Abstract
Colidiarrhea and colienterotoxemia caused by F4+ and/or F18+ enterotoxigenic E. coli (ETEC) strains are the most prevalent infections of suckling and weaned pigs. Here we tested the immunogenicity and protective effectiveness of attenuated F18ac+ non-ETEC vaccine candidate strain against challenge infection with F4ac+ ETEC strain by quantitative phenotypic analysis of small intestinal leukocyte subsets in weaned pigs.We also evaluated levamisole as an immune response modifier (IRM) and its adjuvanticity when given in the combination with the experimental vaccine. The pigs were parenterally immunized with either levamisole (at days -2, -1 and 0) or with levamisole and perorally given F18ac+ non-ETEC strain (at day 0), and challenged with F4ac+ ETEC strain 7 days later.At day 13 the pigs were euthanatized and sampled for immunohistological/histomorphometrical analyses. Lymphoid CD3+, CD45RA+, CD45RC+, CD21+, IgA+ and myeloid SWC3+ cell subsets were identified in jejunal and ileal epithelium, lamina propria and Peyer’s patches using the avidin-biotin complex method, and their numbers were determined by computer-assisted histomorphometry. Quantitative immunophenotypic analyses showed that levamisole treated pigs had highly increased numbers of jejunal CD3+, CD45RC+ and SWC3+ cells (p<0.05) as compared to those recorded in nontreated control pigs.In the ileum of these pigs we have recorded that only CD21+ cells were significantly increased (p<0.01). The pigs that were treated with levamisole adjuvanted experimental vaccine had significantly increased numbers of all tested cell subsets in both segments of the small intestine. It was concluded that levamisole adjuvanted F18ac+ non-ETEC vaccine was a requirement for the elicitation of protective gut immunity in this model; nonspecific immunization with levamisole was less effective, but confirmed its potential as an IRM.
Key words: nonspecific/specific immunization, E. coli, gut immune cells, pigs.
Porcine colidiarrhea and colienterotoxemia induced with F4+ and/or F18+ enterotoxigenic Escherichia coli (ETEC) strains are economically the most significant diseases of swine which account for moderate to high mortality rates and growth retardation, causing death of 5 million pigs per year in the World. Protection from ETEC is a constant challenge due to high genetic flexibility of this widespread bacterial organism.The virulence characteristics of ETEC are strongly dependent on the production of fimbrial adhesins and enterotoxins (Nagy and Fekete, 1999).The ability of adhesion of ETEC to intestinal wall is mainly due to the production of fimbriae. Enterotoxins produced by adherent ETEC strains act locally on enterocytes and stimulate increased water and electrolyte secretion and decreased fluid absorption. Several types of porcine ETEC are known today, including ETEC strain producing F18 fimbriae with their variants “ab” and “ac” (Bretschinger et al., 1990; Nagy and Fekete, 1999; Zang et al., 2007). The ETEC strains causing diarrhea mostly express F4 or F18 adhesins (Fairbrother et al., 2005; Zang et al., 2007). Nearly all known E. coli enterotoxin genes are produced by ETEC strains expressing either F4 or F18 fimbria. Zhang et al. (2007) have conclude that the dominant pathotypes causing diarrhea in weaned pigs are porcine ETEC strains expressing either F4 fimbria and heat-labile (LT) / heat stable (STb) toxins or LT/STb/EAST1 toxins, or F18 fimbria and STa/STb/Stx2e toxins. However, F18ab is more frequently associated with Shiga like toxin 2e, whereas F18ac is more frequently associated with enterotoxin STI (Cheng et al., 2005). Olasz et al. (2005) showed that the 200-kb plasmid, called pF18, contained the genes responsible for F18 fimbriae production.The curing of F18+ ETEC strain, i.e. loss of plasmid carrying the heat stable toxin genes in bacterial mutants has been performed by the plasmid transformation and conjugation following co-culturing of donor and recipient strains as reported earlier (Olasz et al., 2005). New vaccination strategies include the oral immunization of pigs with live avirulent nontoxigenic E. coli strains carrying the fimbrial adhesins F4 and/or F18 (Fairbrother et al., 2005).
Receptors for F18ab and F18ac variants are increasingly produced up to the weaning age and the fimbriae F18ac seem to have more receptors around the ileal Peyer's patches (Nagy et al., 1992). The colonization of the small intestine by an F18+ ETEC strain causes enterotoxemia. The typical clinical symptoms of the disease are neurological signs such as ataxia, convulsions and paralysis (Vögeli et al., 1996). It is well known that enterotoxic colibacillosis produces significant losses in two different age groups of pigs: first among newborn pigs and later at the postweaning age (Nagy and Fekete, 1999). The disease usually starts a few days after lacteal protection completely ceases (within the first 2 weeks after weaning), especially when weaning occurs at 3–4 weeks of age.Thus, the success of a vaccine against porcine colidiarrhea and colienterotoxemia depends upon applying it in the most efficient form at the optimal time and matching the right protective antigens with the type of virulence factors of ETEC present in the given animal population (Nagy and Fekete, 2005).
