Abstract
The protein phosphatase calcineurin mediates many cellular responses to calcium signals. Using a genetic screen in yeast, we identified a new family of proteins conserved in fungi and animals that inhibit calcineurin function when overexpressed. Overexpression of the yeast protein Rcn1p or the human homologs DSCR1 or ZAKI-4 inhibited two independent functions of calcineurin in yeast: The activation of the transcription factor Tcn1p and the inhibition of the H+/Ca2+ exchanger Vcx1p. Purified recombinant Rcn1p and DSCR1 bound calcineurin in vitro and inhibited its protein phosphatase activity. Signaling via calmodulin, calcineurin, and Tcn1p induced Rcn1p expression, suggesting that Rcn1p operates as an endogenous feedback inhibitor of calcineurin. Surprisingly, rcn1 null mutants exhibited phenotypes similar to those of Rcn1p-overexpressing cells. This effect may be due to lower expression of calcineurin in rcn1 mutants during signaling conditions. Thus, Rcn1p levels may fine-tune calcineurin signaling in yeast. The structural and functional conservation between Rcn1p and DSCR1 suggests that the mammalian Rcn1p-related proteins, termed calcipressins, will modulate calcineurin signaling in humans and potentially contribute to disorders such as Down Syndrome.
Keywords: Calcineurin, calcium signaling, Rcn1p, DSCR1, ZAKI-4
The calcium and calmodulin-activated protein phosphatase calcineurin regulates a variety of developmental and cellular processes. Calcineurin helps control T-cell activation (Liu et al. 1992; Crabtree 1999), skeletal and cardiac muscle growth and differentiation (Chin et al. 1998; Hughes 1998; Molkentin et al. 1998; Sussman et al. 1998), memory (Mansuy et al. 1998; Winder et al. 1998), and apoptosis (Shibasaki and McKeon 1995; Krebs 1998). Calcineurin is highly conserved in fungi and animals, becoming activated on binding calcium and calmodulin when cytosolic calcium rises, and inhibited on binding the immunosuppressants Cyclosporin A and FK506 in complexes with their respective cellular receptors (Liu et al. 1991a; Klee et al. 1998; Hemenway and Heitman 1999). Feedback regulators of calcineurin have not yet been identified in any cell type.
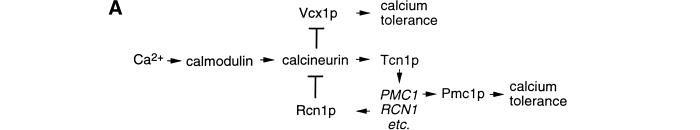
In the budding yeast Saccharomyces cerevisiae, calcineurin regulates gene expression and ion transport in response to calcium signals (Fig. 1A) but the genes encoding calcineurin (CNA1, CNA2, and CNB1) are not essential for viability (Cyert et al. 1991; Kuno et al. 1991; Liu et al. 1991b; Cyert and Thorner 1992; Ye and Bretscher 1992). Calcineurin promotes growth in high calcium environments by dephosphorylating the transcription factor cytoplasmic Tcn1p (also called Crz1p and Hal8p), which then accumulates in the nucleus and induces expression of the calcium ATPases Pmc1p and Pmr1p (Cunningham and Fink 1994b; Cunningham and Fink 1996; Matheos et al. 1997; Stathopoulos and Cyert 1997; Mendizabal et al. 1998; Stathopoulos-Gerontides et al. 1999). Calcineurin also appears to inhibit a vacuolar H+/Ca2+ exchanger Vcx1p by a posttranslational mechanism thereby preventing its function in calcium tolerance (Cunningham and Fink 1996; Pozos et al. 1996). The exogenous inhibitors of calcineurin, FK506 and Cyclosporin A, restore calcium tolerance to pmc1 mutants by permitting Vcx1p function (Cunningham and Fink 1996). We took advantage of this phenomenon to screen for factors that can inhibit calcineurin function when they are overexpressed. We report the identification of a previously uncharacterized family of proteins, conserved in fungi and animals, which can diminish calcineurin function in vivo and can bind and inhibit calcineurin in vitro directly. The Rcn1p family may serve as feedback regulators of calcineurin signaling and thereby modulate a variety of calcineurin-dependent functions.
Figure 1.
(A) Model of the calcium signaling pathway in yeast. Calcineurin activation by binding calcium and calmodulin inhibits the H+/Ca2+ exchanger Vcx1p and induces expression of the Ca2+ pump Pmc1p through activation of the transcription factor Tcn1p. (B) Multiple sequence alignment of Rcn1p from S. cerevisiae and predicted proteins from other fungi (Candida albicans, Schizosaccharomyces pombe, Neurospora crassa, and Aspergillus nidulans), protozoans (Dictyostelium discoideum), and animals (Caenorhabditus elegans, Drosophila melanogaster, and Homo sapiens) was generated using the Clustal algorithm. Residues identical in at least three of the seven sequences are highlighted and the most conserved central motif corresponding to the DS-24 peptide is underlined. GenBank accession numbers are NP_012763 (S.c.), Q09791 (S.p.), P53806 (C.e.), AAD33987 (D.m.), D83407 (H.s. ZAKI-4), AAF01684 (H.s. DSCR1L), and U85266 (H.s. DSCR1). Other sequences were obtained from ongoing genome sequencing projects (C.a., D.d., and N.c.) or from a compilation of EST sequences at GenBank (A.n. and D.d.). Note that some sequences were truncated at amino- and/or carboxyl termini and three small deletions (●) were introduced into the C. albicans sequence.
Results
A genetic screen for endogenous inhibitors of calcineurin
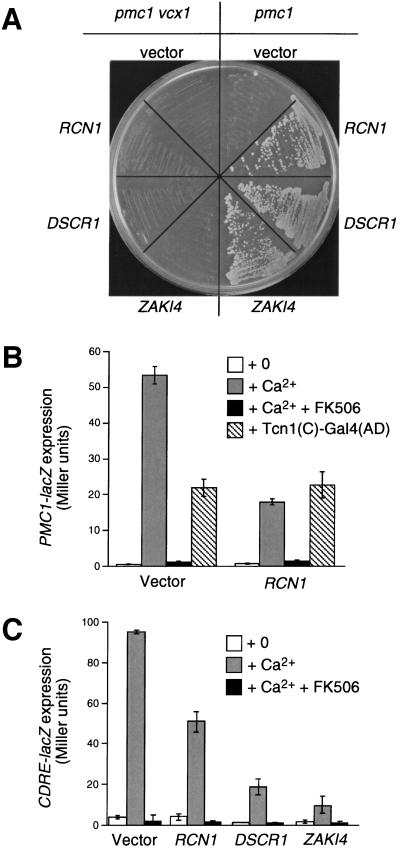
If endogenous inhibitors of calcineurin are produced in yeast, we reasoned that such molecules would confer calcium tolerance phenotypes similar to those observed with FK506 and Cyclosporin A (Cunningham and Fink 1994b). To identify endogenous calcineurin inhibitors or factors that produce them, we screened a high-dosage library of yeast genomic DNA for genes that conferred calcium tolerance to pmc1 mutants in a Vcx1p-dependent manner. From this screen (Cunningham and Fink 1996) we recovered 20 plasmids with overlapping inserts spanning the previously uncharacterized open reading frame (ORF) RCN1 (formerly YKL159c, GenBank accession no. Z28159). Subclones containing only the RCN1 gene conferred strong calcium tolerance to a pmc1 mutant but did not confer calcium tolerance to a pmc1 vcx1 double mutant (Fig. 2A) at any concentration (data not shown). The requirement for VCX1 suggested that overexpression of RCN1 promoted calcium tolerance not through buffering or efflux but through the direct or indirect activation of the H+/Ca2+ exchanger Vcx1p.
