Abstract
Small-diameter cemented Metasul® cups have been previously identified to be at high risk of early loosening. We asked whether this particular mode of failure was associated with a specific histological feature. Periprosthetic tissues were obtained at the time of revision of two aseptically loose cemented Metasul® cups. Each tissue sample was processed for routine histological analysis. A slight metallosis was visible microscopically in all tissue samples. Metallic wear-debris particles were present both extracellularly and within the cytoplasm of macrophages. We noted a perivascular infiltration of lymphocytes accompanied by mature plasma cells. Our observations are compatible with the hypersensitivity-like reaction previously reported, described as an aseptic lymphocytedominated vasculitis-associated lesion (ALVAL). Although wear was within normal reported range limits, this tissue reaction appeared as a consequence of continuous release of metallic ions from the prosthetic articulation. We hypothesise that ALVAL was involved in acetabular component failure, although acetabular loosening may have been initiated by high mechanical stress.
Introduction
Second-generation metal-on-metal (MOM) total hip replacements were introduced in the early 1990s, theoretically to reduce the rates of osteolysis and aseptic loosening associated with polyethylene (PE) wear debris [25]. Clinical studies have shown that a 98.6–99% survival rate could be expected ten years after a contemporary MOM hip replacement with a near absence of osteolysis [4, 5]. Also, a low amount of wear on retrieved implants and in vivo has been reported [20, 21, 23], confirming preliminary hip simulator tests [24] and former clinical data [15, 22]. However, early aseptic failure associated with immunologically determined tissue reactions characterised by intense diffuse and perivascularly lymphocytic infiltration suggests that metal hypersensitivity was involved in the pathogenesis of aseptic loosening [10, 17, 26]. We have reported our observation of early progressive periacetabular radiolucencies in cemented Metasul® acetabular components [19]. In that study, we showed that cemented Metasul® cups with an average diameter of 46 mm were exposed to a higher risk of aseptic loosening compared with acetabular components with a larger diameter. The mechanism for this phenomenon was not fully identified. In accordance with other studies [12], these results led to the abandonment of cemented Metasul® acetabular components in primary total hip replacement in France. We asked whether this particular mode of failure was associated with a specific histological feature. Here we report histological analysis of neocapsule and periprosthetic tissues retrieved from two aseptic failures of cemented Metasul® acetabular components.
Materials and methods
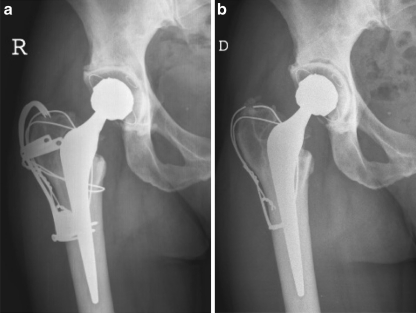
Neocapsules and periprosthetic tissue samples were obtained at the time of revision of two loose Metasul® acetabular components in two healthy female patients. The original devices included a 28-mm-diameter Metasul® head articulated with a cemented Metasul® acetabular component, and a cemented femoral stem. Epidemiological and surgical data are summarised in the Table 1. Primary implantations were performed for advanced osteoarthritis in both cases. Both patients remained symptom free for more than five years after the initial hip replacement. Respective hip function progressively decreased thereafter, associated with an increasing deep pain in the groin. Serial blood analysis of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and full blood count and differential were within normal limits. In patient 1, plain radiographs revealed complete periacetabular radiolucency located at the bone–cement interface (Fig. 1). In patient 2, plain radiographs also showed acetabular component protrusion and periacetabular osteolysis. Based on these investigations, revision procedures of the loose acetabular components were planned. Both hips were revised using a posterior approach. At the time of revision, soft tissues did not appear necrotic or significantly inflamed. Macroscopically, no metallosis or macroscopic inflammatory granuloma was noted in the articulation. In both cases, the acetabular component was loose at the cement–bone interface and easily removed. Some minor scuffing of the taper was noted. In patient 1, it was decided to retain the well-fixed femoral component. A Durasul® acetabular component was cemented in place and articulated with a corresponding 28-mm-diameter CroCo head (Zimmer, Warsaw, IN, USA). In patient 2, periacetabular bone loss necessitated reconstruction with a Kerboull acetabular reinforcement device (Stryker, Hérouville Saint Clair, France) and a bulk allograft. A 52-mm-diameter conventional PE cup with an inner diameter of 22.2 mm was cemented (Stryker). The femoral implant was also revised, and a Kerboull® MKIII stem (Stryker) component was cemented into the femur.
