Abstract
Thymosin beta 4 (Tβ4) is a member of the beta-thymosins family, a family of peptides playing essential roles in many cellular functions. Our recent studies suggested Tβ4 plays a key role in the development of human salivary glands and the gastrointestinal tract. The aim of this study was to analyse the presence of Tβ4 in the human adult and foetal genitourinary tract. Immunolocalization of Tβ4 was studied in autoptic samples of kidney, bladder, uterus, ovary, testicle and prostate obtained from four human foetuses and four adults. Presence of the peptide was observed in cells of different origin: in surface epithelium, in gland epithelial cells and in the interstitial cells. Tβ4 was mainly found in adult and foetal bladder in the transitional epithelial cells; in the adult endometrium, glands and stromal cells were immunoreactive for the peptide; Tβ4 was mainly localized in the glands of foetal prostate while, in the adults a weak Tβ4 reactivity was restricted to the stroma. In adult and foetal kidney, Tβ4 reactivity was restricted to ducts and tubules with completely spared glomeruli; a weak positivity was observed in adult and foetal oocytes; immunoreactivity was mainly localized in the interstitial cells of foetal and adult testis. In this study, we confirm that Tβ4 could play a relevant role during human development, even in the genitourinary tract, and reveal that immunoreactivity for this peptide may change during postnatal and adult life.
Key words: thymosin β4, genitourinary tract, development.
Introduction
Beta-thymosins are N-terminally acetylated peptides of 5 kDa molecular mass, which are composed of 40–44 amino acid residues.1 Thymosyn β4 (Tβ4) is a 43-aminoacid peptide, first isolated in human blood platelets;2 it is probably an important intracellular modulator of actin polymerization3 due to its capacity to interact with monomeric actin.1 As an extracellular factor, Tβ4 has been documented to be involved in hormonal activities and in modulating the immune response.1,4 It has also been found to have anti-inflammatory efficacy5 and to be implicated in cardiac protection,6 angiogenesis,7 wound healing,8 blood coagulation,2 hair growth and apoptosis.1,4
Recently, a role for Tβ4 has been described in developing foetal salivary glands,9 in coronary vasculogenesis,10 and during the development of different neural cell types in rat brains.11 In mouse embryonic kidneys, Tβ4 activity has been shown to increase during development, remaining at high levels during active nephrogenesis.12 In rat foetal kidneys, Tβ4 has been identified in differentiating glomeruli, while in adult rats it has been mainly found in collecting ducts.12,13 High levels of Tβ4 mRNA were detected in normal human embryonic kidneys and in renal tumors.14 Recent studies have also provided new information concerning a role for Tβ4 in human glomerulosclerosis and in kidney fibrosis in adults.15 Tβ4 also plays an important role in the development of the reproductive system. Its secretion has been shown to be modulated by testosterone and gonadotropin in mice and pigs.16–18 Increased levels of Tβ4 in the rat ovary have been correlated with the process of luteinization16 under the influence of human chorionic gonadotropin, which stimulates Tβ4 gene expression.17 The Tβ4 gene is downregulated at transcriptional level by androgen activity in prostate cancer cell culture.19 Presence of Tβ4 has been reported during the formation of actin-based pseudopodia in tumoral cells, facilitating cell migration and prostate cancer progression.20 Our research has also shown high Tβ4 presence in tumour infiltrating mast cells.21 Overall, our research suggests that Tβ4 has an important role in the development of different embryonic and adult organs. Due to controversy in the scientific community regarding the role of Tβ4 in health and disease, this study was aimed at verifying if similarities exist between Tβ4 presence during development and in adult life, and at discussing the putative role played by Tβ4 in both situations. We also found it necessary to better investigate the presence of Tβ4 in the human urinary and reproductive tract of foetuses and adults in order to add new scientific data for a more complete description of the several, and not completely known, functions of this versatile peptide.
Materials and Methods
Four human foetuses, two males and two females, with gestational age ranging from 14 to 27 weeks, and four adults, two males and two females 35, 66, 68 and 72 year-old respectively, were selected for this study. In all cases, no pathological changes were evident in the organs examined in this study. We obtained tissue samples at autopsy from each subject including the following segments of the male and female genitourinary tract: kidney, bladder, uterus, ovary, testicle and prostate. Tissue samples were fixed in 10% formalin, routinely processed and paraffin-embedded. Immunohistochemistry was performed on 5 µm-thick sections, using the labelled streptavidin-biotin complex system (LSAB2, Dako) in a Dako Autostainer (DakoCytomation, Carpintera, CA, USA). Heat-induced antigen retrieval was carried out by steaming unstained sections in Target Retrieval Solution (Dako TRS pH 6.1) for 30 min. Tissue sections were incubated (30 min at room temperature) with the monoclonal anti-thymosin β4 antibody (Bachem, Bubendorf, Switzerland). Sections of a reactive human adult lymph node with activated macrophages were used as positive controls.22 As a negative control the same procedure was applied omitting the primary antibody.
