Abstract
We analyzed the effect of cadmium on corticotropic (ACTH) and prolactin (PRL) cells in the pituitary gland of the Podarcis sicula (P. sicula) lizard under chronic exposure to this metal. Adult lizards were given CdCl2 in drinking water at the dose of 10 µg/10 g body mass for 120 days. Light microscopy was performed after histological and immunohistochemical staining, and the effects were followed at regular time intervals up to 120 days post-treatment. We detected substantial variations in the general morphology of the pituitary: unlike the control lizards in which the gland appeared compact, the treated lizards showed a glandular tissue with dilated spaces that were more extensive at 90 and 120 days. PRL and ACTH cells showed an increase in occurrence and immunostaining intensity in treated lizards in comparison with the same cells of control animals. This cellular increase peaked for PRL at 30 days in the rostral, medial and also caudal pars distalis of the gland. ACTH cells appeared to increase markedly after 60 days of treatment in both the pars distalis and the pars intermedia. Again, at 60 days small, isolated ACTH cells were also found in the caudal pars distalis in which these cells were generally absent. However, at 120 days both these cellular types showed an occurrence, distribution and morphology similar to those observed in the control lizards. In lizards, protracted oral exposure to cadmium evidently involves an alteration of the normal morphology of the gland and an inhibitory effect of ACTH and PRL cells, since they increase in occurrence and immunostaining. Yet in time the inhibitory effect of cadmium on ACTH and PRL cells falls back and their occurrence appears similar to that of the control lizard.
Key words: cadmium, corticotropic and prolactin cells, lizard.
Introduction
Cadmium (Cd) is known to be a potent toxic metal and a significant environmental pollutant. Studies concerning animals exposed to cadmium are of great interest since the presence of this metal in the environment has increased due to industrial activity and agricultural practices, thereby entering the food chain.1
In human populations, cadmium exposure occurs primarily through dietary sources and drinking water as well as cigarette smoking. Cadmium exposure in mammals has been proved to cause bone problems2 and renal dysfunctions,3 alter reproductive functions4 and exert neurotoxic effects.5 It has been shown that Cd is also an endocrine disruptor that may play a role in the aetiology of the pathologies that involve the hypothalamic-pituitary-testicular axis of mammals.6–8 Cadmium mimics the function of steroid hormones and its potential role in the development of hormone-dependent cancers is widely discussed.9 Studies carried out on rats also prove that Cd2+ is absorbed and retained in the pituitary gland,10,11 leading to a decrease in content of luteinizing hormone and alteration of gland functionality.7,8,12 In the mouse Mus platythrix, cadmium induces hypertrophy and hyperplasia of pituitary gonadotrophs.13 In rat, Cd modifies the lactotroph cell activity of the pituitary gland through biochemical, genomic and morphological changes14 and modifies plasma levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH).15–18
Histopathological damage depends on exposure time, dose and administration route of Cd.8 Lower concentrations of CdCl2 do not seem to influence the gonadotrope (GTH) cells in the cyprinid Puntius sarana .19 However, in fish cadmium exposure affects the activity of the endocrine system.19–21
Compared with other classes of vertebrates, reptiles are rarely used in studies on the possible toxic effects of heavy metals.22 However, they are important bioindicators because they are susceptible to the accumulation of persistent pollution which is identified as a major threat to reptile populations worldwide. Although Cd accumulation in various reptile organs has been studied,1,22–25 there has been very little experimental laboratory research on the effects of cadmium in reptiles.26 Particularly lacking are effect-based studies in reptiles exposed to known concentrations of contaminants. Reptiles could be a good model to study the biological effects of cadmium. Although the physiological function of this metal is unknown, there is evidence to suggest that cadmium is a metallohormone with estrogenic and androgenic effects.9
As we have already reported elsewhere, in the lizard an acute treatment with a single high (20 µg/10 g body mass) intraperitoneal dose of CdCl2 not only induces apoptosis, especially in the rostral pars distalis, which appears irreversible,27 but also alters the normal endocrine function of the gland.28 Once again in the lizard, we also reported that chronic exposure to CdCl2 affects the hormonal secretion of GTH cells through an inhibitory effect.29 The aim of this paper was thus to analyse the effects of cadmium on ACTH and PRL cells in the lizard Podarcis sicula (P. sicula) exposed to chronic oral treatment for 120 days at an average dose (10 µg/10 g body mass) of CdCL2.
