Abstract
To assess the potential efficacy of mechano growth factor (MGF) for bone injury, we firstly investigated the effects of growth factors, including MGF, its E peptide (a short 24-amino acid C-terminal peptide, MGF-Ct24E), and insulin-like growth factor 1(IGF-1) on MC3T3-E1 osteoblast-like cell proliferation. MGF-Ct24E had the highest pro-proliferation activity among three growth factors, which was 1.4 times greater than that of IGF-1. Moreover, MGF-Ct24E promoted cell proliferation by inducing cell cycle arrest in the S and G2/M phase of the cell cycle, but also mainly by the activation of the MAPK-Erk1/2 pathway. In vivo, a 5-mm segmental bone defect in the radius of 27 rabbits was treated with MGF-Ct24E by two doses (28.5 and 57 μg /kg body weight) vs. non-growth factor injection for five consecutive days postoperatively. The cumulative rate of radiographically healed defects and histological scores of bone defect-healing revealed a statistical difference between high-dose treatment and non treatment (p < 0.01), which showed the treatment promoted defect healing. This report is the first to demonstrate that MGF-Ct24E possesses positive effects on osteoblast proliferation and bone-defect healing, suggesting a new strategy in fracture healing.
Introduction
Large resections around bone tumors, complications after bone fracture, or inflammatory bone diseases often result in major bone loss or bone defect. Bone defect healing is one of the challenges of orthopaedic surgery, for which bone grafts are widely used [14, 15 and 17]. However, bone grafts have several drawbacks limiting their ude, including the high donor-site morbidity and a high complication rate. In search of alternative therapies, growth factors gain a significant importance. IGF-1 is one of the main growth factors in the human body and has an important role in bone injury repair, among its many functions [15, 16]. The igf-1 gene can be alternatively spliced to generate different IGF-1 isoforms with different functions. MGF is the initial splice-variant of IGF-1 when tissue or cells suffer from damage in some way such as mechanical/press overload, ischemia, hyperthermia, acidification, or myotoxic/ neurotoxic agents [1–6]. And MGF has a unique E domain (MGF-Ct24E) in the C-terminal resulting from a 48 bp insert during the splicing of exons 5 and 6, which distinguishes MGF from other IGF-1 isoforms in peptide sequence and function [7–9].
MGF initially was appreciated as exerting post-mitotic reparative effects in skeletal muscle [9], but more recently reports have demonstrated that MGF or MGF-Ct24E acts as a local tissue repair factor in acute injury models of muscle [3, 7], cardiac muscle [10] and neurons [4, 9].
Our previous study demonstrated that MGF was upregulated in MC3T3-E1 osteoblasts in response to mechanical overload [12]. Moreover, the growth of osteoblasts and bone marrow-derived MSCs (bone marrow mesenchymal stem cells) was improved by IGF-1 treatment [18, 19]. It is thus appealing to speculate whether the splice-variant of IGF-I, MGF or its E peptide, has positive effects on MC3T3-E1 cells action and bone fracture healing. It is well accepted that IGF-1 delivery induced bone growth mainly through the PI3K / Akt pathway [21], while some literature demonstrated that MGF-Ct24E, did not activate Akt signaling [9, 20]. Thus, it appears attractive for the signalling of MGF-Ct24E in osteoblasts. In this study, the effects of MGF, MGF-Ct24E and IGF-1 on MC3T3-E1 cell proliferation were compared, and the signalling pathways activated by MGF-Ct24E were characterised. Then a pilot in vivo study was undertaken to confirm the role of MGF-Ct24E on the bone defect healing.