Ethics statements
The study protocol and written consent forms were approved by the Ethics Human Studies Committee of University Medical Centre of Cagliari (according to the instructions of the Declaration of Helsinki). Full written consent forms were obtained from the parents of the newborns and all rules were respected. For the specimens from adults, we obtained written consent from their next of kin.
Results
The immunostaining for Tβ4 appeared homogeneous or granular and was always restricted to the cytoplasm of positive cells. No nuclear reactivity was observed in this study. No significant differences were found in the immunohistochemical pattern for Tβ4 among the four foetuses analyzed, as well as the four adults observed in this study (Table 1). The immunoreactivity for Tβ4 in foetal kidneys paralleled the immunoreactivity in the adult. A weak diffuse cytoplasmic positivity was detected in developmental and mature ducts while the glomeruli were constantly negative (Figure 1a,b). In the bladder, a weak immunoreactivity of the peptide was observed in the immature transitional epithelium, which changed to a coarse granular reactivity in the mature epithelium of the adult bladder (Figure 2a,b). The surrounding stroma showed mild diffuse immunoreactivity of Tβ4 in the foetus and focal in adults (Figure 2b). No immunoreactivity for Tβ4 was observed in foetal endometrial glands. Only scattered stromal cells showed a weak cytoplasmic immunoreactivity (Figure 3a). A mild, granular cytoplasmic immunoreactivity of Tβ4 was present in the endometrial glands of adults, while the absence of any significant immunoreactivity was found in the stroma (Figure 3b). No reactivity for Tβ4 was detected in the foetal or adult prostatic gland epithelium (Figure 4a,b). We found a diffuse immunoreactivity of Tβ4 in the stroma of adult prostate (Figure 4b), but no reactivity was detected in the foetal prostate stroma (Figure 4a). A fine granular positivity was present in the foetal ovary (Figure 5a), while immunoreactivity of the peptide was homogeneously weak in adult oocytes (Figure 5b). Stroma was negative in both adult and in foetal ovaries (Figure 5a,b). The interstitial cells of the adult testicle were strongly positive for Tβ4 while, in the foetus, the same cells showed a mild immunoreactivity for the peptide (Figure 6a,b). Moreover, a mild reactivity of Tβ4 was observed in the spermatic ducts of the adult testicle (Figure 6b).
Table 1. Immunoreactivity of Tβ4 in different organs and cells of the human genitourinary tract.
| Foetus | Aduld | |
|---|---|---|
| Kidney | ||
| Ducts | Diffuse | Diffuse |
| Glomeruli | Negative | Negative |
| Bladder | ||
| Transitional epithelium | Focal | Diffuse |
| Stroma | Negative | Focal |
| Endometrium | ||
| Glands | Negative | Diffuse |
| Stromal cells | Focal | Diffuse |
| Prostate | ||
| Glands | Negative | Focal |
| Stroma | Negative | Diffuse |
| Isolated cells | Focal | Diffuse |
| Ovary | ||
| Oocyte | Focal | Diffuse |
| Testicle | ||
| Spermatic ducts | Negative | Focal |
| Interstitial cells | Focal | Diffuse |
Figure 1.
