Abstract
Deep-vein thrombosis (DVT) and pulmonary embolism (PE) represent life-threatening postoperative complications frequently responsible for in-hospital mortality following total knee arthroplasty (TKA). Mechanical prophylaxis in the form of a foot pump offers an alternative to pharmacological and physical therapy. The aim of this prospective and randomised study was to examine the clinical efficacy of the A-V Impulse (AVI) system in reduction of soft-tissue swelling of the lower limb following a TKA. A total of 80 patients undergoing cemented TKA between September 2005 and December 2006 were randomised into two groups of 40 patients (n¹ = 40, n² = 40) during the 16-month study period. All patients received a subcutaneous dose of low molecular weight heparin (LMWH) (Enoxaparin/Clexane® 40 mg) once daily beginning 24 hours prior to the operation. The mean age for the groups n¹ and n² were 68.93 and 68.15 years, respectively. The reduction of soft-tissue swelling in the n¹ group was significantly higher (p < 0.05) compared with n². Evaluation of body mass index (BMI) with regard to the average reduction of soft-tissue swelling showed no significant influence (p < 0.05). The better function of the operated knee in group AVI was a significant predictor for improved agility and mobility (p < 0.01). No complications were reported for the application of the AVI. No ultrasonographic evidence of DVT or PE was found in any of the 80 patients during the investigative time period of eight days. After three months, there was no evidence of a symptomatic DVT.
Introduction
The rates of deep-vein thrombosis (DVT), asymptomatic pulmonary embolism (PE) and fatal PE after total knee arthroplasty (TKA) in the absence of any prophylaxis range from 0% to 84% [4, 22]. The incidence of postoperative soft-tissue swelling is another common problem associated with TKA. Mechanical prophylaxis in the form of a foot pump, a device that exerts external pneumatic compression on the foot sole [A-V Impulse (AVI) System™, Novamedix Services Limited, Walworth-Andover, England] [1, 3, 6, 10, 19, 25] offers an alternative to pharmacological and physical therapy, such as compression stockings, and facilitates early postoperative patient mobilisation. Using the AVI system, Gardner and Fox demonstrated a positive effect on the microcirculatory physiology of the leg [5, 23]. Several clinical studies have demonstrated the effectiveness of intermittent impulse compression in cases of skeletal trauma and total hip replacement, both as a stand-alone procedure or in synergy with heparin (add-on therapy) [8, 10, 11, 16–18, 21].
This prospective and randomised study advances the hypothesis that due application of the AVI system leads to a significant reduction of postoperative soft-tissue swelling in comparison with treatment were foot-pump facilities are absent [17]. In addition, our study addresses the issue of whether application of the AVI system influences the incidence of venous thrombosis following elective unilateral TKA.
Methods
The study was designed according to Consolidated Standards of Reporting Trials (CONSORT) policy. Though in our hospital the average number of patients undergoing total knee replacement (TKR) is about 600 annually, only 80 patients agreed to be part of our study. Between September 2005 and December 2006, 80 patients were randomised to two groups of 40 patients (n¹ = 40, n² = 40), of which group 1 was selected for treatment with the AVI system. Surgery on all patients with a primary diagnosis of knee arthritis was performed in the same hospital as the cemented TKA procedure by means of a straight ventral skin incision and an anteromedial capsule incision. All patients were operated upon under general anaesthesia. Exclusion criteria for the study included patients aged younger than 60 years, body mass index (BMI) > 40 (massive adiposis) or <25 (underweight), existing acute DVT, thrombophlebitic varicosis (stages II–IV acc. Marshall), as well as venous insufficiency (stages 2–3 acc. Widmer). Although the operations were performed by more than one surgeon, the same operational technique was used. A tourniquet was always applied to stem blood flow.