The gut mucosal immune system contains specialized lymphoid tissues where environmental antigens are presented inducing B- and T-cell responses (Stokes et al., 1994). These responses are regulated by T cells and cytokines and they lead to plasma cell differentiation and the secretion of IgA antibodies onto intestinal mucosal surfaces.The aggregated lymphoid tissue such as Peyer’s patches and solitary lymphoid cells in the lamina propria both play important roles in the induction and regulation of immune responses in the gut associated lymphoid tissues (GALT) (Lacković et al.,1997b). Such organization of the GALT may provide immune protection at mucosal surfaces where the infection actually occurs (McGhee et al., 1992). Bertschinger et al. (2000) demonstrated the protective effects of a live oral vaccine containing F18 fimbria against porcine postweaning diarrhea and oedema disease.
In this study we have examined the distribution and quantitative patterns of the subsets of T and B cells as well as of macrophages and secretory IgA+ plasma cells within GALT compartments of 4 weeks old pigs perorally immunized with an attenuated F18ac+ non-ETEC vaccine candidate strain against porcine colienterotoxemia. Additionally, we have evaluated adjuvanticity of levamisole in the combination with the experimental vaccine and its immunostimulatory effect when applied as an immune response modifier (IRM). Levamisole (2,3,5,6-tetrahydro-6-phenylimidazole thiazole), was originally described as a highly effective anti-helminthic compound (Thienpont et al., 1966). Subsequent studies have established its ability to restore and enhance depressed immune responses in domestic food animals and to act as an effective adjuvant for parenteral and oral vaccines (Mulcahy and Quinn, 1986; Jenkins and Hurdle, 1989; Božić et al., 2002).
Materials and Methods
Bacterial strains
The vaccine candidate F18ac+ non-ETEC strain 2143 (serotype O157:K119:F18ac) kindly donated by dr. sc. Bela Nagy from the Veterinary Medical Institute of Hungarian Academy of Sciences, Budapest, Hungary, was used for the immunization. The F4ac+ ETEC strain 11-800/1/94 (serotype O149: K91: F4ac: 987P: Hly+ LT+ STb+) was used for the challenge infection.This strain was isolated from diarrheic pigs reared on swine farms in Croatia. Both strains were kept in the glycerin broth at −80°C until used.
The vaccine candidate strain was attenuated by a special culturing procedure as described herein. Briefly, as the strain 2134 was originally described as an ETEC strain we reduced its toxicity with a slightly modified procedure as reported earlier (Gordon et al., 1992). From glycerin the strains were transferred onto trypticase soya agar and within 24 hours onto either glutamine-rich (vaccine candidate strain 2134) trypticase soya broth (TSB) or plain TSB (challenge strain 11-800/1/94). Following incubation overnight at 36°C the cultures were adjusted to at least 1010 colony-forming units (CFU) per ml of TSB. Then we pre-formed growing of these two bacterial organisms together in broth which resulted in the loss of toxicity of 2134 strain without loss of F18ac fimbrial antigens. Prior to the preparation for the immunization/challene both strains were serologically checked for F4 and F18 fimbrial antigens using specific antisera from the collection of Croatian Veterinary Institute, Zagreb, Croatia. The antisera were prepared and absorbed as previously describer (Sojka, 1965; Sojka, 1973). The presence or absence of heat stable enterotoxins (OXOID E. coli ST EIA TD 700) and heat labile enterotoxin (OXOID-VET-RPLA TD 920) were detected using respective test kits by the reversible passive latex agglutination or by competitive immunoassay, respectively.
Monoclonal antibodies
The monoclonal antibodies (mAbs) reactive with porcine leukocyte surface molecules i.e. cluster of differentiation (CD) antigens that we have used to study in situ identification, distribution and quantification patterns of respective cell subsets are listed in Table 1.
Table 1. The mAbs specific for swine leukocyte CD/SWC antigens used in immunohistological demonstration of porcine intestinal lymphoid and myeloid cell subsets.
| Marker | mAbs | Cells | Donor* |
|---|---|---|---|
| CD3a | BB23-8E6 | T cells | Pescovitz |
| CD3b | FY1H2 | T cells | Yang |
| CD45RA | MIL13 | Leukocytes | Haverson |
| CD45RC | MIL5 | Leukocytes | Stokes |
| CD21 | BB6-11c9 | B cells | Pescovitz |
| SWC3 | 74-22-15 | Macrophages,monocytes,granulocytes | Lunney |
| IgA | K61.1B4 | Activated B cells, plasma cells | Haverson |
Kindly donated for research purposes and testing for the Swine CD Workshops held in Davis, CA, USA (1995), Ludhiana, India (1998), and Amsterdam, Netherlands (1999).
