Abstract
Tenascin-X (Tn-X) belongs to the tenascin family of glycoproteins and has been reported to be significantly associated with schizophrenia in a single nucleotide polymorphism analysis in humans. This finding indicates an important role of Tn-X in the central nervous system (CNS). However, details of Tn-X localization are not clear in the primate CNS. Using immunohistochemical techniques, we found novel localizations of Tn-X in the interstitial connective tissue and around blood vessels in the choroid plexus (CP) in macaque monkeys. To verify the reliability of Tn-X localization, we compared the Tn-X localization with the tenascin-C (Tn-C) localization in corresponding regions using neighbouring sections. Localization of Tn-C was not observed in CP. This result indicated consistently restricted localization of Tn-X in CP. Comparative investigations using mouse tissues showed equivalent results. Our observations provide possible insight into specific roles of Tn-X in CP for mammalian CNS function.
Key words: tenascin-X, choroid plexus, monkey, mouse, Ehlers-Danlos syndrome, schizophrenia.
The tenascins (Tn) are a family of four glyco-protein members – tenascin-C (Tn-C), tenascin-R (Tn-R), tenascin-W (Tn-W) and tenascin-X (Tn-X) – found diversely in the extra-cellular matrix of vertebrate organs (Hsia and Schwarzbauer, 2005; Tucker and Chiquet-Ehrismann, 2009). Important functions of Tn have been investigated in developmental cell adhesion modulation and pathological conditions such as wound healing and tumourigenesis (Adams and Watt, 1993; Hsia and Schwarzbauer, 2005; Tucker and Chiquet-Ehrismann, 2009). Tn-C and Tn-R are prominent in the nervous system and play a role in the development of neurite outgrowth and postnatal synaptic plasticity (Yamaguchi, 2000; Chiquet-Ehrismann and Tucker, 2004; Dityatev and Schachner, 2006). Tn-W is found abundantly in the developing bone and stroma of certain tumours (Chiquet-Ehrismann and Tucker, 2004; Tucker and Chiquet-Ehrismann, 2009). Tn-X is the first tenascin member shown to be clearly associated with the human connective tissue disorder Ehlers–Danlos syndrome (EDS; Burch et al., 1997). Patients with a Tn-X deficiency suffer from skin hyperextensibility, joint hypermobility and poor wound healing ability (Bristow et al., 2005). These symptoms are caused by the occurrence of abnormal irregular collagen fibres. Tn-X plays a role in collagen fibrillogenesis by directly binding to collagen (Mao et al. 2002; Minamitani et al. 2004). Mice with a Tn-X deficiency also showed skin symptoms comparable with those of EDS (Mao et al., 2002).
Interestingly, in an analysis of human single nucleotide polymorphisms, Tn-X was reported to be significantly associated with schizophrenia (Wei and Hemmings, 2004; Tochigi et al., 2007). However, thus far, there have been no neuroanatomical reports on the involvement of Tn-X in schizophrenia. In the mammalian central nervous system (CNS), Tn-X mRNA expression has only been shown in the rat meninges of the olfactory bulb (Deckner et al., 2000). Recently, we found novel Tn-X localizations in the adult mouse leptomeninges trabecula in the cerebral cortex and in the connective tissue in the lateral ventricle choroid plexus (CP; Imura and Sato, 2008). Our finding of Tn-X localization in CP, which produces cerebrospinal fluid (CSF), might be a key factor in the investigation of the association between CSF metabolism and enlarged ventricles in schizophrenia. Enlarged ventricles are typical structural abnormalities associated with schizophrenia (Staal et al., 1999). Furthermore, CP secretes biologically active molecules into the CSF for brain development, activity and protection (Strazielle and Ghersi-Egea, 2000; Brown et al., 2004; Thouvenot et al., 2006; Johanson et al., 2008). In these molecules, for instance, there is a brain-derived neurotrophic factor (BDNF), the gene expression level and polymorphism of which have been analysed in relation to the pathogenesis of schizophrenia (Buckley et al., 2007). One study reported that BDNF is able to stimulate Tn-X expression in vitro (Takeda et al., 2005).
