Abstract
Background
The ‘reamer-irrigator-aspirator’ (RIA) is an innovation developed to reduce fat embolism (FE) and thermal necrosis (TN) that can occur during reaming/nailing of long-bone fractures. Since its inception its indications have expanded to include the treatment of long-bone osteomyelitis and as a harvester of bone graft/mesenchymal stem cells (MSCs).
Methods
This study involved a systematic review, via Pubmed® and Google Scholar®, of English language sources (nine non-clinical studies, seven clinical studies and seven case reports) using the keywords: ‘reamer’, ‘irrigator’, ‘aspirator’ (1st May 2010). Sources were reviewed with reference to the RIAs efficacy in (1) preventing FE/TN, (2) treating long-bone osteomyelitis, (3) harvesting bone graft/MSCs, and (4) operating safely. Experimental data supports the use of the RIA in preventing FE and TN, however, there is a paucity of clinical data.
Conclusions
The RIA is a reliable method in achieving high volumes of bone graft/MSCs, and high union rates are reported when using RIA bone-fragments to treat non-unions. Evidence suggests possible effectiveness in treating long-bone osteomyelitis. The RIA appears relatively safe, with a low rate of morbidity provided a meticulous technique is used. When complications occur they respond well to conventional techniques. The RIA demands further investigation especially with respect to the optimal application of MSCs for bone repair strategies.
Introduction
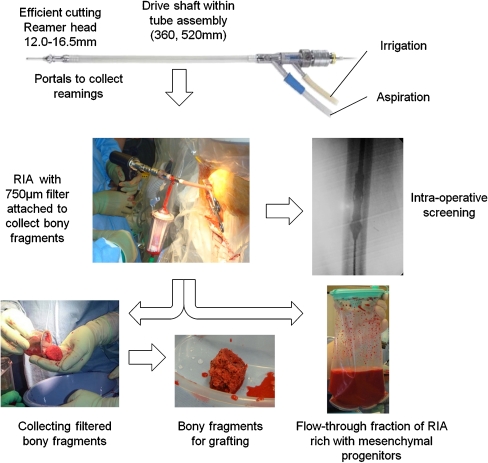
The ‘reamer-irrigator-aspirator’ (RIA) is a novel reaming system (Synthes, Paoli, PA, USA), which provides continuous irrigation and suction during reaming of a long-bone. This system has been developed in an attempt to reduce the incidence of fat embolism (FE) and thermal necrosis (TN) that can complicate reaming/nailing of long-bone fractures. Fat embolism can occur when increased intra-medullary (IM) pressures force depots of fat and reaming debris into the venous system which are then propelled to the lungs and other end-organs [1]. Thermal necrosis can occur when IM temperatures become elevated during reaming, thus causing bone necrosis [2]. The RIA is designed to remove marrow contents and reduce IM pressures whilst operating at decreased reaming temperatures. Irrigation fluid, marrow contents and bony-fragments are aspirated and passed via a coarse filter, which traps the solid bone-fragments, before moving into a closed suction bag. Technical guidance has been previously published [3] and a schematic overview is shown in Fig. 1.
Fig. 1.
The ‘reamer-irrigator-aspirator’ (RIA) system
Reported indications for the RIA have expanded since its inception to include treating postoperative osteomyelitis [4], using the aspirated bone-fragments as bone graft to treat non-unions [5–10] and harvesting mesenchymal-stem-cells (MSCs) [11]. A systematic review of this device is necessary to inform the wider orthopaedic community of the RIAs indications, efficacy and safety.