Pigs and experimental design
Fifteen conventionally reared crossbred pigs (Swedish Landrace x Yorkshire) from a large scale swine farm were weaned at 4 weeks of age and purchased for this experiment.The pigs were housed in the animal facility at the Veterinary Faculty University of Zagreb and fed with a standard weaner diet. They were randomly divided into three groups comprising 5 animals each. After two days of accommodation pigs were treated as follows: control nonvaccinated pigs received saline at day 0, principal pigs were intramuscularly primed with either levamisole (Nilverm®, Pliva, Zagreb, Croatia) at the immunostimulatory dose of 2.5 mg/kg over three consecutive days (−2, −1, 0) or with levamisole over three consecutive days (−2, −1, 0) and intragastrically vaccinated with 1010 CFU/mL of F18ac+ non-ETEC vaccine candidate strain 2143 in 60 mL of TSB at day 0. All pigs were challenged with 1010 CFU/mL of F4ac+ ETEC strain 11-800/1/94 7 days later and three out of each group were euthanatized at day 13 and sampled for immunohistology.
All treatments of pigs were conducted in accordance with the “Directive for the Protection of Vertebrate Animals used for Experimental and other Purposes” (86/609/EEC).
Clinical observations
Clinical observations for signs of colienterotoxemia, such as diarrhea, dehydration, weight loss, oedema, weakness and ataxia were recorded three times daily by the person blinded to given treatments. Pigs were weighed at the beginning of the trial (day −2), and 10 days after the immunization (day 7) or 4 days after the challenge infection.The diarrhea developed by pigs was graded on a scale of intensity where scores (per pig per day of the experiment) were given based on stools consistency: +, soft feces = mild diarrhea; ++, fluid feces = moderate diarrhea; +++, watery feces = severe diarrhea. Pigs with normal firm feces were scored as diarrhea negative (−).
Isolation of vaccine and challege strains from the feces
Biginning with day −2 before the treatments with levamisole, rectal swabs were taken from each pig at day 0 and also at days 7 and 13 following vaccination and challenge. Samples were diluted in serial dilutions up to 1010 in saline and 1 mL of each dilution was placed onto TSB with 5% sheep blood agar (Blood Agar Base, No.2, OXOID CM 271). After incubation at 37°C overnight, the numbers of CFU per mL were determined by counting on an automatic computer-assisted counter. Five E. coli colonies from each plate were serotyped by slide agglutination test using rabbit OK antisera prepared from standard E. coli strains (Croatian Veterinary Institute, Zagreb, Croatia). Hemolytic isolates identified by plating on 5% sheep blood agar with esculine were further serologically checked for F4 and F18 fimbrial antigens.The presence or absence of heat stable and heat labile enterotoxins were confirmed using commercial test kits as afore mentioned.To detect natural infections with other E. coli strains, faecal samples were also plated onto plain agar.
Sampling
As we have observed diarrhea in each group of pigs (although of different intensity/duration, and in different no. of pigs per group) we have selected for euthanasia and sampling 3 pigs per group based on the following criteria: (i) presence or absence of diarrhea (+ or −) and (ii) intensity of diarrhea (mild = +, moderate = ++ or +++ = severe). In order to obtain as much as possible uniform samples of jejunum/ileum for immunohistology we have euthanatized pigs in groups as follows: (A) control - numbers 1(−), 2 (++) and 3 (+), (B) levamisole - numbers 1 (−), 2 (−) and 3 (+) and (C) levamisole + vaccine - numbers 1 (+), 2 (++) and 3 (−).With exception of the group B where only 1 pig developed mild diarrhea (and we had to select for sampling 2 instead of 1 pig without diarrhea), the other two groups were completely uniform regarding diarrhea status of pigs selected for sampling.
Immunohistochemical staining
Immediately after euthanasia the specimens of ileum and jejunum were fixed for 24 hours in 10% neutral buffered formaldehyde (pH 7.0–7.6) and then processed for immunohistochemistry. Paraplast-embedded sections were cut into 5 µm thick serial sections and staining was performed by the avidin/biotin complex (ABC) method. For blocking of endogenous peroxidase activity, the slides were immersed into 3% hydrogen peroxide solution for 30 minutes prior to staining. Blocking of background staining was performed by covering the tissue sections with 5% rabbit serum and 5% pig serum diluted in PBS, for 30 minutes at room temperature. Mouse anti-swine CD antigen-specific mAbs (Table 1) were added over the sections and incubated for 1 hour at room temperature.The secondary antibody, biotinylated rabbit anti-mouse IgG (Sigma, St. Louis, USA) diluted 1:500 in PBS was applied to cover the tissue sections and incubated at room temperature for 1 hour. Streptavidin-peroxidase complex (ICN, ImmunoBiologicals, USA) diluted 1:1000 in PBS was applied for 1 hour at room temperature. All steps were carried out in a humid chamber.The reaction was visualized with a 0.05% solution of 3,3-diaminobenzidine tetrachloride (DAB) in 0.05 M Tris-HCl (pH 7.6) containing 0.01% H2O2. Then, the slides were dehydrated and mounted using standard immunohistological technique.