Materials and methods
Reagents
α-minimum essential meduium (α-MEM) and foetal bovine serum (FBS) were purchased from Gibco (MD, USA). Monoclonal anti-Erk1/2 antibodies, monoclonal anti-P-Erk antibodies, monoclonal anti-Akt antibodies, monoclonal anti-P-Akt antibodies, and goat anti-rabbit IgG were from Santa Cruz (CA, USA). The BCA Protein Assay kit was from Pierce (Washington, USA). PD98059, LY294002 and 3(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kits were from Sigma (St. Louis, USA). The Trizol Reagent Kit was from Invitrogen (CA, USA). The ECL detection system kit was from Amershamm-Pharmacia Biotech (NJ, USA). MGF and MGF-Ct24E were produced as described previously [13].
In vitro experiments
Cell culture
MC3T3-E1 osteoblast-like cells (China Centre for Type Culture Collection, CCTCC) were incubated at 37°C with 5% CO2 in α-MEM containing 10% FBS and 1% antibiotics.
Cell proliferation assay
Cell proliferation was measured by the MTT assay kit. MC3T3-E1 cells were plated in 96-well plates at a density of 104. At 12 hours, the culture medium was replaced with α-MEM containing various concentrations of MGF or MGF-Ct24E or rhIGF-1 (10nM, 1nM, 0.5 nM, and 0.1 nM) and cells were incubated for 24 hours. For Akt or Erk1/2 inhibition, MC3T3-E1 cells were pretreated with LY294002 (50 μM) or PD98059 (100 μM) for 30 minutes prior to the growth factors (1 nM) administration for 24 hours. MTT (0.1 mg/ml) was added to the culture for 4 hours at 37°C, followed by 150 μl DMSO, and the absorbance was read at 570 nm in a Model 550 ELISA reader (Bio-Rad, USA). All the experiments were repeated in triplicate and each concentration arranged six replicates.
Cycle distribution and DNA synthesis
MC3T3-E1 cells incubated with 1nM growth factors for 24 hours were trypsinised and fixed in 70% ethanol at 4°C overnight. Cells were then washed and resuspended in PBS containing 20 μg/ml RNase A and 50 μg/ml PI and incubated on ice for 30 minutes. DNA content and synthesis were analysed by flow cytometry (FACScalibur, Becton Dickinson, USA). Cell cycle analysis was done using the cell quest program by manual setting regions for G0/G1, S and G2/M phases. Data from 2.5 × 105 cells were acquired for each sample. Experiments were repeated in triplicate.
Western blot analysis
MC3T3-E1 cells (1 × 106) were plated in 6-well plates for 24 hours. Then cells were pretreated with LY294002 (50 μM ) or PD98059 (100 μM) for 30 minutes and the medium was replaced with α-MEM containing rhIGF-1 or MGF or MGF-Ct24E (1nM) for acute treatment for 3 hours. Total proteins were extracted according to the manufacturer’s protocol of the Trizol Reagent Kit. Briefly, cells were washed with cold PBS and harvested to lyse in 250 μl of ice-cold 1% Triton X-100 lysis buffer. Cellular lysates were centrifuged at 15,000 rpm for 5 minutes at 4°C. Equal amounts of protein extracts (100 μg) were separated by 10% SDS-PAGE and electrotransferred to nitrocellulose membranes (BIO-RAD Laboratories, USA). The membranes were firstly blocked with 5% (w/v) nonfat dry milk and then incubated with primary antibody of phosphorylated Akt (1:1000), stripped, and reprobed for Akt (1:1000), or phosphorylated Erk1/2(1:1000), stripped, and reprobed for Erk1/2(1:1000). Detection of primary antibody was done by goat anti-rabbit IgG conjugated to horseradish peroxidase (1:2000) and visualised by chemiluminescence.
In vivo experiments
Animals
Twenty seven four-month-old New Zealand white rabbits (the Animal Experiment Centre of Third Military Medical University, China), weighing 2.3–2.8 kg, were used. All animal care and experimental protocols complied with the Animal Management Rule of the Ministry of Public Health, China (documentation 55, 2001).