Table 1.
Epidemiological and surgical data in aseptic loosening of two cemented Metasul® cups
| Patient | Sex | Age at IP (years) | BMI | Initial diagnosis | Cup outer/inner diameters (mm) | Time to revision (months) | New implants | FU (months) | Comment(s) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 42 | 21.5 | Osteoarthritis | 50/28 | 72 | Durasul® cemented cup | 12 | No macroscopic metallosis at the time of revision |
| 2 | F | 57 | 28.7 | Osteoarthritis | 50/28 | 89 | CPE cemented cup | 12 |
F female, IP index procedure, BMI body mass index, CPE conventional polyethylene, FU follow-up
Fig. 1.
Patient 1. An active and healthy 41-year-old woman underwent a bilateral staged cemented Metasul® replacement for hip arthritis subsequent to acetabular dysplasia. A transtrochanteric approach was employed at the time of the total replacement of the right hip. A 50-mm-diameter Metasul® socket was cemented and articulated with a 28-mm Metasul® head. The femoral stem was also cemented. a Standard anteroposterior (AP) postoperative radiograph of the right hip. The trochanteric claw plate was removed 42 months after initial surgery. b Standard AP view of the right hip 70 months after the index hip replacement before the revision procedure. Of note is the complete periacetabular radiolucency located at the bone–cement interface
Postoperatively, both patients recovered uneventfully. There was no bacterial growth from the synovial fluid or tissue samples retrieved at the time of revision. One year after revision, both hip scores were rated very good or excellent according to Postel-Merle d’Aubigné [16]. In patient 1, the unrevised cemented Metasul® hip exhibited a complete periacetabular radiolucency at a 66-month follow-up. However, the patient did not wish to have a revision procedure on her left hip.
Histological analysis
Tissue samples retrieved from the neocapsule and bone–cement interface were obtained at revision and fixed in neutral buffered formalin. Then, they were embedded in paraffin, sectioned into 5-μm-thick serial sections and stained with haematoxylin and eosin (H&E) and Prussian blue. All histological analyses were performed using a light and polarising microscope (Eclipse TE 2000-U, Nikon, Amstelveen, The Netherlands) coupled to a high-resolution colour digital camera (DXM1200F, Nikon), at final magnifications of 20×, 40× and 100×. Metallosis was graded according to the modified Mirra classification [3]. The amounts of metal particles in the tissue and diffuse and perivascular lymphocytes were graded according to the method described by Willert et al. [27, 28]. Lymphocytic infiltration was assessed by counting the individual cells of a diffuse infiltration (magnification 40×) and the number of perivascular agglomerations (magnification 4×) per field of view according to the method previously described [27].
Analysis of wear and bearing surfaces
Wear measurement was carried out on a coordinate-measuring machine (CMM5, SIP, Geneva, Switzerland) with a spatial resolution of <1 μm [20] for explants originated from patient 2. The precision of the wear measurements was estimated to be ± 2 μm. The articulating surface of the Metasul® head was inspected under the microscope at 200× magnification with differential interference contrast (DIC).