Figure 2.
Rcn1p family members inhibit calcineurin function in yeast. (A) Overexpression of yeast RCN1, human DSCR1, or human ZAKI4 genes in yeast blocked calcineurin-dependent inhibition of Vcx1p, thereby restoring growth to pmc1 mutants but not pmc1 vcx1 mutants in high calcium medium (YPD at pH 5.5 + 200 mm CaCl2). Photographs of colonies were taken after two days incubation at 30°C. (B) Overexpression of RCN1 inhibited calcineurin-dependent induction of PMC1–lacZ. Reporter gene expression was monitored in pmc1 vcx1 double mutants carrying either empty vector or RCN1 overexpression plasmid after 4 hr growth in medium supplemented with 100 mm CaCl2 and 0.3 μm FK506 as indicated. Expression was also monitored in wild-type strains containing a constitutively active Tcn1p(C)–Gal4(DB) transcription factor. (C) Overexpression of RCN1, DSCR1, or ZAKI-4 inhibited expression of CDRE–lacZ. Betagalactosidase assays were performed as indicated in B.
If the activation of Vcx1p by RCN1 overexpression was a consequence of calcineurin inhibition, RCN1 overexpression would also be expected to inhibit the activation of Tcn1p, a calcineurin-dependent transcription factor. Indeed, RCN1 overexpression partially blocked calcineurin- and Tcn1p-dependent induction of a PMC1–lacZ reporter gene (Fig. 2B). A pmc1 vcx1 double mutant was used in this experiment to eliminate any differences in calcium tolerance or sequestration secondary to calcineurin inhibition, though similar effects were observed in wild-type strains (data not shown). No effect of RCN1 overexpression was observed when a constitutively active variant of Tcn1p was coexpressed (Fig. 2B), ruling out the possibility that RCN1 might affect the PMC1 promoter independent of calcineurin. Overexpression of RCN1 also inhibited the expression of a CDRE–lacZ reporter gene (Fig. 2C) containing a single Tcn1p binding site upstream of an inert minimal promoter (Stathopoulos and Cyert 1997). These findings suggest RCN1 overexpression disrupts Tcn1p activation. The ability of RCN1 overexpression to inhibit two independent activities of calcineurin suggests that RCN1 exerts its negative effect at the level of calcineurin or further upstream in the calcium-signaling cascade. Experiments described below confirm that the protein product of RCN1 directly binds and inhibits calcineurin in vitro, and therefore is a direct regulator of calcineurin (RCN).
Rcn1p-related proteins
The RCN1 gene encodes a hydrophilic protein (Rcn1p) of 212 amino acids exhibiting significant homology to predicted proteins in other fungi, invertebrate animals, and mammals (aligned in Fig. 1B) all of which express calcineurin homologs. All proteins in the Rcn1p family share a highly conserved central segment containing a novel consensus sequence motif LxxPxxxKxFLISPPxSPPxxW. The human DSCR1 cDNA was identified during analysis of chromosome 21 as a gene mapping near the Down Syndrome critical region (Fuentes et al. 1995). The human ZAKI-4 cDNA was identified in a screen for genes responsive to thyroid hormone in fibroblasts (Miyazaki et al. 1996) and a third closely related human gene termed DSCR1L was recently deposited in GenBank. None of the Rcn1p family members have been characterized functionally.
To determine whether Rcn1p-related proteins retain a similar function, human DSCR1 and ZAKI-4 cDNAs were cloned into yeast expression plasmids and introduced into various yeast strains. As observed with RCN1, overexpression of DSCR1 or ZAKI-4 increased calcium tolerance in pmc1 mutants but not pmc1 vcx1 double mutants (Fig. 2A). Additionally, expression of DSCR1 and ZAKI-4 diminished calcineurin-dependent induction of PMC1–lacZ by 84% and 95%, respectively, and also strongly inhibited induction of the CDRE–lacZ reporter gene (Fig. 2C). Thus, despite their limited sequence similarity to Rcn1p, DSCR1 and ZAKI-4 retained the ability to inhibit calcineurin function when expressed in yeast.
Rcn1p and DSCR1 bind and inhibit calcineurin in vitro
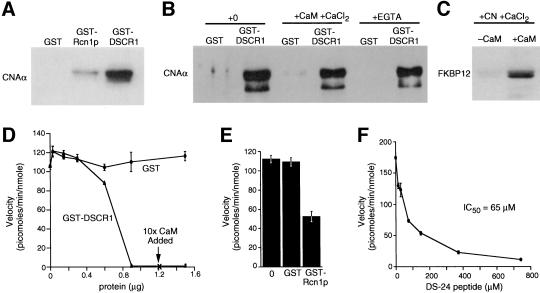
Rcn1p and DSCR1 were expressed as glutathionine S-transferase (GST) fusion proteins in Escherichia coli, purified by affinity chromatography, and tested for interactions with calcineurin purified from bovine brain. Bovine calcineurin specifically bound to both GST–DSCR1 and GST–Rcn1p on glutathione–agarose beads (Fig. 3A), though the crosskingdom interaction between Rcn1p and calcineurin appeared weaker. Calcineurin bound to GST–DSCR1 in buffers containing either 2 mm EGTA or 2 mm CaCl2 + calmodulin (Fig. 3B) and therefore was independent of the calcium concentration. Furthermore, FK506/FKBP12 complexes exhibited a calmodulin-dependent interaction with the DSCR1/calcineurin complex (Fig. 3C). These results suggest DSCR1 binds calcineurin at a site (or sites) distinct from those that bind calmodulin and FK506/FKBP12.
Figure 3.
Binding and inhibition of calcineurin in vitro by Rcn1p, DSCR1, and DS-24 peptide. (A) GST, GST–Rcn1p, and GST–DSCR1 were immobilized on glutathione-sepharose beads and assayed for the ability to bind purified bovine brain calcineurin. (B) Immobilized GST–DSCR1, but not GST alone bound calcineurin in buffer A, or buffer A supplemented with either 2 mm CaCl2 or 2 mm EGTA. (C) GST–DSCR1/calcineurin bound to FK506/FKBP12 in the presence of calmodulin and 2 mm CaCl2. (D–F) Dephosphorylation of RII peptide by calcineurin was inhibited by purified GST–DSCR1, GST–Rcn1p (100 μg/ml), and DS-24 synthetic peptide. Ten-fold excess calmodulin failed to reverse calcineurin inhibition by DSCR1.
The functional significance of DSCR1 and Rcn1p interaction with calcineurin was investigated using standard protein phosphatase assays with phospho-RII peptide as substrate. Addition of purified GST–DSCR1 strongly inhibited the calcium/calmodulin-dependent dephosphorylation of RII peptide by bovine calcineurin, whereas the same quantities of GST had no effect on calcineurin activity (Fig. 3D). Increasing calmodulin concentration 10-fold did not overcome inhibition by GST–DSCR1, confirming that DSCR1 does not compete with calmodulin. Much higher levels of GST–Rcn1p were required to inhibit bovine calcineurin (Fig. 3E) consistent with its lower binding (Fig. 3A). Finally, a synthetic 24-residue peptide surrounding the most conserved motif of DSCR1 (peptide DS-24) also inhibited calcineurin in a dose-dependent manner (Fig. 3F). The DS-24 peptide was much less potent than full-length DSCR1, suggesting that other regions of DSCR1 also contribute to interactions with calcineurin. These results demonstrate that two divergent members of the Rcn1p family of proteins can directly bind and inhibit calcineurin.