Surgical procedure and the rabbit bone defect model
General anaesthesia was administered by intravenous injection of 3% pentobarbital sodium at 30 mg kg−1. After routine skin preparation and disinfection, a unilateral 5-mm segmental bone defect with removal of periosteum and endosteum was created in the middle of the radius. The defect was rinsed with 0.9% saline, and then the deep muscle layer and skin were closed. All the animals received 40,000 units of gentamycin by intramuscular injection per day for two days postoperatively.
At three days postoperatively, the rabbits were randomly assigned to three groups: the control (n = 9), the low dose (n = 9) and the high dose (n = 9). The control group, the low dose group and the high dose group received the injection of 100 μl PBS containing 0 μg kg−1, 28.5 μg kg−1 and 57 μg kg−1 MGF-Ct24E into each bone defect gap for five consecutive days, respectively.
Radiographic examination and histological evaluation
At four, six and eight weeks, the development of new bone in the defects was monitored radiographically. According to the outcome assessment in clinical trials of fracture-healing documented by Morshed, the cortical bridging and loss of the fracture line was the most reliable radiographically to define the union on fracture-healing [22]. Therefore the defect was considered to be healed when the cortical bridging and loss of the fracture line were observed. The number of healed defects was recorded for each group of rabbits at each interval. At eight weeks, the animals were euthanised and nine radii from each group were harvested. Bone specimens were fixed in 10% normal-buffered formalin, decalcified with Gooding and Stewart’s decalcification fluid (10% formic acid and 5% formaldehyde), embedded in paraffin, sectioned parallel to the diaphysis and stained with haematoxylin-eosin (H-E) according to standard protocols. Sections were examined independently scored by three investigators according to the Circi’s histological grading scale [24]. Four individual parameters, including fibroblast density, vascularity, Haversian channel and osteoid synthesis were listed as the histological classification system and were graded on the following scale: for (1) fibroblast density: 3 indicates non appearance, 2 indicates a normal appearance, 1 indicates a slightly abnormal appearance, and 0 a severe abnormal appearance; for (2) vascularity, (3) Haversian channel and (4) osteoid synthesis: 3 indicates a normal or near normal appearance, 2 a moderate appearance, 1 a slight appearance, and 0 no appearance.
Statistical analysis
Data were expressed as mean ± SEM. Statistical analysis was performed using SPSS 11.5 software for Windows student version. Statistical comparisons were analyzed by one-way ANOVA. P < 0.05 was considered statistically significant.
Results
MGF-Ct24E induced the higher MC3T3-E1 cells proliferation compared to other growth factors
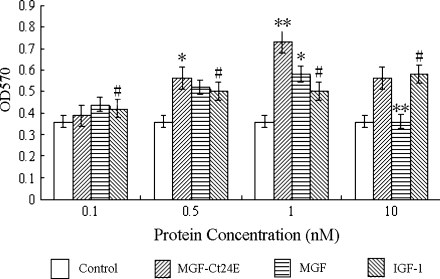
Cells proliferation assay used yellow MTT that is reduced to purple formazan in the mitochondria of living cells. MTT assays demonstrated that the cell proliferation was enhanced gradually following increasing concentrations when it ranged from 0.1 to 1 nM, suggesting a concentration-dependent manner. However, 10 nM growth factor treatment caused the living cell population to decrease, indicating the high-concentration inhibition effect. Compared with the control cells, at the concentration of 1 nM, cell proliferation upregulated about 1-fold caused by MGF-Ct24E (P < 0.001), and increased nearly 67% induced by MGF (P < 0.001), but just increased about 43% by IGF-1 (Fig. 1; P < 0.001), which showed that the pro-proliferation activity of MGF-Ct24E was 1.4-fold that of IGF-1.
Fig. 1.