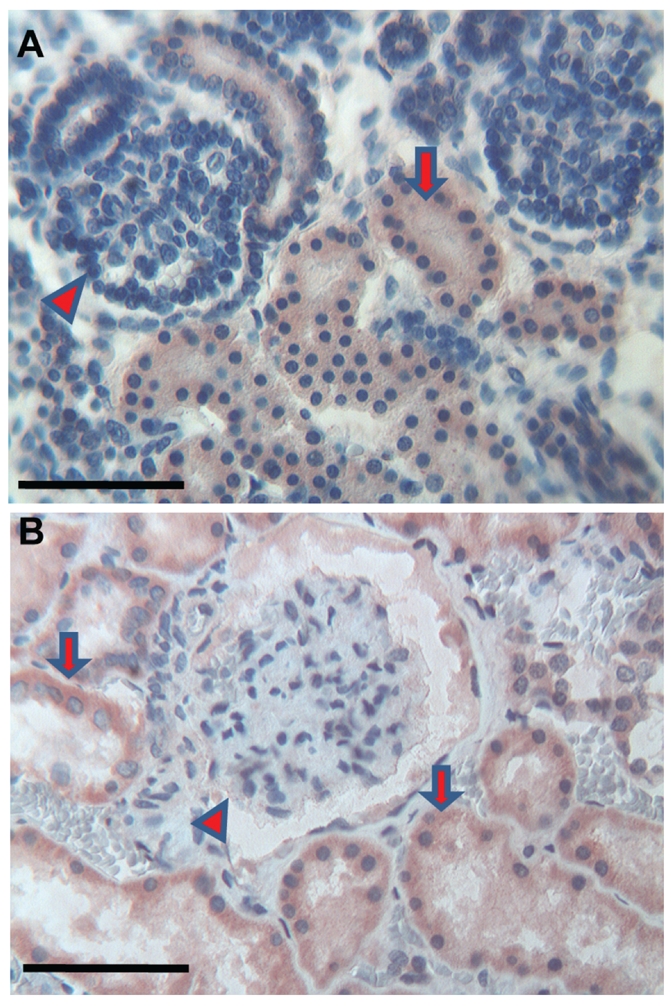
Kidney. (a) Foetuses: a diffuse cytoplasmic immunoreactivity of Tβ4 is present in developing ducts (arrow). The immature glomuruli are negative (arrowhead). Scale bar: 25 µm. (b) Adults: primary and secondary ducts show a diffuse cytoplasmic immunoreactivity of Tβ (arrow). The glomeruli are negative (arrowhead). Scale bar: 25 µm.
Figure 2.
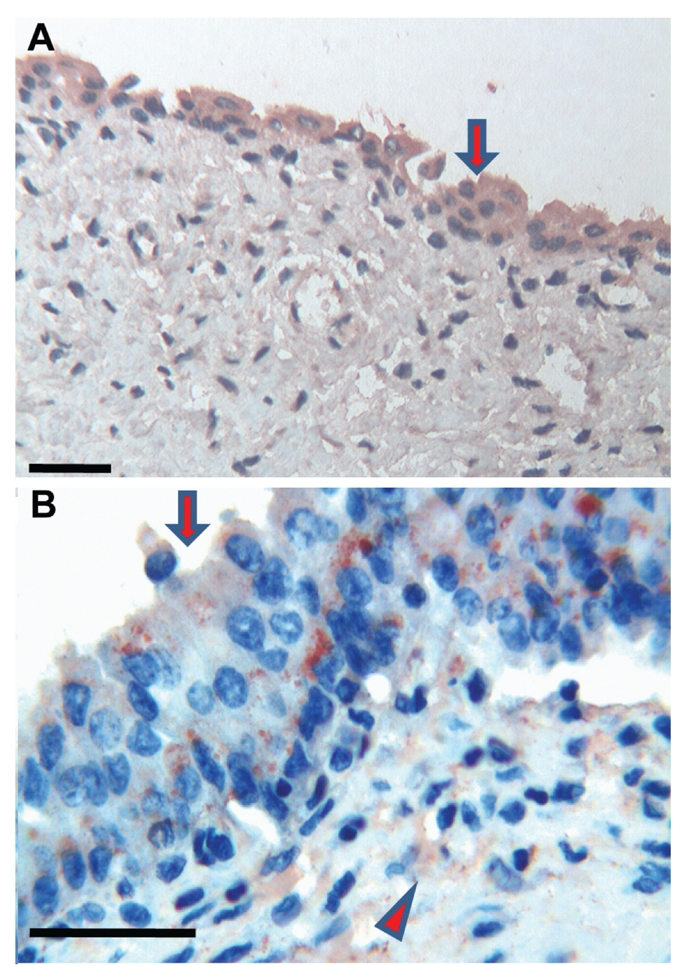
Bladder. (a) Foetuses: the developing transitional epithelium shows a weak and diffuse cytoplasmic immuoreactivity of Tβ4 (arrow). The surrounding stroma shows a mild diffuse immunoreactivity of the peptide. Scale bar: 50 µm. (b) Adults: perinuclear coarse granules, immunoreactive for Tβ4, are detected in the cytoplasm of transitional epithelium (arrow). The stroma shows a focal positivity of the peptide (arrowhead). Scale bar: 50 µm.