Methods
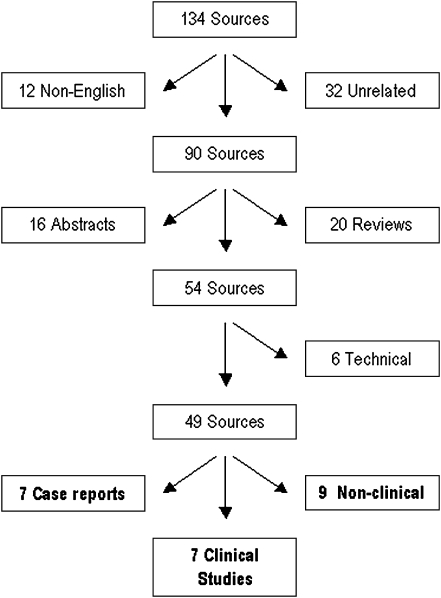
A standard literature search using PUBMED® (http://www.ncbi.nlm.nih.gov/pubmed/) and Google Scholar® (http://scholar.google.co.uk/) was performed using the keywords ‘reamer’, ‘irrigator’ and ‘aspirator’ accessed on 1st May 2010. This identified 22 and 138 sources, respectively. All sources found on PUBMED® were present on the Google Scholar® search. Four sources were duplicated in the search and were excluded, the remainder were examined and sorted as shown in Fig. 2. Unrelated/non-English sources were identified by authors G.C. and P.V.G. and excluded; there was no disagreement in this process. Abstracts of posters and technical reports were further excluded as this evidence was not deemed significantly robust for analysis. Twenty reviews included the words ‘reamer’, ‘irrigator’, ‘aspirator’, and for four of them this device was their focus. There were no systematic reviews. Original papers and case reports were reviewed and indications for procedure, number of cases, endpoint measured and outcomes were studied and recorded. A review of sources’ bibliographies was performed, identifying a further two sources.
Fig. 2.
Flow diagram showing the sorting of sources
Results
From a total of 134 manuscripts, 23 papers fulfilled the inclusion criteria and were the subject of this review [4–28].
Prevention of fat embolism and thermal necrosis
The effect of the RIA on IM pressures was assessed in three studies [12–14] with a further two studies assessing a similar device, the ‘rinsing-suction-reamer’ (RSR) [15, 16]. The RSR is cannulated and flexible allowing continuous suction and irrigation during reaming similar to the RIA; however, it has only been reportedly used in the experimental setting. Three studies used animal models—one sheep [15] and two porcine [13, 16]—with the others using human cadaver specimens [12, 14]. A significant reduction in IM pressures, when the RIA/RSR was used as opposed to conventional reaming, was observed in four of five studies with an unexpected rise in distal pressures seen by Higgins et al. (32.7 ± 39.4 kPa in the RIA group compared with 17.0 ± 32.6 kPa in the standard reaming group; P = 0.019) [12]. Levels of intravenous femoral vein fat were measured by two of the studies, showing a reduced load when using the RSR using the Gurd criteria [15, 16]. The effect of the RIA on systemic response was investigated by Pape et al. using a sheep model with an iatrogenic unilateral pulmonary injury via the measurement of changes in pulmonary permeability [17]. This showed attenuation of the systemic inflammatory response when comparing RIA with conventional reaming with urea/protein ratios of 91.5 compared with 256.7, respectively [17].
The RIAs effect on reducing reaming temperature has been investigated by Higgins et al., using human cadaver tibias, finding a difference in maximal recorded temperatures between the RIA and standard reaming groups which were 42.0±9.1°C and 58.7±15.9°C (P = 0.025), respectively [12].
Treatment of osteomyelitis
The RIA reamer has been reported as an effective adjunct when treating postoperative long-bone osteomyelitis by providing IM debridement and lavage [4, 18]. Zalvras et al. reported an 11 patient case series (eight tibia, three femur) where there was no recurrence of infection, at over six months follow-up, after removal of metalwork and RIA reaming [4].
Harvest of bone graft and MSCs
The RIA has been reported as a source of bone graft with supportive experimental [17] and clinical studies [5, 7–9] and case reports [6, 20–23]. Direct grafting of harvested RIA bone fragments, in addition to ‘autografting’ alone has been shown to improve stiffness (1.21 Nm compared with 0.48 Nm; P = 0.029) and strength (14.09 Nm compared with 6.94 Nm; P = 0.029) in a sheep model [19]. Large volumes of graft can be obtained, with 40–90 ml harvests being reported [5, 7–9]. This graft has been shown to be ‘comparable to iliac crest’ in terms of osteoinductive growth factors [24], and high union rates have been reported in clinical series (50–90%) [5, 7–9]. It is noted that union rates reported by Belthur et al. using RIA derived graft (37 of 41 cases) compared well with an iliac crest bone graft (ICBG) control group (32 of 40 cases) [7].