Results
Histological findings
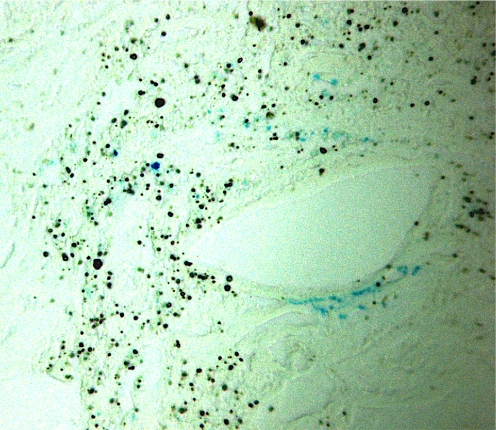
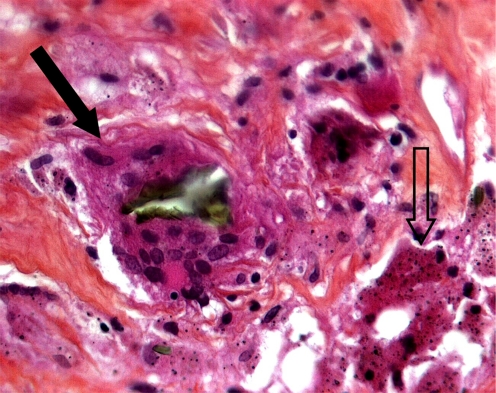
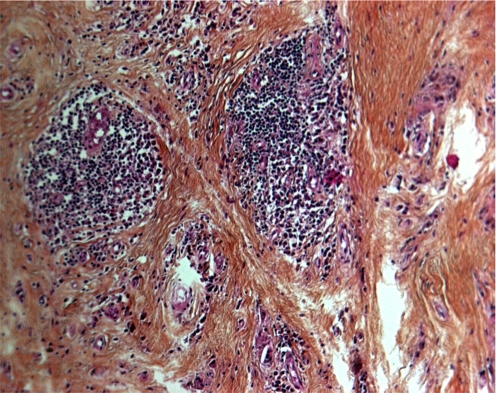
There was slight evidence of metallosis in the neocapsule and the periprosthetic tissues. Microscopic metallic debris <1-μm diameter was identified in all tissues samples. Metallosis was graded 1+ according to the modified Mirra classification [3]. The metal particles mostly appeared as debris in the cytoplasm of histiocytes and macrophages and sometimes as extracellular metal deposits (Fig. 2). The foreign-body reaction to metal-wear particles was rated as “many” in both hips. These findings corresponded to a relatively mild foreign-body reaction. Tissue examination also showed small drop-shaped inclusions within the cytoplasm of the macrophages. Most of them were negative for iron, at least in their centres, after Prussian blue staining. Their size appeared two- to tenfold greater than the microscopic metallic debris. Polyethylene particles were few and always appeared in the cytoplasm of foreign-body giant cells (Fig. 3). Necrosis and ulcerations, characterised by loss of the pseudosynovial lining and fibrin deposition, commonly covered the surface of the neocapsules. All retrieved neocapsule tissues showed a more or less distinct degree of lymphocytic infiltration, sometimes associated with plasma cells. Lymphocytes were either diffusely distributed in the inner capsule layer or concentrated around postcapillary venules in the intermediate vascular layer (Fig. 4). According to Willert [26, 27], perivascular lymphocytic infiltrates were rated as “many” in all analysed tissues. Histological features reported were compatible with the hypersensitivity-like reaction previously described [27].
Fig. 2.
Histological sections of the neocapsule (Prussian blue, ×40). Numerous drop-shaped particles and microscopic debris are seen in the neocapsule, most of which were negative for iron, at least in their centres; some particles stained positive for iron
Fig. 3.
Histological section of the periprosthetic tissues (haematoxylin and eosin ×40) showing metallic particulate debris in the cytoplasm of histiocytes and macrophages (open arrow). Of note: polyethylene particle phagocytised by a giant cell (black arrow)
Fig. 4.
Histology of periarticular soft tissue neocapsule (haematoxylin and eosin ×40) demonstrating vasculitis and dense perivascular cuffs of lymphocytes and perivascular cuff of mature plasma cells
Analysis of wear and bearing surfaces
The articulating surface of the Metasul® inlay exhibited numerous fine scratches. Almost the entire surface of the Metasul® head revealed scratches. In the loaded area, the carbides that protruded slightly in the original surface state were levelled (Fig. 5). There were only two small unloaded areas where the carbides were still visible.
Fig. 5.
Articulation surface of the Metasul® head after approximately 8 years in vivo (loaded bearing area, pole)
The total linear wear value was 15 μm for the head, which gave a yearly wear rate of 1.9 μm. The deviations found for the cup were so small that no clear wear zone could be identified. Therefore, it was not possible to determine a specific wear value for the articulation surface of the inlay.
Discussion
One of the authors previously reported that small-diameter cemented Metasul® acetabular components were exposed to an increased risk of early loosening after observing unusual progressive periacetabular radiolucencies [19]. At the time of the initial review, no cup was found to be loose, and no hip had been revised for aseptic loosening of either component at a mean follow up of 31 months. We, and other authors [12], hypothesised that mechanical stress at the bone–cement interface was increased in small-diameter acetabular components. This feature appeared as a consequence of a thinner PE layer around the metallic surface. To the best of our knowledge, this study is the first to report a retrieval analysis of cemented Metasul® acetabular components after aseptic failure.