Figure 3.
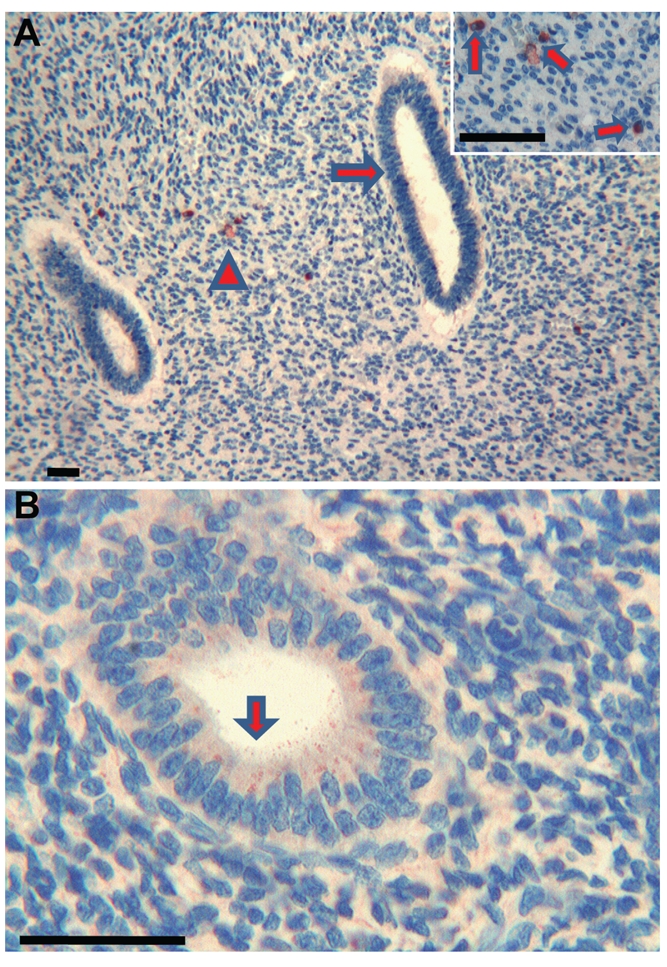
Endometrium. (a) Foetuses: no immunoreactivity of Tβ4 is observed in developing endometrial glands (arrow). Only scattered stromal cells show a weak cytoplasmic immunoreactivity (arrowhead). Scale bar: 100 µm. Inset: scattered stromal cells with a cytoplasmic immunoreactivity (arrows). Scale bar: 25 µm. (b) Adults: a mild granular cytoplasmic immunoreactivity of Tβ4 is observed in the endometrial glands (arrow) while the surrounding stroma has no significant immunoreactivity of the peptide. Scale bar: 25 µm.
Figure 4.
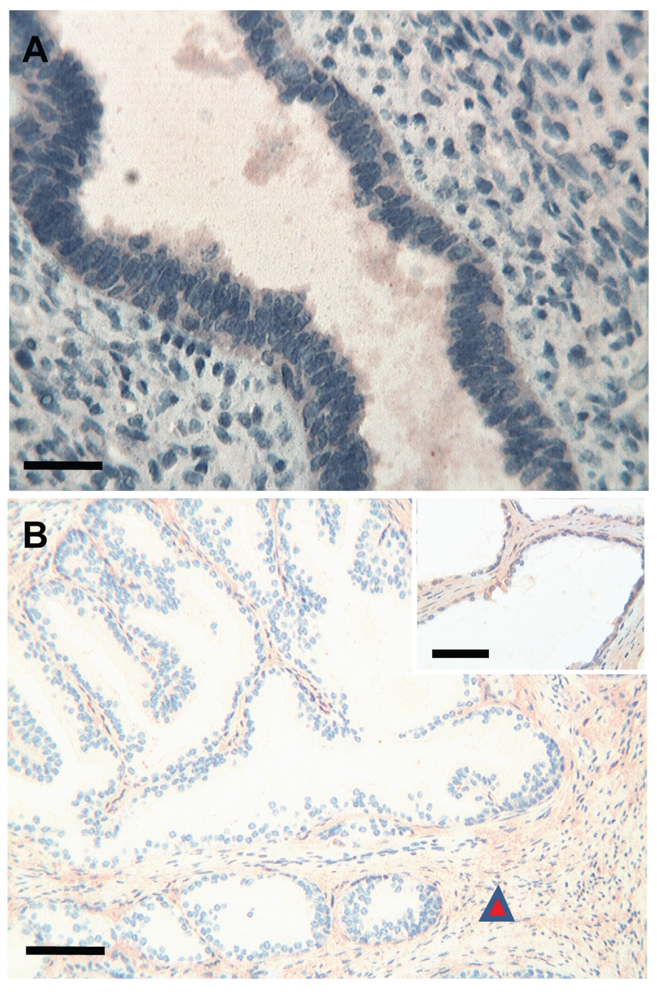
Prostate. (a) Foetuses: no immunoreactivity of Tβ4 is observed in the prostate glands and in the surrounding stroma. Scale bar: 50 µm. (b) Adults: no significant immuoreactivity of Tβ4 is detected in the prostate glands. The surrounding stroma shows a diffuse immunoreactivity for the peptide (arrowhead). Scale bar: 50 µm. Inset: at higher magnification, no significant immunoreactivity for Tβ4 is observed in the prostate gland. Scale bar: 25 µm.