The effects of MGF-Ct24E, MGF and rhIGF-1 at different concentrations on the MC3T3-E1 cell proliferation. Bars represent means ± SEM (n = 6 per concentration), #P < 0.05 versus control; *P < 0.01, **P < 0.001 versus IGF-1 alone
MGF-Ct24E induced cell cycle arrest in the S and G2/M phases of the cell cycle
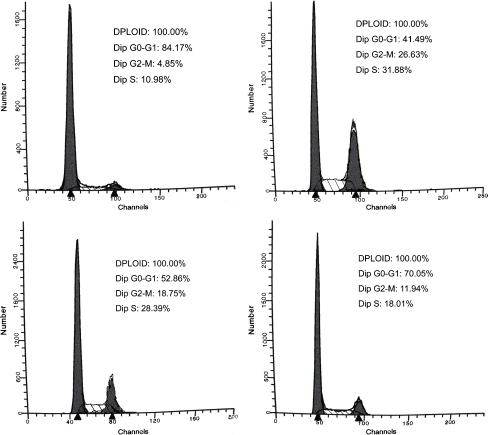
To determine whether the growth promotion of cells was due to the arrest of cell cycle at a certain phase(s), flow cytometry analysis was performed based on DNA content in nuclei stained by PI. Compared with the control, changes in cell cycle distribution were observed when the cells were incubated with three growth factors. But addition of MGF-Ct24E induced the greatest change in cell cycle distribution, resulting in a maximum accumulation of cells in G2/M (rising from 4.85% to 26.63%) or in S (rising from 10.98% to 31.88%), which showed that the synthesis of DNA in the S phase increased twofold and cell mitosis activity was enhanced fivefold by promoting G2/M phase entry (Fig. 2; P < 0.01). The cell proliferation is required for both the initiation of cell DNA synthesis in the S phase and the entry into G2/M phase. Our results indicated that pro-proliferation activity of MGF-Ct24E was induced by the changes in cell cycle progression pattern.
Fig. 2.
The effects of growth factors on MC3T3-E1 cell cycle progression by FACS analysis. Cells were treated with MGF-Ct24E or MGF or IGF-1 at the concentration of 1nM. The cell proportions of the various treatments are presented in the top right corner. n > 3 per group
MGF-Ct24E promoted the cell proliferation via MAPK-Erk1/2 pathway
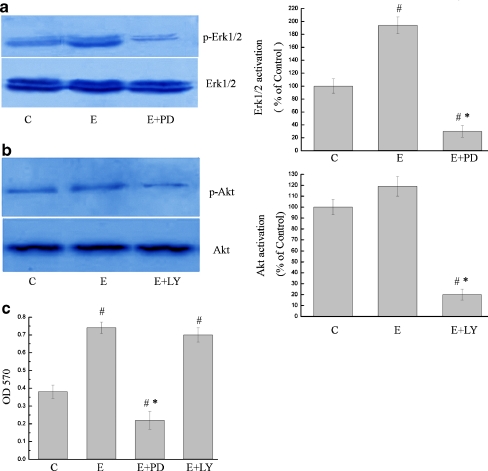
We found that 24-hour incubation with MGF-Ct24E upregulated robustly phosphorylated Erk1/2 expression but upregulated little phosphorylated Akt expression (Fig. 3a and b). The addition of PD (inhibitor of the Erk1/2 pathway) to MGF supplemented cultures remarkably reduced cell proliferation by 70% (Fig. 3c; P < 0.05), and the addition of LY (inhibitor of the PI3K pathway) to MGF supplemented cultures reduced proliferation by 4% (Fig. 3c; P > 0.05). These results indicate that the PI3K/Akt pathway had little effect on the cell proliferation induced by MGF-Ct24E, and MGF-Ct24E promoted the cell proliferation via the MAPK-Erk1/2 pathway.
Fig. 3.