According to our standard venous thromboembolism prevention, all patients received a subcutaneous dose of low molecular weight heparin (LMWH) (Enoxaparin/Clexane® 40 mg) once daily beginning 24 hours prior to the operation. In addition, all patients were fitted with thigh-length anti-embolic or compression stockings. The AVI system was attached in the recovery room to both feet of group n¹ patients only shortly after completion of the operation. The use of the device was aimed to continue for 24 hours per day. The AVI system consists of two inflatable pneumatic pads that wrap around both feet. An air chamber incorporated in the pad is inflated at intermittent intervals with compressed air. To optimise device function, the legs were positioned lower than the body at an angle of 25° in the reverse Trendelburg position. The patients in group n¹ were free to discontinue the use of the AVI system at will. Circumference of the leg at different prespecified points, range of knee motion, postoperative and patient compliance (duration of application of AVI system in hours per day) were the parameters measured for both groups beginning from day one preoperative to day eight postoperative. Leg circumference was determined by measuring the distance between prespecified points on the leg with a tape measure. These points were previously marked using a permanent ink marker pen on the thigh, knee joint, lower leg and both feet. Using this protocol, repeatable and accurate measurements of up to ±1 mm could be achieved. These measurements were performed at 20 cm above the knee to the inside knee joint line, at 10 cm above the knee to the inside knee joint line, at circumference of the knee centre, at 15 cm under the knee to the inside knee joint line, at minimum circumference of calf or lower leg, at circumference of ankles, at instep and at the ball of the foot daily at 08.00 hours Central European Time (CET) and documented from preoperative day one. For all measurements, values for soft-tissue swelling are defined by the difference between measurements on day one and day eight.
Using a goniometer, daily measurements of the postoperative mobility of the knee joint were undertaken. Patient mobilisation with 50% partial weightbearing began on the second postoperative day and was continued up to the sixth postoperative week. During the period of mobilisation, the AVI system was removed. A recording of patient compliance with regard to the period of AVI system application was undertaken on a daily basis at 08.00 hours CET.
In order to preclude DVT, an ultrasound screening test was performed in both groups by the same internist on day eight. For the duplex ultrasound examinations, a Toshiba SSA-660 A (Xario) 4D ultrasound system was used. The ultrasonographs were interpreted on a blinded basis by a single physician unaware of the thromboprophylactic regime. A DVT event would also be acceptable when relevant clinical symptoms of pulmonary arterial embolism (PAE) in the lung were confirmed using scintigraphy or thoracic computed tomography (CT) [2]. Follow-up on an outpatient basis was performed at three months to exclude a thrombotic event.
Statistical evaluation was carried out using SPSS 17.0 software (Statistical Package for Social Sciences, SPSS Inc. 2008). As it could not be established whether data from this study came from the family of normal distributions, nonparametric tests of significance were used at chosen significance level of α = 0.05. To conclude whether or not data were consistent with the null hypothesis that the two patient groups came from the same distribution, we compared α with the critical or p level attained, which is the smallest significance level at which the null hypothesis would be rejected for a given set of observations. Thus, is the probability of a test statistic arising that was as or more extreme than that observed if the null hypothesis were true. The lower the attained p level in comparison with the chosen α, the stronger the evidence against the null hypothesis, indicating the power of the test to detect a false null hypothesis. A p level greater than α indicated failure to reject the null hypothesis. Preoperative data were tested for homogeneity by the Mann–Whitney U test. This test was also used for evaluating soft-tissue swelling at prespecified measuring points as well as for a possible difference in average flexion of the operated knee joint between the two patient groups. Group sizes n¹ = n² = 40 were chosen to ensure an excellent approximation of the Mann–Whitney test statistic to a normal distribution if the null hypothesis was true, and also to detect a significant difference in soft-tissue swelling if there was one. The Kruskal–Wallis test was used to test for a significant influence of BMI on soft-tissue swelling at the prespecified measuring points in three equally sized BMI groups. The correlation between the parameter BMI versus the value for a decrease in soft-tissue swelling for both patient groups was evaluated according to Pearson/Bravais.
