Abstract
In the current study, we aimed at investigating the presence of nitric oxide synthase (NOS) positive nerve fibers in rat meibomian glands (MGs) at various stages of development. There is good evidence to suggest that nicotinamide adenine dinucleotide phosphate diaphorase (NADPH-d) is a surrogate for neuronal nitric oxide synthase (NOS). Sections of the central, upper eyelids of Wistar rats were processed histochemically for NADPH-d to investigate the presence and distribution of NOS-positive nerve fibers at the following time points: day 1 and weeks 1, 2 and 3 post partum, and in adult controls. At day 1, MG acini were lightly stained and located at a distance from the mucosal border. Vessels were accompanied by intensely stained NADPH-d positive nerve fibers. At the week 1 time point, both the vessels and the NADPH-d positive fibers were still present, but less numerous. MGs were now closer to the mucosa, so that the submucosa was thinner. The acini were mostly pale but occasionally darker. At week 3, there were fewer blood vessels in both the sub-mucosa and within the septa. Darker acini were more common than lightly stained acini. NADPH-d positive dots were observed in the vicinity of the MGs. At the week 3 time point, MGs were adjacent to the mucosal border and stained more intensely than at earlier times; almost all acini were stained. The microscopic appearances were almost identical with those of adult palpebra. Submucosal and septal blood vessels and NADPH-d positive nerve fibers were less numerous. NADPH-d histochemical staining confirmed differences in the density of stained nerve fibers at different developmental stages. The greatest density of NADPH-d -positive nerve fibers occurred in 1-day-old rats whereas they were less numerous in adult rat eyelids. Nerves innervating MGs utilize nitric oxide (NO) as a neurotransmitter mostly in early developmental stages and this need thereafter decreases and stabilizes at 3 weeks postnatally.
Key words: meibomian gland, NADPH-d, histochemistry, nitric oxide synthase, development.
Introduction
Meibomian glands (MGs) are sebaceous glands which lipid secretions are essential for the ocular health. Meibomian oil forms the tear film lipid layer that retards evaporative water loss from the eye.1 Several studies have reported the innervation of the Meibomian glands in different species. In the guinea pig, Seifer and Spitznas found nerve fibers in the Meibomian glands immunoreactive for neuropeptide Y, substance P and vasoactive intestinal polypeptide (VIP).2 In humans, nerves are positive for calcitonin gene-related peptide (CGRP) and substance P. In cynomolgus monkeys, the nerve fibers in the Meibomian glands show positivity for substance P, CRGP, neuropeptide Y and VIP. In addition, axons stained for tyrosine hydrolase, dopamine-beta-hydroxylase, nitric oxide synthase and nicotinamide adenine dinucleotide phosphate (NADPH)-diaphorase.3 Recently, neuropeptide Y has been localized in the tarsal muscle of rat eyelids and nitric oxide synthase has been found in a second population of nerve terminals that lack immunoreactivity to enkephalin.4
Nitric oxide (NO) is an unconventional neurotransmitter involved in many physiological and pathological processes.5,6 Although its localization has been described in various tissues, its full biological importance is still not completely clear. The production of NO is indicated immunohistochemically by the demonstration of nitric oxide synthases (NOS), of which there are 3 isoforms. Two of these, neuronal NOS (nNOS; NOS-I) and endothelial NOS (eNOS; NOS-III), are constitutive and their presence may be inferred by positive NADPH-d staining, which is specific for the depiction of blood vessels and nerve structures.7,8 Staining of other structures is non-specific NADPH-d stain that is not equivalent to the presence of NOS. The third isoform is inducible NOS (iNOS; NOS-II), which is induced in macrophages in response to various stimuli and cannot be visualized by the NADPH-d histochemical method.5
The NADPH-diaphorase (NADPH-d) staining method is widely used in the investigation of the nervous system.9–11 Previous studies have demonstrated sympathetic innervation of the Meibomian gland, associated with blood vessels and acini and also the presence of parasympathetic axons and nerve terminals in close contact with the basal lamina of Meibomian acini.12–13 LeDoux et al. studied the parasympathetic innervation of the Meibomian glands in rats and it has been suggested that there is parasympathetic regulation of MG secretion.13 In the present study we confirmed the presence of NADPH-d-positive fibers in Meibomian glands, thereby implying participation of NO in its innervation. In order to discover when this system appears, we investigated the presence of NADPH-d activity in and around the Meibomian glands at various stages of postnatal development.