MGF-Ct24E mainly enhanced the cell proliferation via the MAPK-Erk1/2 pathway. Immunoblotting analysis demonstrated that MGF-Ct24E treatment induced a significant enhancement of phosphorylated Erk1/2 expression (a) but had a blur effect on Akt compared to the vehicle control (b). Cells were incubated with MGF-Ct24E containing inhibitor of the MAPK-Erk1/2 or PI3K pathway (a and b), and the addition of PD or LY to MGF-Ct24E supplemented cultures reduced cell proliferation by 70% or 4%, respectively (c). # P < 0.01 versus C, *P < 0.05 versus MGF-Ct24E. C: The vehicle control containing α-MEM and 0.1% DMSO, PD: PD98059, LY: LY294002, E: MGF-Ct24E, n > 3
MGF-Ct24E accelerated bone-defects healing
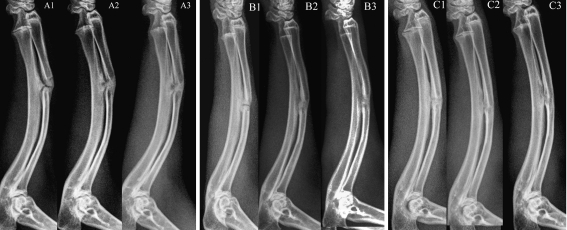
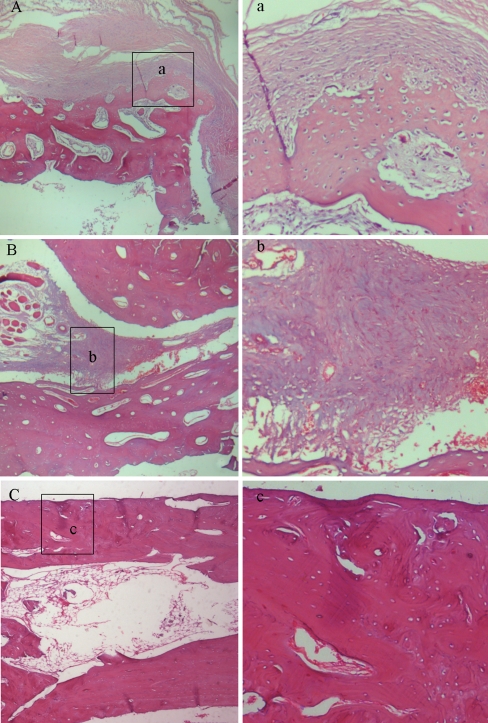
In the segmental ostectomy model, radiographs revealed a marked positive effect of MGF-Ct24E at high dose on new bone formation. At four weeks, the detectable calluses around the defect could be seen in three groups, but no visible gap was found in the high-dose group, which was different from the clear gap in the other groups (Fig. 4a1, b1 and c1). At six weeks, the defects were completely filled by calluses in the low-dose group and control group, and more new bone formation and the blur fracture line were shown in the high-dose group (Fig. 4a2, b2 and c2). At eight weeks, radiographs suggested the bone defect was healed in the high-dose group, indicated by cortical bridging and loss of the fracture line, and the reopening of the medullary cavity. Increased fracture callus density was shown in the other two groups, but compared to the control, more new bone formation near the side of the ulna was detectable in the low-dose group (Fig. 4a3, b3 and c3). In addition, as Table 1 shows, four of nine defects in the high-dose group had healed radiographically at eight weeks. No healed defect was observed in either the control or the low-dose group. The cumulative rate of healed defects suggested that high-dose treatment had significantly greater healing effect than non-treatment (p < 0.01). Figure 5 C suggests bone remodelling in the high-dose treated defects, as indicated by increased lamellar bone and the restored marrow cavity. But the defects of the other two groups were in the phase of fibrous callus. Compared to the control, the low-dose group showed much more vascularity and less fibroblast density (Fig. 5a, b). Meanwhile the histological scores at eight weeks postoperatively showed the statistical differences between the high-dose treated and the non-treated (P < 0.01; Table 2). These results revealed that the treatment of MGF-Ct24E could accelerate bone-defect healing.
Fig. 4.