Calcineurin-dependent expression of Rcn1p
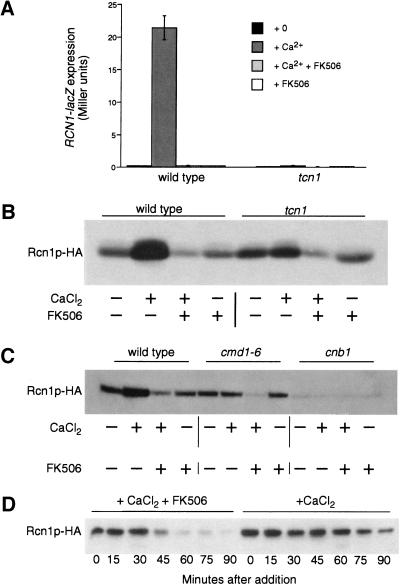
Calcium signals increased the expression of DSCR1 mRNA in mammalian cell lines (Crawford et al. 1997; Leahy et al. 1999; Fuentes et al. 2000). Therefore, we tested whether RCN1 transcription and Rcn1p accumulation was regulated in yeast. A RCN1–lacZ reporter gene containing 2-kb of the RCN1 promoter region was expressed at low levels in wild-type cells during growth in standard medium and was induced >20-fold after shift to high calcium conditions (Fig. 4A). Induction of RCN1–lacZ in response to calcium was completely blocked by addition of FK506 or by deletion of the genes encoding Tcn1p (Fig. 4A) or calcineurin (data not shown), suggesting RCN1 may be another downstream target of the calcineurin-dependent transcription factor. Western blot analysis of cells expressing an epitope-tagged Rcn1p–HA protein from a low-dosage plasmid confirmed this pattern of expression. Growth in high calcium stimulated Rcn1p–HA accumulation in wild-type cells (Figs. 4B, lanes 1,2) but not in tcn1 mutants (lanes 5,6). Induction of Rcn1p–HA was also blocked in cna1 cna2 double mutants that lack the two catalytic A subunits of calcineurin (not shown), in cnb1 mutants that lack the single regulatory B subunit of calcineurin (Fig. 4C, lanes 9,10), and in cmd1-6 mutants that express a defective calmodulin (Fig. 4C, lanes 5,6) that is unable to bind calcium or activate calcineurin (Geiser et al. 1991). Thus, calcium signaling through calmodulin, calcineurin, and Tcn1p was required for stimulation of Rcn1p expression above a basal level. Strong up-regulation of Rcn1p in yeast and DSCR1 in mammals in response to calcineurin signaling may therefore constitute a negative-feedback mechanism modulating calcineurin activity in vivo.
Figure 4.
Calcineurin regulates RCN1 transcription and Rcn1p stability in yeast. (A) Induction of a RCN1–lacZ reporter gene required calcium, calcineurin, and Tcn1p. A RCN1–lacZ reporter gene was introduced into wild-type and tcn1 mutants. β-Galactosidase activity was assayed in three independent transformants following 4 hr growth at 30°C in YPD at pH 5.5 medium supplemented with 100 mm CaCl2 and 0.3 μm FK506 as indicated. (B) Rcn1p–HA protein levels increased in response to calcium via the activation of calcineurin and Tcn1p. A low-dosage plasmid containing the epitope-tagged Rcn1p–HA gene was introduced into wild-type yeast and tcn1 mutants. Cells were grown as in A and then total cell protein was fractionated by SDS-PAGE and analyzed by Western blotting. (C) Basal accumulation of Rcn1p–HA required calcineurin but not calcium, calmodulin, or Tcn1p. Total cell extracts were prepared and analyzed as in B using wild-type cells, cnb1 mutants lacking the regulatory B subunit of calcineurin, or cmd1–6 mutants lacking Ca2+-binding sites in calmodulin. (D) FK506 destabilized Rcn1p–HA in high calcium conditions. Wild-type yeast expressing Rcn1p–HA were treated with 100 μm cycloheximide for 20 min and then treated with 100 mm CaCl2 in either the presence or absence of FK506. Total cell protein was extracted at 15 min intervals and analyzed by Western blotting as in B.
An additional effect of calcineurin on Rcn1p stability was revealed through analysis of Rcn1p–HA levels in calcineurin-deficient mutants. In nonsignaling conditions, accumulation of Rcn1p–HA was greatly reduced in cnb1 mutants and cna1 cna2 double mutants, relative to wild-type and tcn1 mutants and cmd1-6 mutants (Fig. 4B,C; data not shown). These results reveal a role of calcineurin A/B heterodimers on Rcn1p stability or basal expression that is independent of calcium, calmodulin, and Tcn1p. Basal expression of RCN1–lacZ was identical in wild-type and cnb1 mutants, suggesting that Rcn1p stability might be increased by the presence of calcineurin in the cell (data not shown). Stability of Rcn1p–HA was difficult to quantitate in cnb1 mutants because of the low initial levels of the protein. However, we noticed that addition of FK506 plus calcium caused Rcn1p–HA levels to decline in wild type, tcn1 mutants, and cmd1-6 mutants to levels approaching that of cnb1 mutants whereas FK506 alone had no effect (Fig. 4B,C). Treatment with FK506 plus calcium accelerated the disappearance of Rcn1p–HA in wild-type cells that had been pretreated with cycloheximide to inhibit protein synthesis (Fig. 4D). These findings demonstrate that calcineurin stabilizes Rcn1p in vivo, even in the absence of activation by Ca2+/calmodulin.