Materials and Methods
Animal treatment and tissue preparation
The animals were treated in accordance with the regulations of the Association for Research in Vision and Ophthalmology. Seventeen Wistar rats of both genders were used in this study at the following time points: day 1 (P1) and weeks 1, 2 and 3 post partum (P7, P14 and P21) and adult controls (P90). The number of eyelids was in total 34: six eyelids were used in each experimental group P7, P14, P21 and P90. In experimental group P1, ten eyelids were used because of difficulties with mechanical treatment. Experimental animals were sacrificed with an overdose of diethyl ether. The upper eyelids were carefully dissected out and stored in the 4% paraformaldehyde + 0.1% glutaraldehyde buffered with 0.1 M sodium phosphate, pH 7.4 for 3–4 h. The fixatives were freshly made up immediately prior to perfusion. Then eyelids were placed in ascending concentrations of sucrose (15–30%) in the same phosphate buffer, for cry-oprotection, and stored overnight at 4°C. Both upper eyelids were sampled at their centre and sectioned parasagittaly in a freezing microtome at a thickness of 45 µm. Three animals were used per time point and 10 sections/eyelid. In addition, the 5 sections of eyelids from each experimental group were embedded in paraffin, sectioned to 8 µm and stained with hematoxylin and eosin (H&E).
Histochemical procedure
Sections were processed for nicotinamide adenine dinucleotide phosphate diaphorase (NADPH-d). NADPH-d histochemical detection was performed as reported in our previous studies.14 Sections were incubated for 1 h at 37°C in a solution of 1.5 mM nitroblue tetrazolium (NBT, Sigma Chemicals. N-6876, Perth, WA, Australia), 1,0 mM β-nicotinamide adenine dinucleotide phosphate (NADPH, Sigma Chemicals, N-1630, Perth, WA, Australia), 0,5% Triton X-100 dissolved in 0.1 M phosphate buffer (pH 7.4), 10.0 mM monosodium malate (Malic acid, Sigma Chemicals, M-1125, Perth, WA, Australia). Control sections were treated in the same way but without NADPH in the reaction medium. This was to test for endogenous reduction activity in the corresponding blue formazan product.15 Following the reaction, sections were rinsed in 0.1 M phosphate buffer (pH 7.4), mounted on slides, air-dried overnight and coverslipped with Entellan.
Assessment
The slides were evaluated on day 1, weeks 1, 2 and 3. We performed qualitative analysis of the slides for the presence of NADPH-d positive structures.
Results
Histochemical staining with NADPH-d was used to indicate the presence of NOS.7 The histochemical procedure results in the deposition of a dark blue formazan reaction product. Sections stained with H&E were used for comparison. We observed dark blue NADPH-d positive staining in nerve fibers and endothelial cells of blood vessels coursing around the Meibomian glands (Figure 1, 2 and 3). Staining around the MG acini depended on the developmental stage. The following stages were compared with sections from adult rats, of two months of age or older.
Figure 1.