The validity and limitations of animal models (rodents and monkeys) for use in the study of schizophrenia have been discussed (Tordjman et al., 2007). The authors concluded that monkeys appear to be an interesting social interaction model, more so than rodents, because of their complex well-organized social structure. In addition to differences in social structure, the dopaminergic system of rats and monkeys is quite different (García-Cabezas et al., 2009), and dysfunction of the dopaminergic system is related to schizophrenia (Wang et al. 2008).
The CSF outflow system has been studied in some animal models (Kapoor et al., 2008). An anatomical difference in arachnoid granulations has been shown between rodents and monkeys (Krisch, 1988). Arachnoid granulations in monkeys are structurally similar to those in humans (Cooper, 1958; Krisch, 1988). In contrast, arachnoid granulations in rodents are similar to those of cats and dogs (Krisch, 1988). It is possible that Tn-X localization in CP is different between rodents and monkeys.
Therefore, details concerning Tn-X localization in monkey CP need to be clarified. In the present study, we compared the immunohistochemistry of Tn-X in monkey CP with that in mouse CP. Subsequently, to verify the reliability of Tn-X localization, we compared it with Tn-C localization in corresponding regions using neighbouring sections.
Materials and Methods
Two male monkeys (Macaca mulatta) weighing 3.3 and 8.0 kg and five male ICR mice (11 weeks of age) were used as experimental animals in this study. The monkey tissues were a gift of Dr Kathleen Rockland (RIKEN Brain Science Institute).This study was conducted in accordance with of the official Japanese regulations for research on animals (according to institutionally approved protocols; RIKEN Institute and the Nippon Dental University, approval no. 07-04) and in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication no. 80-23, revised 1996). The animals were perfused with 4% paraformaldehyde in 0.1 M phosphate buffered saline after being deeply anaesthetized with nembutal as previously reported (Imura and Rockland, 2007; Imura and Sato, 2008). The monkey CP was carefully dissected from the lateral and fourth ventricles. Mouse brains were removed from the skull. The tissues were rapidly frozen by immersing them in a cold acetone solution with dry ice and were cut transversely or horizontally at 30 µm with a cryostat. We used a previously described immunohistochemical to analyse Tn-X and Tn-C (Imura and Sato 2008). Briefly, the sections were processed for either Tn-X or Tn-C single immunoperoxidase staining (goat polyclonal anti-Tn-X antibody, catalogue no. sc-5498 or anti-Tn-C antibody, catalogue no. sc-9871; Santa Cruz Biotechnology, Santa Cruz, CA). The sections were incubated for 2 days at 4°C. Finally, the sections were incubated with biotinylated anti-goat secondary rabbit antibody (Vector Laboratories, Burlingame, CA) and visualized using avidin-biotin complex conjugated horseradish peroxidase (ABC Elite kits, Vector Laboratories) with diaminobenzidine. Routine controls for immunostaining were used during these experiments, by omission of the two primary antibodies. Photographs were taken as digital images with a digital camera (VB7010; Keyence, Japan) equipped with a microscope (DM-RE; Leica Microsystems, Germany). The images were adjusted for contrast and/or brightness to match the real image using standard image software (Adobe Photoshop 5.5, Adobe, San Jose, CA).