Results
Eighty patients were included during the 16-month study period. Mean ages for the n¹ and n² groups were 68.93 and 68.15, respectively, and BMI values were 31.32 and 31.48, respectively. The tourniquet was kept on the lower limb during the operation for an average of 80.13 min (range 53–129 min) in the n¹ group and 80.68 min (range 40–156 min) in the n² group. Since no normal distribution could be established for either group, homogeneity was assessed by the Mann–Whitney U test. At the 0.05 level, no significant difference was found between the two groups. Thus, it was assumed that both groups were homogenous preoperatively. Significant differences in soft-tissue swelling for the thigh region as well as the centre of the knee joint were demonstrated postoperatively for the operated limbs in both groups. The mean reduction of soft-tissue swelling for those variables in group n¹ was significantly higher compared with n² (p < 0.05). Accordingly, the improved reduction in swelling for both the thigh region and the centre of the knee joint were statistically confirmed in group n¹. For variables in the lower-leg region (15 cm below the knee joint line and the ball of the foot), values for a reduction in swelling were very close, and no significant difference was established for these variables (Table 1).
Table 1.
Mean reduction of soft-tissue swelling (cm) from the measuring point 20 cm above the knee joint line to the point on the ball of the foot for group n¹ [A-V Impulse (AVI) system] and the control group n² (non-AVI)
| Point of measurement | Group n¹ (AVI) mean value | Group n² (non-AVI) mean value | P value |
|---|---|---|---|
| 20 cm above knee joint line | 2.013 | 1.650 | p < 0.05 |
| 10 cm above knee joint line | 1.563 | 1.450 | p < 0.05 |
| Knee centre | 2.438 | 1.575 | p < 0.05 |
| 15 cm below knee joint line | 0.713 | 0.825 | p > 0.05 |
| Smallest circumference, lower leg | 0.650 | 0.775 | p > 0.05 |
| Ankle | 0.438 | 0.425 | p > 0.05 |
| Instep | 0.188 | 0.300 | p > 0.05 |
| Ball of the foot | 0.138 | 0.250 | p > 0.05 |
The average BMI values were 31.32 and 31.48 for groups n¹ and n², respectively. The distribution function for BMI was calculated for 100% of the patients and classified in three equally sized BMI groups (<30, 30–33, >33). Evaluation of the BMI with regard to the average reduction of soft-tissue swelling for the defined measuring points using the Kruskal–Wallis test showed no significant influence of BMI on soft-tissue swelling at a significance level of 0.05 for group n¹ (Table 2). In contrast, the results for group n² showed a significant influence of each variable 10 cm above the inner knee joint line and at the knee centre.
Table 2.
Evaluation of body mass index (BMI) with regard to the average reduction of soft-tissue swelling (cm) according to Kruskal–Wallis test for group n¹ [ball of foot preoperatively is for the BMI groups A-V Impulse (AVI) system; BMI was constant at >33 and is omitted here] and for group n² (ball of foot preoperatively is for the BMI groups non-AVI, 30 < BMI < 33 is constant and is omitted here)
| Average reduction of soft-tissue swelling cm | Group | 20 cm above knee joint line | 10 cm above knee joint line | Knee centre | 15 cm below knee joint line | Smallest circumference lower leg | Ankle | Instep | Ball of the foot |
|---|---|---|---|---|---|---|---|---|---|
| BMI < 30 | n¹ | 2.385 | 1.885 | 2.538 | 0.346 | 0.615 | 0.769 | 0.385 | 0.385 |
| n² | 2.000 | 2.111 | 2.000 | 0.611 | 0.667 | 0.556 | 0.556 | 0.111 | |
| 30 < BMI < 33 | n¹ | 1.607 | 1.214 | 2.107 | 1.036 | 0.714 | 0.321 | 0.036 | 0.036 |
| n² | 1.800 | 0.900 | 0.900 | 1.000 | 1.000 | 0.300 | ─ | 0.500 | |
| BMI > 33 | n¹ | 2.077 | 1.615 | 2.692 | 0.731 | 0.615 | 0.231 | 0.154 | ─ |
| n² | 1.000 | 0.917 | 1.500 | 1.000 | 0.750 | 0.333 | 0.167 | 0.250 | |
| P value | n¹ | 0.218 | 0.116 | 0.727 | 0.293 | 1.000 | 0.320 | 0.204 | 0.040 |
| n² | 0.117 | 0.001 | 0.038 | 0.450 | 0.515 | 0.568 | 0.094 | 0.588 |
In addition, the correlation between BMI and reduction of soft-tissue swelling was determined by Pearson/Bravais correlation coefficients R to be significant for both patient groups 20 cm above the knee joint line, 10 cm above the knee joint line and at the knee centre (Table 3). Results in group n¹ showed that here the BMI did not correlate with a reduction in soft-tissue swelling. In contrast, a negative correlation was found between the BMI and a reduction in soft-tissue swelling at the three points mentioned above. A higher BMI was associated with a comparatively weaker reduction in soft-tissue swelling.