X-ray photography at four, six and eight weeks postoperatively. A, B and C represent the control group, low-dose group and high-dose group, respectively. 1, 2 and 3 represent four, six and eight weeks respectively; n = 9 per group
Table 1.
Numbers of radiographically healed defectsa
| Week | Control | Low dose | High dose |
|---|---|---|---|
| 4 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 |
| 8 | 0 | 0 | 4 |
| Total | 0 of 9 | 0 of 9 | 4 of 9 |
a Comparison of the cumulative rate of healed defects (control compared with low-dose group, p > 0.05; control compared with high-dose group, p < 0.01; and low-dose compared with high-dose group, p < 0.01)
Fig. 5.
The histological microphotographs of the defects in the control group (A, a), low-dose group (B, b) and high-dose group (C, c) at eight weeks postoperatively. H-E staining; original magnification: 40× (A, B and C) and 200× (a, b and c); n = 9 per group. The defects in the control group (A) and the low-dose group (B) were filled by fibrous callus, but more vascularity and less fibroblast density appeared in the low-dose group (b), and numerous blue-violet fibroblasts were formed in the control group (a). In the dark red lamellar bone areas, no visible margin of osteotomy was seen in high-dose group, and the restored marrow cavity suggested bone remodelling (C). No fibroblast and restored blood supply were seen in c
Table 2.
Histological scores of bone defect-healing at eight weeks postoperatively
| Score parameter | Control | Low dose | High dose |
|---|---|---|---|
| Fibroblast density | 0.8 ± 0.6 | 1.3 ± 0.5 | 2.3 ± 0.6 |
| Vascularity | 1.1 ± 0.6 | 1.2 ± 0.4 | 2.4 ± 0.5 |
| Haversian channels | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.4 ± 0.5 |
| Osteoid synthesis | 0.1 ± 0.3 | 0.2 ± 0.4 | 2.2 ± 0.7 |
| Total | 2.0 ± 1.1 | 2.7 ± 1.3 ** | 8.4 ± 2.0* |
All values are given as the mean and standard deviation for each parameter
*p < 0.01 vs. the control; ** p > 0.05 vs. the control
Discussion
IGFs are the most important regulators of bone cell function because of their abundance and their proven anabolic effects on the skeleton [11, 23]. MGF and its E peptide, as the splice-variant of IGF-I, which were documented to promote some tissue injury healing, have drawn increasing attention in recent years [4, 7, 9, 10]. The similar positive role on bone injury healing was firstly confirmed in this study. These results demonstrated that MGF and MGF-Ct24E improved osteoblast proliferation, but also the pro-proliferation activity of MGF-Ct24E was 1.4-fold that of IGF-1. Pro-proliferation activity of MGF-Ct24E acted via a promotion of DNA synthesis and mitosis.
MacRae et al. documented that the addition of PD or LY to IGF-I supplemented cultures reduced bone cell proliferation by 32% or 66%, respectively [19]. However, our results showed that the addition of PD or LY to MGF-Ct24E supplemented cultures reduced cell proliferation by 70% or 4%, respectively. So MGF-Ct24E mainly exerted pro-proliferation through activation of the MAPK-Erk1/2 pathway, which was different from the mechanism activated by IGF-1, although MGF and IGF-1 share a common genetic basis. This finding is intriguing, as it indicates that the action of MGF-Ct24E may be forecasted via its activated pathways.
We used an animal model of 5-mm segmental bone defect with removal of periosteum and endosteum. This surgery deprived the most important sources of osteoprogenitor cells at the defect site [14], and offered a favoured bone-defect healing environment for MGF-Ct24E, which reduced MGF-Ct24E to show the advantage of pro-proliferation activity. The results of the in vivo experiment also demonstrated that over eight weeks, the defects treated with the high-dose MGF-Ct24E in advance reached up to the radiographically healed which was confirmed by clinicians, with respect to the control group.