The findings we report in this work are characterised by the presence of metallic wear debris, a few PE particles phagocytosed by foreign-body giant cells and diffuse and perivascular lymphocytic infiltrates and plasma cells. Also, the wear rates measured should be considered low (1.9 μm/year), since retrievals explanted after two or more years in vivo reportedly had an average wear rate of 6.2 μm/year [22]. In agreement with data reported by other authors [1, 3, 8, 10, 13, 27], these results support the idea that metallic particulate debris released from the bearing couple initiated a specific immunological reaction similar to type IV delayed hypersensitivity. It is speculated that metallic particles in the inframicrometric range were phagocytised by histiocytes and macrophages, initiating sensitisation. Willert and Semlitsch [26] described infiltrates of lymphocytes and plasma cells in tissues retrieved from hips with contemporary MOM prostheses that had failed at an average of 30 months after implantation. In contrast, infiltrates of such cells have not been described in tissues retrieved from failed metal-on-polyethylene or ceramic-on-ceramic total hip arthroplasties. A relationship has been established between debris release from the all-metal cobalt–chromium articulation and the diagnosis of hypersensitivity reaction, described as an aseptic lymphocyte-dominated vasculitis-associated lesion (ALVAL). Such changes have been observed in both total hip replacements and hip surface replacements using metallic bearing surfaces [13]. In addition, it has been suggested that histological changes of surface ulceration and lymphocytic perivascular cuffs were more commonly seen in aseptic loosening after contemporary MOM replacement [1].
However, it is still unclear whether this reaction was responsible for acetabular component failure. The observations by Willert et al. [27], and Korovessis et al. [10] support the hypothesis that hypersensitivity to the cobalt–chromium metal of the articulation is involved in the pathogenesis of periprosthetic osteolysis and subsequent aseptic loosening. However, ALVAL has also been described in periprosthetic tissues surrounding well-fixed implants with a contemporary MOM-bearing couple [17]. Conversely, high rates of periacetabular osteolysis have been reported in a recent series of MOM hip replacements, with no argument in favour of tissue reaction to metallic debris [7]. In contrast to with previous studies [10, 27], other authors failed to correlate the presence of lymphocyte aggregates and the appearance of osteolysis [17]. Thus, it has been suggested that distinct lymphocytic infiltration should be considered as a characteristic histological pattern of tissue reactions on metal particles and/or ions around MOM bearings, not necessarily associated with implant failure [28]. Last, it should be noted that no massive foreign-body reaction was observed in the explants in our analysis. Therefore, we cannot definitively conclude that ALVAL, although present, generated aseptic loosening of the cemented cups in these cases.
High mechanical stress has been regarded as a potential cause of cemented acetabular component failure in hip replacements using a hard-on-hard bearing [6, 18]. In our study, only the acetabular side of the replacement was loose, and no osteolysis surrounded the femoral component, indicating a local problem rather than a problem affecting the entire hip replacement. Not all authors noted a high rate of progressive radiolucencies around cemented acetabular components containing a metallic-bearing surface. Eswaramoorthy et al. [4] employed a cemented Stuehmer-Weber cup with a Metasul® bearing in half of 104 primary MOM total hip replacements. These authors did not report alarming periacetabular changes. However, the cups’ mean diameter used in that study was somewhat larger than in our previous work, and thus, mechanical forces about the cemented cups may have been different when compared with our study. In line with our observations, early failure of small-diameter uncemented Metasul® cups have also been reported, with acetabular component diameter <49 mm identified at high risk of loosening [14].
We do acknowledge that this study has some limitations. First, we did not perform hypersensitivity tests (e.g. the patch test) or serum metallic ion (cobalt–chromium) levels. However, the significance of these tests is still debated in the literature [2, 9, 11]. Then, we did not compare our histological findings with tissues retrieved from well-fixed implants containing an MOM articulation. However, our findings are consistent with previous observations [27] and support the idea that a type IV hypersensitivity consistently occurred in these cases. We found that a hypersensitivity-like reaction, described as ALVAL, was associated with aseptic loosening of cemented Metasul® acetabular components as a result of continuous release of metal ions from the MOM articulation. Taken in combination with our previous clinical data, we hypothesise that histological findings in these two cases were involved in acetabular component failure, although mechanical stress may have initiated loosening.
