previously reported.4,11 Briefly, aggregates made up of at least 150 lymphocytes and a variable number of plasma cells per high-power field, were considered as lymphoid aggregates and scored from 0 to 3 (0=no lymphoid aggregate or at most one single, small lymphoid aggregate without germinal center in each section; 1=occasional, usually small lymphoid aggregates with rare or absent germinal centers in each section; 2=several, usually mixed, small and large lymphoid aggregates with some germinal center in each section; 3=numerous, large lymphoid aggregates with frequent germinal centers in each section).
Cellular morphology was classified according to the nuclear and cytoplasm features at light microscopy, as previously reported.4,39 Based on these morphological features, the thyrocytes of each lesion were classified into three different cellular types: i) dark nucleus and eosinophilic cytoplasm (DN-EC); ii) dark nucleus and oncocytic cytoplasm (DN-OC or Hurtle cells); iii) clear nucleus and eosinophilic cytoplasm (CN-EC) (Table 1).
Immunohistochemistry
The 55 blocks were cut into serial sections of five-micrometers each. Immunohistochemistry was performed, separately, using rabbit polyclonal antibodies against HGF-α (H-145, working dilution (w.d.) 1:100), c-met (p140 anti h-met, w.d. 1:100) and STAT3 (h-190, w.d.1:100), all three purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA); a mouse monoclonal antibody against PI3K p85α (B-9, w.d. 1:200), purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA), and a rabbit monoclonal antibody against RHO (Y486, w.d.1:100), purchased from Abcam plc., Cambridge, UK. Tissue sections were deparaffinized in xylene and rehydrated in alcohol. Next, the endogenous biotin was inactivated by the addition of a 0.05% (v/v) solution of streptavidin in phosphate-buffered saline (PBS), and the endogenous peroxidase activity was quenched by adding a 0.3% (v/v) solution of 3% H2O2/methanol for 30 min. Staining was obtained with the LSAB system (kit from Dako, Carpinteria, CA, USA). 3,3′-diaminobenzidine (DAB, Sigma) activated with 0.05% hydrogen peroxide was used to develop the end reactions. Sections were counterstained with Mayer's hematoxylin, dehydrated and mounted.
Specificity of the antibody binding was assessed by omitting the primary antiserum or by replacing the primary antiserum with normal rabbit or mouse serum. In each of these conditions, no staining was evident. In addition, an immunoabsorption test was performed to confirm the specific immunoreactivity of each antibody. Specimens of normal tissues of bladder and liver were used as positive controls for HGF and c-met immunoreaction, respectively. Positive controls for STAT3, PI3K and RHO immonoreaction were performed by using specimens of lung, breast and liver carcinomas, respectively. The evaluation of the results was based on: i) number of positive cases; ii) number of reactive epithelial and stromal cells per case, based on counting 1000 cells/case at 50X magnification; iii) subcellular location of the staining: membrane, cytoplasm and nucleus; iv) staining grade using a semiquantitave score system from 0 to 4+ (0, absent; 1+, weak but distinct; 2+, moderate; 3+ , intense; 4+, very intense). Histological and immunohistochemical evaluations were done twice and blindly by two pathologists (M.T., G.B.) with an inter-observer concordance of nearly 100%. Where minimal inter-observer discrepancies were present, data were averaged.
Statistical analysis
Data were tested for normal distribution and variance (mean ± SD) and analyzed by the two-tailed Student's t-test, ANOVA and χ2 test with Yates' correction for continuity, when appropriate. The association between two variables was analyzed by the linear regression analysis. The level of statistical significance was always set at P<0.05.
Results
Histopathology and cellular morphology
All the 50 benign NGs showed the histological features of colloid nodules characterized by either large or small colloid-filled follicles built by cells with small flat or cubic shape. Instead, cellular hallmarks of these lesions were the epithelial follicular cells with DN-EC features, even if in most cases of HT-associated NGs, DN-OC cells and seldom CN-EC cells too were observed (Table 1). The predominant cellular type in HT showed features of DN-EC cells. Intra-nodular lymphoid aggregates were absent (grade 0) in all 50 NGs. Extra-nodular lymphoid aggregates were the main histological evidence of HT. These aggregate were graded as 1 (10/25 or 40%), 2 (12/25 or 48%) or 3 (3/25 or 12%). As previously specified into the Materials and Methods paragraph, the 25 HT specimens were from patients diagnosed at our Endocrine Unit, and all of them have circulating anti-thyroid antibodies.