Multipotential marrow stromal cells also known as mesenchymal stem cells (MSCs) have been isolated from outgrowths of RIA bony-fragments [11]. These cells have the characteristics of MSCs and a phenotype that is consistent with other established sources. These cells have also been identified within the filtrate fraction, with a reported robust chondrogenic ability [11]. The filtrate, containing MSCs, has been combined with RIA bone fragments and dexamethasone, a synthetic steroid shown to induce osteogenesis in vitro, within the clinical setting (six tibia, four femur and three humerus fractures) with some positive results (12 of 13 partial to full union) [8].
Safety of the RIA reamer
When combining all 87 patients from initial studies [5, 7–9], only three complications are reported: (1) femoral neck fracture, (2) perforation of the anterior cortex of the femur, and (3) hypertrophic scar. All complications were successfully managed, with the femoral neck fracture being fixed and the other two managed non-operatively [5, 7]. A more recent case series (six patients) of RIA related complications reported three diaphyseal fractures, one trochanteric fracture and two cortical breeches. These were all successfully treated with intra-medullary nail (four of six cases) and cephallomedullary nail (one of six cases) fixation, and in one case the cortical breech was deemed sufficiently small to manage non-operatively [25]. Belthur et al. found a reduction in postoperative pain when compared with conventional iliac crest bone graft (ICBG) harvest and overall the RIA appears well tolerated [5]. The rate of heterotopic ossification following this procedure is unknown.
Discussion
The RIA is an innovative device with indications that have expanded to treating osteomyelitis, harvesting bone graft and MSCs, as well reducing the incidence of FE/TN. This review assesses the RIAs effectiveness in performing these tasks and its safety. Given its novelty only a limited number of sources have reported on this device, with many using animal or cadaver models, as shown by our searches. Nonetheless, given the immense potential offered, a review is necessary at this point to inform the wider orthopaedic community of this significant development.
Prevention of fat embolism and thermal necrosis
The data supporting the use of the RIA/RSR to prevent FE is encouraging but is limited in a number of ways. All studies used animal/human cadaveric models, which are beneficial in gaining ethical approval for studies but must be translated to the clinical setting, in which the environment may be different. It is noted that Pape et al. attempted to validate the sheep model and concluded that ‘the effect of IM instrumentation in sheep is less pronounced than in humans’ [29]. Several of the studies [12–16] measured IM pressures as a measure of FE, and whilst elevated IM pressures have been shown to be associated with FE [30], this is an indirect measurement and some studies have suggested this per se may not be directly related [31]. In addition, the threshold at which FE in humans occurs is unknown, although we note the work from Wenda et al. suggesting a 200-mmHg threshold in the sheep model [32]. It is concerning that one paper recorded elevated distal pressures compared to conventional reaming but this is out of step with other studies [12]. Quantifying the embolic load, using the femoral vein fat levels [15, 16] and systemic response [17] suggest a protective effect of the RIA reamer; however, how this relates clinically is debatable. Only one paper refers to the cooling effect of the RIA and whilst a significant difference was found, this should be supported by additional studies [12].
Treatment of osteomyelitis
The data from Zalavras et al. examining the RIA as a treatment for postoperative long-bone osteomyelitis holds great promise but must be supported with further studies given the small numbers (11 cases) and six month follow-up [4].
Harvest of bone graft and MSCs
Experimental work carried by Hammer et al. supports the use of RIA bone-fragments in improving callus formation but does not look directly at its use in the non-union situation [19]. The union rates presented by the different sources [5, 7–9] are compelling but are in the majority of cases limited in their power, given their low numbers, case-series design [7–9] and the use of additional factors to attempt to induce bone union [5, 8, 26]. Belthur et al. attempted to compare RIA bone fragments with ICBG; this paper however, is subject to confounding factors given that the cohorts were unmatched, the lengths of follow-up varied significantly and the majority of cases were treated concurrently with bone morphogenic protein-2 (BMP-2) [5].
Reamings generated by conventional techniques increase callus formation when implanted at fracture sites [33] and are associated with increased levels of heterotopic bone formation [34]. Additionally, ‘autografting’ that can be observed with conventional reaming, when reamings become lodged at the fracture site [35], contributes to the increased rates of union seen with reamed as opposed to unreamed nail stabilisation [36]. Thus it ‘makes sense’ that RIA bone fragments would be beneficial in promoting fracture healing, and despite the noted limitations in the current data, the weight of evidence supports this assertion. A randomised control trial comparing the ‘gold-standard’ ICBG with RIA bone-fragments would further elucidate this issue.