Figure 5.
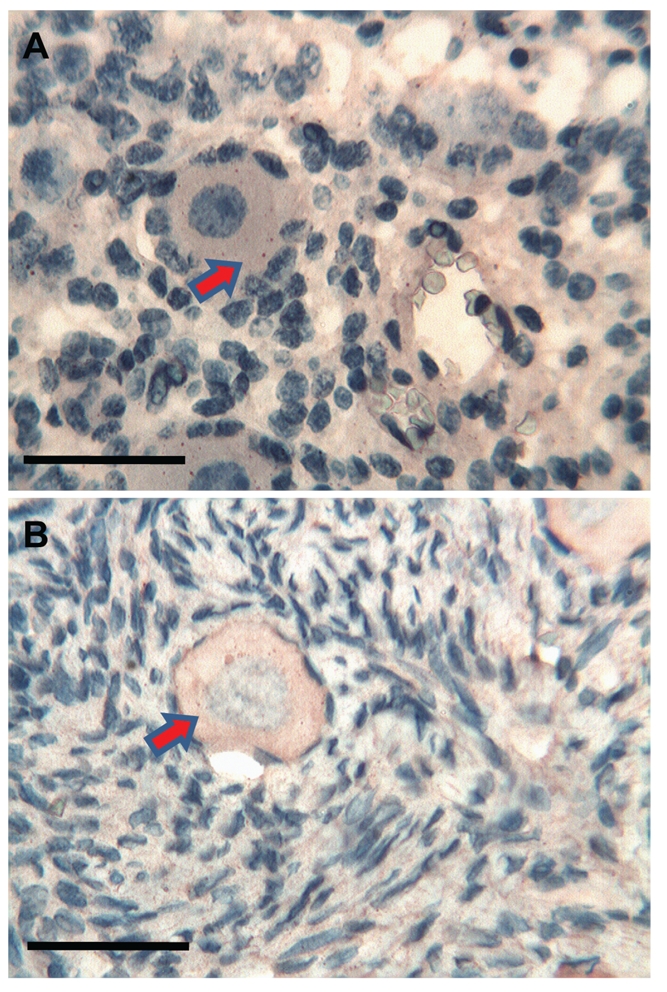
Ovary. (a) Foetuses: scattered Tβ4 reactive fine granules are present in the cytoplasm of the oocyte (arrow). A weak diffuse immunoreactivity of the surrounding stroma is detected. Scale bar: 25 µm. (b) Adults: a weak and homogeneous cytoplasmic immunoreactivity for Tβ4 is detected in the oocyte of the primary follicle (arrow). A weak diffuse immunopositivity in the surrounding stroma is observed. Scale bar: 25 µm.
Figure 6.
