Abstract
It is believed that multiple effectors independently control the checkpoints permitting transitions between cell cycle phases. However, this has not been rigorously demonstrated in mammalian cells. The p53-induced genes p21 and 14-3-3ς are each required for the G2 arrest and allow a specific test of this fundamental tenet. We generated human cells deficient in both p21 and 14-3-3ς and determined whether the double knockout was more sensitive to DNA damage than either single knockout. p21−/− 14-3-3ς−/− cells were significantly more sensitive to DNA damage or to the exogenous expression of p53 than cells lacking only p21 or only 14-3-3ς. Thus, p21 and 14-3-3ς play distinct but complementary roles in the G2/M checkpoint, and help explain why genes at the nodal points of growth arrest pathways, like p53, are the targets of mutation in cancer cells.
Keywords: checkpoint, p53, 14-3-3ς, p21, cell cycle
The p53 tumor suppressor gene is mutated in a large fraction of human cancers, suggesting that wild-type p53 function is required to limit tumor growth (Hollstein et al. 1991). p53 is thought to inhibit growth through two mechanisms. First, expression of p53 causes arrest in both the G1 and G2 phases of the cell cycle (El-Deiry et al. 1994; Agarwal et al. 1995; Stewart et al. 1995; Levine 1997). The G1 arrest is in large part due to transcriptional activation of p21, a cyclin-dependent kinase inhibitor (El-Deiry et al. 1993; Harper et al. 1993; Xiong et al. 1993; Brugarolas et al. 1995; Deng et al. 1995; Waldman et al. 1995). p53-induced G2 arrest appears to be due to the induction of both p21 and 14-3-3ς, a protein that normally sequesters cyclinB1–cdc2 complexes in the cytoplasm (Hermeking et al. 1997; Bunz et al. 1998; Chan et al. 1999). The second mechanism through which p53 acts involves the induction of apoptosis (Yonish-Rouach et al. 1991; Shaw et al. 1992). p53 activates transcription of a variety of apoptosis-associated genes, and programmed cell death (PCD) in response to genotoxic stress is impaired in the absence of p53 (Clarke et al. 1994; Miyashita et al. 1994; Oren 1994; Polyak et al. 1997; Yu et al. 1999).
Cells deficient in either p21 or 14-3-3ς are unable to maintain stable G2 arrest and die when exposed to DNA-damaging agents (Bunz et al. 1998; Chan et al. 1999). However, it is unclear whether these two genes play complementary roles in checkpoint control, with each independently contributing to the arrest that occurs after DNA damage. To address this question, we have generated human colorectal cancer (CRC) cells lacking both p21 and 14-3-3ς through targeted homologous recombination. These experiments constitute the first successful attempt to create double knockouts in human cells and provide definitive data about the nonredundant roles of p21 and 14-3-3ς in controlling the G2/M checkpoint following DNA damage.
Results and Discussion
Creation of double knockout cells
Targeting vectors for p21 and 14-3-3ς were constructed (Waldman et al. 1995; Chan et al. 1999) and sequentially used to create cells deficient in both genes. The CRC cell line HCT116 was chosen for these studies because it has intact p53, p21, and 14-3-3ς genes and apparently normal DNA damage checkpoint responses. Southern blot analyses confirmed targeting of all four alleles (Fig. 1), and Western blot analysis showed that the double knockout cells did not produce either p21 or 14-3-3ς proteins (data not shown). Two independently derived clones were obtained and compared with the p21−/− and 14-3-3ς−/− cells described previously (Waldman et al. 1995; Chan et al. 1999).
Figure 1.
Generation of human cells lacking 14-3-3ς and p21. HCT116 CRC cells were transfected with 14-3-3ς targeting constructs, and knockout cells were identified as described in Chan et al. (1999). Southern analysis was used to confirm the successful deletion of both 14-3-3ς alleles (top). 14-3-3ς−/− cells were transfected with p21 targeting vectors, and clones with successfully targeted alleles were identified by PCR and confirmed by Southern blot as in Waldman et al. (1995) (bottom). Wild-type and targeted alleles are labeled and marked with arrows.