Previous researchers have established the role of the RIA as a harvester of MSCs [12, 27, 28]. MSCs have previously been isolated from reaming debris [37, 38], but what is significant about the RIA, is that MSCs have been extracted from bony-fragments and the filtrate bag. The bony fragments already have a purported role in providing bone graft, whereas the filtrate bag is generally seen as waste. Thus, finding the filtrate to be a high volume, high concentration source of MSCs is extremely significant [12].
MSCs have long promised benefits of synthesising bone [39–41] and cartilage [41], treating non-unions [42] and possibly accelerating fracture repair. One of the challenges to achieving this has been isolating sufficient numbers of MSCs. Mesenchymal stem cells are scarce within the ‘gold standard’ iliac crest aspirate forming only 0.001–0.01% of cells [43, 44] and the tissues with more abundant levels need enzymatic digestion [45]. This has potentiated the in vitro culture of MSCs for cell expansion; however, this leads to cells with reduced differentiation capacity [46]. The enrichment and possible concentration of the MSC fraction in filtrate bag offers the possibility of large numbers of fresh MSCs being isolated without enzymatic digestion. This is especially relevant since this fraction might be otherwise discarded.
The phenotype, numbers and in vitro functionality of MSCs purified from the iliac crest using the cluster of differentiation (CD) CD271+/CD45dim is established [47, 48]. There is a need to better characterise the RIA derived populations including cell numbers, phenotypes, and possible heterogeneity with respect to osteogeneic capabilities in order to further optimise tissue repair strategies.
Safety of the RIA
The case series of complications reported by Lowe et al. is a concern; however, it is unclear as to the incidence of these events from this report [25]. The other evidence suggests that the RIA is relatively safe, with a relatively low rate of complications (three of 87 cases) [5, 7–9]. All papers identify the need for a meticulous technique, emphasise the need for care when identifying entry point and that screening should be continuous to prevent/identify any complication [5, 7–9]. Reported complications were treated successfully using conventional techniques, with the majority (six of nine cases) requiring surgical stabilisation. Assessing the safety of a new device at an early stage in its use is fraught with danger as complications may take time to become evident.
Alternative sites that can provide similar volumes of bone graft such as the anterior/posterior ICBG and the fibula have well documented morbidity attached to them [49] and the RIA appears to be comparatively well tolerated [5]. The reported reduction in postoperative pain, when compared with ICBG harvest, is consistent with our own experience [5].
Limitations of the study
This review is limited principally by the relative scarcity of clinical data regarding the RIA. This is seen particularly when examining the incidence of TN and FE, as no clinical studies have examined this, and it raises questions as to the clinical relevance of the experimental findings. The exclusion of abstracts of posters was made in our data collection as we believe this data is insufficiently robust and lacking in detail to draw meaningful conclusions. This, along with the exclusion of non-English sources, has reduced the number of sources of data for our review, potentially biasing our findings.
Conclusion
In conclusion, the RIA appears well tolerated and relatively safe. When complications occur they respond well to conventional techniques. Experimental data suggest that the RIA is successful in preventing TN and FE although clinical evidence is lacking. Encouraging, albeit limited, clinical evidence supports the RIAs role in treating long-bone osteomyelitis. There is weight of evidence supporting RIA bone-fragments as a graft in promoting fracture union and it has been shown to be an effective harvester of MSCs. Well-designed higher power studies are warranted to further investigate the RIAs potential.
Acknowledgments
Conflict of interest The authors declare that there is no conflict of interest.