Results
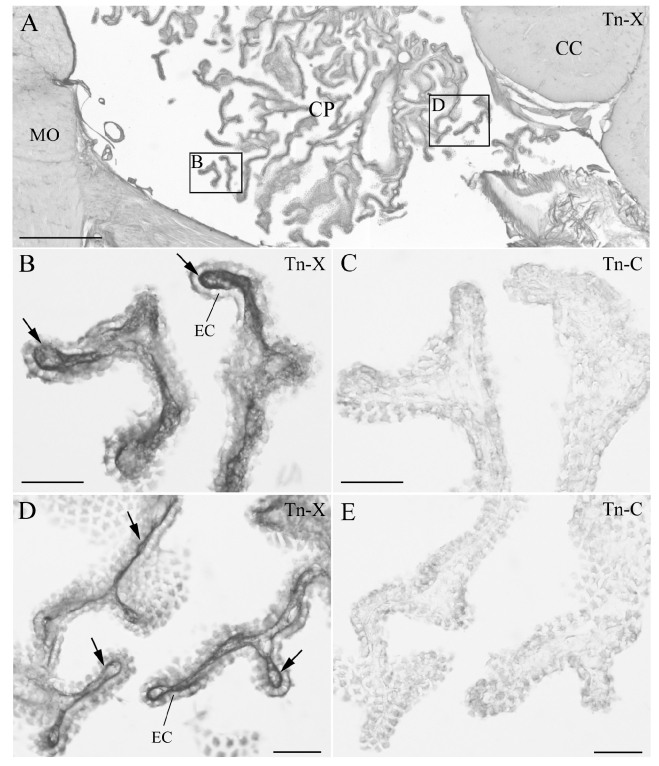
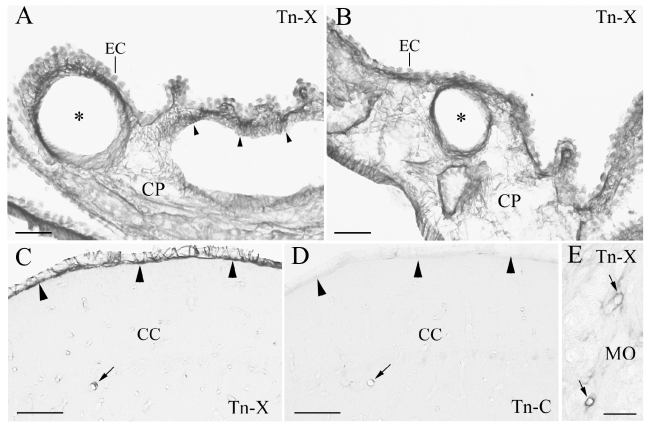
We observed Tn-X and Tn-C localizations in the fourth and lateral ventricles of monkey CP (Figures 1 and 2A, B). Tn-X- positive (Tn-X+) fibrous structures were clearly observed in CP (Figures 1 and 2A, B) of both ventricles. The Tn-X+ fibrous structures were restricted to the interstitial connective tissue in CP (Figures 1 and 2A, B). In neighbouring sections, Tn-X+ fibrous structures were clearly confined to the connective tissue and did not colocalize with Tn-C in CP (Figure 1B, E). Tn-X was notably distributed around the blood vessels in CP. Dense Tn-X+ fibrils surrounded blood vessels in CP (Figure 2A, B). The presence of Tn-X+ and Tn-C+ was confirmed in some blood vessels in the parenchyma of the cerebellum and medulla oblongata (Figure 2C, E). These results were consistent with previous reports of vascular localization of Tn-X and Tn-C (Matsumoto et al., 1994; Hasegawa et al., 1997; Ballard et al., 2006). In addition, a leptomeningeal Tn-X+ trabecular structure was also observed in the cerebellum (Figure 2C). In contrast, no leptomeningeal localization of Tn-C was detected (Figure 2D). The Tn-X+ trabecula might correspond to the leptomeninges trabecula in the subarachnoid space (Haines, 1991; Imura and Sato, 2008). These observations in monkeys were similar to those of our previous findings of Tn-X localization in mouse CP of lateral ventricles and leptomeninges (Imura and Sato, 2008).
Figure 1.
Immunohistochemistry (IHC) of tenascin-X (Tn-X) and tenascin-C (Tn-C) in monkey choroid plexus (CP) in fourth ventricle. (A) Low magnification of IHC of Tn-X in the lateral recess of fourth ventricle. Two boxed regions are re-photographed at higher magnification in B and D. (B, D) Tn-X positive fibrous structures are clearly observed within connective tissue of CP (arrows). (C, E) IHC of the Tn-C in neighboring sections to the B and D. Tn-C positive fibrous structures are not detected within the connective tissue of CP. CC: cerebellar cortex, EC: epithelial cell, MO: medulla oblongata. Scale bars=500 µm in A, 50 µm in B–E.