Morphometry
Immunophenotypes of lymphoid and myeloid cell subsets within jejunal/ileal mucosa as demonstrated by immunohistochemical ABC method were quantitatively analyzed using software program Lucia G for digital image analysis (DIA). Morphometric analyses were performed by counting of specifically marked immune cells in 12 randomly selected tissue section fields at ×200 on screen magnification. Such counting included the areas of lamina propria and adjacent epithelium, the areas of villous and crypts and also the Peyer’s patches.The results are expressed as the mean values and standard deviations of the number of cells per µm2 of an average tissue section field of 672387,5 µm2. Data were analyzed by Student’s t test and differences between number of cells recorded in principals and controls were considered as significant at p<0.05 and lower values.
Results
Clinical observations
None of the pigs developed signs of colienterotoxemia, and all were clinically normal at the time of treatments. The mean weight (kg ±SD) per group of pigs at day −2 were: control = 7.4±0.6; levamisole-primed = 6.5±0.4; and levamisole-primed vaccinated = 6.5±0.8. All these body weights were statistically similar.Ten days after the treatments, levamisole-primed non-immunized pigs (9.0±1.2) gain weight significantly (p<0.05) as compared to their weight at day −2. In the control pigs (8.7±1.0) as well as in levamisole-primed vaccinated pigs (7.4±0.5) body weight also increased 10 days after the treatments. However, such increases were not significantly different between days −2 and 10. All pigs gained body weight 4 days following challenge inoculation, but the values were not significantly different between days −2 and 13 of the experiment.
Two of five control pigs became diarrheic two days after the treatment (at day 3) and the third piglet developed moderate diarrhea at day 4 (Table 2). The diarrhea, ranging from mild to severe and other correlating signs of colienterotoxemia continued for three to five days. Transient diarrhea was apparent in one levamisole-primed pig at days 3 and 4 after the treatments, otherwise these pigs were in good health and feeding conditions during the entire study period. Although one pig from levamisole-primed vaccinated group developed moderate diarrhea at day 1 and two other pigs from that group developed mild diarrhea at day 3, other pigs remained clinically normal. With the exception of two pigs that had moderate diarrhea at day 8, none of other pigs developed diarrhea following challenge infection. As diarrheic pigs developed diarrhea at day 1 or 3 we assume it is as consequence of rather natural infection than the vaccination.
Table 2. Extent of diarrhea intensity expressed as scores based on stools consistency.*.
| Treatment of pigsa | Pig no. | Day of experiment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9–13 | ||
| Noneb | 1 | |||||||||
| 2 | ++ | ++ | +++ | ++ | ++ | |||||
| 3 | ++ | + | + | |||||||
| 4 | ++ | + | + | |||||||
| 5 | ||||||||||
| Levamisole | 1 | |||||||||
| 2 | ||||||||||
| 3 | + | + | ||||||||
| 4 | ||||||||||
| 5 | ||||||||||
| Levamisole +F18ac+non-ETEC | 1 | + | ||||||||
| 2 | ++ | ++ | ++ | + | + | ++ | ||||
| 3 | ||||||||||
| 4 | ||||||||||
| 5 | + | + | ||||||||
Groups comprised five 4-weeks-old pigs each.
Control pigs received saline as a placebo.
-, firm feces = no diarrhea;
, soft feces = mild diarrhea;
, fluid feces = moderate diarrhea;
, watery feces = severe diarrhea.
Fecal shedding of the vaccine/challege strain
The isolated E. coli strains and their numbers were assesed from the rectal swabs before and after the treatments as shown in Table 3.The vaccine candidate strain (O157:K119:F18ac) was isolated from 4/5 control (1×106 CFU/mL) and 5/5 levamisole primed challenged pigs (8.0×105 CFU/mL) at day 13 of the experiment.Thus, between 99.90% and 99.92% of vaccinal bacterial strain (applied in the concentration of 1010 CFU/mL at day 0) could not be cultured 14 days after the specific immunization. This strain could not be recovered from the fecal material obtained by rectal swabs in levamisole treated pigs. However, we were able to isolate challenge strain (O149:K91:F4ac) from 2/5 pigs in this group (5.5×106 CFU/mL) at day 13 of the experiment. We are speculating that challenge strain cold not be recovered from other two groups of pigs due to the prolonged presence (from day −2 to day 7) of homologous farm strain (O8:K87:F4ac: Hly.) in the gastrointestinal tract of these pigs, which resulted in its more rapid passage through the intestines following challenge infection. Although in minor quantities, we were able to isolate both vaccinal (0.08–0.10%) and challenge strain (0.55%) 14 days or 7 days following the inoculation, respectively. It seems that challenge strain was passing through the intestines almost three times slower than did the vaccinal strain. Accordingly, it would be more appropriate to vaccinate pigs on at least three consecutive days, starting at weaning with much higher doses of vaccine candidate strain.