Immunohistochemistry
Illustrative immunohistochemistry for HGF, c-met, PI3K and RHO is presented in Figure 1. No expression of HGF, c-met, STAT3, PI3K and RHO was detected in normal thyroid tissue, namely the control normal thyroids and the contralateral lobe to the 25 NGs not associated with HT. Similarly, no immunostaining for STAT3 was observed in all nodular lesions.
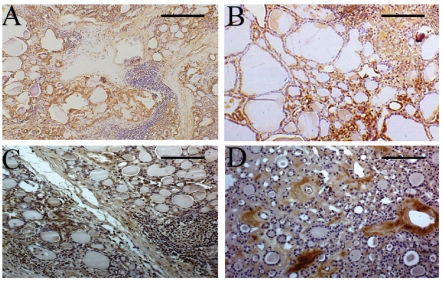
Figure 1.
HGF, c-met, PI3K and RHO immunostaining in specimens of Hashimoto's thyroditis (HT) and nodular goiters (NGs). Panel A: very intense HGF immunoreaction in a sample of HT with lymphoid aggregates defined as grade 2. The HGF immunostaining is located on the cytoplasm of thyrocytes (brown deposits) showing morphological characteristics of DN-EC cells. Note the absence of HGF reactivity in the lymphocytes (original magnification: X 150, reference bar = 133 µm). Panel B: intense c-met immunostaining in a sample of HT with grade 2 lymphoid aggregates. The c-met reaction is observed on the membrane and cytoplasm (brown deposits) of follicular DN-EC cells. No c-met reactivity is detected in lymphocytes (original magnification: X 150, reference bar = 133 µm). Panel C: moderate PI3K immunoreaction in a sample of HT with grade 2 lymphoid aggregates. The immunoreaction is located in the cytoplasm of epithelial DN-EC cells (brown deposits). Note the absence of PI3K reactivity in lymphocytes (original magnification: X 130, reference bar = 153 µm). Panel D: moderate RHO immunoreaction in a sample of NG not associated with HT. The RHO immunostaining is located on the cytoplasm of stromal cells (original magnification: X 130, reference bar = 153µm).
Expressions of HGF/ c-met/PI3K/RHO in NGs
The ligand/tyrosine kinase receptor pair HGF/c-met and the two trasducers PI3K and RHO were expressed in NGs, regardless of association with HT. However, significant differences in the distribution of immunoreactions could be detected by comparing the NGs arising in the context of HT with the HT-unassociated NGs. The expression of HGF, c-met, PI3K and RHO was detected more frequently in the former than in the latter nodules (25/25 or 100% vs 7/25 or 28%; P<0.001) (Table 1). However, there were no differences in the localization of immunostaining between the two types of lesions. Thus, in all 32 reactive NGs, HGF, PI3K and RHO expressions were only stromal (fibroblasts and endothelial cells) and cytoplasmic, while the localization of c-met was consistently epithelial (thyrocyes), both membranous and cytoplasmic. The percentage of reactive cells and the intensity of their immunoreactivity were similar in both types of NGs. Thus, immunoreaction for HGF, PI3K and RHO concerned 5–10% of stromal cells, and intensity of immunostaining was weak or moderate. In the same 32 nodules, a weak c-met immunoreaction was restricted to 3–8% of thyrocytes (Table 1). The c-met reactive epithelial cells of all 32 nodules showed the cytological features of DN-EC cells.
Expressions of HGF/ c-met/ STAT3/PI3-K/RHO in the non-nodular tissue of HT
Immunoreactions for HGF, c-met, PI3K and RHO were also observed in the non-nodular tissue of HT specimens, but their expression differed from what observed in NGs (Table 1).