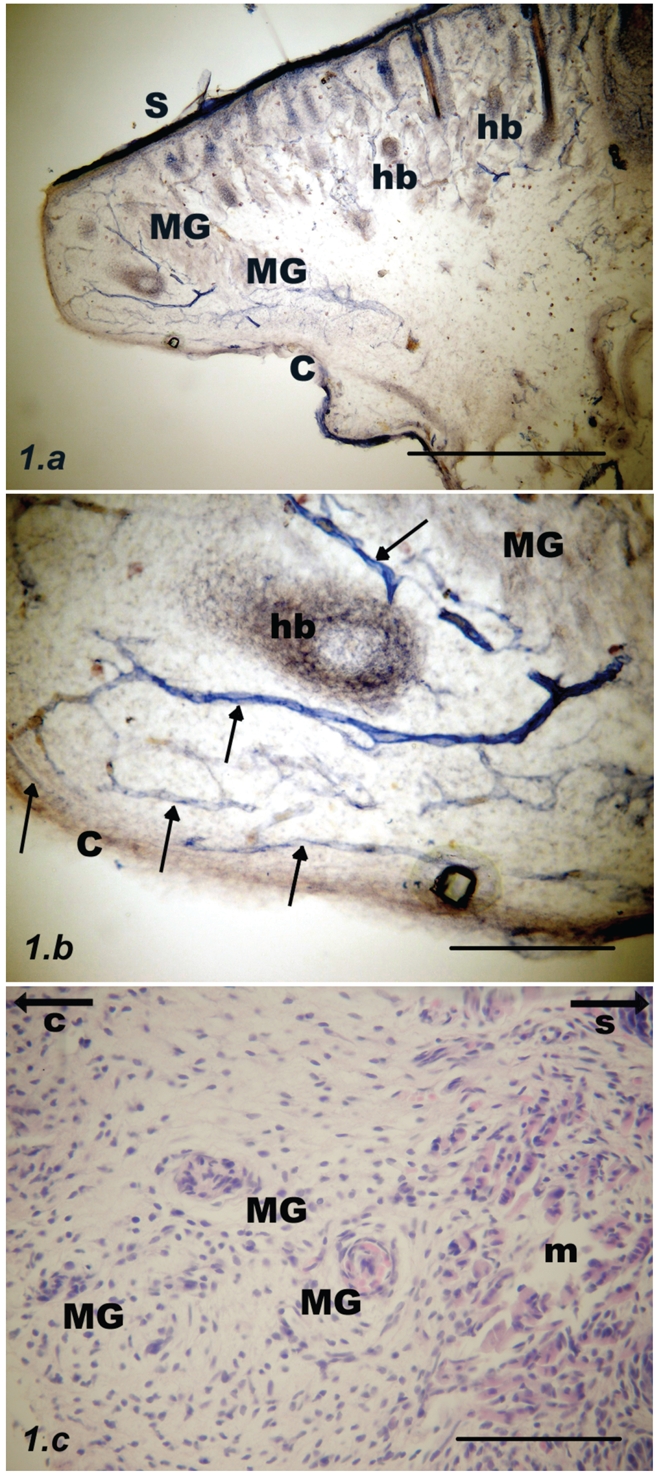
Parasagittal section through the upper rat eyelid at postnatal day #1. (a) Meibomian glands (MG) are barely NADPH-d stained and are located at a distance from the tarsal conjunctival surface (C). Deep to the skin (S) there are numerous hair bulbs (hb); scale bar = 500 µm. (b) Inset from previous figure: Non-uniformly stained blood vessels (arrows) with accompanying nerve fibers. The submucous layer is relatively wide and heavily vascularised, scale bar = 100 µm. (c) Developing Meibomian glands (MG) stained with haematoxylin and eosin. Orbicularis oculi muscle fibers (m) are visible on the right. Arrows point towards the conjunctiva (C) or skin (S) respectively; scale bar = 100 µm.
Figure 2.
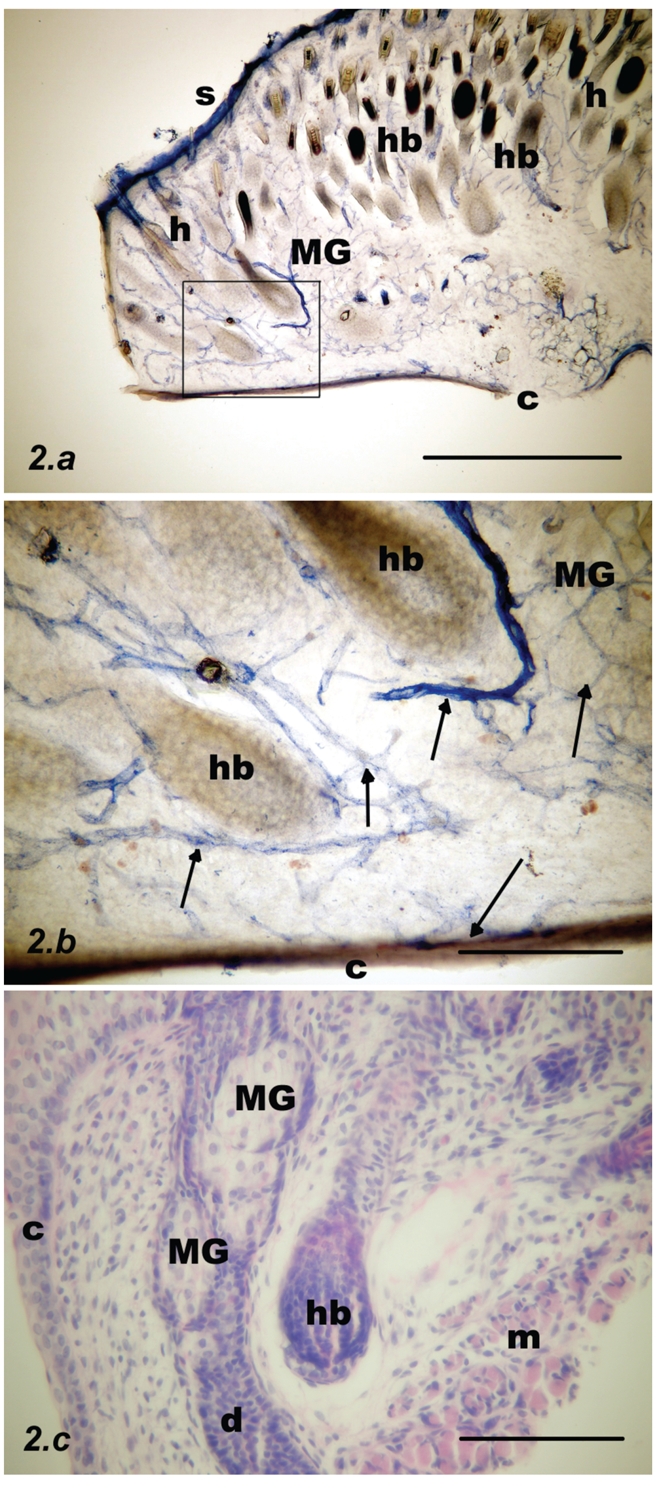
Parasagittal section through the upper rat eyelid at postnatal week #1. (a) NADPH-d stain. Acini of Meibomian glands are mostly pale (MG). Deep to the skin (S) there are many hair bulbs and hairs (hb, h); scale bar = 500 µm. (b) Inset from previous figure: NADPH-d positive nerve fibers were visualised, running along the blood vessels (arrows) and in addition, single nerve fibers in the submucosa under the conjunctival epithelium (C); scale bar = 100 