Table 3. Isolation and quantification of E. coli strains from rectal swabs of weaned pigs before and after the treatments; numerical data are expressed as either average values of CFU/mL or percent values of hemolytic colonies in the isolates for each group of pigs.
| Treatment of pigsa | Day of experiment | No. of E. coli positive pigs/total no. of pigs | E. coli isolate | CFU/mL | Hemolytic isolates (%) |
|---|---|---|---|---|---|
| Noneb | −2 | 3/5 | O8:K87:F4acc | - | 10 |
| 0 | 1/5 | O8:K87:F4ac | - | 10 | |
| 7 | 5/5 | E. colid | - | - | |
| 13 | 4/5 | O138:K81, | 3.0 × 107 | 20 | |
| O157:K119:F18ac | 1.0×106 | - | |||
| Levamisole | −2 | 1/5 | O8:K87:F4ac | - | 75 |
| 0 | 0/5 | - | - | - | |
| 7 | 1/5 | E. coli | 7.0×106 | - | |
| 13 | 2/5 | O149:K91:F4ac | 5.5×106 | 22.5 | |
| Levamisole + F18ac+ non-ETEC | −2 | 1/5 | O8:K87:F4ac | - | 15 |
| 0 | 1/5 | O8:K87:F4ac | - | 15 | |
| 7 | 3/5 | E. coli | - | - | |
| 13 | 5/5 | O157:K119:F18ac | 8.0 × 105 | 13.3 |
Groups comprised five 4-weeks-old pigs each.
Control pigs received saline as a placebo.
“Farm strain”.
Nonpathogenic strain.
Immunohistochemical data
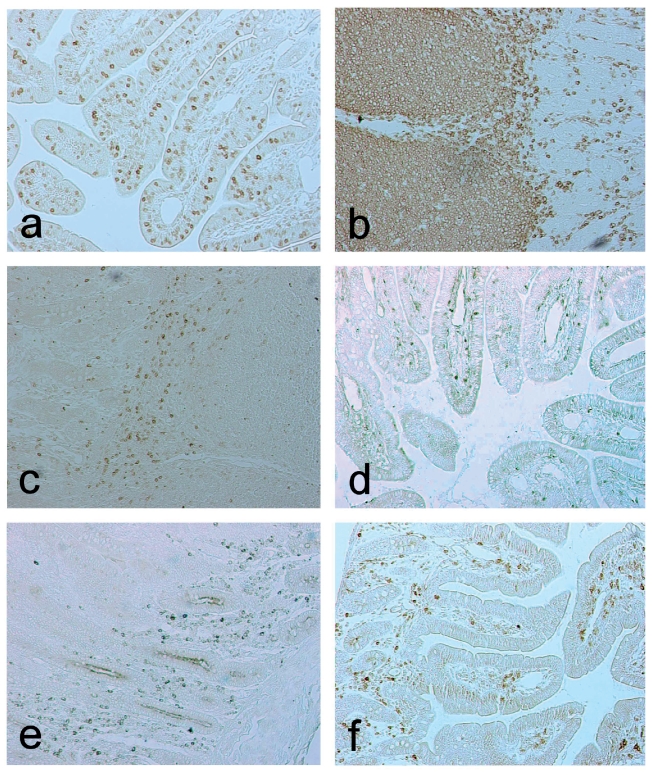
The in situ localization/distribution of intestinal lymphoid and myeloid cells is shown in Figure 1. We have demonstrated that CD3+ T cells are abundant within the villous epithelium, in the lamina propria and between ileal Peyer's patches. Small number of these cells was also found inside the follicles. They were uniformly distributed between crypt and villous areas whereas naive CD45RA+ lymphoid cells were more abundant in the crypts area. Predominance of these cells was found in the Peyer’s patches and also they were numerous in the extrafollicular areas. The CD45RC+ isoforms were mostly found in the villous lamina propria and in the interfollicular areas, but quite scarcely in the Peyer’s patches. Predominance of CD21+ B cells was observed in the villi and inside the follicles of Peyer's patches.The most frequent localization of IgA+ plasma cells was visible in the crypts zone. Species-specific SWC3+ macrophages were particularly distributed in lamina propria, in the areas directly below the epithelium of the villi, but also could be found between the crypts.These cells were also rarely scattered in the ileal Peyer's patches.
Figure 1.
Immunohistochemical localization of CD3+(a), CD45RA+(b), CD45RC+(c), CD21+(d), IgA+(e) and SWC3+(f) cells in the lamina propria and Peyer’s patches of small intestine from 6-weeks-old pig as demonstrated by ABC method; ×200.
Histomorphometric data
Numbers of CD3+, CD21+, IgA+ and SWC3+ lymphoid and myeloid cells in the sections of porcine jejunum and ileum were determined by computer-assisted histomorphometry and presented in Table 4 and Table 5, respectively. When we analyze quantitative data obtained by histomorphometry it is obvious that the presence or absence of diarrhea or its mild to moderate intensity in sampled pigs did not influence neither the expression of CD/SWC antigens on lymphoid/myeloid cell subsets tested nor their quantitative/distribution patterns. Thus, we assume that numerically expressed data may objectively reflect the effects of agents applied on development of the intestinal immunity in control pigs as well as in two groups of principals. Quantitative immunophenotypic analyses showed that levamisole treated pigs had highly increased numbers of jejunal CD3+, CD45RC+ and SWC3+ cells (p<0.05) as compared to those recorded in nontreated control pigs (Table 4). In the ileum of these pigs (Table 5) we have recorded that only CD21+ cells were significantly increased (p<0.01).The pigs that were treated with levamisole adjuvanted experimental vaccine had significantly increased numbers of all tested cell subsets in both segments of the small intestine (Table 4 and Table 5).