Indeed, while stromal expression of HGF, PI3K and RHO matched the corresponding stromal expression in NGs, the cytoplasm of thyrocytes also expressed HGF and PI3K, an epithelial immunoreactivity that was absent in all NGs. HGF and c-met were detected in thyrocytes with DN-EC, DN-OC and CN-EC features, while PI3K was expressed by epithelial follicular cells with the sole DN-EC features (10–35%) (Table 1 and Figure 1). Hence, thyrocytes with DN-EC features expressed the three molecules (HGF, c-met and PI3K), while both DN-OC and CN-EC thyrocytes expressed only HGF and its receptor c-met. Among thyrocytes immunoreacting for both HGF and c-met, the major proportion of HGF and c-met +ve cells showed DN-EC features (HGF+ve DN-EC cells: 58±20%, range 30 to 80%; c-met +ve DN-EC cells: 19±5%, range 10 to 25%; P<0.001 vs the corresponding percentages of DN-OC and CN-EC cells) (Table 1). Moreover, all HT lesions showed an intense stromal immunostaining for HGF (which concerned 5–40% of cells) as well as PI3K and RHO (5–15% of cells) (Table 1). Finally, the staining grade observed for the four proteins in HT was higher than that observed in NGs, because it was moderate to very intense as compared with weak to moderate (data non shown).
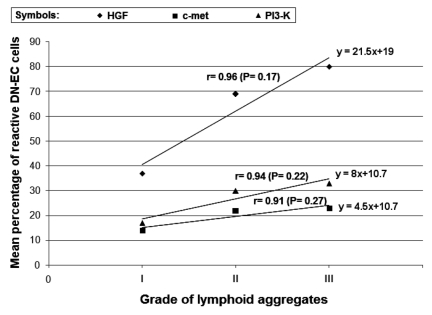
Comparing the proportion of DN-EC cells expressing the HGF, c-met and PI3K with the grade of lymphoid aggregates, a positive correlation was observed for the three protein [HGF: r=0.96, P=0.17; c-met: r=0.91, P=0.22; PI3K: r=0.94, P=0.27] (Figure 2). Moreover, by splitting the HT cases into two subgroups, according to the presence or absence of germinal centers in the lymphocytic infiltrate (Table 2), we found that the expression of c-met, PI3K and especially HGF in epithelial cells was much more pronounced in the 15 cases with presence of germinal centers (lymphocytic infiltrate grade 2 and 3), as compared to the 10 cases with lymphocytic infiltrate grade 1, in which the lymphoid aggregates were occasional with rare or absent germinal centers (P<0.001). This observation reinforces the hypothesis that the expression of such molecules in HT may be regulated by lymphoid infiltrate.
Figure 2.
Positive correlation between HGF, c-met and PI3K expressions and the grade of lymphoid aggregates, which is scored as indicated in Materials and Methods.
Table 2. Expression of HGF, c-met, PI3K and RHO on epithelial and stromal cells in the extra-nodular tissue of the 25 cases of Hashimoto's thyroditis (HT), split into two subgroups (A and B) according to the presence or absence of germinal centers in the lymphoid infiltrate*.
| HT (n=25) | Positive cases | Reactive cases° | Percentage of positive cells (mean ±SD)# | |||
|---|---|---|---|---|---|---|
| HGF | c-met | PI3K | RHO | |||
| Group A | #1–10 | epithelial | ||||
| (Lymphoid aggregate grade 1) (n=10) | DN-EC | 37±8 | 14±4 | 17±4 | 0 | |
| DN-OC | 4±8 | 2±4 | 0 | 0 | ||
| CN-EC | 14±12 | 5±4 | 0 | 0 | ||
| stromal | 15±11 | 0 | 9±4 | 8±3 | ||
| Group B | #11–25 | epithelial | ||||
| (Lymphoid aggregate grade 2 and 3) ( n=15) | DN-EC | 71±11 | 22±2 | 30±5 | 0 | |
| DN-OC | 38±22 | 13±7 | 0 | 0 | ||
| CN-EC | 26±13 | 10±4 | 0 | 0 | ||
| P<0.001 vs Group A | ||||||
| stromal | 7±5 | 0 | 8±3 | 7±2 | ||
| P<0.025 vs Group A | ||||||
Lymphoid aggregates were scored from 0 to 3 (0=no lymphoid aggregate; 1=occasional lymphoid aggregates with rare or absent germinal centers in each section; 2=several lymphoid aggregates with some germinal center; 3=numerous lymphoid aggregates with frequent germinal centers), as detailed in Material and Methods.