Acknowledgments
The authors thank Dr. C. Rieker (Zimmer, Winterthur, Switzerland) for providing component wear measurement and explant surface analysis, Pr. T.S. Vinh (Service de Chirurgie Orthopédique B, Hôpital Cochin) for his case contribution, and Mr. V. Myrtille (Laboratoire de Biomécanique et Biomatériaux Ostéo-Articulaires) for histological analysis.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Davies AP, Willert HG, Campbell PA, Learmonth ID, Case CP. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J Bone Joint Surg Am. 2005;87:18–27. doi: 10.2106/JBJS.C.00949. [DOI] [PubMed] [Google Scholar]
- 2.Delaunay CP. Metal-on-Metal bearings in cementless primary total hip arthroplasty. J Arthroplasty. 2004;19(Suppl 1):35–40. doi: 10.1016/j.arth.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Doorn PF, Mirra JM, Campbell PA, Amstutz HC. Tissue reaction to metal on metal total hip prostheses. Clin Orthop Relat Res. 1996;329(Suppl):187–205. doi: 10.1097/00003086-199608001-00017. [DOI] [PubMed] [Google Scholar]
- 4.Eswaramoorthy V, Moonot P, Kalairajah Y, Biant LC, Field RE. The Metasul metal-on-metal articulation in primary total hip replacement: clinical and radiological results at ten years. J Bone Joint Surg Br. 2008;90:1278–1283. doi: 10.1302/0301-620X.90B10.20378. [DOI] [PubMed] [Google Scholar]
- 5.Grübl A, Marker M, Brodner W, Giurea A, Heinze G, Meisinger V, Zehetgruber H, Kotz R. Long-term follow-up of metal-on-metal total hip replacement. J Orthop Res. 2007;25:841–848. doi: 10.1002/jor.20381. [DOI] [PubMed] [Google Scholar]
- 6.Hamadouche M, Boutin P, Daussange J, Bolander ME, Sedel L. Alumina-on-alumina total hip arthroplasty: a minimum 18.5-year follow-up study. J Bone Joint Surg Am. 2002;84:69–77. [PubMed] [Google Scholar]
- 7.Holloway I, Walter WL, Zicat B, Walter WK. Osteolysis with a cementless second generation metal-on-metal cup in total hip replacement. Int Orthop. 2009;33:1537–1542. doi: 10.1007/s00264-008-0679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huber M, Reinisch G, Trettenhahn G, Zweymüller K, Lintner F. Presence of corrosion products and hypersensitivity-associated reactions in periprosthetic tissue after aseptic loosening of total hip replacements with metal bearing surfaces. Acta Biomater. 2009;5:172–180. doi: 10.1016/j.actbio.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs JJ, Urban RM, Hallab NJ, Skipor AK, Fischer A, Wimmer MA. Metal-on-metal bearing surfaces. J Am Acad Orthop Surg. 2009;17:69–76. doi: 10.5435/00124635-200902000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Korovessis P, Petsinis G, Repanti M, Repantis T. Metallosis after contemporary metal-on-metal total hip arthroplasty. J Bone Joint Surg Am. 2006;88:1183–1191. doi: 10.2106/JBJS.D.02916. [DOI] [PubMed] [Google Scholar]
- 11.Lazennec JY, Boyer P, Poupon J, Rousseau MA, Roy C, Ravaud P, Catonné Y. Outcome and serum ion determination up to 11 years after implantation of a cemented metal-on-metal hip prosthesis. Acta Orthop. 2009;80:168–173. doi: 10.3109/17453670902947408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levai JP, Descamps S, Roch G, Boisgard S. Early aseptic loosening of cemented cup in metal-on-metal total hip arthroplasties. Rev Chir Orthop Reparatrice Appar Mot. 2006;92:575–580. doi: 10.1016/s0035-1040(06)75915-3. [DOI] [PubMed] [Google Scholar]
- 13.Mabilleau G, Kwon YM, Pandit H, Murray DW, Sabokbar A. Metal-on-metal hip resurfacing arthroplasty: a review of periprosthetic biological reactions. Acta Orthop. 2008;79:734–747. doi: 10.1080/17453670810016795. [DOI] [PubMed] [Google Scholar]