Figure 2.
Tenascin-X (Tn-X) positive vascular and leptomenigeal structures in monkey choroid plexus (CP), cerebellar cortex (CC), and medulla oblongata (MO). (A, B) Tn-X positive fibrous structures are clearly observed around blood vessels (asterisks) and within the connective tissue of CP in lateral ventricle (arrowheads). (C) Leptomeninges on the CC is strongly Tn-X positive (arrowheads). An arrow indicates Tn-X positive blood vessel. (D) Immunohistochemistry of tenascin -C in a neighboring section to the C. Tn-C positive leptomeninges is not observed in the CC (arrowheads). An arrow indicates Tn-C positive blood vessel, which is corresponding to the Tn-X positive blood vessel in C (arrow). E: Tn-X positive blood vessels are also observed in MO (arrows). EC: epithelial cell. Scale bars=50 µm.
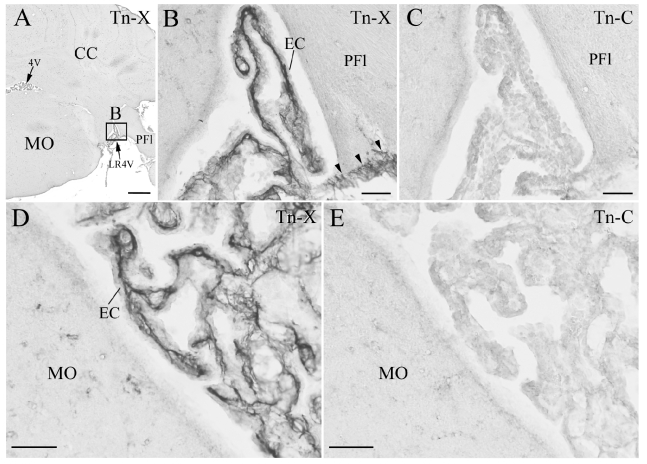
In the fourth ventricle of mouse CP, Tn-X+ fibrils were restricted to the interstitial connective tissue and leptomeninges in the cerebellum, and no localization of Tn-C was observed (Figure 3). These findings in the fourth ventricle were homologous in the monkeys described above.Tn-X localization in CP was consistently confirmed using immunohistochemical techniques in the lateral and fourth ventricles of monkeys and mice.
Figure 3.
Immunohistochemistry (IHC) of the tenascin-X (Tn-X) and tenascin-C (Tn-C) in mouse choroid plexus (CP) in fourth ventricle. (A) Low magnification of IHC of Tn-X in a coronal section at fourth ventricle level. One boxed region is re-photographed at higher magnification in B. (B, D) Tn-X positive fibrous structures are clearly observed within connective tissue of CP. Three arrowheads in B indicate Tn-X positive leptomeninges on PFl (paraflocculus). The panel D is photographed from an another section. (C, E) IHC of the Tn C in neighboring sections to the B and D. Tn C positive fibrous structures are not detected within the connective tissue of CP and leptomeninges. 4V: fourth ventricle, CC: cerebellar cortex, EC: epithelial cell, LR4V: lateral recess of 4V, MO: medulla oblongata. Scale bars=500 µm in A, 50 µm in B–E.
Discussion
We observed Tn-X localization in monkey CP in the present study. These findings were not previously reported in primates.
In mouse CP, we confirmed our previous reports of Tn-X localization in the lateral and fourth ventricles (Imura and Sato, 2008; present study). Tn-X localization in both species was confined to the interstitial connective tissue in CP. Tn-X directly binds to type I collagen and regulates type VI collagen for collagen fibrillogenesis (Mao et al., 2002; Minamitani et al., 2004). Collagen fibril in the CP connective tissue has been clearly observed in monkeys (Ling 1981). Furthermore, the connective tissue of CP consists of type I collagen in mice (Masuda et al. 1998) and of type VI collagen in humans (Roggendorf et al., 1988). Because of the presence of collagen fibres and the role of Tn-X in collagen fibrillogenesis, Tn-X localization in CP has a functional role, although the type of collagen in monkey CP is unknown.