The phenotype of rcn1 null mutants
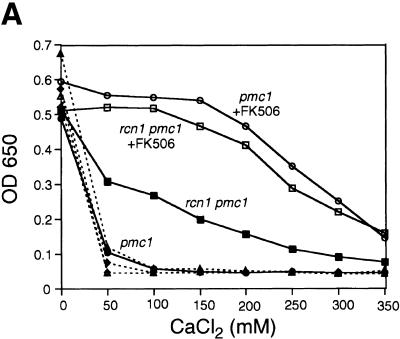
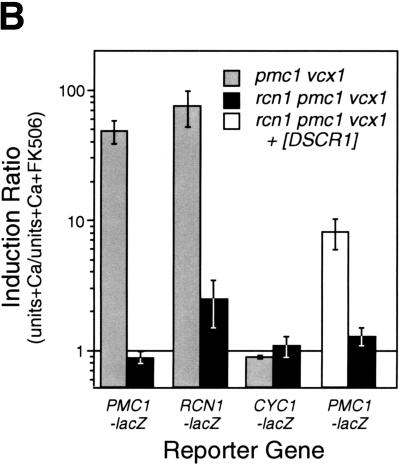
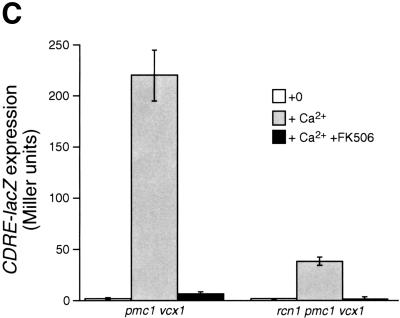
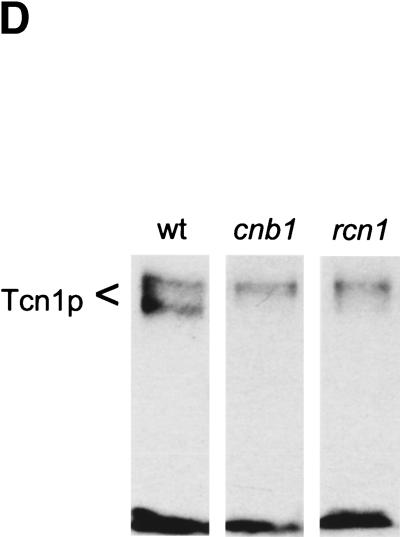
The above results all suggest that Rcn1p may operate as a feedback inhibitor of calcineurin signaling in vivo. If this were the only role of Rcn1p in yeast, mutants lacking Rcn1p would likely exhibit characteristics of enhanced calcineurin activity. To test this hypothesis, a rcn1 null mutant was constructed by homologous recombination and assayed for Tcn1p activation and Vcx1p inhibition. Surprisingly, both assays revealed reduced calcineurin activity in rcn1 null mutants. First, the low calcium tolerance of pmc1 mutants but not pmc1 vcx1 double mutants was suppressed partially by the deletion of RCN1 (Fig. 5A). The addition of FK506 increased the calcium tolerance of pmc1 mutants to the same level with or without Rcn1p, indicating that Rcn1p and calcineurin function within a common pathway. Secondly, calcineurin-dependent induction of PMC1–lacZ, RCN1–lacZ, and FKS2–lacZ in rcn1 mutants decreased by 93%, 87%, and 62%, respectively, relative to wild type. Similar effects were observed in pmc1 vcx1 double mutants (Fig. 5B). Induction of CDRE–lacZ was also largely dependent on Rcn1p (Fig. 5C) but expression of a calcineurin-independent CYC1–lacZ reporter was unaffected by Rcn1p function (Fig. 5B). Calcineurin-dependent dephosphorylation of Tcn1p causes a shift in its mobility on SDS gels even in wild-type cells (Stathopoulos and Gerontides 1999). Using this method, we found that epitope-tagged Tcn1p–HA from rcn1 mutants migrated similar to that of cnb1 mutants (Fig. 5D), indicating that Rcn1p was required for calcineurin-dependent dephosphorylation of Tcn1p. Thus, at least two independent outputs of calcineurin were specifically impaired in rcn1 mutants. Interestingly, the positive role of Rcn1p on calcineurin may also be conserved in mammalian cells because expression of DSCR1 in rcn1 mutants partially complemented the defect in PMC1–lacZ expression (Fig. 5B).
Figure 5.
Rcn1p promotes calcineurin function and expression. (A) Rcn1p promotes calcineurin-dependent inhibition of Vcx1p. The optical density of pmc1 mutant cultures grown for 20 hr at 30°C was measured at 650 nm and plotted as a function of added CaCl2. Solid lines represent growth of pmc1 or rcn1 pmc1 mutants and dashed lines represent growth of pmc1 vcx1 or rcn1 pmc1 vcx1 mutants in the presence and absence of 0.3 μm FK506. (B) Calcineurin-dependent induction ratios were calculated for various reporter genes expressed in pmc1 vcx1 double mutants or rcn1 pmc1 vcx1 triple mutants as indicated. Expression of DSCR1 partially complemented the rcn1 defect for PMC1–lacZ expression. (C) Induction of the calcineurin-dependent minimal CDRE–lacZ reporter gene was reduced in the absence of Rcn1p. The CDRE–lacZ reporter gene was introduced into pmc1 vcx1 and rcn1 pmc1 vcx1. β-Galactosidase activity was assayed in three independent transformants following 4 hr growth at 30°C in YPD medium at pH 5.5 supplemented with 100 mm CaCl2 and 0.3 μm FK506 as indicated. (D) Western blot analysis of Tcn1p–HA extracted from wild type and rcn1 mutants. Total protein was extracted from log phase cells grown in YPD medium at pH 5.5 and analyzed by Western blotting using 12CA5 monoclonal antibody.
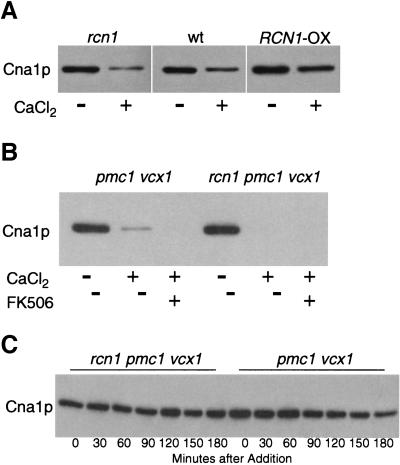
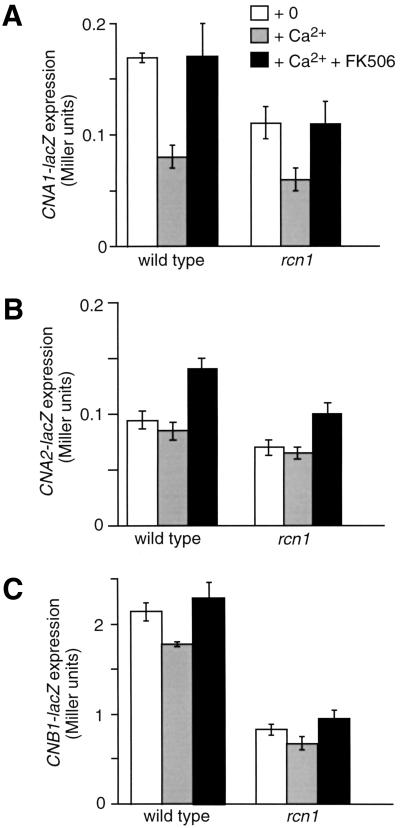
The nature of Rcn1p's positive contribution to calcineurin signaling was investigated further by monitoring calcineurin expression and stability. Neither deletion nor overexpression of Rcn1p affected expression of an epitope-tagged Cna1p–MYC protein in nonsignaling conditions (Fig. 6A). In high calcium conditions however, Cna1p–MYC consistently declined to lower levels in rcn1 mutants compared to wild type and remained higher in Rcn1p-overexpressing strains. The calcium-dependent decline of Cna1p–MYC was more pronounced in pmc1 vcx1 mutants (Fig. 6B) where cytosolic calcium increases to higher levels than wild type (Miseta et al. 1999). Inhibition of calcineurin through FK506 addition did not prevent the loss of Cna1p–MYC in the presence of high calcium. To determine if Rcn1p affected Cna1p–MYC stability, Cna1p expression was assayed in the presence of cycloheximide. Surprisingly, cycloheximide blocked the down-regulation of Cna1p–MYC with or without Rcn1p and/or FK506 (Fig. 6C; data not shown). Thus, Cna1p stability appeared to be insensitive to calcium, FK506, and Rcn1p. Instead, the elevated accumulation of Cna1p–MYC caused by Rcn1p during signaling conditions may reflect a positive effect of Rcn1p on calcineurin expression. This hypothesis was confirmed through analysis of CNA1–lacZ, CNA2–lacZ, and CNB1–lacZ expression (Fig. 7). In standard medium with or without calcium and FK506, these reporter genes were expressed respectively at ∼50%, ∼35%, and ∼150% higher levels in wild-type cells relative to rcn1 mutants. Interestingly, high calcium conditions diminished expression of all three reporter genes in a FK506-sensitive fashion. These findings reveal a significant role for Rcn1p in stimulating calcineurin expression. This effect provides at least a partial explanation for the positive role of Rcn1p on calcineurin signaling in yeast.