Table 4. Morphometric data of lymphoid and myeloid cell subsets in jejunum of pigs immunized with either levamisole or with combination of levamisole and vaccine candidate F18ac+non-ETEC. The results are expressed as the mean values and standard deviations of the number of cells per µm2 of tissue section field; in every sample the cells were counted in 12 randomly chosen fields with the average area of 672387,5 µm2.
| Treatment of pigsa Mean±SD(×10−5) number of lymphoid and myeloid cells residing jejunal mucosa of 6-weeks-old pigs | ||||||
| CD3 | CD45RA | CD45RC | CD21 | IgA | SWC3 | |
| Noneb | 1.54±0.61 | 6.84 ± 2.06 | 2.25±0.50 | 1.7±1.19 | 1.13±0.23 | 1.39±0.09 |
| Levamisole | 5.06±1.44* | 16.8±6.98 | 5.28±1.15* | 30.1±19.3 | 0.84±0.06 | 2.33±0.38* |
| Levamisole +F18ac+non-ETEC | 19.2±10.1* | 95±2.67*** | 16.6±10.0* | 23.7±12.3* | 6.97±1.96** | 11.5±4.15* |
Groups comprised five 4-weeks-old pigs each.
Control pigs received saline as a placebo.
Significantly different at p<0.05,
<0,01 or
<0.001 than in the control nontreated pigs.
Table 5. Morphometric data of lymphoid and myeloid cell subsets in ileum of pigs immunized with either levamisole or with combination of levamisole and vaccine candidate F18ac+non-ETEC. The results are expressed as the mean values and standard deviations of the number of cells per µm2 of tissue section field; in every sample the cells were counted in 12 randomly chosen fields with the average area of 672 387,5 µm2.
| Treatment of pigsa Mean±SD(×10−5) number of lymphoid and myeloid cells residing jejunal mucosa of 6-weeks-old pigs | ||||||
| CD3 | CD45RA | CD45RC | CD21 | IgA | SWC3 | |
| Noneb | 3.14±0.47 | 12.2 ±4.17 | 2.98±0.7 | 4.93± 1.67 | 1.12±0.21 | 1.42±0.86 |
| Levamisole | 6.37±6.17 | 45.4±24.7 | 9.45±4.86 | 21.5±5.8** | 1.32±0.45 | 2.81±0.44 |
| Levamisole +F18ac+non-ETEC | 31.8±1.04** | 192±98.2* | 28.6±13.1* | 82.7±19.7** | 8.14±2.44** | 8.73±1.38*** |
Groups comprised five 4-weeks-old pigs each.
Control pigs received saline as a placebo.
Significantly different at p<0.05,
<0,01 or
<0.001 than in the control nontreated pigs.
Discussion
Protection of weaned pigs from colonization of their intestines by adherent ETEC strains remains a formidable challenge. Due to great genetic flexibility of these bacteria and their ability to create the new factors of virulence or to mask the phenotypic characteristics that represent survival defects in gastrointestinal tract of the host. Stress of the young pigs during the period after weaning also influences the occurrence and the development of the disease (Nagy, 1999). The effects of dietary factors on intestinal physiology and mucosal immunology by changes in the gastrointestinal microbiology (numbers of favorable bacteria such as lactobacilli or bifidobacteria and numbers of potentially pathogenic bacteria i.e. E. coli, Clostridia, etc.) may also predispose to the incidence of infections by ETEC strains.
So far there is no safe and effective commercial vaccine which would provide adequate protection against such infections of weaned pigs. The best results are obtained from the research with the live oral vaccines that were applied before weaning. However, for now, not even one of these studies has led to preparation of the effective and commercial vaccine. Bertschinger et al., (2000) described the effectiveness of these vaccines alongside the application of low-energy diet. Further studies of these authors showed success in the protection of weaned pigs with live oral vaccines that comprised F18 fimbrial antigens.
Selection and breeding of the line of pigs (recessive homozygote), which do not express the receptors for F18 fimbrial antigens on small intestinal enterocytes, would probably have a lot more defects than advantages, because it would lead to the occurrence and proliferation of the new ETEC phenotypes and pathotypes. We also have to take into consideration the possibility of co-selection of the unwanted properties, for example, the gene for enhanced sensitivity to stress (Nagy, 1999).