Thyroid follicular cells were categorized as: i) dark nucleus and eosinophilic cytoplasm (DN-EC); ii) dark nucleus and oncocytic cytoplasm (DN-OC); iii) clear nucleus and eosinophilic cytoplasm (CN-EC), based on nuclear and cytoplasm features at light microscopy,4,39 as detailed in Material and Methods.
The count of the reactive epithelial and stromal cells was based on evaluation of 1000 cells/case, using a 50X magnification. Values pertaining to stromal cells are typed in italics.
Discussion
True nodular lesions frequently arise in the context of HT, as consequence of a hyperplastic follicular growth, which leads to an increase in number and size of follicles. Several growth factors and cytokines are known to cooperate with TSH in thyroid nodular growth, but few data are available on their expression in nodular lesions associated with HT.10,11
In the present study, we report that the expression of the HGF/c-met system is associated with that of the tyrosine kinase receptors trasducers PI3K and RHO in both NGs and HT lesions, while the normal thyroids do not express any. These findings are in line with the known ability of HGF to induce cellular growth, as well as with our previous reports in which we observed that HGF and its receptor c-met are expressed in goitrous samples as well as in thyroid tissues with HT, but undetectable in normal thyroid tissue.4,15 Our present study offers some new knowledge.
First, we demonstrated the expression of the tyrosine kinase receptors trasducers PI3K and RHO in both HNs and HT lesions expressing the HGF/cmet system, but we detected a more frequent expression of HGF, c-met, PI3K and RHO in the NGs associated with HT, either with scarce lymphoid aggregates or with germinal centers, as compared to non HT-associated NGs.
Another major result of our study is the different localization of HGF and PI3K expressions in NGs and HT, even when occurring in the same thyroid gland. In the NGs, approximately 10% of stromal cells expressed HGF, thus providing the ligand for a paracrine interaction with the c-met expressed on a limited number of epithelial cells. However, this possible interaction was not accompanied by the epithelial expression of any of the three trasducers investigated (STAT3, PI3K and RHO). In fact, both PI3K and RHO expression was restricted to approximately 8% of the stromal cells of NGs regardless of coexisting HT. Therefore, the cellular localization of such molecules seems to be independent from the HT lesion associated with the nodular goiter. Further, the expression of PI3K and RHO in stromal cells appears to be independent from activation of the HGF/c-met signaling in this cellular type, since there was no stromal expression of c-met. In HT, HGF and PI3K immunoreactions involved both stromal and epithelial cells, raising the possibility of c-met activation through an autocrine loop with subsequent involvement of PI3K.
The characterization of the follicular epithelial cells of HT based on the nuclear and cytoplasmic features allowed us to identify the follicular cells with DN-EC features as the cellular type specifically involved in the epithelial pathway of HGF, c-met and PI3K. Indeed, the epithelial co-expression of HGF, c-met and PI3K was observed only in such cells, while the other reactive epithelial cellular types expressed only the ligand and the receptor.
These evidences suggest that the HGF/c-met system may play a role in the development of nodular goiter, and more specifically in the goitrous growth of HT. Moreover, these data suggest that HGF acts as a goitrous growth factor in a paracrine fashion in HT-associated or not HT-associated NGs, but both in a paracrine and autocrine fashion in HT. The expression of the three molecules (c-met, PI3K and especially HGF) is much more pronounced in nodules associated with diffuse HT, proved by the presence of germinal centers, rather than in HT with scarce lymphoid aggregates. In those latter, however, the expression of the investigated molecules is significantly higher with respect to non HT-NGs.