Figure 6.
Down-regulation of calcineurin during calcium signaling conditions. (A) Cna1p expression correlates with Rcn1p in high calcium conditions. Western blots of Cna1p–MYC in rcn1 mutants, wild type, and Rcn1p-overexpressing strains were performed on total cell protein after 4 hr growth in YPD medium at pH 5.5 supplemented with 100 mm CaCl2 as indicated. (B) Endogenous Rcn1p increases Cna1p expression in pmc1 vcx1 mutants in high calcium conditions. Experimental conditions were as described in A except 0.3 μm FK506 was added as indicated. (C) Rcn1p is not required to stabilize Cna1p. Cna1p–MYC levels were monitored in pmc1 vcx1 double and rcn1 pmc1 vcx1 triple mutants after a pretreatment with 100 μm cycloheximide for 20 min followed by addition of 100 mm CaCl2 and 0.3 μm FK506. Total cell protein was extracted at 30 min intervals and analyzed by Western blotting as in Figure 3.
Figure 7.
Involvement of Rcn1p in expression of calcineurin structural genes. (A) CNA1–lacZ, (B) CNA2–lacZ, and (C) CNB1–lacZ reporter genes were introduced into wild type and rcn1 deletion mutant. β-Galactosidase activity was assayed in three independent transformants following 4 hr growth at 30°C in YPD medium at pH 5.5 supplemented with 100 mm CaCl2 and 0.3 μm FK506 as indicated.
Discussion
This study reports the identification of a conserved family of proteins that appear to function as feedback inhibitors of calcineurin during calcium signaling. Recombinant Rcn1p and DSCR1 proteins bound and inhibited bovine calcineurin activity in vitro while overexpression of Rcn1p and DSCR1 inhibited at least two independent functions of yeast calcineurin in vivo, including the activation of Tcn1p and the inhibition of Vcx1p (see Fig. 1A). RCN1 transcription and Rcn1p accumulation in yeast were strongly induced by calcineurin-dependent activation of Tcn1p, supporting the hypothesis that Rcn1p operates as an endogenous feedback inhibitor of calcineurin signaling. Recent studies suggest that DSCR1 functions as a feedback inhibitor of calcineurin signaling in human cells. DSCR1 transcription in human astrocytoma cells was strongly stimulated by calcineurin signaling and DSCR1 overexpression inhibited calcineurin-dependent activation of NFAT (Rothermel et al. 2000; Fuentes et al. 2000). Together these findings suggest broad conservation of the Rcn1p-related proteins as feedback inhibitors of calcineurin.
The analysis of rcn1 null mutants also revealed a stimulatory role of Rcn1p on calcineurin signaling. Calcineurin-dependent regulation of both Tcn1p and Vcx1p was clearly reduced in rcn1 mutants but not completely abolished as judged by the more severe consequences of adding FK506. The apparent deficiency of calcineurin signaling in rcn1 mutants may be the result of decreased calcineurin expression. For example, Cna1p levels were lower in rcn1 mutants and higher in Rcn1p-overexpressing strains as compared to wild-type strains grown in high calcium conditions. We detected no obvious effects of Rcn1p on either Cna1p accumulation in nonsignaling conditions or on Cna1p stability in any conditions tested. Additionally, we could not detect any effect of Rcn1p on Vcx1p or Tcn1p function when calcineurin had been inactivated by FK506, although Rcn1p is unstable under these conditions. The simplest model consistent with these results is one where Rcn1p stimulates calcineurin expression during calcium signaling.
Calcineurin expression in yeast has not yet been studied in detail. Rcn1p increased expression of CNA1, CNA2, and CNB1 reporter genes whereas calcium decreased expression through an FK506-sensitive mechanism (Fig. 7). Cna1p levels also declined during growth in high calcium conditions, an effect that was enhanced in rcn1 mutants (Fig. 6) and diminished in cmd1-6 mutants (data not shown). These results suggest calcineurin activation may down-regulate expression of its structural genes, an effect that would be stimulated by calmodulin and inhibited by Rcn1p. However, the decline of Cna1p was not blocked by FK506 addition as if another calcium-dependent mechanism contributed to calcineurin down-regulation in yeast. Therefore, calcineurin expression, accumulation, and function appear to be regulated by calcium at multiple levels. Further analysis of calcineurin dynamics is warranted in order to understand the significance of this unexpected complexity and to fully explain the calcineurin-deficient phenotype of rcn1 mutants.
Additional roles for Rcn1p in promoting calcineurin function in yeast can not be ruled out. For example, Rcn1p may actually stimulate calcineurin signaling to some degree in vivo through a mechanism that was not reconstituted or detectable in our in vitro assays, even at levels 100-fold lower than those required to inhibit calcineurin activity. Low doses of these proteins might increase calcineurin activity in vivo but such effects might escape detection in vitro if oxidative inactivation of calcineurin was also stimulated (Wang et al. 1996). Alternatively, these proteins might promote interactions between calcineurin and its natural substrates, much like the targeting or scaffolding subunits of type 1 protein phosphatase (Hubbard and Cohen 1993; Sim and Scott 1999). For example, the inhibitor-2 proteins prevent some activities of PP1 while stimulating others (Alessi et al. 1993), possibly by altering the partitioning of active PP1 molecules. Remarkably, genetic analysis of inhibitor-2 function in yeast revealed positive and negative effects on PP1 function (Tung et al. 1995) much like the effects of Rcn1p on calcineurin function reported here. Two proteins in mammals, AKAP79 (Coghlan et al. 1995; Kashishian et al. 1998) and Cabin1/cain (Lai et al. 1998; Sun et al. 1998; Youn et al. 1999), are known to bind calcineurin at sites distinct from the FK506/FKBP12 binding sites, to inhibit calcineurin phosphatase activity, and to also bind other cellular factors which may include substrates. The calcineurin-binding domains of both proteins are rather basic in character and not obviously related to the conserved domains of the Rcn1p family members. These structural differences and the dramatic up-regulation of Rcn1p and DSCR1 in response to calcineurin signaling distinguish this new family of calcineurin regulators from proteins described previously.
Finally, the possibility that Rcn1p and DSCR1 act as downstream effectors of calcineurin signaling remains to be fully explored. We have been unable to detect any effect of Rcn1p on Vcx1p or Tcn1p function in various contexts, but negative results of this nature do not rule out the possibility that Rcn1p mediates the regulation of other factors that respond to calcineurin signaling. The most conserved segment KxFLISPPxSPPx bears some resemblance to the conserved SPxxSPxxSPxx motifs repeated several times in NFAT proteins (Rao et al. 1997). These SP repeats are thought to be functionally important for NFAT regulation by calcineurin, serving as substrates of calcineurin after phosphorylation by protein kinases in the nucleus (Beals et al. 1997; Chow et al. 1997; Zhu et al. 1998; Crabtree 1999). Therefore, it is conceivable that calcineurin activation dephosphorylates Rcn1p family members as a mechanism for regulating additional downstream factors. Because Rcn1p stability and expression depend on interactions with calcineurin, any effector functions of Rcn1p would also be affected by FK506.