Table 3.
Correlation of body mass index (BMI) versus reduction of soft-tissue swelling in groups n¹ [A-V Impulse (AVI) system] and n² (non-AVI)
| Point of measurement | 20 cm above knee joint line | 10 cm above knee joint line | Knee centre |
|---|---|---|---|
| Group n¹ Pearson -R | -0.023 | -0.166 | 0.031 |
| Group n² Pearson -R | -0.516 | -0.501 | -0.273 |
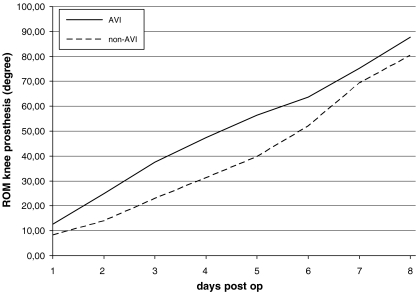
The improved and rapid flexion of the operated knee joint in the AVI group in contrast to the control group was a highly significant predictor for improved agility and mobility (p < 0.01) for the AVI- roup (Fig. 1).
Fig. 1.
Passive mobility of the operated knee joint after total knee arthroplasty (TKA) in degrees of flexion. Depiction of groups n¹ [A-V Impulse (AVI) system and n² (non-AVI)]. ROM range of motion
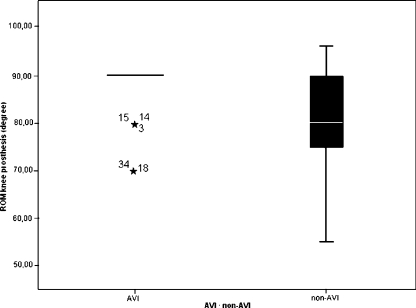
The box plot (Fig. 2) shows homogeneous mobility values of flexion at the operated knee joint for the majority of patients in group n¹. The control group (n²) demonstrated relatively strong fluctuations between a minimum of 55° and a maximum of 95° flexion of knee joint mobility.
Fig. 2.
Range of motion (ROM) in degree of knee flexion following total knee arthroplasty (TKA) in groups n¹ [A-V Impulse (AVI) system and n² (non-AVI)]
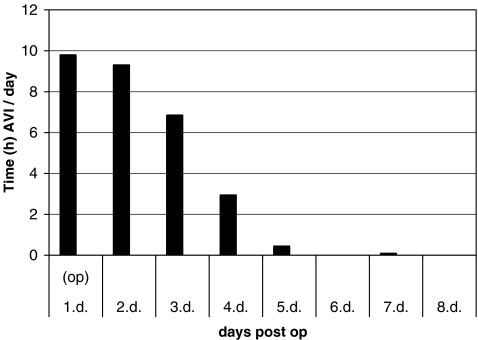
The longest effective average daily application of the AVI system was 9.79 hours on day one of the operation and 9.30 hours on day two, the first day postoperation. The best compliance for the AVI system was achieved between day one and day four (Fig. 3), thereafter compliance gradually decreased.