Finally, the positive correlation we found between the immunoistochemical expression of HGF, c-met and PI3K in DN-EC cells and the lymphoid aggregates lead us to postulate that HGF, c-met and PI3K signals may have a role also in the mechanism of thyroid autoimmunity, as supported by two recent studies relating PI3K expression/activation with the mechanisms of the immunity response.35,36 In this way, the expression of PI3K in DN-EC thyrocytes could be involved in the cascade of immune events, such as in presenting antigens and recalling up lymphocytes.
Acknowledgements:
the authors thank Giuseppe Trombetta, Ph.D, for collaboration with his skillness.
References
- 1.Weetman AP. Autoimmune thyroid disease: propagation and progression. Eur J Endocrinol. 2003;148:1–9. doi: 10.1530/eje.0.1480001. [DOI] [PubMed] [Google Scholar]
- 2.Weetman AP. Autoimmune thyroid disease. Autoimmunity. 2004;37:337–40. doi: 10.1080/08916930410001705394. [DOI] [PubMed] [Google Scholar]
- 3.Rago T, Chiovato L, Grasso L, Pinchera A, Vitti P. Thyroid ultrasonography as a tool for detecting thyroid autoimmune diseases and predicting thyroid dysfunction in apparently healthy subjects. J Endocrinol Invest. 2001;24:763–9. doi: 10.1007/BF03343925. [DOI] [PubMed] [Google Scholar]
- 4.Ruggeri RM, Sciacchitano S, Trimarchi F, Barresi G, Trovato M. Expression of hepatocyte growth factor in Hashimoto's thyroiditis with nodular lesions. Eur J Histochem. 2007;51:193–8. [PubMed] [Google Scholar]
- 5.Benvenga S, Trimarchi F. Changed presentation of Hashimoto's thyroiditis in North-Eastern Sicily and Calabria (Southern Italy) based on a 31-year experience. Thyroid. 2008;18:429–41. doi: 10.1089/thy.2007.0234. [DOI] [PubMed] [Google Scholar]
- 6.Ruggeri RM, Sciacchitano S, Vitale A, Cardelli P, Galletti M, Vitarelli E, et al. Serum Hepatocyte Growth Factor (HGF) is increased in Hashimoto's thyroiditis either or nor associated with nodular goiter as compared with healthy non-goitrous individuals. J Endocrinol Invest. 2009;32:465–9. doi: 10.1007/BF03346487. [DOI] [PubMed] [Google Scholar]
- 7.Bidey SP, Hill DJ, Eggo MC. Growth factors and goitrogenesis. J Endocrinol. 1999;160:321–32. doi: 10.1677/joe.0.1600321. [DOI] [PubMed] [Google Scholar]
- 8.Kimura T, Van Keymeulen A, Golstein J, Fusco A, Dumont JE, Roger PP. Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocr Rev. 2001;22:631–56. doi: 10.1210/edrv.22.5.0444. [DOI] [PubMed] [Google Scholar]
- 9.Eggo MC, Quiney VM, Campbell S. Local factors regulating growth and function of human thyroid cells in vitro and in vivo. Mol Cell Endocrinol. 2003;213:47–58. doi: 10.1016/j.mce.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 10.Ruggeri RM, Villari D, Simone A, Scarfì R, Attard M, Orlandi F, et al. Co-expression of interleukin-6 (IL-6) and interleukin-6 receptor (IL-6R) in thyroid nodules is associated with co-expression of CD30 ligand/CD30 receptor. J Endocrinol Invest. 2002;25:959–66. doi: 10.1007/BF03344068. [DOI] [PubMed] [Google Scholar]
- 11.Ruggeri RM, Barresi G, Sciacchitano S, Trimarchi F, Benvenga S, Trovato M. Immunoexpression of the CD30 ligand/ CD30 and IL-6/IL-6R signals in thyroid autoimmune diseases. Histol Histopathol. 2006;21:249–56. doi: 10.14670/HH-21.249. [DOI] [PubMed] [Google Scholar]