Materials and Methods
This study was performed on 30 adult females of P. sicula, captured near Naples (Italy) and kept under controlled conditions of light and temperature. Twenty specimens were subjected to chronic treatment and the others were used as control. CdCl2 was administered to the lizards in drinking water for four months at a daily dose of 10 µg/10 g body mass while control lizards received cadmium-free water. The above dosage was chosen in accordance with previous reports.24,29 No mortality or altered animal behaviour was recorded during the experiments. Groups of four treated and two control animals were killed at 10, 30, 60, 90 and 120 days. Experiments were performed in accordance with the Guidelines for Animal Experimentation of the Italian Department of Health under the supervision of a veterinarian, and organised to minimise stress and the number of lizards used. All animals were killed under ice anaesthesia by a cervical cut. In lizards the pituitary gland is extremely small and almost completely enclosed in the sella turcica, which makes its removal difficult and may cause damage to the gland. For this reason, we analysed the hypophysis in toto with the brain. After removal of the skullcap, the brains were fixed in Bouin’s solution for 48 h at room temperature and then decalcified in a solution of 5% EDTA in 10% formalin for 25–30 days, dehydrated and enclosed in paraffin. This was the only procedure able to preserve not only the morphology but also the antigenicity of the cells. Serial 6 µm sections were processed for routine histological and immunohistochemical staining. Mallory’s trichromic stain was used for the study of the general morphology while the immunohistochemical procedure was applied to identify and observe the adenohypophyseal cells. For immunohistochemical staining,30 the sections were processed according to the ABC technique31 using the following heterologous antisera at specific working dilutions: anti-human PRL (1/300, Signet Laboratories, Dedham, MA, USA) and anti-synthetic ACTH1-24 (1/600, Biogenesis, Poole, UK). Visualization was carried out using the Vectastain Elite ABC kit (Vector Labs, Inc., Burlingame, CA, USA) and revealed by 3 mg 3,3′-diaminobenzidine-tetrahydrochloride (Sigma, St. Louis, MO, USA) in 10 mL PBS and 150 µL 3% H2O2. The sections were then contrasted with haemalum for one minute. Antibody specificity was assessed by omitting the primary antisera and absorbing each antiserum with the specific hormone. The images were examined and acquired by a Kontron Electronic Imaging System KS300 (Zeiss, Oberkochen, Germany). Quantification of the two cellular types was carried out on at least 300 cells, with a visible nucleus, on serial sections per animal and relative to specific regions, namely the rostral, medial and caudal pars distalis and pars intermedia. Data were expressed as the number of immunostained cells × 100/number of total cells. The data obtained were pooled and analysed performing Student’s t-test to determine the significance (P<0.05) between control and cadmium exposed groups.
Results
General morphology
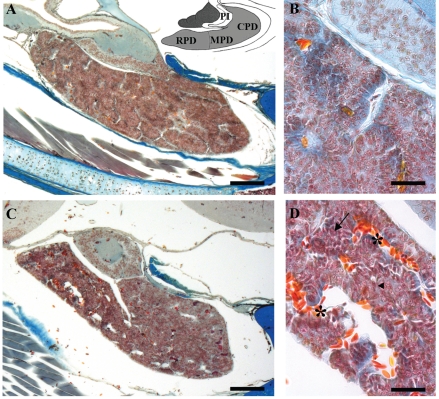
In all control specimens the pituitary gland appeared compact and extended in the cephalic-caudal direction in which the pars distalis (PD) was divided into a rostral part (RPD), a caudal part (CPD) and a medial part (MPD) (Figure 1 A). The whole PD consisted of homogeneous vascularised cellular cordons with an evident basal lamina surrounding them and with the cells clearly identifiable by Mallory stain (Figure 1 B). In the treated animals the pituitary gland tissue appeared atrophied in some areas, with wide irregular intercellular spaces, which appeared more extensive at 60 (Figure 1 C) and evident also at 90 and 120 days. The gland also showed greater vascularisation; a basal lamina surrounded the cellular cordons only partially and several cells appeared altered in shape (Figure 1 D).
Figure 1.
Mallory stain. Sagittal sections of the P. sicula pituitary gland. (A) Control lizard showing the extension of the gland. At the top the subdivision of the gland into rostral (RPD), medial (MPD), caudal (CPD) pars distalis and pars intermedia (PI) is reported. (B) Detail of panel A showing the organization of the cellular cordons. (C) Treated lizard at 60 days. The gland tissue shows wide irregular spaces. (D) Detail of panel C: the cellular cordons show a major vascularisation (*), a basal lamina only partly surrounding them (◂) and several cells with altered shape (←). Bar 140 µm in A and C; Bar 40 µm in B and D.