µm. (c) Contours of the Meibomian acini (MG) and Meibomian duct (d) shown with haematoxylin and eosin staining. A hair bulb (hb) is visible at the centre of picture and orbicularis oculi muscle fibers (m) are visible to the right; scale bar = 100 µm.
Figure 3.
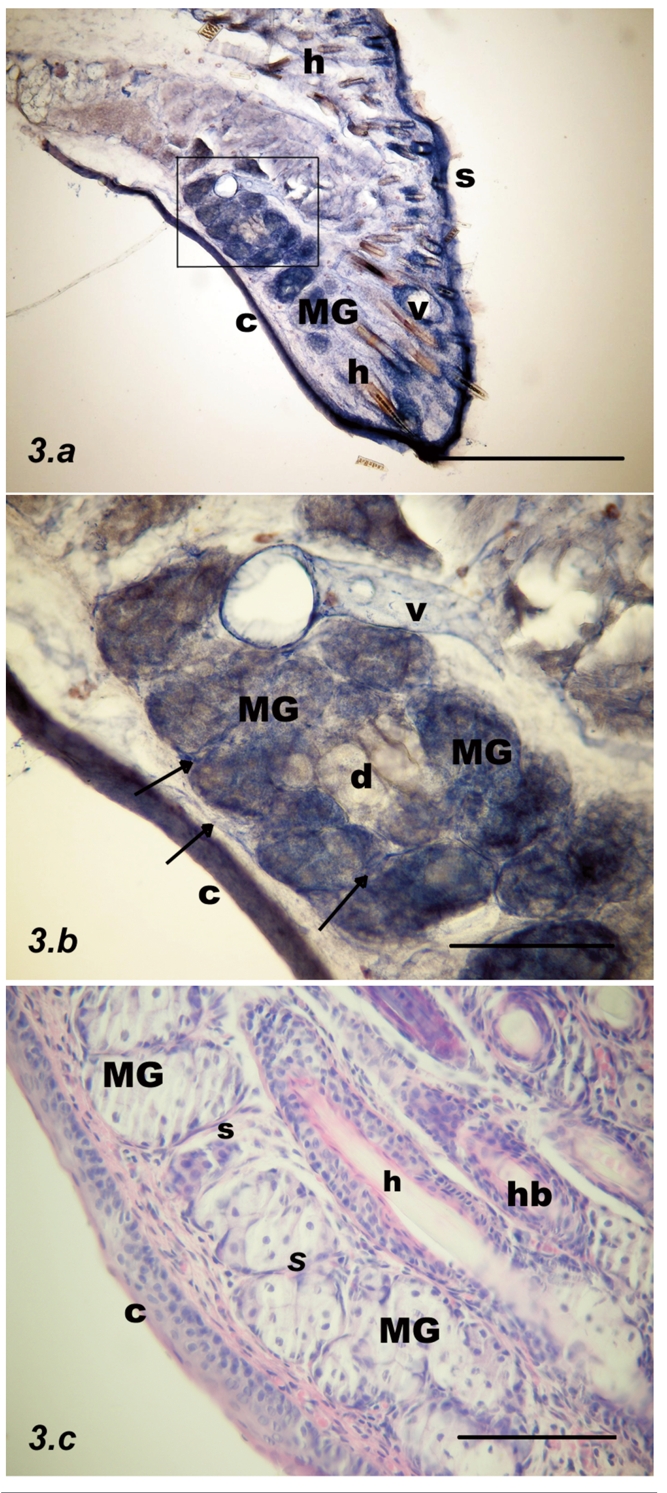
Parasagittal section through the rat upper eyelid at postnatal week #2. (a) Darker NADPH-d stained acini (MG) are clearly seen at this period of development close to the conjunctival surface (C); hairs (h) are brown; scale bar = 500 µm. (b) Inset from previous figure: Blood vessels, accompanied by nerve fibers (arrows), are running in the septa between the Meibomian glands and at their surface and are moderately NADPH-d positive in both the sub-mucosa and within the septa. A blood vessel lumen is visible centrally (v); scale bar = 100 µm. (c) Almost mature Meibomian glands (MGs) with septa between acini (s) are seen with haematoxylin and eosin staining; scale bar = 100 µm.
Day #1
MG acini were lightly stained or almost unstained and located at a distance from the tarsal conjunctival surface. The submucous layer was relatively wide and heavily vascularised by tortuous, branching blood vessels. Vessels were accompanied by intensely stained NADPH-diaphorase positive nerve fibers (Figure 1).
Week #1
Both the vessels and the NADPH-d positive fibers were still present, but less numerous. NADPH-d positive nerve fibers were visualised running along the blood vessels and in addition, single nerve fibers in the submucosa deep to the conjunctival epithelium were present. MGs were now closer to the mucosa and the submucosa was thinner. The acini were mostly pale, but occasionally darker (Figure 2).
Week #2
There were fewer blood vessels in both the submucosa and within the septa. Darker acini were more common than lightly stained acini. NADPH-d positive dots were observed in the vicinity of the MGs. Moderately NADPH-d stained vessels with nerve fibers were running in the septa between acini (Figure 3).
Week #3
MGs were adjacent to the mucosal border and stained more intensely than at earlier times; almost all acini were stained, while ducts were unstained and appeared yellow. The microscopic appearances were almost identical with those of adult palpebra. Submucosal and septal blood vessels and NADPH-d positive nerve fibers were less numerous, although fine nerve fibers were present, running in the septa between the acini (Figure 4).
Figure 4.