The resistance of pigs to colienterotoxemia caused by ETEC strains relies on stimulation of active mucosal immunity at weaning. In the current study a significant reduction in colonization and clinical signs of infection with ETEC strain was achieved by the use of the vaccine with F18 fimbrial antigens in the combination with levamisole as an adjuvant. Recent research has confirmed the hypothesis that levamisole can have immunostimulatory effect on lymphocytes and macrophages in mesenteric lymph node (MLN) of weaned pigs vaccinated against colibacillosis. Božić et al. (2003) suggested that levamisole affects the enhanced proliferation and activation of the immune cells that participated in cellular immunity in the MLN. Snoeck et al. (2006) reported that jejunal Peyer’s patches play a role as the major inductive site for development of mucosal immune response after intestinal immunization of pigs with F4 fimbriae.
Although live oral vaccines seem to be relatively effective, it is necessary to evaluate the local immune responsiveness within both inductive and effector sites of intestinal mucosa. Therefore, we have studied by histomorphometric analyses the effect of an oral immunization of weaned pigs with non-ETEC strain that produces F18ac fimbrial antigens on quantitative and distribution patterns of lymphoid and myeloid cells in their small intestinal mucosa. According to our results it is likely that nonspecific immunostimulatory effect of levamisole, as well as its synergistic effect with F18ac+ non-ETEC vaccine candidate strain may provide an effective immune protection of weaned pigs against experimentally induced colienterotoxemia. These results showed that levamisole adjuvanted vaccine candidate non-ETEC strain strongly stimulates proliferation of CD3+ T-lymphocytes, naive CD45RA+ and memory CD45RC+ lymphoid cells, CD21+ B-lymphocytes, IgA+ plasma cells and SWC3+ macrophages in the lamina propria and Peyer’s patches of ileum/jejunum in weaned pigs. The effector phase of protective immune response was achieved 4–6 days after challenge infection as shown by the increased number of IgA+ plasma cells in the intestinal mucosa. Tissue distribution of SWC3+ macrophages, especially in the areas directly below the epithelium of the villi and inside the follicles of Peyer’s patches indicates their function in presenting the antigens to the helper T-lymphocytes during the inductive phase of immune response stimulated by adjuvanted experimental vaccine. Localization of naive CD45RA+ and memory CD45RC+ T cells showed their different distribution patterns within particular areas of mucosa and submucosa which indicated their different functions in intestinal immune response.The expression of both isoforms was mostly increased in the ileum of pigs primed with levamisole and immunized with F18ac+ non-ETEC strain.The number of CD21+ B cells was also strongly increased in the ileum of pigs that were immunized with adjuvanted vaccine candidate non-ETEC strain. The immunogenicity of experimental vaccine applied may be also validated by increased number of CD3+ T cells in small intestinal mucosa. It is well known that T cells can be further phenotyped into inductor/helper (CD4+) and effector/cytolytic (CD8+) subsets, but we did not analyze the expression of their surface antigens and their numbers in this model system. Beside strong adjuvanticity of levamisole, we have observed its effectiveness as an IRM since the numbers of CD3+ T cells, CD45RC+ memory cells and SWC3+ macrophages were highly elevated in the lamina propria of jejunum in nonspecifically primed pigs. However, in the ileum of these pigs only CD21+ B cells were significantly increased as compared to the control values.
The findings of our study as compared with the findings of others in weaned pigs inoculated with a live oral vaccine containing F18 fimbrial antigen (Bertschinger et al., 2000) showed that levamisole adjuvanted F18ac+ non-ETEC vaccine may exhibit an additive immunostimulatory effect on the host. Indeed, this effect is well documented by increased expression of all tested gut immune cell subsets in weaned pigs pretreated with levamisole and immunized with F18ac+ non-ETEC vaccine candidate strain.These two agents seem to act synergistically on intestinal immunity and provide better protection of weaned pigs against experimentally induced ETEC infection. Our data suggest that effective immune protection against infections with porcine F4ac+ and/or F18ac+ ETEC strains could be achieved by dually immunized weaned pigs that enables targeting of antigens of vaccine candidate homologous non-ETEC strains to the major mucosal inductor and effector sites,i. e. ileal Peyer’s patches and jejunal/ileal lamina propria, respectively.
Acknowledgements
This study was supported by the grants nos. 053-0532265-2255, 053-0532265-2248 and 053-0532265-2242 from the Ministry of Science, Education and Sport of Croatia. The excellent technical assistance of Zrinka Benčina and valuable suggestions for image analysis of Gordana Gregorović, B.S., M. S. are gratefully acknowledged.