Immunohistochemistry
In the control lizards, ACTH and PRL cells appeared clearly through immunohistochemical detection as distinct cellular populations with a specific distribution in the PD or PI (pars intermedia). In treated specimens we observed the increase in occurrence and immunostain both for ACTH and for PRL cells. ACTH and PRL cells already at 10 days revealed a small increase, albeit with a different time course: PRL cells appeared more abundant at 30 days from treatment, while ACTH were more copious at 60 days. In all these cells we also observed greater cytoplasmic immunostaining intensity.
Prolactin cells
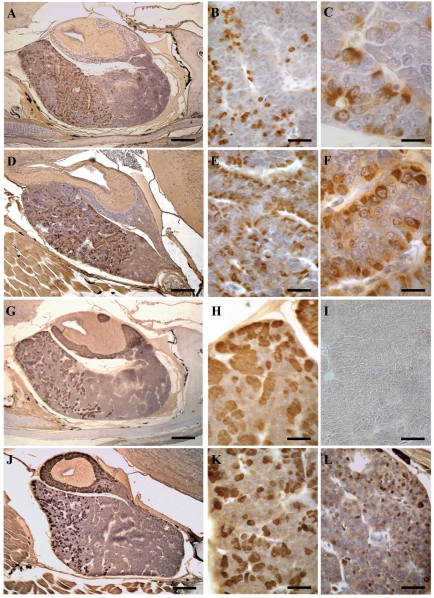
In control lizards PRL cells were found essentially in the RPD (24.0±0.5%) but also in the MPD (20.1±0.24%) (Figure 2 A). They were generally isolated or clustered in small cellular cordons of three-five cells (Figure 2 B). PRL cells appeared pyriform or ovoidal in shape, with an eccentric and ovoidal nucleus and a moderately dense cytoplasm (Figure 2 C). A few PRL cells were also observed in the CPD (1.2±0.05%), but they were always absent in the PI. At 10 days of treatment PRL cells had slightly increased in the RPD and MPD, but they were more numerous in the CPD (Table 1). Their occurrence peaked at 30 days from treatment (Figure 2 D): in the RPD (34.0±1.7%), MPD (39.8±1.2%) and CPD (17.3±0.67%). In all these regions they also showed greater immunostaining intensity (Figure 2 E,F). At 60 and 90 days of treatment PRL cells still appeared numerous, albeit with diminishing values, and at 120 days close to those of the control animals as reported in Table 1. No other differences were observed between PRL cells in control and treated specimens.
Figure 2.
ABC technique. Sagittal sections. (A–F) PRL cells (in brown). (A) Control lizard, showing the occurrence of PRL cells in the RPD and in the MPD. (B, C) Details of panel A showing these isolated cells (B) or organized into small cordons (C). (D) Treated lizard at 30 days, showing the increase in PRL cells extending also into the CPD. (E, F) Details of panel D showing the greater intensity of immunostain of the cytoplasm in the MPD (E) and the CPD (F). (G–L) ACTH cells (in brown). (G) Control lizard, showing the occurrence of ACTH cells in the RPD, the MPD and also in the PI. (H, I) Details of panel G to show their elongated shape and the cytoplasm moderately marked in the RPD (H) and their absence in CPD (I). (J) Treated lizard at 60 days with an evident strong immunostain of ACTH cells, which also appears in the CPD. (K, L) Details of panel J highlighting the marked immunostain of these cells in the RPD (K), as well as their occurrence in the CPD (L). Bar 150 µm in A, D, G, J; Bar 33 µm in B, E, I, L; Bar 15 µm in C, F; Bar 38 µm in H, K.
Table 1. Percentage immunopositive prolactin cells in pituitary gland of control and treated lizards.
| Control | 10 days | 30 days | 60 days | 90 days | 120 days | |
|---|---|---|---|---|---|---|
| RPD | 24.0±0.5 | 26.8±1.5 | 34.0±1.7* | 31.2±1.1 | 29.7±0.66 | 24.8±1.1 |
| MPD | 20.1±0.24 | 29.2±1.35 | 39.8±1.2° | 25.4±1.045 | 25.7±1.11 | 23.1±0.9 |
| CPD | 1.2±0.05 | 8.3±0.56* | 17.3±0.67° | 5.1±0.33* | 3.9±0.4 | 2.1±0.2 |
| PI | 0 | 0 | 0 | 0 | 0 | 0 |
Immunopositive cells are expressed as mean ± SEM (%);
P<0.05,
P<0.01, compared with control groups.