References
- 1.Glover P, Worthley LI. Fat embolism. Crit Care Resusc. 1999;1(3):276–284. [PubMed] [Google Scholar]
- 2.Giannoudis PV, Snowden S, Matthews SJ, et al. Temperature rise during reamed tibial nailing. Clin Orthop Relat Res. 2002;395:255–261. doi: 10.1097/00003086-200202000-00031. [DOI] [PubMed] [Google Scholar]
- 3.Giannoudis PV, Tzioupis C, Green J. Surgical techniques: how I do it? The reamer/irrigator/aspirator (RIA) system. Injury. 2009;40(11):1231–1236. doi: 10.1016/j.injury.2009.07.070. [DOI] [PubMed] [Google Scholar]
- 4.Zalavras CG, Singh A, Patzakis MJ. Novel technique for medullary canal debridement in tibia and femur osteomyelitis. Clin Orthop Relat Res. 2007;461:31–34. doi: 10.1097/BLO.0b013e318098673f. [DOI] [PubMed] [Google Scholar]
- 5.Belthur MV, Conway JD, Jindal G, et al. Bone graft harvest using a new intramedullary system. Clin Orthop Relat Res. 2008;466(12):2973–2980. doi: 10.1007/s11999-008-0538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobbe P, Tarkin IS, Pape HC. Use of the 'reamer irrigator aspirator' system for non-infected tibial non-union after failed iliac crest grafting. Injury. 2008;39(7):796–800. doi: 10.1016/j.injury.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Newman JT, Stahel PF, Smith WR, et al. A new minimally invasive technique for large volume bone graft harvest for treatment of fracture nonunions. Orthopedics. 2008;31(3):257–261. doi: 10.3928/01477447-20080301-29. [DOI] [PubMed] [Google Scholar]
- 8.Miller MA, Ivkovic A, Porter R, Harris MB, Estok DM, 2nd, Smith RM, Evans CH, Vrahas MS. Autologous bone grafting on steroids: preliminary clinical results. A novel treatment for nonunions and segmental bone defects. Int Orthop. 2010 doi: 10.1007/s00264-010-1013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCall TA, Brokaw DS, Jelen BA, Scheid DK, Scharfenberger AV, Maar DC, Green JM, Shipps MR, Stone MB, Musapatika D, Weber TG. Treatment of large segmental bone defects with reamer-irrigator-aspirator bone graft: technique and case series. Orthop Clin North Am. 2010;41(1):63–73. doi: 10.1016/j.ocl.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Stafford PR, Norris B. Reamer-irrigator-aspirator as a bone graft harvester. Tech Foot Ankle Surg. 2007;6:100–107. doi: 10.1097/btf.0b013e331806213b3. [DOI] [Google Scholar]
- 11.Porter RM, Liu F, Pilapil C, et al. Osteogenic potential of reamer irrigator aspirator (RIA) aspirate collected from patients undergoing hip arthroplasty. J Orthop Res. 2009;27(1):42–49. doi: 10.1002/jor.20715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins TF, Casey V, Bachus K. Cortical heat generation using an irrigating/aspirating single-pass reaming vs conventional stepwise reaming. J Orthop Trauma. 2007;21(3):192–197. doi: 10.1097/BOT.0b013e318038d952. [DOI] [PubMed] [Google Scholar]
- 13.Husebye EE, Lyberg T, Madsen JE, et al. The influence of a one-step reamer-irrigator-aspirator technique on the intramedullary pressure in the pig femur. Injury. 2006;37(10):935–940. doi: 10.1016/j.injury.2006.06.119. [DOI] [PubMed] [Google Scholar]
- 14.Gorp CC, Falk JV, Kmiec SJ, Jr, et al. The reamer/irrigator/aspirator reduces femoral canal pressure in simulated TKA. Clin Orthop Relat Res. 2009;467(3):805–809. doi: 10.1007/s11999-008-0258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joist A, Schult M, Ortmann C, et al. Rinsing-suction reamer attenuates intramedullary pressure increase and fat intravasation in a sheep model. J Trauma. 2004;57(1):146–151. doi: 10.1097/01.TA.0000100379.54339.0E. [DOI] [PubMed] [Google Scholar]
- 16.Schult M, Küchle R, Hofmann A, et al. Pathophysiological advantages of rinsing-suction-reaming (RSR) in a pig model for intramedullary nailing. J Orthop Res. 2006;24(6):1186–1192. doi: 10.1002/jor.20106. [DOI] [PubMed] [Google Scholar]
- 17.Pape HC, Zelle BA, Hildebrand F, et al. Reamed femoral nailing in sheep: does irrigation and aspiration of intramedullary contents alter the systemic response? J Bone Joint Surg Am. 2005;87(11):2515–2522. doi: 10.2106/JBJS.D.02024. [DOI] [PubMed] [Google Scholar]