Our results suggest that MGF-Ct24E promoted bone-defect healing in rabbits by enhancing osteoblast proliferation. Meanwhile, we found MGF-Ct24E mediated osteoblast proliferation through an IGF-1R independent mechanism, confirming a similar conclusion that MGF-Ct24E does not activate the IGF-1 receptor by Stavropoulou et al. [20], Dluzniewska et al. [5] and Quesada et al. [9], so MGF-Ct24E may avoid the potential disadvantages of IGF-1. In addition, its small size simplifies synthesis and purification as a drug for injury healing [9] and its positive effect on bone-defect healing may contribute to some further studies as a therapy of bone injury healing.
Acknowledgements
This work was supported by the National Nature Science Foundation of China (1032012, 30870609); Natural Science Foundation of Chongqing (CSTC2009BB4382CSTC2010BB5225); Foundation for Science & Technology Research Project of Chongqing (CSTC 2009AB517) and Project Foundation of Chongqing Municipal Education Committee (KJ091415).
Contributor Information
Moyuan Deng, Email: dengmoyuan@yahoo.cn.
Yuanliang Wang, Email: wyl@cqu.edu.cn.
References
- 1.Skarli M, Yang SY, Bouloux P, Goldspink G. Upregulation and alternative splicing of the IGF-I gene in the rabbit heart following a brief pressure/volume overload. J Physiol. 1998;9:92–193. [Google Scholar]
- 2.Yang S, Alnaqeeb M, Simpson H, Goldspink G. Cloning and characterization of an IGF-1 isoform expressed in skeletal muscle subjected to stretch. J Muscle Res Cell Motil. 1996;17:487–495. doi: 10.1007/BF00123364. [DOI] [PubMed] [Google Scholar]
- 3.Hill M, Wernig A, Goldspink G. Muscle satellite (stem) cell activation during local tissue injury and repair. J Anat. 2003;203:89–99. doi: 10.1046/j.1469-7580.2003.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dluzniewska J, Sarnowska A, Goldspink G, Zabłocka B. A strong neuroprotective effect of the autonomous C-terminal peptide of IGF-1 Ec (MGF) in brain ischemia. FASEB J. 2005;19:1896–1898. doi: 10.1096/fj.05-3786fje. [DOI] [PubMed] [Google Scholar]
- 5.Dimitrios M, Haralampos K, Protopapas MS, Stelios GM, Michael K. Insulin-like growth factor-1 isoform mRNA expression in women with endometriosis: eutopic endometrium versus endometriotic cyst. Ann NY Acad Sci. 2006;1092:434–439. doi: 10.1196/annals.1365.042. [DOI] [PubMed] [Google Scholar]
- 6.Olesen JL, Heinemeier KM, Flyvbjerg A, Kjaer M. Expression of insulin-like growth factor I, insulin-like growth factor binding proteins and collagen mRNA in mechanically loaded plantaris tendon. Appl Physiol. 2006;101:183–188. doi: 10.1152/japplphysiol.00636.2005. [DOI] [PubMed] [Google Scholar]
- 7.Ates K, Yang SY, Orrell RW, Simons P. The IGF-I splice variant MGF increases progenitor cells in ALS, dystrophic, and normal muscle. FEBS Lett. 2007;581:2727–2732. doi: 10.1016/j.febslet.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 8.Mills P, Lafreniere JF, Tremblay JP. A new pro-migratory activity on human myogenic precursor cells for a synthetic peptide within the E domain of the mechano growth factor. Exp Cell Res. 2007;313:5275–5237. doi: 10.1016/j.yexcr.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 9.Quesada A, Micevych P, Handforth A. C-terminal mechano growth factor protects dopamine neurons: A novel peptide that induces heme oxygenase-1. Exp Neurol. 2009;220:255–266. doi: 10.1016/j.expneurol.