Tn-X deficiency is consistent with structural defects in human collagen. Patients with the connective tissue disorder EDS have been found to lack Tn-X (Burch et al., 1997); incomplete collagen fibere bundles are responsible for the symptoms. In addition to skin symptoms, vascular fragility and an abnormal perivascular structure have also been observed in EDS patients (Burch et al. 1997). In mouse and human blood vessels, Tn-X was confirmed by immunohistochemistry (Matsumoto et al. 1994; Hasegawa et al. 1997). Type VI collagen was observed in CP blood vessels in humans (Roggendorf et al., 1988). These previous reports might support our finding of dense Tn-X localization around blood vessels in CP (Figure 2).
Recently, Tn-X was shown to be significantly associated with schizophrenia in a single nucleotide polymorphism analysis in humans (Wei and Hemmings, 2004; Tochigi et al., 2007). Previous reports and our present findings possibly indicate a novel important role of Tn-X in the CNS. Tn-X interacts with vascular endothelial growth factor B (VEGF-B) and BDNF in endothelial cells and enhances the function of VEGF-B in endothelial cell proliferation (Ikuta et al., 2000), and Tn-X expression is stimulated by BDNF (Takeda et al., 2005). In the nervous system, VEGF-B stimulates neurogenesis in the adult brain (Sun et al., 2006), and BDNF signalling is impaired in patients with schizophrenia (Buckley et al., 2007). The secretion of BDNF in CP (Borlongan et al., 2004) and the strong expression of VEGF-B in CP of mouse embryo (Aase et al., 1999) suggest the possibility of an interaction between Tn-X and these two molecules in CP. To clarify this hypothesized interaction of Tn-X in CP, further investigation is needed in future studies.
Acknowledgements
We thank Dr Kathleen Rockland of RIKEN BSI for donating the precious monkey tissue and members of the Department of Anatomy in NDU for their general cooperation.
References
- Aase K, Lymboussaki A, Kaipainen A, Olofsson B, Alitalo K, Eriksson U. Localization of VEGF-B in the mouse embryo suggests a paracrine role of the growth factor in the developing vasculature. Dev Dyn. 1999;215:1–25. doi: 10.1002/(SICI)1097-0177(199905)215:1<12::AID-DVDY3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–98. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- Ballard VLT, Sharma A, Duignan I, Holm JM, Chin A, Choi R, Hajjar KA, Wong SC, Edelberge JM. Vascular tenascin-C regulates cardiac endothelial phenotype and neovascularization. FASEB J. 2006;20:717–9. doi: 10.1096/fj.05-5131fje. [DOI] [PubMed] [Google Scholar]
- Brown PD, Davies SL, Speake T, Millar ID. Molecular mechanisms of cerebrospinal fluid production. Neuroscience. 2004;129:957–70. doi: 10.1016/j.neuroscience.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Skinner SJM, Geaney M, Vasconcellos AV, Elliott RB, Emerich DF. Intracerebral transplantation of porcine choroid plexus provides structural and functional neuroprotection in a rodent model of stroke. Stroke. 2004;35:2206–10. doi: 10.1161/01.STR.0000138954.25825.0b. [DOI] [PubMed] [Google Scholar]