Corticotropic cells
ACTH cells were observed in both the RPD (29.0±0.7%), and the MPD (12.0±0.13%), as well as in the PI (59.0±0.12%), but were absent in the CPD of the control specimens (Figure 2 G,I). ACTH cells were elongated in shape, with a generally central nucleus and higher cytoplasmic density in the PD (Figure 2 H) compared with PI.
At 10 days of treatment their occurrence appeared similar to that of control lizards, except for the presence of a few cells also in the CPD (Table 2). ACTH cells increased markedly after 60 days of treatment with strong immunostaining of the cytoplasm in all the regions of the gland (Figure 2 J,K,L): in the RPD (40.3±1.11%), MPD (23.8±0.9%), PI (76.2±1.25%) and CPD (16.1±0.85%). In the caudal region they appeared small and isolated cells, with no cordonal organization (Figure 2 L). At 90 and 120 days of treatment ACTH cells showed a similar distribution and morphology to the control; in the CPD as well their occurrence had considerably decreased, appearing nearly absent at 120 days of treatment.
Table 2. Percentage immunopositive corticotropic cells in pituitary gland of control and treated lizards.
| Control | 10 days | 30 days | 60 days | 90 days | 120 days | |
|---|---|---|---|---|---|---|
| RPD | 29.0±0.7 | 26.3±1.1 | 30.3±0.9 | 40.3±1.11* | 29.8±0.95 | 29.3±0.13 |
| MPD | 12.0±0.13 | 14.0±0.8 | 15.8±0.8 | 23.8±0.9* | 14.5±0.21 | 12.8±0.69 |
| CPD | 0 | 0 1.5±0.36 | 3.7±0.18* | 16.1±0.85° | 5.1±0.6* | 0.35±0.3 |
| PI | 59.0±0.12 | 60.1±1.84 | 73.2±1.9 | 76.2±1.25* | 69.5±1.11 | 63.2±1.18 |
Immunopositive cells are expressed as mean ± SEM (%);
P<0.05,
P<0.01, compared with control groups.
Discussion
Our findings indicate the toxic effect of cadmium upon the pituitary gland of the lizard P. sicula exposed to chronic oral treatment with an average CdCl2 dosage. Cytotoxic action may be inferred both from the morphological alteration of the gland and from the dysregulatory process in ACTH and PRL cells, effects which are nonetheless subject to different time courses. In terms of morphology, we observed the progressive disorganisation of hypophyseal tissue due to the appearance of atrophied areas with wide intercellular spaces, which were more expressed at 90 and 120 days of treatment. Similar effects have been reported in other glandular tissues such as the thyroid of the catfish Clarias batrachus 20 and the testis of the cyprinid Puntius sarana 19 and of the monkey Presbytis entellus entellus.32
However, in lizard exposed to acute treatment with a single high intraperitoneal dose of CdCl2 we previously noted that this metal induces apoptosis, as also reported in the anterior pituitary cells of the rat,33 and that this effect is irreversible.27 In this chronic treatment, apart from morphological damage, a parallel increase in the vessel network was also observed. The protraction of such tissue alterations and the enhanced vascularization of the pituitary gland agree with previous findings that chronic exposure to Cd can lead to elevations in blood pressure. Considerable evidence suggests that hypertensive effects of Cd result from complex actions on both the vascular endothelium and vascular smooth muscle.34 The same increase in blood pressure could partly explain the alterations in the tissue architecture which we found in this gland.
This heavy metal is also known to affect the endocrine system in mammals.7,8,12
Likewise, we also observed in the lizard the inhibitory action of cadmium in both ACTH and PRL cells, just as we previously reported for gonadotrope cells.29 Indeed, both these cell types during chronic treatment are more numerous and show marked immunoreactivity. However, we observed that the cellular increase peaked for PRL at 30 days, while for ACTH it peaked after 60 days of treatment. This finding is also supported by a concomitant increase in immunostaining of the cytoplasmic granules of all these cells. The increase in occurrence and in cytoplasmic density is indicative of a hormonal accumulation in these cells due to the inhibiting effect induced by cadmium. It has been proved elsewhere that divalent cations, such as Cd2+, inhibit in vitro release of GH and PRL from bovine adenohypophyseal secretory granules.35 Further, the inhibitory effect of cadmium on the hormonal secretion of many adenohypophyseal cells has been found in mammals by virtue of biochemical studies: the levels of LH and FSH in serum of rat7,12 and pig36 exposed to cadmium decrease.