Effects of DNA damage
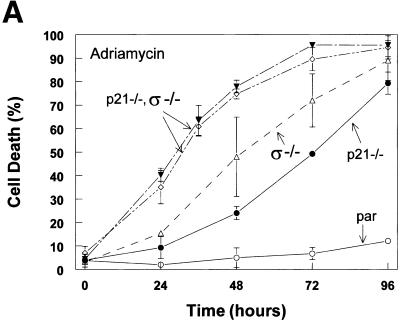
To assess the functions of p21 and 14-3-3ς following DNA damage, we first treated cells of various genotypes with adriamycin, a DNA-damaging agent and commonly used chemotherapeutic agent. Cells were scored for apoptosis and mitotic death at sequential times after initiation of treatment (Fig. 2A) and subjected to analysis by flow cytometry (Fig. 2B). Parental cells stably arrested in both G1 and G2 and did not undergo cell death when treated with adriamycin (Fig. 2). The disruption of p21 had a marked effect on the responses of the cells to DNA damage, as described previously (Waldman et al. 1996; Bunz et al. 1998). The cells failed to arrest in G1 (Fig. 2B), failed to maintain a stable G2 arrest, and eventually underwent PCD after failed cytokinesis. Cells lacking 14-3-3ς were able to arrest in G1 but could not maintain a stable G2 arrest. These cells underwent mitotic death (Fig. 2), a process associated with chromosome condensation and DNA degradation (Heald et al. 1993; Chan et al. 1999). Cells lacking both p21 and 14-3-3ς died significantly sooner than cells lacking only one of the two genes (Fig. 2A) and this death was associated with marked DNA degradation (see 48 hr time point in Fig. 2B).
Figure 2.
Cells lacking 14-3-3ς and p21 are more sensitive to DNA damage than cells lacking either 14-3-3ς or p21. (A) Cells were treated with 0.2 μg/ml adriamycin for the times indicated. Cells were harvested and scored for death after staining with Hoechst 33258. (▾,⋄) p21−/−, ς−/−; (▵) ς−/−; (●) p21−/−; (par) parental cells containing normal 14-3-3ς and p21 genes. Error bars correspond to one standard deviation; the points represent the mean cell death determined from at least three independent experiments. (B) Compromised cell cycle arrest in cells deficient in p21 and 14-3-3ς. Cells of the indicated genotypes were treated with adriamycin, harvested at the indicated times, stained with Hoechst 33258, and subjected to flow cytometry. Peaks corresponding to 2N and 4N are labeled accordingly.
Colony-forming ability
Checkpoint defects in Saccharomyces cerevisiae and Schizosaccharomyces pombe result in cells that are more sensitive to DNA-damaging agents than wild-type yeast when studied with colony formation assays (Weinert 1992; Iede et al. 1994). Interestingly, loss of checkpoint function in human cells results in increased apoptosis, but this is often not associated with greater drug sensitivities when measured with standard colony formation assays (Slichenmyer et al. 1993). Lack of such checkpoints in mammalian cells may cause them to preferentially undergo cell death instead of arrest, with little net effect on colony-forming ability. Loss of the p21 gene, for example, causes cells to die more readily after DNA damage, but there is no significant effect on colony formation (Waldman et al. 1997). To determine whether a similar phenomenon was applicable to the double knockout cells, colony formation assays were performed on the various mutants with varying concentrations of adriamycin. Cells were treated with the drug for 12 hr, replated in new flasks, and allowed to grow for 10 days, at which time clonogenic survival was assessed. Cells lacking both 14-3-3ς and p21 were dramatically more sensitive than cells lacking only one of the genes (Fig. 3). To our knowledge, this is the first demonstration that cells with defective checkpoints are actually more sensitive to DNA-damaging agents when their growth potential is measured in a standard assay for measuring drug sensitivities. Thus, human cells lacking both the p21 and 14-3-3ς genes, but not either alone, behaved like checkpoint deficient yeast mutants when exposed to DNA damaging agents.
Figure 3.
Decreased clonogenic survival of p21−/− 14-3-3ς−/− cells following DNA damage. Cells with indicated genotypes were treated with 0.02 μg/ml adriamycin and replated in new flasks (ADR). Colonies were stained with crystal violet 7–10 days later. (Control) Cells were not treated with adriamycin.