- Bristow J, Carey W, Egging D, Schalkwijk J. Tenascin-X, collagen, elastin, and the Ehlers-Danlos syndrome. Am J Med Genet C Semin. 2005;139:24–30. doi: 10.1002/ajmg.c.30071. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Mahadik S, Pillai A, Terry A., Jr Neurotrophins and schizophrenia. Schizophr Res. 2007;94:1–11. doi: 10.1016/j.schres.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Burch GH, Gong Y, Liu W, Dettman RW, Curry CJ, Smith L, Miller WL, Bristow J. Tenascin-X deficiency is associated with Ehlers–Danlos syndrome. Nature Genet. 1997;17:104–8. doi: 10.1038/ng0997-104. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Tucker RP. Connective tissues: signalling by tenascins. Int J Biochem Cell Biol. 2004;36:1085–89. doi: 10.1016/j.biocel.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Cooper ERA. Arachnoid granulations in man. Acta Anat. 1958;34:187–200. doi: 10.1159/000141382. [DOI] [PubMed] [Google Scholar]
- Deckner M, Lindholm T, Cullheim S, Risling M. Differential expression of tenascin-C, tenascin-R, tenascin-/J1, and tenascin-X in spinal cord scar tissue and in the olfactory system. Exp Neurol. 2000;166:350–62. doi: 10.1006/exnr.2000.7543. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Schachner M. The extracellular matrix and synapses. Cell tissue Res. 2006;326:647–54. doi: 10.1007/s00441-006-0217-1. [DOI] [PubMed] [Google Scholar]
- García-Cabezas MA, Martínez-Sánchez P, Sánchez-González MA, Garzón M, Cavada C. Dopamine innervation in the thalamus: monkey versus rat. Cereb Cortex. 2009;19:424–34. doi: 10.1093/cercor/bhn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines DE. On the question of a subdural space. Anat Rec. 1991;230:3–21. doi: 10.1002/ar.1092300103. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Yoshida T, Matsumoto K, Katsuta K, Waga S, Sakakura T. Differential expression of tenascin-C and tenascin-X in human astrocytomas. Acta Neuropathol. 1997;93:431–7. doi: 10.1007/s004010050636. [DOI] [PubMed] [Google Scholar]
- Hsia HC, Schwarzbauer JE. Meet the tenascins: multifunctional and mysterious. J Biol Chem. 2005;280:26641–4. doi: 10.1074/jbc.R500005200. [DOI] [PubMed] [Google Scholar]
- Ikuta T, Ariga H, Mtsumoto K. Extracellular matrix tenascin-X in combination with vascular endothelial growth factor B enhances endothelial cell proliferation. Genes Cell. 2000;5:913–27. doi: 10.1046/j.1365-2443.2000.00376.x. [DOI] [PubMed] [Google Scholar]
- Imura K, Rockland KS. Giant neurons in the macaque pulvinar: a distinct relay subpopulation. Front Neuroanat. 2007;1:2–2. doi: 10.3389/neuro.05.002.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura K, Sato I. Novel localization of tenascin-X in adult mouse leptomeninges and choroid plexus. Ann Anat. 2008;190:324–8. doi: 10.1016/j.aanat.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Duncan JA, III, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:1–32. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor KG, Katz SE, Grzybowski DM, Ludow M. Cerebrospinal fluid outflow: an evolving perspective. Brain Res Bull. 2008;77:327–34. doi: 10.1016/j.brainresbull.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Krisch B. Ultrastructure of the meninges at the site of penetration of veins through the dura mater, with particular reference to pacchionian granulations investigations in the rat and two species of new-world monkeys (Cebus apella, Callitrix jacchus) Cell Tissue Res. 1988;251:621–31. doi: 10.1007/BF00214011. [DOI] [PubMed] [Google Scholar]
- Ling EA. Ultrastructure and mode of formation of epiplexus cells in the choroid plexus in the lateral ventricles of the monkey (Macaca fascicularis) J Anat. 1981;133:555–569. [PMC free article] [PubMed] [Google Scholar]
- Mao JR, Taylor G, Dean WB, Wagner DR, Afzal V, Lotz JC, Rubin EM, Bristow J. Tenascin-X deficiency mimics Ehlers–Danlos syndrome in mice through alteration of collagen deposition. Nature Genet. 2002;30:421–5. doi: 10.1038/ng850. [DOI] [PubMed] [Google Scholar]