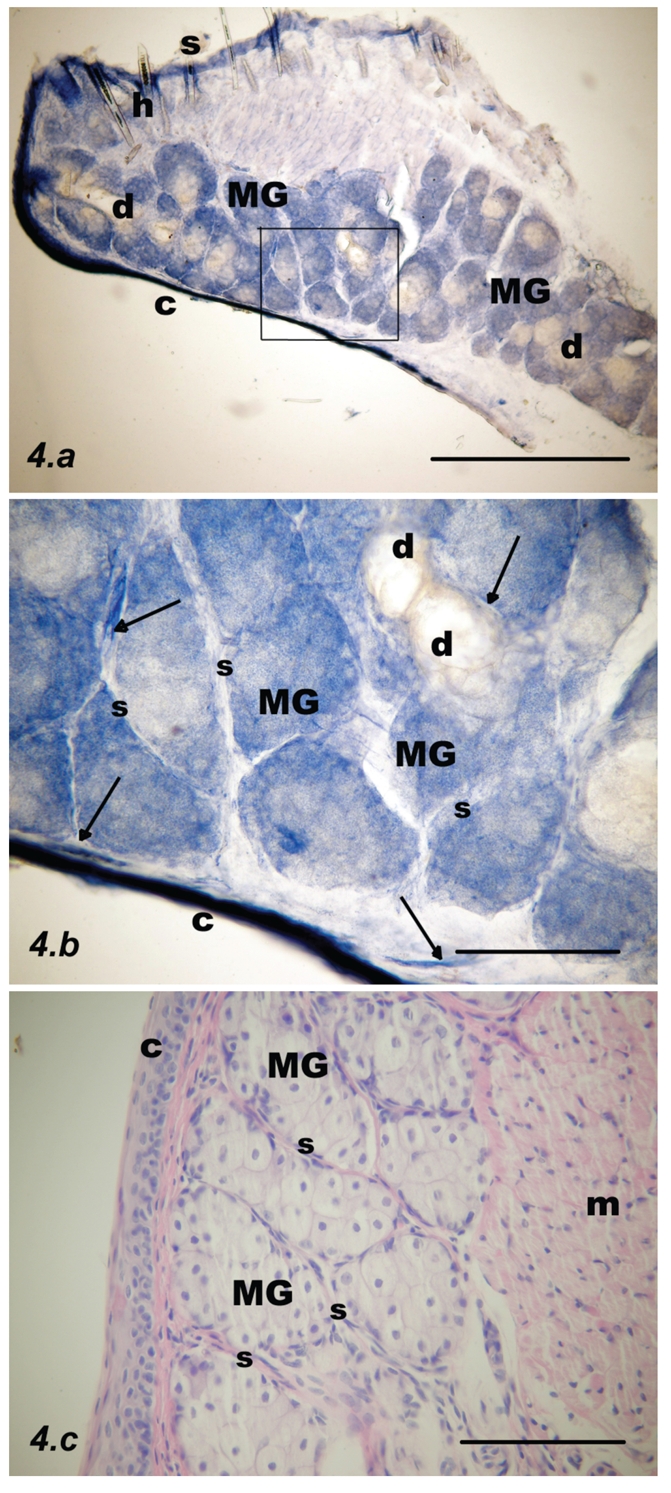
Parasagittal section through the upper rat eyelid at postnatal week 3. The microscopic appearances were almost identical with those of adult lids. (a) NADPH-d stain. Meibomian glands (MGs) are adjacent to the mucosal border (C) and like adult glands, are intensely NADPH-d positive, except the ducts (d) which stain yellow; scale bar = 500 µm. (b) Inset from the previous figure: NADPH-d positive nerve fibers (arrows) and submucosal and septal blood vessels are less numerous. Nerves are visible as fine fibers running in the septa (s) between the acini; scale bar = 100 µm. (c) Fully developed Meibomian gland acini (MGs) and septa (s). Orbicularis oculi muscle fibers (m) are visible on the right. Haematoxylin and eosin staining; scale bar = 100 µm.
Discussion
We studied the presence of NOS in rat eyelids using the NADPH-diaphorase method of staining which indicates the presence of NOS in cells, fibres and blood vessel endothelia. In other tissues, positive staining for NADPH-d is non-specific. Staining of Meibomian acini was noted from the second postnatal week when the majority of acini showed a uniform dark blue staining or staining at their margins. At postnatal week #3, the NADPH-d staining of the MGs was intense and identical to that found in adult rats. Until now, little was known about Meibomian gland development, the most extensive study focusing on human Meibomian gland.16 A recent report provides the evidence that Meibomian gland development bears similarities to sebaceous gland development. The observations indicate that Meibomian gland development in the mouse is initiated around embryonic day 18.5. By postnatal day 8 the developing Meibomian gland shows extensive ductal branching and the formation of distinct acini with mature Meibomian glands present by P15.17 The intensity of NADPH-d staining may be a guide to the presence of NOS activity. Thus in neural tissues such as the mouse, cat, monkey, rabbit and human retina,18–21 where the intensity of staining is low, positive staining is not considered to be evidence for the presence of NOS.6–8,11,14
In the present study, NADPH-d reactivity was found in nerve fibers and in the walls of blood vessels within the eyelid and is considered to represent the presence of NOS22 activity in these tissues. In many regions of the central and peripheral nervous system,6 the presence of NOS is taken to indicate a functional role for NO in the involved tissues.23 This is likely to be the case here and we conclude from our study that there is a nitrergic innervation of the rat MG and a role for NO as a neurotransmitter in this gland. This innervation appears in early development and is fully established by three weeks postnatally.