Fig. 3.
Average daily application of the A-V Impulse (AVI) System™ for group n¹ measured in hours (h)
No complications were reported for the application of the AVI system, and none of the patients needed to be operated upon for haemarthrosis. Moreover, no ultrasonographic evidence of DVT or PE was found in any of the 80 patients during the investigative time period of eight days. The clinical evaluation at three months showed no evidence of symptomatic DVT.
Discussion
Our findings confirm the effectiveness of the AVI system in combination with LMWH, in particular, as add-on therapy to facilitate rapid mobilisation of patients following TKA. No evidence of a negative impact of the AVI system on DVT was found. This rapid mobilisation is possible due to a reduction of postoperative soft-tissue swelling in the region of the thigh and knee centre compared with the control group. Similar results were obtained in a study by Westrich and Sculco, who also showed significant reduction in soft-tissue swelling in the lower and upper leg regions following a unilateral TKA and application of a foot compression system in comparison with their control group [24].
The mobility values of knee flexion in group n¹ showed highly significant homogeneity compared with the control group. In addition, BMI for group n¹ did not correlate with the reduction values in soft-tissue swelling. In contrast, for group n², a negative correlation of BMI with reduction of soft-tissue swelling was recorded for measurements taken at 20 cm and 10 cm above the knee joint line as well as at the knee centre. Thus, a higher BMI was associated with a comparatively weaker reduction in soft-tissue swelling. Due to the effect of BMI on soft-tissue swelling, this negative correlation can be attributed to the AVI system manufacturer’s recommended usage of medical compression stockings. The extremely tight compression stockings could, in fact, lead to a decrease of the venous blood velocity that is generated by the pneumatic compression device. A study by Higgs et al. demonstrated a negative influence on the venous flow velocity upon application of the AVI system in combination with compressions stockings compared with treatment with just the foot device [9]. Similarly, a further study by Warwick et al. showed a reduction in the venous peak velocity in the popliteal vein with the simultaneous use of compression stockings and foot pump [20]. In a study by Pitto and Young involving total hip and knee replacement, the combined use of a foot pump and compression stockings compared with sole deployment of the foot pump revealed no reduction in the effectiveness of thromboprophylaxis in the group without compression stockings. In fact, there was an improvement in patient compliance in this group [15]. Generally, however, the manufacturer of pneumatic compression devices (Novamedix) recommends the use of medical compression stockings in combination with the foot device.
Regarding compliance, the best compliance for the AVI system was achieved between day one and the third postoperative day. A significant reduction in patient compliance was documented thereafter. We assume that reduced patient compliance after day three was associated with an improvement in patient mobility, which was due to the application of the foot device.
The incidence of venous thrombosis following unilateral TKA was demonstrated in 86% of patients during the first 24 hours by Maynard et al. [14] by means of early and late postoperative phlebography. Thus, additional use of the AVI system for the management of thromboprophylaxis in patients immobilised during the first postoperative days following TKA should be undertaken promptly.
It has been presumed that the highest incidence for DVT occurs around the eighth postoperative day [13]. On ultrasound examination, no evidence of DVT incidence at eight days was found for the AVI or control group in our study. However, Doppler ultrasonography examination is not the best means of testing for evidence of all thrombotic events following a TKA [7]. On the other hand, Kraay et al. showed the applicability of Doppler ultrasound examinations for the identification of asymptomatic thrombosis following TKA [12]. A study by Colwell et al. compared a mobile compression device for prophylaxis against venous thromboembolic events following total hip arthroplasty with LMWH [2]. In that study, patients also underwent bilateral ultrasound examinations to screen for thrombotic events. The authors demonstrated that the rates of venous thromboembolic events were similar in both groups. They also reported that the use of the mobile compression device resulted in a significant decrease in major bleeding events. In our study, a physician with more than ten years of experience performed the ultrasound examinations under blinded conditions. None of the patients demonstrated a DVT three months postoperation.