- 12.Bottaro DP, Rubin JS, Faletto D, Chan AM, Kmiecik TE, Vande Woude GF, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–4. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 13.Weidner KM, Sachs M, Birchmeier W. The Met receptor tyrosine kinase transduces motility, proliferation, and morphogenic signals of scatter factor/hepatocyte growth factor in epithelial cells. J Cell Biol. 1993;121:145–54. doi: 10.1083/jcb.121.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eccles N, Ivan M, Wynford-Thomas D. Mitogenic stimulation of normal and oncogene transformed human thyroid epithelial cells by hepatocyte growth factor. Mol Cell Endocrinol. 1996;117:247–51. doi: 10.1016/0303-7207(95)03757-8. [DOI] [PubMed] [Google Scholar]
- 15.Trovato M, Villari D, Bartolone L, Spinella S, Simone A, Violi MA, et al. Expression of the hepatocyte growth factor and c-met in normal thyroid, non-neoplastic, and neoplastic nodules. Thyroid. 1998;8:125–31. doi: 10.1089/thy.1998.8.125. [DOI] [PubMed] [Google Scholar]
- 16.Trovato M, Grosso M, Vitarelli E, Ruggeri RM, Alesci S, Trimarchi F, et al. Distinctive expression of STAT3 in papillary thyroid carcinomas and a subset of follicular adenomas. Histol Histopathol. 2003;18:393–9. doi: 10.14670/HH-18.393. [DOI] [PubMed] [Google Scholar]
- 17.Scarpino S, D'Alena FC, Di Napoli A, Ballarini F, Prat M, Ruco LP. Papillary carcinoma of the thyroid: evidence for a role for hepatocyte growth factor (HGF) in promoting tumour angiogenesis. J Pathol. 2003;199:243–50. doi: 10.1002/path.1278. [DOI] [PubMed] [Google Scholar]
- 18.Mineo R, Costantino A, Frasca F, Sciacca L, Russo S, Vigneri R, et al. Activation of the hepatocyte growth factor (HGF)-Met system in papillary thyroid cancer: biological effects of HGF in thyroid cancer cells depend on Met expression levels. Endocrinology. 2004;145:4355–65. doi: 10.1210/en.2003-1762. [DOI] [PubMed] [Google Scholar]
- 19.Graziani A, Gramaglia D, Cantley LC, Comoglio PM. The tyrosine-phosphorylated hepatocyte growth factor/scatter factor receptor associates with phosphatidylinositol 3-kinase. J Biol Chem. 1991;266:22087–90. [PubMed] [Google Scholar]
- 20.Ponzetto C, Zhen Z, Audero E, Maina F, Bardelli A, Basile ML, et al. Specific uncoupling of GRB2 from the met receptor. Differential effects on transformation and motility. J Biol Chem. 1996;271:14119–23. doi: 10.1074/jbc.271.24.14119. [DOI] [PubMed] [Google Scholar]
- 21.Boccaccio C, Ando M, Tamagnone L, Bardelli A, Michieli P, Battistini C, et al. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature. 1998;391:285–8. doi: 10.1038/34657. [DOI] [PubMed] [Google Scholar]
- 22.Okano J, Shiota G, Matsumoto K, Yasui S, Kurimasa A, Hisatome I, et al. Hepatocyte growth factor exerts a proliferative effect on oval cells through the PI3K/AKT signaling pathway. Biochem Biophys Res Commun. 2003;309:298–304. doi: 10.1016/j.bbrc.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Vandeput F, Perpete S, Coulonval K, Lamy F, Dumont JE. Role of the different mitogen-activated protein kinase subfamilies in the stimulation of dog and human thyroid epithelial cell proliferation by cyclic adenosine 5'-monophosphate and growth factors. Endocrinology. 2003;144:1341–9. doi: 10.1210/en.2001-211316. [DOI] [PubMed] [Google Scholar]
- 24.Ridley AJ, Comoglio PM, Hall A. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–22. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potempa S, Ridley AJ. Activation of both MAP kinase and phosphatidylinositide 3-kinase by Ras is required for hepatocyte growth factor/scatter factor-induced adherens junction disassembly. Mol Biol Cell. 1998;9:2185–200. doi: 10.1091/mbc.9.8.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bardelli A, Basile ML, Audero E, Giordano S, Wennström S, Ménard S, et al. Concomitant activation of pathways downstream of Grb2 and PI 3-kinase is required for MET-mediated metastasis. Oncogene. 1999;18:1139–46. doi: 10.1038/sj.onc.1202607. [DOI] [PubMed] [Google Scholar]