- 14.Maezawa K, Nozawa M, Matsuda K, Yuasa T, Shitoto K, Kurosawa H. Early failure of modern metal-on-metal total hip arthroplasty using a Wagner standard cup. J Arthroplasty. 2006;21:522–526. doi: 10.1016/j.arth.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 15.McKellop H, Park SH, Chiesa R, Doorn P, Lu B, Normand P, Grigoris P, Amstutz H. In vivo wear of three types of metal on metal hip prostheses during two decades of use. Clin Orthop Relat Res. 1996;329(Suppl):128–140. doi: 10.1097/00003086-199608001-00013. [DOI] [PubMed] [Google Scholar]
- 16.Merle d’Aubigné RM, Postel M. Functional results of hip arthroplasty with acrylic prostheses. J Bone Joint Surg Am. 1954;36:451–457. [PubMed] [Google Scholar]
- 17.Milosev I, Trebse R, Kovac S, Cör A, Pisot V. Survivorship and retrieval analysis of Sikomet metal-on-metal total hip replacements at a mean of seven years. J Bone Joint Surg Am. 2006;88:1173–1182. doi: 10.2106/JBJS.E.00604. [DOI] [PubMed] [Google Scholar]
- 18.Nich C, Sariali el-H, Hannouche D, Nizard R, Witvoet J, Sedel L, Bizot P. Long-term results of alumina-on-alumina hip arthroplasty for osteonecrosis. Clin Orthop Relat Res. 2003;417:102–111. doi: 10.1097/01.blo.0000096820.67494.bf. [DOI] [PubMed] [Google Scholar]
- 19.Nich C, Rampal V, Vandenbussche E, Augereau B. Metal-metal-backed polyethylene cemented hip arthroplasty: mid-term results. Rev Chir Orthop Reparatrice Appar Mot. 2006;92:118–124. doi: 10.1016/s0035-1040(06)75696-3. [DOI] [PubMed] [Google Scholar]
- 20.Rieker C, Shen M, Köttig P. In-vivo tribological performance of 177 metal-on-metal hip articulations. In: Rieker C, Oberholzer S, Wyss U, editors. World tribology forum in arthroplasty. Bern: Hans Huber; 2001. pp. 137–142. [Google Scholar]
- 21.Rieker CB, Schön R, Köttig P. Development and validation of a second-generation metal-on-metal bearing: laboratory studies and analysis of retrievals. J Arthroplasty. 2004;19(Suppl 3):5–11. doi: 10.1016/j.arth.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Schmalzried TP, Peters PC, Maurer BT, Bragdon CR, Harris WH. Long-duration metal-on-metal total hip arthroplasties with low wear of the articulating surfaces. J Arthroplasty. 1996;11:322–331. doi: 10.1016/S0883-5403(96)80085-4. [DOI] [PubMed] [Google Scholar]
- 23.Sieber HP, Rieker CB, Köttig P. Analysis of 118 second-generation metal-on-metal retrieved hip implants. J Bone Joint Surg Br. 1999;81:46–50. doi: 10.1302/0301-620X.81B1.9047. [DOI] [PubMed] [Google Scholar]
- 24.Streicher RM, Semlitsch M, Schön R, Weber H, Rieker C. Metal-on-metal articulation for artificial hip joints: laboratory study and clinical results. Proc Inst Mech Eng H. 1996;210:223–232. doi: 10.1243/PIME_PROC_1996_210_416_02. [DOI] [PubMed] [Google Scholar]
- 25.Weber BG. Experience with the Metasul total hip bearing system. Clin Orthop Relat Res. 1996;329(Suppl):569–577. doi: 10.1097/00003086-199608001-00007. [DOI] [PubMed] [Google Scholar]
- 26.Willert HG, Semlitsch M. Tissue reactions to plastic and metallic wear products of joint endoprostheses. Clin Orthop Relat Res. 1996;333:4–14. [PubMed] [Google Scholar]
- 27.Willert HG, Buchorn GH, Fayyazi A, Flury R, Windler M, Koster G, et al. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87:28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 28.Witzleb WC, Hanisch U, Kolar N, Krummenauer F, Guenther KP. Neo-capsule tissue reactions in metal-on-metal hip arthroplasty. Acta Orthop. 2007;78:211–220. doi: 10.1080/17453670710013708. [DOI] [PubMed] [Google Scholar]