Response to exogenous p53
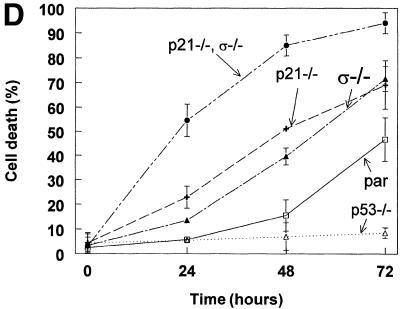
One way to view the effects of p53 is as a competition between cell arrest and cell death (Oren 1994; Polyak et al. 1996). Accordingly, the absence of genes that result in growth arrest may allow the death-inducing effects of p53 to be more apparent. To determine the effects of the loss of p21 and 14-3-3ς genes on the response to p53, cells were infected with a recombinant adenovirus engineered to express wild-type p53 (Adp53) (Yu et al. 1999). Green fluorescent protein (GFP) was coexpressed along with p53 to serve as a marker of infection. As shown in Figure 4A, all cell types were infected by Adp53 at similar efficiencies. In addition, after infection with Adp53, cells of all the genotypes were analyzed by Western blot using anti-p53 antibody. The Western blot analysis indicated that infection with Adp53 resulted in similar levels of p53 protein in all the cell lines used (data not shown). After infection, cells were harvested and stained with Hoechst 33258 to assess cell death. On expression of p53, parental cells with intact p21 and 14-3-3ς genes underwent cell cycle arrest (Polyak et al. 1996). In contrast, cells lacking either p21 or 14-3-3ς underwent cell death (Fig. 4B). p21−/− 14-3-3ς−/− cells died significantly more quickly than cells lacking only one of the two genes (Fig. 4B). As expected, when the cells were infected with an adenovirus expressing mutant p53 (R175H), they did not die. As with DNA damaging agents, it thereby seemed that p21 and 14-3-3ς cooperated to prevent cell death.
Figure 4.
Cooperative effect of p21 and 14-3-3ς in determining cell cycle arrest vs. death following expression of p53. (A) Cells of the indicated genotypes were infected with an adenovirus that directed the expression of p53 and GFP. (Top) GFP expression of the cells 20 hr following start of infection; (Bottom) the same field of cells under bright field. Cells of all the genotypes became infected with the p53 adenovirus to a similar extent. Similar infection frequencies were obtained with the mutant p53 adenovirus (data not shown). (B) Cells lacking both p21 and 14-3-3ς died more readily following expression of p53 than cells lacking only one or none of the two genes. (●) p21−/−, ς−/−; (▴) ς−/−; (+) p21−/−; □, (par) parental cells containing normal 14-3-3ς and p21 genes. (C) No cell death was observed in any of the cells upon infection with an adenovirus expressing inactive mutant p53 (R175H). Labels as in B. (D) Cells lacking both p21 and 14-3-3ς are sensitive to 5-FU. 5-FU was used to induce endogenous p53 expression. Cells were scored for death at the indicated time points. Error bars correspond to one standard deviation; the points represent the mean cell death determined from at least three independent experiments.(▵) p53−/−; other labels as in B.
Response to endogenous p53 induction
5-Fluorouracil (5-FU) is the most important drug used for the treatment of colorectal cancer. Previous work has demonstrated that 5-FU activates a p53-dependent apoptotic program (Bunz et al. 1999). Therefore, if 14-3-3ς and p21 protect cells from p53-mediated PCD, the double knockout cells should be more sensitive to 5-FU. Parental, p21−/−, 14-3-3ς−/−, and double mutant cells were treated with 5-FU, cells were harvested at various time points, and cell death was scored (Fig. 4D). 5-FU treatment induced cell cycle arrest but not death in p53−/− cells, confirming that p53 was required for PCD following 5-FU treatment (Bunz et al. 1999). Parental cells with wild-type p53 died, with ∼50% of the cells apoptotic at 72 hr post-treatment. Cells lacking either p21 or 14-3-3ς were more sensitive to 5-FU than parental or p53−/− cells. And, cells lacking both p21 and 14-3-3ς were significantly more sensitive than either of the single mutants. Of the double mutant cells, >90% were dead at 72 hr post-treatment.