- 27.Kodama A, Matozaki T, Fukuhara A, Kikyo M, Ichihashi M, Takai Y. Involvement of an SHP-2-Rho small G protein pathway in hepatocyte growth factor/scatter factor-induced cell scattering. Mol Biol Cell. 2000;11:2565–75. doi: 10.1091/mbc.11.8.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Royal I, Lamarche-Vane N, Lamorte L, Kaibuchi K, Park M. Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol Biol Cell. 2000;11:1709–25. doi: 10.1091/mbc.11.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coulonval K, Vandeput F, Stein RC, Kozma SC, Lamy F, Dumont JE. Phosphatidylinositol 3-kinase, protein kinase B and ribosomal S6 kinases in the stimulation of thyroid epithelial cell proliferation by cAMP and growth factors in the presence of insulin. Biochem J. 2000;348:351–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Z, Hou P, Ji M, Guan H, Studeman K, Jensen K, et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-Kinase/Akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab. 2008;93:3106–16. doi: 10.1210/jc.2008-0273. [DOI] [PubMed] [Google Scholar]
- 31.Paes JE, Ringel MD. Dysregulation of the phosphatidylinositol 3-kinase pathway in thyroid neoplasia. Endocrinol Metab Clin North Am. 2008;37:375–87. doi: 10.1016/j.ecl.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santarpia L, El-Naggar AK, Cote GJ, Myers JN, Sherman SI. Phosphatidylinositol 3-kinase/akt and ras/raf-mitogen-activated protein kinase pathway mutations in anaplastic thyroid cancer. J Clin Endocrinol Metab. 2008;93:278–84. doi: 10.1210/jc.2007-1076. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Hou P, Yu H, Wang W, Ji M, Zhao S, et al. High prevalence and mutual exclusivity of genetic alterations in the phosphatidylinositol-3-kinase/AKT pathway in thyroid tumors. J Clin Endocrinol Metab. 2007;92:2387–90. doi: 10.1210/jc.2006-2019. [DOI] [PubMed] [Google Scholar]
- 34.Larson SD, Jackson LN, Riall TS, Uchida T, Thomas RP, Qiu S, et al. Increased incidence of well-differentiated thyroid cancer associated with Hashimoto's thyroiditis and the role of the PI3K/Akt pathway. J Am Coll Surg. 2007;204:764–73. doi: 10.1016/j.jamcollsurg.2006.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hazeki K, Nigorikawa K, Hazeki O. Role of phosphoinositide 3-kinase in innate immunity. Biol Pharm Bull. 2007;30:1617–23. doi: 10.1248/bpb.30.1617. [DOI] [PubMed] [Google Scholar]
- 36.Matheu MP, Deane JA, Parker I, Fruman DA, Cahalan MD. Class IA phosphoinositide 3-kinase modulates basal lymphocyte motility in the lymph node. J Immunol. 2007;179:2261–9. doi: 10.4049/jimmunol.179.4.2261. [DOI] [PubMed] [Google Scholar]
- 37.Caturegli P, Kimura H, Rocchi R, Rose NR. Autoimmune thyroid diseases. Curr Opin Rheumatol. 2007;19:44–8. doi: 10.1097/BOR.0b013e3280113d1a. [DOI] [PubMed] [Google Scholar]
- 38.Li Volsi V. Lymphocytes in the thyroid. In: Li Volsi V, editor. Surgical Pathology of the Thyroid. W.B. Saunders Company; Philadelphia: 1990. pp. 68–97. [Google Scholar]
- 39.Trovato M, Ulivieri A, Dominici R, Ruggeri RM, Vitarelli E, Benvenga S, et al. Clinicopathological significance of cell-type-specific loss of heterozygosity on chromosome 7q21: analysis of 318 microdissected thyroid lesions. Endocr Relat Cancer. 2004;11:365–76. doi: 10.1677/erc.0.0110365. [DOI] [PubMed] [Google Scholar]