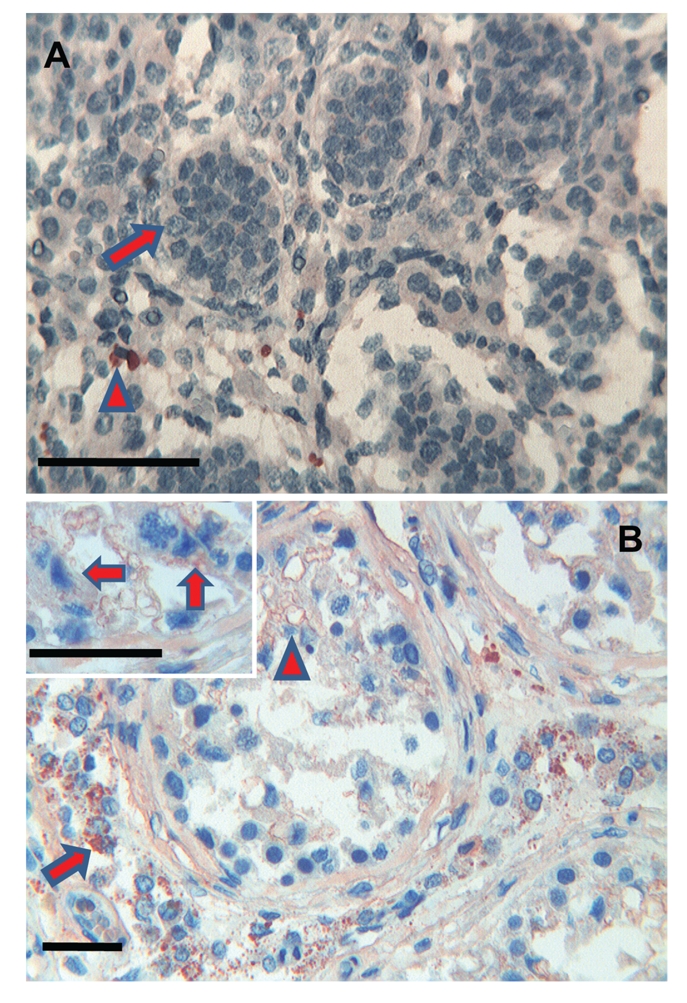
Testicle. (a) Foetuses: no immunoreactivity for Tβ4 is observed in the immature spermatic ducts (arrow). A mild cytoplasmic immunoreactivity of the peptide is observed in isolated interstitial cells (arrowhead). Scale bar: 25 µm. (b) Adults: a granular cytoplasmic immunoreactivity of Tβ4 is detected in the interstitial cells of the testicle (arrow). A weak immunoreactivity is observed in the cells of the spermatic ducts (arrowhead). Scale bar: 50 µm Inset: cells of the spermatic ducts with a weak immunoreactivity (arrows). Scale bar: 25 µm.
Discussion
This is the first comprehensive study that analyzes Tβ4 presence in the human genitourinary tract, and compares the presence of this peptide during development with its reactivity in the adult. We convincingly demonstrate that Tβ4 presence is particular for every organ and, inside each organ, it appears restricted to certain structures or to particular cell types. Many studies in experimental animals and recently in humans suggest that Tβ4 could play a relevant role during development.9,23 Our data confirms this hypothesis. In this study, we find that Tβ4 is present during intrauterine life even in the genitourinary tract, and reveal that immunoreactivity for this peptide may change during postnatal life with marked differences between foetal and adult organs. Our previous studies on Tβ4 protein presence in salivary glands9 and in the gastrointestinal tract23 first disclosed the uneven distribution of the peptide in different organs of the same system and within each organ in the different epithelial and mesenchymal structures and cells. Moreover, in recent years, Tβ4 presence has been reported to increase in different tumors,24–26 mainly in its infiltrative borders and in their metastastic stage (Nemolato et al, unpublished data). The uneven distribution of Tβ4 among different organs as well as among different cell types within each organ is confirmed by this study also in the genitourinary tract. Tβ4 was clearly present in all organs examined in this study; it was more diffuse in kidney, adult bladder and in interstitial cells of the testis.
Interestingly, we found differences in this study between foetal and mature organs. In general, we found direct associations between presence of Tβ4 and the degree of development in all the organs tested. The presence of a relationship between Tβ4 presence and the developmental status does not coincide with our previous data regarding the gastrointestinal tract.23 In previous studies, Tβ4 presence patterns were characterized by a higher presence in the foetus and by a marked decrease in the adult suggesting a major role of Tβ4 during embryogenesis. This hypothesis was confirmed by our studies of Tβ4 content in human saliva,9 which clearly showed high Tβ4 levels during gestation followed by the disappearance of the peptide in the adult saliva. Our study reinforces our hypothesis that Tβ4 functions are not totally known and stresses the different function this peptide could play in different human organs, not only during development, but also even during adult life. Some peculiar features related to the patchy Tβ4 distribution in different organs deserve to be noted in: i) kidney restriction of Tβ4 reactivity to ducts and tubules with completely spared glomeruli (Figure 1a,b); ii) a positivity in the oocytes of the ovary (Figure 5a,b); iii) immunoreactivity restricted to interstitial cells in the testis (Figure 6a,b). What is the relationship between these very different types of cells? For the time being, we have no clear answer. The immunoreactivity of oocytes of Tβ4 in the foetal ovary and its increase in the adult oocytes indicates a role of the peptide not only in germ cell development but also in its maintenance during adult life.