The findings reported here and elsewhere (Fuentes et al. 2000) suggest that feedback inhibition of calcineurin is conserved from yeast to humans. The function of this feedback mechanism may be to fine-tune calcineurin signaling over a spectrum of intervals and conditions. Improper regulation of Rcn1p family members might lead to the disruption of calcineurin function in humans and contribute disease. For example, the increased dosage of DSCR1 in trisomy-21 individuals may contribute to the neurological, cardiac, or immunological defects observed in Down syndrome patients (Epstein 1995) through inhibition of calcineurin signaling. It will be interesting to determine if the interactions between DSCR1 and calcineurin in human cells are as complex as those we have observed for Rcn1p in yeast. It is not yet known if endogenous levels of DSCR1 are required to promote calcineurin function in vivo, or if calcineurin expression is down-regulated during prolonged calcium signaling. If FK506 and Cyclosporin A destabilize DSCR1 or its homologs in human cells, the efficacy or side effects of these drugs in transplantation therapies might be attributed to loss of these proteins. Understanding the relationship between the human Rcn1p family members and calcineurin in vivo will not only enhance our understanding of calcineurin function but also potentially provide novel therapeutic targets to control calcineurin function in humans.
Materials and methods
Genetic procedures
A library of yeast genomic DNA carried on high-dosage plasmids was screened for potential inhibitors of calcineurin by selecting for plasmids that could restore growth of a pmc1 null mutant (strain K473) on solid YPD medium at pH 5.5 supplemented with 200 mm CaCl2 (Cunningham and Fink 1996). Of 24 plasmids that were recovered, two carried the Ca2+ pumps Pmc1p and Pmr1p, two carried Vcx1p, and 20 carried the uncharacterized yeast gene RCN1/YKL159c plus flanking sequences. Subcloning demonstrated the active gene was RCN1. The entire RCN1 coding sequence was deleted from the genome by homologous recombination using plasmid pTJK39 linearized by EcoRI digestion. The resulting rcn1::HIS3 null mutant was crossed to other mutants in an isogenic background (Matheos et al. 1997) to generate strains bearing multiple mutations (see Table 1).
Table 1.
S. cerevisiae strains used in this study
| Strains
|
Genotype
|
Reference
|
|---|---|---|
| DMY14 | tcn1::G418 | Matheos et al. (1997) |
| JGY148 | cmd1-6 | Moser et al. (1996) |
| K482 | pmc1::TRP1 | Cunningham and Fink (1994) |
| K537 | cna1::URA3 cna2::HIS3 | this study |
| K601 | + | Cunningham and Fink (1994) |
| K603 | cnb1::LEU2 | Cunningham and Fink (1994) |
| K651 | pmc1::TRP1 vcx1Δ | Cunningham and Fink (1996) |
| K665 | pmc1::TRP1 vcx1Δ | Cunningham and Fink (1996) |
| TKY268 | rcn1::HIS3 pmc1::TRP1 | this study |
| TKY275 | rcn1::HIS3 | this study |
| TKY278 | rcn1::HIS3 pmc1::TRP1 vcx1Δ | this study |
All strains are isogenic to W303-1A (ade2-1 can1-100 his3-11, 15 leu2-3, 112 trp1-1 ura3-1).
Recombinant DNA
All recombinant DNA work was conducted with standard techniques with enzymes purchased from New England Biolabs or GIBCO BRL. The rcn1::HIS3 disruption plasmid pTJK39 was constructed by sequentially ligating two PCR products corresponding to the 5′ and 3′ flanking regions of RCN1 that had been amplified from genomic DNA using the primers CCGAATTCGCCATACTATCAAATG and GGGGATCCCTGCAGTTCTGTGTTT (for 5′ sequences) and CCCTCGAGGATGGCGAGGCGATTTG and CCGAATTCGAATAGTAATAAAGAT (for 3′ sequences) into vector pRS303 digested with EcoRI + BamHI and EcoRI + XhoI, respectively. The RCN1–HA expression plasmid pTJK29 was generated by subcloning the 3xHA tag from pBSHA3 (Cunningham and Fink 1994a) into pRS316 (Sikorski and Hieter 1989) containing 5′ regulatory and coding sequences from the RCN1 gene lacking a stop codon that had been amplified by PCR using primers GATCTTCACAAATCTTGGGG and GCCATCTTATCTAGAATCATCGTCATCAG. A downstream stop codon was reconstructed by generating a frameshift at the SpeI site in the polylinker. Plasmids bearing CNA1–lacZ, CNA2–lacZ, CNB1–lacZ, and RCN1–lacZ reporter genes were constructed by subcloning PCR-generated DNA segments corresponding to nucleotides −2000 and +3 relative to the initiator codon of each gene into plasmid pLGΔ178 using the following primers: for CNA1, CTCGAGAACGGAAGTGGCAACTTG and GGATCCCATTGGCGTTGAGAGTGT; for CNA2, CTCGAGTAAAGCTGGAGCCAAGAC and GGATCCCATTGCGGGTTCAAGAAG; for CNB1, CTCGAGGACTAGTTCAAAGGTAAA and AGATCTCATTTTAAGAAATAAAAATGC; and for RCN1, CTGCTCGAGCAGAAAATTCGTGAAC and GGGATCCCATCTGCAGTTCTGTGT. The ZAKI4 expression plasmid pTJK1 was constructed by subcloning the XmnI–NsiI fragment of phZAKI-4-3.2 (courtesy of H. Seo, Nagoya University, Japan) into pRS425MET digested with SmaI + PstI. The DSCR1 expression plasmid pTJK37 was constructed by subcloning a PCR product from DSCR1-1pBS (courtesy of X. Estivill, I.R.O., Barcelona, Spain) using primers GCGAGGATCCGTATGGAGGAGGTGGACCTG and CCCTCACTCGAGGCTGAGGTGGATCGGCGTGTA into pRS426MET digested with BamHI + XhoI. The GST–DSCR1 expression plasmid pTJK92 was constructed from the same PCR product after subcloning a BamHI–BglII fragment into the BamHI site of pGEX3X. The GST–Rcn1p expression plasmid pTJK93 was constructed by subcloning into the BamHI site of pGEX-3X a BglII + BamHI-digested PCR product using primers CTGCAGGGGATCCGTATGGGTAATATTATAAC and CGAAATAGATCTGATGAAGAGGAGGT. The Cna1p–MYC expression plasmid pTJK91 was constructed by subcloning the 3xMYC sequences from pKB241 into pRS315 containing the promoter and coding sequences of CNA1 that had been amplified using the primers CTCGAGAACGGAAGTGGCAACTTG plus GGATCCCATTGGCGTTGAGAGTGT and AGATCTAGAATGTCGAAAGACTTGAATTCT plus GGCTGATGGTGGTGTTCA. Plasmid pAMS342 (Stathopoulos and Cyert 1997) harboring the CDRE–lacZ reporter gene and pDM16 harboring the activated TCN1 allele (Matheos et al. 1997) were described previously.
Protein analysis and purification
The consensus Aspergillis nidulans and Dictyostelium discoideum sequences were compiled from overlapping cDNA and gDNA sequences at GenBank and the D. discoideum Genome Project. Protein sequences were aligned using the Clustal program from DNAstar based on the PAM250 weight table. GST–DSCR1, GST–Rcn1p, and GST were expressed in TOPP2 cells as suggested by the manufacturer (Stratagene) and purified on glutathione-agarose beads according to instructions (Pharmacia Biotech). β-Galactosidase assays were conducted as described previously (Cunningham and Fink 1996). The average β-galactosidase activity measured from three independent transformants is plotted (±s.d.).