Several neurotransmitters have been studied in Meibomian glands. Perra et al.24 used histochemistry to study acetylcholinesterase activity in human Meibomian glands. With light microscopy they showed a dense network of AChE-positive nerve fibers around the acini and ducts of the glands. No discrete nerve endings were observed, whereas a strong reaction was elicited in some fibers closely associated with blood vessels. These observations suggest that the cholinergic system is involved in the regulation of MG secretory function.24
Comparison of those results with the current study of the histochemical picture of NADPH-d activity showed very similar picture.
In samples of adult rats we typically observed intensely stained nerve fibers running as a network around blood vessels. They were mostly seen in the septa around the acinar ducts. There was, however, a difference between developing and adult animals. In the early phases of development (up to postnatal week #1) we observed single nerve fibers running separately from blood vessels. These were seen in the submucosa, not in the septa between the acini.
Several glands contribute to the tears ensuring the health of the ocular surface by maintaining a moist condition of the exposed epithelia. These are the lacrimal gland, the Meibomian glands and the goblet cells of the conjunctiva. Each of these glands receives an autonomic innervation, either directly to the glands, in close proximity to them, or to neighbouring vessels. There is good evidence to indicate that this protective system is maintained, at least for the lacrimal gland, by a reflex, sensory, trigeminal drive from the ocular surface, which regulates lacrimal secretion according to environmental circumstances. This is part of the so-called lacrimal functional unit.25 Because of the technical difficulties in measuring Meibomian secretion there is less certainty that the Meibomian glands participate in this homeostatic process26 and it may be that innervation is more related to regulating the periacinar vascular network and meeting nutritional demands. Further studies are needed to explore the role of NO in these processes.
References
- 1.Bron A, Tripathi R, Tripathi B. Wolff's Anatomy of the Eye and Orbit. Chapman & Hall; London, UK: 1997. [Google Scholar]
- 2.Seifert P, Spitznas M. Immunocytochemical and ultrastructural evaluation of the distribution of nervous tissue and neuropeptides in the meibomian gland. Graefes Arch Clin Exp Ophthalmol. 1996;234:648–56. doi: 10.1007/BF00185300. [DOI] [PubMed] [Google Scholar]
- 3.Kirch W, Horneber M, Tamm ER. Characterization of Meibomian gland innervation in the cynomolgus monkey (Macaca fascicularis) Anat Embryol (Berl) 1996;193:365–75. doi: 10.1007/BF00186693. [DOI] [PubMed] [Google Scholar]
- 4.Chanthaphavong RS, Murphy SM, Anderson CR. Chemical coding of sympathetic neurons controlling the tarsal muscle of the rat. Auton Neurosci. 2003;105:77–89. doi: 10.1016/S1566-0702(03)00045-6. [DOI] [PubMed] [Google Scholar]
- 5.Bredt DS, Snyder SH. Nitric oxide, a novel neuronal messenger. Neuron. 1992;8:3–11. doi: 10.1016/0896-6273(92)90104-l. [DOI] [PubMed] [Google Scholar]
- 6.Christopherson KS, Bredt DS. Nitric oxide in excitable tissues: Physiological roles and disease. J Clin Invest. 1997;100:2424–9. doi: 10.1172/JCI119783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hope BT, Michael GJ, Knigge KM, Vincent SR. Neuronal NADPH diaphorase is a nitric oxide synthase. PNAS USA. 1991;88:2811–4. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grozdanovic Z, Baumgarten HG, Bruning G. Histochemistry of NADPH diaphorase, a marker for neuronal nitric oxide synthase, in the peripheral autonomic nervous system of the mouse. Neuroscience. 1992;48:225–35. doi: 10.1016/0306-4522(92)90351-2. [DOI] [PubMed] [Google Scholar]