Refrences
- Bertschinger HU, Nief V, Tschape H. Active oral immunization of suckling piglets to prevent colonization after weaning by enterotoxigenic Escherichia coli with fimbriae F18. Vet Microbiol. 2000;7:255–67. doi: 10.1016/s0378-1135(99)00166-2. [DOI] [PubMed] [Google Scholar]
- Bertschinger HU, Bachmann M, Mettler C, Pospischil A, Schrane E, Stamm M, Sadler T, Wild P. Adhesive fimbriae produced in vivo by Escherichia coli O139:K12(B):H1 associated with enterotoxemia in pigs. Vet Microbiol. 1990;15:267–81. doi: 10.1016/0378-1135(90)90083-8. [DOI] [PubMed] [Google Scholar]
- Božić F, Biliç V, Valpotić I. Levamisole mucosal adjuvant activity for a live attenuated Escherichia coli oral vaccine in weaned pigs. J Vet Pharm Therap. 2003;26:225–31. doi: 10.1046/j.1365-2885.2003.00458.x. [DOI] [PubMed] [Google Scholar]
- Božić F, Bilić V, Valpotić I. Modulation by levamisole of CD45RA and CD45RC isoforms expression in the gut. J Vet Pharm Therap. 2002;25:69–72. doi: 10.1046/j.1365-2885.2002.00379.x. [DOI] [PubMed] [Google Scholar]
- Cheng D, Sun H, Xu J, Gao S. Prevalence of fimbrial colonization factors F18ab and F18ac in Escherichia coli isolates from weaned piglets with edema and/or diarrhea in China. Vet Microbiol. 2005;110:35–9. doi: 10.1016/j.vetmic.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Fairbrother JM, Nadeau E, Gyles CL. Escherichia coli postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim Health Res. 2005;6:17–39. doi: 10.1079/ahr2005105. [DOI] [PubMed] [Google Scholar]
- Gordon VM, Whipp SC, Moon HW, O’Brien AD, Samuel JE. An enzymatic mutant of Shiga-like toxin-II variant is a vaccine candidate for oedema disease of swine. Infect Immunity. 1992;60:485–90. doi: 10.1128/iai.60.2.485-490.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins EM, Hurdle C. Effect of levamisole on parenteral vaccine for swine dysentery. Brit Vet J. 1989;145:565–72. doi: 10.1016/0007-1935(89)90119-X. [DOI] [PubMed] [Google Scholar]
- Lacković G, Rode B, Tomašković M, Balaban K, Bilić V, Valpotić I. Distribution of cytolytic and suppressor T cell subsets in the small intestine of pigs demonstrated by immunohistochemical methods. Per Biol. 1997;99:437–40. [Google Scholar]
- McGhee JR, Mestecky J, Dertzbaugh MT, Eldridge JH, Hirasawa M, Kiyono H. The mucosal immune system from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- Mulcahy G, Quinn PJ. A review of immunomodulators and their application in veterinary medicine. J Vet Pharm Therap. 1986;9:119–39. doi: 10.1111/j.1365-2885.1986.tb00022.x. [DOI] [PubMed] [Google Scholar]
- Nagy B, Fekete PZS. Enterotoxigenic Escherichia coli in veterinary medicine. I J Med Microbiol. 2005;295:443–54. doi: 10.1016/j.ijmm.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Nagy B, Fekete P. Enterotoxigenic Escherichia coli (ETEC) in farm animals. Vet Res Commun. 1999;30:259–84. [PubMed] [Google Scholar]
- Nagy B, Casey TA, Whipp SC, Moon HW. Susceptibility of porcine intestine to pilus-mediated adhesion by some isolates of piliated enterotoxigenic Escherichia coli increases with age. Infect Immun. 1992;60:1285–94. doi: 10.1128/iai.60.4.1285-1294.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olasz F, Fekete PZ, Blum-Oehler G, Boldogkoi Z, Nagy B. Characterization of an F18+ enterotoxigenic Escherichia coli strain from post weaning diarrhoea of swine, and of its conjugative virulence plasmid pTC. FEMS Microbiol Lett. 2005;244:281–9. doi: 10.1016/j.femsle.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Snoeck V, Verfaillie T, Verdonck F, Goddeeris BM, Cox E. The jejunal Peyer’s patches are the major inductive sites of the F4-specific immune response following intestinal immunization of pigs with F4 (K88) fimbriae. Vaccine. 2006;24:3812–20. doi: 10.1016/j.vaccine.2005.07.025. [DOI] [PubMed] [Google Scholar]
- Sojka WJ. Commonwealth Agricultural Bureaux; Farnham Royal, UK: 1965. Escherichia coli in domestic animals and poultry. [Google Scholar]
- Sojka WJ. Central Veterinary Laboratory; Weybridge, Surrey, UK: 1973. Enteric colibacillosis in pigs. Lectures in Mexico City. [Google Scholar]
- Stokes CR, Bailey M, Wilson AD. Immunology of the porcine gastrointestinal tract. Vet Immunol Immunopathol. 1994;43:143–50. doi: 10.1016/0165-2427(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Thienpont D, Vanparijs OFJ, Raeymaekers AHM, Vandenberk J, Demoen JA, Allewijn FT, Marsboom RP, Niemegeers CJ, Schellekens KH, Janssen PA. Tetramizole (Ar 8299), a new, potent broad spectrum antihelminthic. Nature. 1966;209:1084–6. doi: 10.1038/2091084a0. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhaoa M, Ruescha L, Omota A, Francisa D. Prevalence of virulence genes in Escherichia coli strains recently isolated from young pigs with diarrhea in the US. Vet Microbiol. 2007;123:145–52. doi: 10.1016/j.vetmic.2007.02.018. [DOI] [PubMed] [Google Scholar]