Log-phase cell cultures were harvested, extracted with trichloroacetic acid, and processed for SDS-PAGE, Western blotting, and ECL detection as described previously (Cunningham and Fink 1996). Monoclonal antibodies 12CA5 (Boehringer) or 9E10 (Santa Cruz Biotechnology) were used to detect HA and MYC-tagged proteins respectively. Calcineurin was detected using polyclonal antibodies (gift of Claude Klee, NIH). For studies of Rcn1p–HA stability, yeast cells were grown to log phase overnight in SC minus uracil medium and then shifted to YPD medium at pH 5.5. After 1 hr incubation, 100 μg/ml cycloheximide was added and cultures were split and diluted into the same medium containing 100 mm CaCl2 with or without 0.3 μm FK506. Samples were processed for Western blotting as described above.
Purified bovine brain calcineurin, calmodulin, and FKBP12 were obtained from Sigma Chemical Corp. Polyclonal anti-FKBP12 was from BIOMOL. Calcineurin binding experiments were performed as described (Lai et al. 1998) followed by SDS-PAGE and Western blot detection. Calcineurin activity assays using 32P-labeled RII peptide as substrate were performed as described (Fruman et al. 1996; Sagoo et al. 1996) except 25 nm calcineurin was used in 15 min reactions at 30°C. The DS-24 peptide HLAPPNPDKQFLISPPASPPVGWKC was synthesized at Pocono Rabbit Farm and Laboratory.
Acknowledgments
We are grateful to Claude Klee for antibodies, to Xavier Estivill, Martha Cyert and Hisao Seo for plasmids, to Fujisawa USA Inc. for gifts of FK506, and to Liz O'Sullivan for technical assistance. This research was jointly supported by the Basil O'Connor Starter Scholar Research Award (FY96-1131) from the March of Dimes Birth Defects Foundation, the Searle Scholars Program/The Chicago Community Trust, a Research Grant from the National Institutes of Health (GM53082) and a grant from the American Heart Association.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL kwc@jhunix.hcf.jhu.edu; FAX (410) 516-5213.
References
- Alessi DR, Street AJ, Cohen P, Cohen PT. Inhibitor-2 functions like a chaperone to fold three expressed isoforms of mammalian protein phosphatase-1 into a conformation with the specificity and regulatory properties of the native enzyme. Eur J Biochem. 1993;213:1055–1066. doi: 10.1111/j.1432-1033.1993.tb17853.x. [DOI] [PubMed] [Google Scholar]
- Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF–ATc enhanced by glycogen synthase kinase-3. Science. 1997;275:1930–1934. doi: 10.1126/science.275.5308.1930. [DOI] [PubMed] [Google Scholar]
- Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes & Dev. 1998;12:2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CW, Rincon M, Cavanagh J, Dickens M, Davis RJ. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science. 1997;278:1638–1641. doi: 10.1126/science.278.5343.1638. [DOI] [PubMed] [Google Scholar]
- Coghlan VM, Perrino BA, Howard M, Langeberg LK, Hicks JB, Gallatin WM, Scott JD. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science. 1995;267:108–111. doi: 10.1126/science.7528941. [DOI] [PubMed] [Google Scholar]
- Crabtree GR. Generic signals and specific outcomes: Signaling through Ca2+, calcineurin, and NF–AT. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- Crawford DR, Leahy KP, Abramova N, Lan L, Wang Y, Davies KJ. Hamster adapt78 mRNA is a Down syndrome critical region homologue that is inducible by oxidative stress. Arch Biochem Biophys. 1997;342:6–12. doi: 10.1006/abbi.1997.0109. [DOI] [PubMed] [Google Scholar]
- Cunningham KW, Fink GR. Ca2+ transport in Saccharomyces cerevisiae. J Exp Biol. 1994a;196:157–166. doi: 10.1242/jeb.196.1.157. [DOI] [PubMed] [Google Scholar]
- ————— Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J Cell Biol. 1994b;124:351–363. doi: 10.1083/jcb.124.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in yeast. Mol Cell Biol. 1996;16:2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert MS, Thorner J. Regulatory subunit (CNB1 gene product) of yeast Ca2+/calmodulin-dependent phosphoprotein phosphatases is required for adaptation to pheromone. Mol Cell Biol. 1992;12:3460–3469. doi: 10.1128/mcb.12.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert MS, Kunisawa R, Kaim D, Thorner J. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc Natl Acad Sci. 1991;88:7376–7380. doi: 10.1073/pnas.88.16.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein CJ. In: Down Syndrome (Trisomy 21) Scriber CR, Beaudet AL, Sly WS, Vaile D, editors. McGraw-Hill, Inc; 1995. [Google Scholar]
- Fruman DA, Pai SY, Klee CB, Burakoff SJ, Bierer BE. Measurement of calcineurin phosphatase activity in cell extracts. Methods. 1996;9:146–154. doi: 10.1006/meth.1996.0020. [DOI] [PubMed] [Google Scholar]
- Fuentes, J.J., Genescà, L., Kingsbury, T.J., Cunningham, K.W., Pérez-Riba, M., Estivill, X., and de la Luna, S. 2000. DSCR1, overexpressed in Down syndrome, is an inhibitor of calcineurin-mediated signaling pathways. Hum. Mol. Gen. (in press). [DOI] [PubMed]
- Fuentes JJ, Pritchard MA, Planas AM, Bosch A, Ferrer I, Estivill X. A new human gene from the Down syndrome critical region encodes a proline-rich protein highly expressed in fetal brain and heart. Hum Mol Genet. 1995;4:1935–1944. doi: 10.1093/hmg/4.10.1935. [DOI] [PubMed] [Google Scholar]
- Geiser JR, van Tuinen D, Brockerhoff SE, Neff MM, Davis TN. Can calmodulin function without binding calcium? Cell. 1991;65:949–959. doi: 10.1016/0092-8674(91)90547-c. [DOI] [PubMed] [Google Scholar]
- Hemenway CS, Heitman J. Calcineurin: Structure, function, and inhibition. Cell Biochem Biophys. 1999;30:115–151. doi: 10.1007/BF02737887. [DOI] [PubMed] [Google Scholar]
- Hubbard MJ, Cohen P. On target with a new mechanism for the regulation of protein phosphorylation. Trends Biochem Sci. 1993;18:172–177. doi: 10.1016/0968-0004(93)90109-z. [DOI] [PubMed] [Google Scholar]
- Hughes SM. Muscle development: Electrical control of gene expression. Curr Biol. 1998;8:892–894. doi: 10.1016/s0960-9822(07)00554-4. [DOI] [PubMed] [Google Scholar]
- Kashishian A, Howard M, Loh C, Gallatin WM, Hoekstra MF, Lai Y. AKAP79 inhibits calcineurin through a site distinct from the immunophilin-binding region. J Biol Chem. 1998;273:27412–27419. doi: 10.1074/jbc.273.42.27412. [DOI] [PubMed] [Google Scholar]
- Klee CB, Ren H, Wang X. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J BiolChem. 1998;273:13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- Krebs J. The role of calcium in apoptosis. Biometals. 1998;11:375–382. doi: 10.1023/a:1009226316146. [DOI] [PubMed] [Google Scholar]