- 9.Racekova E, Martoncikova M, Mitruskova B, Cizkova D, Orendacova J. Age related changes of NADPH-diaphorase positivity in the rat rostral migratory stream. Cell Mol Neurobiol. 2005;25:1093–105. doi: 10.1007/s10571-005-8191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munoz M, Marin O, Gonzales A. Localization of NADPH diaphorase, nitric oxide synthase and choline acetyltransferase in the spinal cord of the frog, Rana perezi. J Comp Neurol. 2000;419:451–70. doi: 10.1002/(sici)1096-9861(20000417)419:4<451::aid-cne4>3.3.co;2-d. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto T, Nakane M, Pollock JS, Kuk JE, Forstermann UA. Correlation between soluble brain nitric oxide synthase and NADPH-diaphorase activity is only seen after exposure of the tissue to fixative. Neurosci Lett. 1993;155:61–4. doi: 10.1016/0304-3940(93)90673-9. [DOI] [PubMed] [Google Scholar]
- 12.Simons E, Smith PG. Sensory and autonomic innervation of the rat eyelid: neuronal origins and peptide phenotypes. J Chem Neuroanat. 1994;7:35–47. doi: 10.1016/0891-0618(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 13.LeDoux MS, Zhou Q, Murphy RB, Greene MI, Ryan P. Parasympathetic innervation of the Meibomian glands in rats. Invest Ophtalmol Vis Sci. 2001;42:2434–41. [PubMed] [Google Scholar]
- 14.Kluchova D, Klimcik R, Kloc P. Neuronal nitric oxide synthase in the rabbit spinal cord visualised by histochemical NADPH-diaphorase and immunohistochemical NOS methods. Gen Physiol Biophys. 2002;21:163–74. [PubMed] [Google Scholar]
- 15.Hope BT, Vincent SR. Histochemical characterization of neuronal NADPH-diaphorase. J Histochem Cytochem. 1989;37:653–661. doi: 10.1177/37.5.2703701. [DOI] [PubMed] [Google Scholar]
- 16.Andersen H, Ehlers N, Mathiessen ME. Histochemistry and development of the human eyelids. Acta Ophtalmol. 1965;43:642–68. doi: 10.1111/j.1755-3768.1965.tb00335.x. [DOI] [PubMed] [Google Scholar]
- 17.Nien ChJ, Massei S, Lin G, Liu H, Paugh JR, Liu Ch, et al. The development of meibomian glands in mice. Mol Vis. 2010;16:1132–40. [PMC free article] [PubMed] [Google Scholar]
- 18.Sagar SM. NADPH-diaphorase histochemistry in the rabbit retina. Brain Res. 1986;373:153–8. doi: 10.1016/0006-8993(86)90325-2. [DOI] [PubMed] [Google Scholar]
- 19.Sandell JH. NADPH diaphorase cells in the mammalian inner retina. J Comp Neurol. 1985;238:466–72. doi: 10.1002/cne.902380410. [DOI] [PubMed] [Google Scholar]
- 20.Bhattacharjee J. Sequential differentiation of retinal cells in the mouse studied by diaphorase staining. J Anat. 1997;123:273–82. [PMC free article] [PubMed] [Google Scholar]
- 21.Obata H. Anatomy and histopathology of human Meibomian glands in 72 autopsy cases. Nippon Ganka Gakkai Zasshi. 2002;98:765–71. [PubMed] [Google Scholar]
- 22.Kozak I, Bron AJ, Kucharova K, Kluchova D, Marsala M, Heichel C, et al. Morphologic and volumetric studies of the Meibomian glands in elderly human eyelids. Cornea. 2007;26:610–4. doi: 10.1097/ICO.0b013e318041f0d2. [DOI] [PubMed] [Google Scholar]
- 23.Lovasova K, Kluchova D, Bolekova A, Dorko F, Spakovska T. Distribution of NADPH-diaphorase and AChE activity in the anterior leaflet of rat mitral valve. Eur J Histochem. 2010;54:25–9. doi: 10.4081/ejh.2010.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perra MT, Serra A, Sirigu P, Turno F. Histochemical demonstration of cetylcholinesterase activity in human Meibomian glands. Eur J Histochem. 1996;40:39–44. [PubMed] [Google Scholar]
- 25.Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004;78:409–16. doi: 10.1016/j.exer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Bron AJ, Yokoi N, Gaffney E, Tiffany JM. Predicted Phenotypes of Dry Eye: Proposed Consequences of Its Natural History. Ocul Surf. 2009;7:78–92. doi: 10.1016/s1542-0124(12)70299-9. [DOI] [PubMed] [Google Scholar]