In mammals Cd differentially affects the secretory mechanisms of the pituitary hormones: the effects of this metal are dosedependent only for prolactin and ACTH.8 In the fish Puntius sarana,19 only high concentrations of CdCl2 influence the pituitary gonadotropins with a gradual accumulation of secretory granules. Also in the lizard Cd could well compete with calcium at pituitary level through the membrane channels or change intracellular calcium mobilization, as postulated for mammals,37 and inhibit hormone secretion. However, Cd could also cause alterations in receptor binding and secretory mechanisms of pituitary hormones as reported by Pillai et al.38 in female rats: cadmium generates free radicals which change the biophysical properties of the pituitary membranes with an inhibitory effect on hormone secretion.
However, in the present study we observed that, unlike tissue alteration which persists in time, the inhibitory action both on ACTH and PRL cells diminishes: at 120 days the occurrence of these cells returned to values similar to those observed in the control lizards. This reaction could be viewed as a probable adaptation to the toxic action of cadmium in time, when the Cd dosage is at moderate levels. It may also be attributed to the activation of defence mechanisms such as the action of metallothionein, given that Cd in chronic intoxication stimulates de novo synthesis of MTs; toxicity in the cells is assumed to start when Cd ion loading exceeds the buffering capacity of intracellular MTs.39 That said, the lizard appears to be a good experimental model for studying the action of heavy metals on the endocrine system.
References
- 1.Burger J, Campbell KR, Murray S, Campbell TS, Gaines KF, Jeitner C, et al. Metal levels in blood, muscle and liver of water snakes (Nerodia spp.) from New Jersey, Tennessee and South Carolina. Sci Total Environ. 2007;373:556–63. doi: 10.1016/j.scitotenv.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Brzoska MM, Moniuszko-Jakoniuk J. Bone metabolism of male rats chronically exposed to cadmium. Toxicol Appl Pharmacol. 2005;207:195–211. doi: 10.1016/j.taap.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Uriu K, Kaizu K, Komine N, Ikeda M, Qie YL, Hashimoto O, et al. Renal hemodynamics in rats with cadmium-induced nephropathy. Toxicol Appl Pharmacol. 1998;150:76–85. doi: 10.1006/taap.1998.8411. [DOI] [PubMed] [Google Scholar]
- 4.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–13. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonio MT, Corpas I, Leret ML. Neurochemical changes in newborn rat’s brain after gestational cadmium and lead exposure. Toxicol Lett. 1999;104:1–9. doi: 10.1016/s0378-4274(98)00125-8. [DOI] [PubMed] [Google Scholar]
- 6.Lafuente A, Esquifino AI. Cadmium effects on hypothalamic activity and pituitary hormone secretion in the male. Toxicol Lett. 1999;110:209–18. doi: 10.1016/s0378-4274(99)00159-9. [DOI] [PubMed] [Google Scholar]
- 7.Lafuente A, Marquez N, Pazo D, Esquifino AI. Cadmium effects on hypothalamicpituitary-testicular axis in male rats. Exp Biol Med (Maywood) 2001;226:605–11. doi: 10.1177/153537020122600615. [DOI] [PubMed] [Google Scholar]
- 8.Lafuente A, Pilar C, Esquifino AI. Are cadmium effects on plasma gonadotropins, prolactin, ACTH, GH and TSH levels, dosedependent? Biometals. 2003;16:243–50. doi: 10.1023/a:1020658128413. [DOI] [PubMed] [Google Scholar]