- Kuno T, Tanaka H, Mukai H, Chang CD, Hiraga K, Miyakawa T, Tanaka C. cDNA cloning of a calcineurin B homolog in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1991;180:1159–1163. doi: 10.1016/s0006-291x(05)81188-x. [DOI] [PubMed] [Google Scholar]
- Lai MM, Burnett PE, Wolosker H, Blackshaw S, Snyder SH. Cain, a novel physiologic protein inhibitor of calcineurin. J Biol Chem. 1998;273:18325–18331. doi: 10.1074/jbc.273.29.18325. [DOI] [PubMed] [Google Scholar]
- Leahy KP, Davies KJ, Dull M, Kort JJ, Lawrence KW, Crawford DR. adapt78, a stress-inducible mRNA, is related to the glucose-regulated protein family of genes. Arch Biochem Biophys. 1999;368:67–74. doi: 10.1006/abbi.1998.1059. [DOI] [PubMed] [Google Scholar]
- Liu J, Farmer J, Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991a;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Liu J, Albers MW, Wandless TJ, Luan S, Alberg DG, Belshaw PJ, Cohen P, MacKintosh C, Klee CB, Schreiber SL. Inhibition of T-cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry. 1992;31:3896–3901. doi: 10.1021/bi00131a002. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ishii S, Tokai M, Tsutsumi H, Ohki O, Akada R, Tanaka K, Tsuchiya E, Fukui S, Miyakawa T. The Saccharomyces cerevisiae genes (CMP1 and CMP2) encoding calmodulin-binding proteins homologous to the catalytic subunit of mammalian protein phosphatase 2B. Mol Gen Genet. 1991b;227:52–59. doi: 10.1007/BF00260706. [DOI] [PubMed] [Google Scholar]
- Mansuy IM, Mayford M, Jacob B, Kandel ER, Bach ME. Restricted and regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell. 1998;92:39–49. doi: 10.1016/s0092-8674(00)80897-1. [DOI] [PubMed] [Google Scholar]
- Matheos DP, Kingsbury TJ, Ahsan US, Cunningham KW. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes & Dev. 1997;11:3445–3458. doi: 10.1101/gad.11.24.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendizabal I, Rios G, Mulet JM, Serrano R, de Larrinoa IF. Yeast putative transcription factors involved in salt tolerance. FEBS Lett. 1998;425:323–328. doi: 10.1016/s0014-5793(98)00249-x. [DOI] [PubMed] [Google Scholar]
- Miseta A, Kellermayer R, Aiello DP, Fu L, Bedwell DM. The vacuolar Ca2+/H+ exchanger Vcx1p/Hum1p tightly controls cytosolic Ca2+ levels in S. cerevisiae. FEBS Lett. 1999;451:132–136. doi: 10.1016/s0014-5793(99)00519-0. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Kanou Y, Murata Y, Ohmori S, Niwa T, Maeda K, Yamamura H, Seo H. Molecular cloning of a novel thyroid hormone-responsive gene, ZAKI-4, in human skin fibroblasts. J Biol Chem. 1996;271:14567–14571. doi: 10.1074/jbc.271.24.14567. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MJ, Geiser JR, Davis TN. Ca2+-calmodulin promotes survival of pheromone-induced growth arrest by activation of calcineurin and Ca2+-calmodulin-dependent protein kinase. Mol Cell Biol. 1996;16:4824–4831. doi: 10.1128/mcb.16.9.4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozos TC, Sekler I, Cyert MS. The product of HUM1, a novel yeast gene, is required for vacuolar Ca2+/H+ exchange and is related to mammalian Na+/Ca2+ exchangers. Mol Cell Biol. 1996;16:3730–3741. doi: 10.1128/mcb.16.7.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: Regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- Rothermel B, Vega RB, Yang J, Wu H, Bassel-Duby R, Williams RS. A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J Biol Chem. 2000;275:8719–8725. doi: 10.1074/jbc.275.12.8719. [DOI] [PubMed] [Google Scholar]
- Sagoo JK, Fruman DA, Wesselborg S, Walsh CT, Bierer BE. Competitive inhibition of calcineurin phosphatase activity by its autoinhibitory domain. Biochem J. 1996;320:879–884. doi: 10.1042/bj3200879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki F, McKeon F. Calcineurin functions in Ca2+-activated cell death in mammalian cells. J Cell Biol. 1995;131:735–743. doi: 10.1083/jcb.131.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim AT, Scott JD. Targeting of PKA, PKC, and protein phosphatases to cellular microdomains. Cell Calcium. 1999;26:209–217. doi: 10.1054/ceca.1999.0072. [DOI] [PubMed] [Google Scholar]
- Stathopoulos AM, Cyert MS. Calcineurin acts through the CRZ1/TCN1 encoded transcription factor to regulate gene expression in yeast. Genes & Dev. 1997;11:3432–3444. doi: 10.1101/gad.11.24.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos-Gerontides A, Guo JJ, Cyert MS. Yeast calcineurin regulates nuclear localization of the Crz1p transcription factor through dephosphorylation. Genes & Dev. 1999;13:798–803. doi: 10.1101/gad.13.7.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Youn HD, Loh C, Stolow M, He W, Liu JO. Cabin 1, a negative regulator for calcineurin signaling in T lymphocytes. Immunity. 1998;8:703–711. doi: 10.1016/s1074-7613(00)80575-0. [DOI] [PubMed] [Google Scholar]
- Sussman MA, Lim HW, Gude N, Taigen T, Olson EN, Robbins J, Colbert MC, Gualberto A, Wieczorek DF, Molkentin JD. Prevention of cardiac hypertrophy in mice by calcineurin inhibition. Science. 1998;281:1690–1693. doi: 10.1126/science.281.5383.1690. [DOI] [PubMed] [Google Scholar]
- Tung HY, Wang W, Chan CS. Regulation of chromosome segregation by Glc8p, a structural homolog of mammalian inhibitor 2 that functions as both an activator and an inhibitor of yeast protein phosphatase 1. Mol Cell Biol. 1995;15:6064–6074. doi: 10.1128/mcb.15.11.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Culotta VC, B. KC. Superoxide dismutase protects calcineurin from inactivation. Nature. 1996;383:434–437. doi: 10.1038/383434a0. [DOI] [PubMed] [Google Scholar]
- Winder DG, Mansuy IM, Osman M, Moallem TM, Kandel ER. Genetic and pharmacological evidence for a novel, intermediate phase of long-term potentiation suppressed by calcineurin. Cell. 1998;92:25–37. doi: 10.1016/s0092-8674(00)80896-x. [DOI] [PubMed] [Google Scholar]
- Ye RR, Bretscher A. Identification and molecular characterization of the calmodulin-binding subunit gene (CMP1) of protein phosphatase 2B from Saccharomyces cerevisiae. An alpha-factor inducible gene. Eur J Biochem. 1992;204:713–723. doi: 10.1111/j.1432-1033.1992.tb16686.x. [DOI] [PubMed] [Google Scholar]
- Youn HD, Sun L, Prywes R, Liu JO. Apoptosis of T cells mediated by Ca2+-induced release of the transcription factor MEF2. Science. 1999;286:790–793. doi: 10.1126/science.286.5440.790. [DOI] [PubMed] [Google Scholar]
- Zhu J, Shibasaki F, Price R, Guillemot JC, Yano T, Dotsch V, Wagner G, Ferrara P, McKeon F. Intramolecular masking of nuclear import signal on NF–AT4 by casein kinase I and MEKK1. Cell. 1998;93:851–861. doi: 10.1016/s0092-8674(00)81445-2. [DOI] [PubMed] [Google Scholar]