Mitotic death
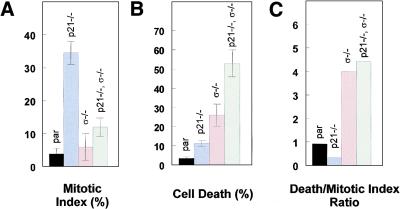
A mitotic trap assay was used to directly assess whether the cells entered mitosis following DNA damage. Parental, p21−/−, 14-3-3ς−/−, and double knockouts were treated with adriamycin for 12 hr, the adriamycin-containing medium was removed, and colcemid containing medium was added. After 12 hr, cells were harvested and cell death and mitotic index was scored as described (Cahill et al. 1998; Chan et al. 1999). After such treatment, parental cells remained arrested in either G1 or G2. The parental cells did not progress into mitosis, as indicated by the low mitotic index (Fig. 5A) and low incidence of cell death (Fig. 5B). Those 14-3-3ς−/− cells that escaped the G2 arrest appeared to die quickly thereafter, as evidenced by the higher fraction of dead cells in Figure 5B. That 14-3-3ς is required to prevent mitotic cell death was strongly supported by the results in the double knockout cells. It has been shown previously that p21−/− cells fail to maintain a stable G2 arrest and progress into mitosis, as indicated by their high mitotic index score (Fig. 5A; Bunz et al. 1998). In the presence of wild-type 14-3-3ς, p21−/− cells can remain in colcemid-stablized mitosis for long periods without dying (Fig. 5A,B). In the absence of 14-3-3ς, this state is unstable and the cells rapidly die after they enter mitosis. As a result, the double knockout cells have a much higher ratio of dead to mitotic cells than cells that lack only p21 (Fig. 5C).
Figure 5.
Distinct functions of p21 and 14-3-3ς following DNA damage. (A) Cells of the indicated genotypes were treated with 0.2 μg/ml adriamycin for 12 hr and subsequently with colcemid for 20 hr. The y-axis corresponds to the percentage of cells in mitosis (mitotic index). Error bars correspond to one standard deviation; the bars represent the means determined from at least three independent experiments. (B) Cells were treated as in A and cell death was scored. The y-axis corresponds to percentage of dead cells. (C) Cells were treated as in A. The y-axis corresponds to the ratio between the fraction of dead cells to the fraction of cells arrested in mitosis.
If p21 and 14-3-3ς were redundant with regards to G2 arrest, one would expect that the phenotype of double knockout cells would be identical to that of one of the two single knockouts. Our results exclude this possibility, as the double knockouts were significantly different from either single knockout, in terms of sensitivities to the chemotherapeutic drugs adriamycin and 5-FU as well as in their response to exogenous p53. The markedly different cell death/mitosis ratios observed in Figure 5C further illustrate these differences and suggest that the absence of p21 simply leads to an inability to remain in G2, with cells progressing into a reasonably normal mitosis. In contrast, the absence of 14-3-3ς is incompatible with stable G2 arrest and normal mitosis, and cells without p21 rapidly die once they pass through G2 in the absence of 14-3-3ς.
Our finding that p21 and 14-3-3ς cooperate to achieve arrest is consistent with the known biochemical activities of these two proteins. The 14-3-3ς protein sequesters cdc2–cyclin B1 in the cytoplasm during a G2 checkpoint initiated by DNA damage (Chan et al. 1999). Any cdc2–cyclin B1 that escapes this sequestration and migrates to the nucleus is “mopped up” and presumably inactivated by p21, which is permanently nuclear. It therefore is not difficult to understand why cells without p21 and without 14-3-3ς have a more defective checkpoint than cells with defects in either alone. However, although this may be the case in CRC cells, the generality of our results remain to be determined. Further work is required to determine whether our model applies to other cell types and organisms.
Epithelial cells have thereby evolved the ability to induce two strong effectors of G2 arrest following p53 expression. This provides some insights into the nature of the genes that are mutated in human cancers and thereby drive the process. Mutations of p21 or 14-3-3ς are rarely, if ever, observed in human cancers, whereas inactivation of p53, either directly through mutation or indirectly through binding to amplified MDM2 genes or viral proteins, is the rule rather than the exception (Kinzler and Vogelstein 1992). It makes sense that the most critical genes to disable during tumor progression are those that control numerous downstream pathways and thereby function as nodal points of growth control. Based on the data provided here, it is clear that mutation of p21 or 14-3-3ς genes would not have as dramatic an effect on control of the cell cycle as mutation of p53, as p53 mutation leads to loss of control of the expression of both these downstream targets. Moreover, it is known that p53 not only controls passage through the cell cycle, but also controls apoptosis. It is likely that most, if not all, commonly mutated genes in human tumors will reside at such nodal points, controlling the expression or function of numerous downstream targets (Yu et al. 1999).