- 9.Byrne C, Divekar SD, Storchan GB, Parodi DA, Martin MB. Cadmium - A metallohormone? Toxicol Appl Pharmacol. 2009;238:266–71. doi: 10.1016/j.taap.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kollmer WE. Uptake and retention of cadmium-109 in the pituitary, the adrenals and the thyroid of the laboratory rat. Int J Appl Radiat Isot. 1980;31:607–9. doi: 10.1016/0020-708x(80)90016-2. [DOI] [PubMed] [Google Scholar]
- 11.Varga B, Paksy K, Naray M. Distribution of cadmium in ovaries, adrenals and pituitary gland after chronic administration in rats. Acta Physiol Hung. 1991;78:221–6. [PubMed] [Google Scholar]
- 12.Pillai A, Priya L, Gupta S. Effects of combined exposure to lead and cadmium on the hypothalamic-pituitary axis function in proestrous rats. Food Chem Toxicol. 2003;41:379–84. doi: 10.1016/s0278-6915(02)00247-8. [DOI] [PubMed] [Google Scholar]
- 13.Madhyastha NA, Gopal Dutt NH. Cadmium induced hypertrophy & hyperplasia of pituitary gonadotrophs of the brown spiny mouse Mus platythrix (Bennett) Indian J Exp Biol. 1979;17:637–9. [PubMed] [Google Scholar]
- 14.Calderoni AM, Biaggio V, Acosta M, Oliveros L, Mohamed F, Giménez MS. Cadmium exposure modifies lactotrophs activity associated to genomic and morphological changes in rat pituitary anterior lobe. Biometals. 2010;23:135–43. doi: 10.1007/s10534-009-9274-8. [DOI] [PubMed] [Google Scholar]
- 15.Zylber-Haran EA, Gershman H, Rosenmann E, Spitz IM. Gonadotropin, testosterone and prolactin interrelationships in cadmium treated rats. J Endocrinol. 1982;92:123–30. doi: 10.1677/joe.0.0920123. [DOI] [PubMed] [Google Scholar]
- 16.Paksy K, Varga B, Horwath E, Tatrai E, Ungvary G. Acute effects of cadmium on preovulationary serum FSH, LH and prolactin levels on ovulation and ovarian hormone secretion in estrus rats. Reprod Toxicol. 1989;3:241–47. doi: 10.1016/0890-6238(89)90018-x. [DOI] [PubMed] [Google Scholar]
- 17.Lafuente A, Blanco A, Marquez N, Alvarez-Damanuel E, Esquifino AI. Effects of acute and subchronic cadmium administration on pituitary hormone secretion in rat. Rev Esp Fisiol. 1997;53:265–9. [PubMed] [Google Scholar]
- 18.Lafuente A, Marquez N, Piquero S, Esquifino AI. Cadmium affects the episodic luteinizing Hormone secretion in male rats: possible age dependent effects. Toxicol Lett. 1999;104:27–33. doi: 10.1016/s0378-4274(98)00349-x. [DOI] [PubMed] [Google Scholar]
- 19.Kumari M, Gopal Dutt NH. Cadmiuminduced histomorphological changes in the testis and pituitary gonadotrophic hormone secreting cells of the cyprinid Puntius sarana. Ital J Zool. 1991;58:71–6. [Google Scholar]
- 20.Jadhao AG, Paul PL, Rao PD. Effect of cadmium chloride on the pituitary, thyroid and gonads in the catfish, Clarias batrachus (Linn) Funct Dev Morphol. 1994;4:39–44. [PubMed] [Google Scholar]
- 21.Norris DO, Felt SB, Woodling JD, Dores RM. Immunocytochemical and histological differences in the interregnal axis of feral brown trout, Salmo trutta, in metal-contaminated waters. Gen Comp Endocrinol. 1997;108:343–51. doi: 10.1006/gcen.1997.7000. [DOI] [PubMed] [Google Scholar]
- 22.Campbell KR, Campbell TS. Lizard contaminant data for ecological risk assessment. Rev Environ Contam Toxicol. 2000;165:39–116. doi: 10.1007/978-1-4612-1172-3_2. [DOI] [PubMed] [Google Scholar]
- 23.Campbell KR, Campbell TS, Burger J. Heavy Metal Concentrations in Northern Water Snakes (Nerodia sipedon) from East Fork Poplar Creek and the Little River, East Tennessee, USA. Arch Environ Contam Toxicol. 2005;49:239–48. doi: 10.1007/s00244-004-0200-3. [DOI] [PubMed] [Google Scholar]