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter V, Matthews K, Yang SY, Goldspink G. Mechano-growth factor reduces loss of cardiac function in acute myocardial infarction. Heart Lung Circ. 2008;17:33–39. doi: 10.1016/j.hlc.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Abbaspour A, Takata S, Matsui Y, Yasui N. Continuous infusion of insulin-like growth factor-I into the epiphysis of the tibia. Int Orthop. 2008;32:395–402. doi: 10.1007/s00264-007-0336-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang LL, Xian CY, Wang YL. The MGF expression of osteoblasts in response to mechanical overload. Arch Oral Biol. 2006;51:1080–1085. doi: 10.1016/j.archoralbio.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Zhang B, Jiang P, Xian C, Tang L, Wang Y. Expression of mechano-growth factor in Eschericha coli and activity analysis. Sheng Wu Gong Xue Bao. 2008;24:1180–1185. [PubMed] [Google Scholar]
- 14.Eckardt H, Christensen KS, Lind M. Recombinant human bone morphogenetic protein 2 enhances bone healing in an experimental model of fractures at risk of non-union. Injury. 2005;36:489–494. doi: 10.1016/j.injury.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Meinel L, Zoidis E, Schneider R. Localized insulin-like growth factor I delivery to enhance new bone formation. Bone. 2003;33:660–672. doi: 10.1016/S8756-3282(03)00207-2. [DOI] [PubMed] [Google Scholar]
- 16.Yao W, Yao W, Jun Yu, Bozic T. IGF-I improved bone mineral density and body composition of weaver mutant mice. Growth Horm IGF Res. 2008;18:517–525. doi: 10.1016/j.ghir.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forriola F, Longob UG, Denaro V. Platelet-rich plasma, rhOP-1® (rhBMP-7) and frozen rib allograft for the reconstruction of bony mandibular defects in sheep. A pilot experimental study. Injury Int J Care Injured. 2009;40(S3):S44–S49. doi: 10.1016/S0020-1383(09)70011-7. [DOI] [PubMed] [Google Scholar]
- 18.McMahon LA, Prendergast PJ, Campbell VA. A comparison of the involvement of p38, ERK1/2 and PI3K in growth factor-induced chondrogenic differentiation of mesenchymal stem cells. Biochem Biophys Res Commun. 2008;18:990–995. doi: 10.1016/j.bbrc.2008.01.160. [DOI] [PubMed] [Google Scholar]
- 19.Vicky E, MacRae S, Ahmed F, Farquharson C. IGF-I signalling in bone growth: Inhibitory actions of dexamethasone and IL-1b. Growth Horm IGF Res. 2007;7:435–439. doi: 10.1016/j.ghir.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Stavropoulou A, Halapas A, Sourla A. IGF-1 expression in infarcted myocardium and MGF E peptide actions in rat cardiomyocytes in vitro. Mol Med. 2009;15:127–135. doi: 10.2119/molmed.2009.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raucci A, Bellosta P, Grassi R, Basilico C, Mansukhani A. Osteoblast proliferation or differentiation is regulated by relative strengths of opposing signaling pathways. J Cell Physiol. 2008;215:442–451. doi: 10.1002/jcp.21323. [DOI] [PubMed] [Google Scholar]
- 22.Morshed S, Corrales L, Miclau T. Outcome assessment in clinical trials of fracture-healing. J Bone Joint Surg. 2008;90:62–67. doi: 10.2106/JBJS.G.01556. [DOI] [PubMed] [Google Scholar]
- 23.Mohan S. Insulin-like growth factor binding proteins in bone cell regulation. Growth Reg. 1993;3:67–70. [PubMed] [Google Scholar]
- 24.Circi E, Akpinar S, Tuncay IC. Biomechanical and histological comparison of the influence of oestrogen deficient state on tendon healing potential in rats. Int Orthop. 2009;33:1461–1466. doi: 10.1007/s00264-009-0778-1. [DOI] [PMC free article] [PubMed] [Google Scholar]