Materials and methods
Targeted deletion of p21 and 14-3-3ς
Targeting constructs for p21 and 14-3-3ς, p21−/− cells, and 14-3-3ς−/− cells were described previously (Waldman et al. 1997; Chan et al. 1999). To generate p21−/− 14-3-3ς−/− cells, 14-3-3ς−/− cells were transfected with a p21 targeting construct containing a neomycin resistance gene and selected in 0.4 mg/ml geneticin (GIBCO). Clones with a successfully targeted allele were identified by PCR using the primers 5′-cggattcgccgaggcaccg-3′ and 5′-gttgtgcccagtcatagccg-3′ and Taq Platinum (GIBCO). 14-3-3ς−/− p21+/− cells were then transfected with a second p21 targeting vector containing a hygromycin resistance gene and selected in 0.1 mg/ml hygromycin B. Clones with successfully targeted p21 alleles were again identified using PCR. Southern analysis was used to confirm that the clones had a 14-3-3ς−/− p21−/− genotype, and Western blot analysis was used to confirm that the double mutants did not produce p21 or 14-3-3ς proteins (Waldman et al. 1997; Chan et al. 1999).
Cell culture and transfection
HCT116 cells were obtained from the American Type Culture Collection (ATCC). Cells were cultured in McCoy's medium supplemented with 10% fetal bovine serum (FBS; GIBCO). Tranfections were performed with lipofectamine as directed by the manufacturer (GIBCO). Clonal selection following transfection with the knockout construct was carried out in McCoy's medium with 10% FBS and 0.4 mg/ml geneticin or 0.1 mg/ml hygromycin B (GIBCO). Following transfection, cells were diluted in selection media and plated out in 96-well plates. After selection, genomic DNA was prepared from the drug-resistant clones using the QiaAmp column system (Qiagen). Adriamycin was used at a concentration of 0.2 μg/ml for time courses. For the colony formation assays, adriamycin was used at concentrations of 0.2 μg/ml, 0.1 μg/ml, or 0.02 μg/ml. 5-FU was used at a concentration of 50 μg/ml. Assays for cell death and mitotic catastrophe were as described previously (Chan et al. 1999).
Flow cytometry
Cells were trypsinized, washed with HBSS (GIBCO), and resuspended in 40 μl of HBSS. The cells were then added to 360 μl of a solution containing 1% NP-40 (Sigma), 4.7% formaldehyde (J.T. Baker), and 11 μg/ml Hoechst 33258 in PBS. These fixed and stained cells were stored at 4°C and analyzed within three days by flow cytometry.
Colony formation assays
Cells were grown to 50% confluency. Adriamycin was added to the media for 18 hr. Cells were then trypsinized and replated in new flasks with fresh media. Cells were grown for 10 days and then stained with crystal violet.
Mitotic trap assay
Cells were treated with 0.2 μg/ml adriamycin for 12 hr. Subsequently, adriamycin was removed and media containing 0.1 μg/ml colcemid was added to the cells. After 20 hr, cells were collected, stained with Hoechst 33258, and mitotic cells were scored as described previously (Cahill et al. 1998).
Acknowledgments
We thank the members of the Vogelstein/Kinzler Laboratories for helpful discussions. T.A.C. would like to thank D. Tomasallo for helpful advice. T.A.C. received financial support from the Medical Scientist Training Program. K.W.K. and B.V. are consultants to Genzyme. The University and researchers (K.W.K. and B.V.) own Genzyme stock, which is subject to certain restrictions under University policy. The terms of this arrangement are being managed by the University in accordance with its conflict of interest policies. This work was supported by the Clayton Fund and by National Institutes of Health Grants CA57345 and CA62924. B.V. is an Investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL vogelbe@welch.jhu.edu; FAX (410) 955-0548.