- 24.Trinchella F, Riggio M, Filosa S, Volpe MG, Parisi E, Scudiero R. Cadmium distribution and metallothionein expression in lizard tissues following acute and chronic cadmium intoxication. Comp Biochem Physiol C Toxicol Pharmacol. 2006;144:272–8. doi: 10.1016/j.cbpc.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 25.Simoniello P, Filosa S, Riggio M, Scudiero R, Tammaro S, Trinchella F, et al. Responses to cadmium intoxication in the liver of the wall lizard Podarcis sicula. Comp Biochem Physiol C Toxicol Pharmacol. 2010;151:194–203. doi: 10.1016/j.cbpc.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Favorito R, Grimaldi MC, Ferrandino I. Effects of a chronic exposure to cadmium on the glial architecture in the lizard brain: an immunocytochemical study. Eur J Histochem. 2009;53(supplement 1):21–21. [Google Scholar]
- 27.Ferrandino I, Favorito R, Annunziata M, Grimaldi MC. Cadmium induces apoptosis in the pituitary gland of Podarcis sicula. Ann N Y Acad Sci. 2009;1163:386–8. doi: 10.1111/j.1749-6632.2008.03667.x. [DOI] [PubMed] [Google Scholar]
- 28.Favorito R, Grimaldi MC, Coppola M, Ferrandino I. Effects of Acute Cadmium Exposure on the Pituitary Gland of Podarcis sicula. Open Zool J. 2010;3:30–6. [Google Scholar]
- 29.Ferrandino I, Favorito R, Grimaldi MC. Cadmium effects on GTH cells in the pituitary gland of Podarcis sicula. In: Bologna MA, Capula M, Carpaneto GM, Luiselli L, Marangoni C, Venchi A, editors. Edizioni Belvedere; Latina, Italy, “Le Scienze”. Proc. 6th Congr. Naz. Societas Herpetologica Italica 2007.pp. 151–8. [Google Scholar]
- 30.Ferrandino I, Viscardi G, Grimaldi MC. An immunohistochemical study of adenohypophysial cells in the viviparous reptile Chalcides chalcides. Histochem J. 2001;33:1–8. doi: 10.1023/a:1017564211097. [DOI] [PubMed] [Google Scholar]
- 31.Hsu SM, Raine L, Fanger H. Use of avidinbiotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–80. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 32.David GF, Ramaswami LS. Changes observed in the FSH and LH cells of the adenohypophysis of Presbytis entellus entellus following cadmium induced testicular necrosis. Experientia. 1971;27:342–3. doi: 10.1007/BF02138186. [DOI] [PubMed] [Google Scholar]
- 33.Poliandri AH, Cabilla JP, Velardez MO, Bodo CC, Duvilanski BH. Cadmium induces apoptosis in anterior pituitary cells that can be reversed by treatment with antioxidants. Toxicol Appl Pharmacol. 2003;190:17–24. doi: 10.1016/s0041-008x(03)00191-1. [DOI] [PubMed] [Google Scholar]
- 34.Prozialeck WC, Edwards JR, Nebert DW, Woods JM, Barchowsky A, Atchison WD. The Vascular System as a Target of Metal Toxicity. Toxicol Sci. 2008;102:207–18. doi: 10.1093/toxsci/kfm263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lorenson MY, Robson DL, Jacobs LS. Divalent cation inhibition of hormone release from isolated adenohypophysial secretory granules. J Biol Chem. 1983;258:8618–22. [PubMed] [Google Scholar]
- 36.Han XY, Xu ZR, Wang YZ, Du WL. Effects of cadmium on serum sex hormone levels in pigs. J Anim Physiol Anim Nutr (Berl) 2006;90:380–84. doi: 10.1111/j.1439-0396.2006.00607.x. [DOI] [PubMed] [Google Scholar]
- 37.Waalkes MP, Poirier LA. In vitro cadmium-DNA interactions: cooperativity of cadmium binding and competitive antagonism by calcium, magnesium, and zinc. Toxicol Appl Pharmacol. 1984;75:539–46. doi: 10.1016/0041-008x(84)90190-x. [DOI] [PubMed] [Google Scholar]
- 38.Pillai A, Priya L, Gupta S. Effects of combined exposure to lead and cadmium on pituitary membrane of female rats. Arch Toxicol. 2002;76:671–75. doi: 10.1007/s00204-002-0399-6. [DOI] [PubMed] [Google Scholar]
- 39.Sabolic I, Breljak D, Skarica M, Herak-Kramberger CM. Role of metallothionein in cadmium traffic and toxicity in kidneys and other mammalian organs. Biometals. 2010;23:897–926. doi: 10.1007/s10534-010-9351-z. [DOI] [PubMed] [Google Scholar]