- Masuda H, Hosokawa N, Nagata K. Expression and localization of collagen-binding stress protein Hsp47 in mouse embryo development: comparison with types I and II collagen. Cell Stress Chaperones. 1998;3:256–64. doi: 10.1379/1466-1268(1998)003<0256:ealocb>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Saga Y, Ikemura T, Sakakura T, Chiquet-Ehrismann R. The distribution of tenascin-X is distinct and often reciprocal to that of tenascin-C. J Cell Biol. 1994;125:483–93. doi: 10.1083/jcb.125.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamitani T, Ikuta T, Saito Y, Takebe G, Sato M, Sawa H, Nishimura T, Nakamura F, Takahashi K, Ariga H, Matsumoto K. Modulation of collagen fibrillogenesis by tenascin-X and type VI collagen. Exp Cell Res. 2004;298:305–15. doi: 10.1016/j.yexcr.2004.04.030. [DOI] [PubMed] [Google Scholar]
- Roggendorf W, Opitz H, Schuppan D. Altered expression of collagen type VI in brain vessels of patients with chronic hypertension. Acta Neuropathol. 1988;77:55–60. doi: 10.1007/BF00688243. [DOI] [PubMed] [Google Scholar]
- Staal WG, Pol HEH, Kahn RS. Outcome of schizophrenia in relation to brain abnormalities. Schizophr Bull. 1999;25:337–48. doi: 10.1093/oxfordjournals.schbul.a033382. [DOI] [PubMed] [Google Scholar]
- Strazielle N, Ghersi-Egea JF. Choroid plexus in central nervous system: biology and physiopathology. J Neuropathol Exp Neurol. 2000;59:561–74. doi: 10.1093/jnen/59.7.561. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Childs JT, Xie L, Mao XO, Greenberg DA. Vascular endothelial growth factor-B (VEGFB) stimulates neurogenesis: evidence from knockout mice and growth factor administration. Dev Biol. 2006;289:329–35. doi: 10.1016/j.ydbio.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Takeda K, Shiba H, Mizuno N, Hasegawa N, Mouri Y, Hirachi A, Yoshino H, Kawaguchi H, Kurihara H. Brain-derived neurotrophic factor enhances periodontal tissue regeneration. Tissue Eng. 2005;11:1618–29. doi: 10.1089/ten.2005.11.1618. [DOI] [PubMed] [Google Scholar]
- Thouvenot E, Lafon-Cazal M, Dementtre E, Jouin P, Bockaert J, Marin P. The proteomic analysis of mouse choroid plexus secretome reveals a high protein secretion capacity of choroidal epithelial cells. Proteomics. 2006;6:5941–52. doi: 10.1002/pmic.200600096. [DOI] [PubMed] [Google Scholar]
- Tochigi M, Zhang X, Ohashi J, Hibino H, Otowa T, Rogers M, Kato T, Okazaki Y, Kato N, Tokunaga K, Sasaki T. Association study between the TNXB locus and schizophrenia in a Japanese population. Am J Med Genet. 2007;144B:305–9. doi: 10.1002/ajmg.b.30441. [DOI] [PubMed] [Google Scholar]
- Tordjman S, Drapier D, Bonnot O, Graignic R, Fortes S, Cohen D, Millet B, Laurent C, Roubertoux PL. Animal models relevant to schizophrenia and autism: validity and limitations. Behav Genet. 2007;37:61–78. doi: 10.1007/s10519-006-9120-5. [DOI] [PubMed] [Google Scholar]
- Tucker RP, Chiquet-Ehrismann R. The regulation of tenascin expression by tissue microenvironments. Biochim Biophys Acta. 2009;1793:888–92. doi: 10.1016/j.bbamcr.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Wang M, Lee FJS, Liu F. Dopamine receptor interacting proteins (DRIPs) of dopamine d1-like receptors in the central nervous system. Mol Cell. 2008;25:149–57. [PubMed] [Google Scholar]
- Wei J, Hemmings GP. TNXB locus may be a candidate gene predisposing to schizophrenia. Am J Med Genet. 2004;125B:43–4. doi: 10.1002/ajmg.b.20093. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–89. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]