In conclusion, this study demonstrates the safety and versatility of the AVI system in combination with the use of compression stockings and LMWH following elective unilateral TKA. The foot device is easy to apply and is not influenced by postoperative immobility of the patient. Finally, intermittent impulse compression is associated with reduced soft-tissue swelling that, in turn, leads to early patient mobilisation and, thus, to a shorter stay in hospital or the rehabilitation unit, resulting in lower treatment costs.
References
- 1.Asano H, Matsubara M, Suzuki K, et al. Prevention of pulmonary embolism by a foot sole pump. J Bone Joint Surg Br. 2001;83:1130–1132. doi: 10.1302/0301-620X.83B8.11938. [DOI] [PubMed] [Google Scholar]
- 2.Colwell CW, Jr, Froimson MI, Mont MA, Ritter MA, Trousdale RT, Buehler KC, Spitzer A, Donaldson TK, Padgett DE. Thombosis prevention after total hip Arthroplasty: a prospective, randomized trial comparing a mobile compression device with low-molecular-weight heparin. J Bone Joint Surg Am. 2010;92:527–535. doi: 10.2106/JBJS.I.00047. [DOI] [PubMed] [Google Scholar]
- 3.Eisele R, Kinzl L, Koelsch T. Rapid-inflation intermittent pneumatic compression for prevention of deep venous thrombosis. J Bone Joint Surg Am. 2007;89:1050–1056. doi: 10.2106/JBJS.E.00434. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgerald RH, Jr, Spiro TE, Trowbridge AA, Gardiner GA, Jr, Whitsett TL, O’Connell MB, Ohar JA, Young TR. Prevention of venous thromboembolic disease following primary total knee arthroplasty. A randomized, multicenter, open-label, parallel-group comparison of enoxaparin and warfarin. J Bone Joint Surg Am. 2001;83:900–906. [PubMed] [Google Scholar]
- 5.Gardner AM, Fox RH. The venous pump of the human foot: preliminary report. Bristol Med Chir J. 1983;98:109–112. [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner AM, Fox RH, Lawrence C, Bunker TD, Ling RS, MacEachern AG. Reduction of post-traumatic swelling and compartement pressure by impulse compression of the foot. J Bone Joint Surg Br. 1990;72:810–815. doi: 10.1302/0301-620X.72B5.2211762. [DOI] [PubMed] [Google Scholar]
- 7.Grady-Benson JC, Oishi CS, Hannson PB, et al. Postoperative surveillance for deep venous thrombosis with duplex ultrasound after total knee arthroplasty. J Bone Joint Surg Am. 1994;76A:1649–1657. doi: 10.2106/00004623-199411000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Haas S. Thrombosis in trauma and orthopedic surgery. Prevention, diagnosis, therapy. Unfallchirurg. 2004;100(4):307–319. [PubMed] [Google Scholar]
- 9.Higgs D, Uglow M, Fail T. Graduated compression stockings reduce the venous velocity augmentation of foot pumps. J R Nav Med Serv. 2004;90:142–146. [PubMed] [Google Scholar]
- 10.Hooker JA, Lachiewicz PF, Kelley SS. Efficacy of prophylaxis against thromboembolism with intermittend pneumatic compression after primary and revision total hip arthroplasty. J Bone Joint Surg Am. 1999;81:690–696. doi: 10.2106/00004623-199905000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Ivanic G, Moser I, Homann NC, et al. Die intermittierende Impulskompression zur Abschwellung und Thromboseprophylaxe – Pilotstudie nach Hüft-TEP: Ist die Minimalanwendungsdauer von 2 h täglich ausreichend? Unfallchirurg. 2006;109(9):786–792. doi: 10.1007/s00113-006-1140-3. [DOI] [PubMed] [Google Scholar]