In this study, Tβ4 was frequently detected not only inside of cells but also in the stroma surrounding gland structures both in foetuses and in adults. Considering the fact that the extracellular forms of beta-thymosins have been shown to selectively prevent neuronal cell death,27 we can speculate that the same role could be played by Tβ4 in the genitourinary tract during development. This role should be elucidated in further studies. Finally, our study clearly shows that further work is required to better understand the presence of Tβ4 and its impacts on development and on biological behaviour of adult cells in different human organs, which confirms Tβ4 as a versatile peptide with several, mostly unknown important functions in human health and disease.
Acknowledgements:
the authors thank Mr. Iganzio Ferru for his secretarial assistance, and Mrs. Sandra Serra and Mrs. Simonetta Paderi for their technical support. They also would like to acknowledge the financial support of the “Fondazione Banco di Sardegna”, Cagliari, Sardinia, Italy.
References
- 1.Mannherz HG, Hannappel E. The beta-thymosins: intracellular and extracellular activities of a versatile actin binding protein family. Cell Motil Cytoskeleton. 2009;66:839–51. doi: 10.1002/cm.20371. [DOI] [PubMed] [Google Scholar]
- 2.Safer D, Golla R, Nachmias VT. Isolation of a 5-kilodalton actin-sequestering peptide from human blood platelets. PNAS USA. 1990;87:2536–40. doi: 10.1073/pnas.87.7.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominguez R. The β-Thymosin/WH2 fold. Multifunctionality and structure. Ann N Y Acad Sci. 2007;1112:86–94. doi: 10.1196/annals.1415.011. [DOI] [PubMed] [Google Scholar]
- 4.Paulussen M, Landuyt B, Schoofs L, Luyten W, Arckens L. Thymosin beta 4 mRNA and peptide expression in phagocytic cells of different mouse tissues. Peptides. 2009;30:1822–32. doi: 10.1016/j.peptides.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Young JD, Lawrence AJ, MacLean AG, Leung BP, McInnes IB, Canas B, et al. Thymosin beta-4 sulfoxide is an anti-inflammatory agent generated by monocytes in the presence of glucocorticoids. Nat Med. 1999;5:1424–7. doi: 10.1038/71002. [DOI] [PubMed] [Google Scholar]
- 6.Wessels A, Perez-Pomares JM. The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:43–57. doi: 10.1002/ar.a.10129. [DOI] [PubMed] [Google Scholar]
- 7.Smart N, Rossdeutsch A, Riley PR. Thymosin β4 and angiogenesis: modes of action and therapeutic potential. Angiogenesis. 2007;10:229–41. doi: 10.1007/s10456-007-9077-x. [DOI] [PubMed] [Google Scholar]
- 8.Sosne G, Szliter EA, Barrett R, Kernacki KA, Kleinman H, Hazlett LD. Thymosin beta-4 promotes corneal wound healing and decreases inflammation in vivo following alkali injury. Exp Eye Res. 2002;74:293–9. doi: 10.1006/exer.2001.1125. [DOI] [PubMed] [Google Scholar]
- 9.Nemolato S, Messana I, Cabras T, Manconi B, Inzitari R, Fanali C, et al. Thymosin beta (4) and beta (10) levels in preterm newborn oral cavity and foetal salivary glands evidence a switch of secretion during foetal development. PLoS One. 2009;4:e5109. doi: 10.1371/journal.pone.0005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Kodolitsch Y, Ito WD, Franzen O, Lund GK, Koschyk DH, Meinertz T. Coronary artery anomalies Part I: recent insights from molecular embryology. Z Kardiol. 2004;93:929–37. doi: 10.1007/s00392-004-0152-7. [DOI] [PubMed] [Google Scholar]
- 11.Lin SC, Morrison-Bogorad M. Developmental expression of mRNAs encoding thymosins beta-4 and beta 10 in rat brain and other tissues. J Mol Neurosci. 1990;2:35–44. doi: 10.1007/BF02896924. [DOI] [PubMed] [Google Scholar]