References
- Agarwal ML, Agarwal A, Taylor WR, Stark GR. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc Natl Acad Sci. 1995;92:8493–8497. doi: 10.1073/pnas.92.18.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Radiation-induced cell arrest compromised by p21 deficiency. Nature. 1995;377:552–557. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L, Williams J, Lengauer C, Kinzler KW, Vogelstein B. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104:263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill DP, Lengauer C, Yu J, Riggins GJ, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- Chan TA, Hermeking H, Lengauer C, Kinzler KW, Vogelstein B. 14-3-3ς is required to prevent mitotic catastrophe after DNA damage. Nature. 1999;401:616–620. doi: 10.1038/44188. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Gledhill S, Hooper ML, Bird CC, Wyllie AH. p53 dependence of early apoptotic and proliferative responses within the mouse intestinal epithelium following gamma-irradiation. Oncogene. 1994;9:1767–1773. [PubMed] [Google Scholar]
- Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- El-Deiry WS, Harper JW, O'Connor PM, Velculescu VE, Canman CE, Jackman J, Pietenpol JA, Burrell M, Hill DE, Wang Y, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–1174. [PubMed] [Google Scholar]
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Heald R, McLoughlin M, McKeon F. Human wee1 maintains mitotic timing by protecting the nucleus from cytoplasmically activated cdc2 kinase. Cell. 1993;74:463–474. doi: 10.1016/0092-8674(93)80048-j. [DOI] [PubMed] [Google Scholar]
- Hermeking H, Lengauer C, Polyak K, He T-C, Zhang L, Thiagalingam S, Kinzler KW, Vogelstein B. 14-3-3ς is a p53-regulated inhibitor of G2/M progression. Mol Cell. 1997;1:3–11. doi: 10.1016/s1097-2765(00)80002-7. [DOI] [PubMed] [Google Scholar]
- Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Iede W, Friedberg AS, Dianova I, Friedberg EC. Characterization of G1 checkpoint control in the yeast Saccharomyces cerevisiae following exposure to DNA-damaging agents. Genetics. 1994;138:271–281. doi: 10.1093/genetics/138.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B. The colorectal cancer gene hunt: Current findings. Hosp Pract (Off Ed) 1992;27:51–58. doi: 10.1080/21548331.1992.11705522. [DOI] [PubMed] [Google Scholar]
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B, Reed JC. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994;9:1799–1805. [PubMed] [Google Scholar]
- Oren M. Relationship of p53 to the control of apoptotic cell death. Semin Cancer Biol. 1994;5:221–227. [PubMed] [Google Scholar]
- Polyak K, Waldman T, He T-C, Kinzler KW, Vogelstein B. Genetic determinants of p53 induced apoptosis and growth arrest. Genes & Dev. 1996;10:1945–1952. doi: 10.1101/gad.10.15.1945. [DOI] [PubMed] [Google Scholar]
- Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53 induced apoptosis. Nature. 1997;389:300–304. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- Shaw P, Bovey R, Tardy S, Sahli R, Sordat B, Costa J. Induction of apoptosis by wild-type p53 in a human colon tumor-derived cell line. Proc Natl Acad Sci. 1992;89:4495–4499. doi: 10.1073/pnas.89.10.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slichenmyer WJ, Nelson WG, Slebos RJ, Kastan MB. Loss of a p53-associated G1 checkpoint does not decrease cell survival following DNA damage. Cancer Res. 1993;15:4164–4168. [PubMed] [Google Scholar]
- Stewart N, Hicks GG, Paraskevas F, Mowat M. Evidence for a second cell cycle block at G2/M by p53. Oncogene. 1995;10:109–115. [PubMed] [Google Scholar]
- Waldman T, Kinzler KW, Vogelstein B. p21 Is necessary for the p53-mediated G(1) arrest in human cancer cells. Cancer Res. 1995;55:5187–5190. [PubMed] [Google Scholar]
- Waldman T, Lengauer C, Kinzler KW, Vogelstein B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature. 1996;381:713–716. doi: 10.1038/381713a0. [DOI] [PubMed] [Google Scholar]
- Waldman T, Zhang Y, Dillehay L, Yu J, Kinzler K, Vogelstein B, Williams J. Cell-cycle arrest versus cell death in cancer therapy. Nat Med. 1997;3:1034–1036. doi: 10.1038/nm0997-1034. [DOI] [PubMed] [Google Scholar]
- Weinert TA. Dual cell cycle checkpoints sensitive to chromosome replication and DNA damage in the budding yeast Saccharomyces cerevisiae. Radiat Res. 1992;132:141–143. [PubMed] [Google Scholar]
- Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991;352:345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang L, Hwang PM, Rago C, Kinzler KW, Vogelstein B. Identification and classification of p53-regulated genes. Proc Natl Acad Sci. 1999;96:14517–14522. doi: 10.1073/pnas.96.25.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]