- 12.Kraay MJ, Goldberg VM, Herbener TE. Vascular ultrasonography for deep venous ultrasonography for deep venous thrombosis after total knee arthroplasty. Clin Orthop. 1993;286:18–26. [PubMed] [Google Scholar]
- 13.Lachiewicz PF, Kelley SS, Haden RN. Two mechanical devices for prophylaxis of thromboembolism after total knee arthroplasty. Aprospective, randomised study. J Bone Joint Surg Br. 2004;86:1137–1141. doi: 10.1302/0301-620X.86B8.15438. [DOI] [PubMed] [Google Scholar]
- 14.Maynard MJ, Sculco TP, Ghelman B. Progression and regression of deep venous thrombosis after total knee arthroplasty. Clin Orthop. 1991;273:125–130. [PubMed] [Google Scholar]
- 15.Pitto RP, Young S. Foot pumps without graduated compression stockings for prevention of deep-vein thrombosis in total joint replacement: efficacy, safty and patient compliance. Int Orthop. 2008;32:331–336. doi: 10.1007/s00264-007-0326-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitto RR, Hamer H, Heiss-Dunlop W, et al. Mechanical prophylaxis of deep-vein thrombosis after total hip replacement: a randomised clinical trial. J Bone Joint Surg Br. 2004;86:639–642. doi: 10.1302/0301-620X.86B5.14763. [DOI] [PubMed] [Google Scholar]
- 17.Santori FS, Vitullo A, Stopponi M, et al. Prophylaxis against deep-vein thrombosis in total hip replacement: comparison of heparin and foot impulse pump. J Bone Joint Surg Br. 1994;76:579–583. [PubMed] [Google Scholar]
- 18.Stannard JP, Lopez-Ben RR, Volgas DA, Aderson ER, Busee M, Karr DK, GR McGwin JR, Alonso JE. Prophylaxis against deep-vein thrombosis following trauma: a prospective, randomized comparison of mechanical and Pharmacologic prophylaxis. J Bone Joint Surg Am. 2006;88:261–266. doi: 10.2106/JBJS.D.02932. [DOI] [PubMed] [Google Scholar]
- 19.Stöckle U, Hoffmann R, Raschke M, et al. Intermittierende Impulskompression – die alternative in der therapie des posttraumatischen und postoperativen Ödems. Chirurg. 1996;67:539–545. [PubMed] [Google Scholar]
- 20.Warwick DJ, Hertmant P, Shewale S, Sulkin T. Venous impulse foot pumps. Should graduated compression stockings be used? J Arthroplasty. 2002;17:446–448. doi: 10.1054/arth.2002.31248. [DOI] [PubMed] [Google Scholar]
- 21.Warwick D, Harrison J, Glew D, et al. Comparison of the use of a foot pump with the use of low-molecular-weight heparin for the prevention of deep- vein thrombosis after total hip replacement. J Bone Joint Surg Am. 1998;80:1158–1166. doi: 10.2106/00004623-199808000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Westrich GH, Haas SB, Mosca P, Peterson M. Meta-analysis of thromboembolic prophylaxis after total knee arthroplasty. J Bone Joint Surg Br. 2000;82:795–800. doi: 10.1302/0301-620X.82B6.9869. [DOI] [PubMed] [Google Scholar]
- 23.Westrich GH, Specht LM, Sharrock NE, et al. Venous heamodynamics after total knee arthroplasty: evaluation of active dorsal to plantar flexion and several mechanical compression devices. J Bone Joint Surg Br. 1998;80:1057–1066. doi: 10.1302/0301-620X.80B6.8627. [DOI] [PubMed] [Google Scholar]
- 24.Westrich GH, Sculco TP P. Prophylaxis against deep venous thrombosis after total knee arthroplasty. Pneumatic plantar compression and aspirin compared with aspirin alone. J Bone Joint Surg Am. 1996;78:826–834. doi: 10.2106/00004623-199606000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Wilson NV, Das SK, Kakkar VV, et al. Thrombo-embolic prophylaxis in total knee replacement. J Bone Joint Surg Br. 1992;74:50–52. doi: 10.1302/0301-620X.74B1.1732265. [DOI] [PubMed] [Google Scholar]