- 12.Guinobert I, Viltard M, Piquemal D, Elalouf JM, Marti J, Lelièvre-Pégorier M. Identification of differentially expressed genes between fetal and adult mouse kidney:candidate gene in kidney development. Nephron Physiol. 2006;102:81–91. doi: 10.1159/000090054. [DOI] [PubMed] [Google Scholar]
- 13.Mora CA, Baumann CA, Paino JE, Goldstein AL, Badamchian M. Biodistribution of synthetic thymosin beta 4 in the serum, urine, and major organs of mice. Int J Immunopharmacol. 1997;19:1–8. doi: 10.1016/s0192-0561(97)00005-2. [DOI] [PubMed] [Google Scholar]
- 14.Hall AK. Differential expression of thymosin genes in human tumors and in developing human kidney. Int J Cancer. 1991;48:672–7. doi: 10.1002/ijc.2910480507. [DOI] [PubMed] [Google Scholar]
- 15.Ma LJ, Fogo AB. PAI-1 and kidney fibrosis. Front Biosci. 2009;14:2028–41. doi: 10.2741/3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall AK, Aten R, Behrman HR. Thymosin gene expression is modulated by pregnant mare’s serum gonadotropin, human chorionic gonadotropin, and prostaglandin F2 alpha in the immature rat ovary. Endocrinology. 1991;128:951–7. doi: 10.1210/endo-128-2-951. [DOI] [PubMed] [Google Scholar]
- 17.Hall AK, Aten R, Behrman HR. Differential modulation of thymosin genes in the immature rat ovary by gonadotropins. Mol Cell Endocrinol. 1991;79:37–43. doi: 10.1016/0303-7207(91)90093-8. [DOI] [PubMed] [Google Scholar]
- 18.Wise TH. Developmental changes of thymosin alpha 1 and beta 4 in male and male castrated pigs: modulation by testosterone and human chorionic gonadoropin. Biol Reprod. 1992;46:892–7. doi: 10.1095/biolreprod46.5.892. [DOI] [PubMed] [Google Scholar]
- 19.Iguchi K, Ito M, Usui S, Mizokami A, Namiki M, Hirano K. Downregolation of thymosin beta 4 expression by androgen in prostate cancer LNCaP cells. J Androl. 2008;29:207–12. doi: 10.2164/jandrol.107.003608. [DOI] [PubMed] [Google Scholar]
- 20.Ito M, Iguchi K, Usui S, Hirano K. Overexpression of thymosin beta 4 increases pseudopodia formation in LNCaP prostate cancer cells. Biol Pharm Bull. 2009;32:1101–4. doi: 10.1248/bpb.32.1101. [DOI] [PubMed] [Google Scholar]
- 21.Nemolato S, Cabras T, Fanari MU, Cau F, Fraschini M, Manconi B, et al. Thymosin beta 4 expression in normal skin, colon mucosa and in tumor infiltrating mast cells. Eur J Histochem. 2010;54:e3. doi: 10.4081/ejh.2010.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kannan L, Rath NC, Liyanage R, Lay JO. Identification and characterization of thymosin β-4 in chicken macrophages using whole cell MALDI-TOF. Ann N Y Acad Sci. 2007;1112:425–34. doi: 10.1196/annals.1415.028. [DOI] [PubMed] [Google Scholar]
- 23.Nemolato S, Cabras T, Cau F, Fanari MU, Fanni D, Manconi B, et al. Different Thymosin Beta 4 Immunoreactivity in Foetal and Adult Gastrointestinal Tract. PLoS One. 2010;5:e9111. doi: 10.1371/journal.pone.0009111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang WS, Chen PM, Su Y. Colorectal carcinoma: from tumorigenesis to treatment. Cell Mol Life Sci. 2006;63:663–71. doi: 10.1007/s00018-005-5425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otto AM, Muller CSG, Huff T, Hannappel E. Chemotherapeutic drugs change actin skeleton organization and the expression of beta thymosins in human breast cancer cells. J Cancer Res Clin Oncol. 2002;128:247–56. doi: 10.1007/s00432-002-0332-7. [DOI] [PubMed] [Google Scholar]
- 26.Ji P, Diederichs S, Wang W, Boing S, Metzger R, Schneider PM, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–41. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 27.Choi SY, Kim DK, Eun B, Kim K, Sun W, Kim H. Anti-apoptotic function of thymosin beta in developing chick spinal motoneurons. Biochim Biophys Res Commun. 2006;346:872–8. doi: 10.1016/j.bbrc.2006.05.207. [DOI] [PubMed] [Google Scholar]