- 18.Bellapianta J, Gerdeman A, Sharan A, et al. Use of the reamer irrigator aspirator for the treatment of a 20-year recurrent osteomyelitis of a healed femur fracture. J Orthop Trauma. 2007;21(5):343–346. doi: 10.1097/BOT.0b013e318051532d. [DOI] [PubMed] [Google Scholar]
- 19.Hammer TO, Wieling R, Green JM, et al. Effect of re-implanted particles from intramedullary reaming on mechanical properties and callus formation. A laboratory study. J Bone Joint Surg Br. 2007;89(11):1534–1538. doi: 10.1302/0301-620X.89B11.18994. [DOI] [PubMed] [Google Scholar]
- 20.Pape HC, Zelle BA, Pfeiffer R, et al. Retrograde autologous bone grafting through the intramedullary canal using the reamer irrigator aspirator. Injury Extra. 2008;39:398–400. doi: 10.1016/j.injury.2008.07.009. [DOI] [Google Scholar]
- 21.Cole P, Hollier L. An alternative source of autograft bone for spinal fusion: the femur. J Craniofac Surg. 2009;20:266. [Google Scholar]
- 22.Nichols TA, Sagi HC, Weber TG et al (2008) An alternative source of autograft bone for spinal fusion: the femur: technical case report. Neurosurgery. 62(3 Suppl 1) [DOI] [PubMed]
- 23.Huffman LK, Harris JG, Suk M. Using the bi-masquelet technique and reamer-irrigator-aspirator for post-traumatic foot reconstruction. Foot Ankle Int. 2009;30(9):895–899. doi: 10.3113/FAI.2009.0895. [DOI] [PubMed] [Google Scholar]
- 24.Schmidmaier G, Herrmann S, Green J, et al. Quantitative assessment of growth factors in reaming aspirate, iliac crest, and platelet preparation. Bone. 2006;39(5):1156–1163. doi: 10.1016/j.bone.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 25.Lowe JA, Della Rocca GJ, Murtha Y, Liporace FA, Stover MD, Nork SE, Crist BD. Complications associated with negative pressure reaming for harvesting autologous bone graft: a case series. J Orthop Trauma. 2010;24(1):46–52. doi: 10.1097/BOT.0b013e31819c0ccb. [DOI] [PubMed] [Google Scholar]
- 26.Bajada S, Mofidi A, Richardson JB. Comment on: Kobbe P, et al. Use of the 'reamer irrigator aspirator' system for non-infected tibial non-union after failed iliac crest grafting [Injury 2008;39(7):796-800] Injury. 2009;40(6):678. doi: 10.1016/j.injury.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Kwong FN, Richardson SM, Evans CH. Chordin knockdown enhances the osteogenic differentiation of human mesenchymal stem cells. Arthritis Res Ther. 2008;10(3):R65. doi: 10.1186/ar2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wehling N, Palmer GD, Pilapil C, et al. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009;60(3):801–812. doi: 10.1002/art.24352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pape HC, Hildebrand F, Krettek C, et al. Experimental background: review of animal studies. Injury. 2006;37(suppl 4):S25–S38. doi: 10.1016/j.injury.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 30.Giannoudis PV, Tzioupis C, Pape HC. Fat embolism: the reaming controversy. Injury. 2006;37(Suppl 4):S50–S58. doi: 10.1016/j.injury.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 31.Wozasek GE, Simon P, Redl H, et al. Intramedullary pressure changes and fat intravasation during intramedullary nailing: an experimental study in sheep. J Trauma. 1994;36(2):202–207. doi: 10.1097/00005373-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Wenda K, Runkel M, Degreif J, et al. Pathogenesis and clinical relevance of bone marrow embolism in medullary nailing: demonstrated by intraoperative echocardiography. Injury. 1993;24(suppl 3):S73–S81. doi: 10.1016/0020-1383(93)90011-T. [DOI] [PubMed] [Google Scholar]
- 33.Frölke JP, Bakker FC, Patka P, et al. Reaming debris in osteotomized sheep tibiae. J Trauma. 2001;50(1):65–69. doi: 10.1097/00005373-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Furlong AJ, Giannoudis PV, Smith RM. Heterotopic ossification: a comparison between reamed and unreamed femoral nailing. Injury. 1997;28(1):9–14. doi: 10.1016/S0020-1383(96)00147-7. [DOI] [PubMed] [Google Scholar]
- 35.Frölke JP, Krol H, Bakker FC, et al. Destination of debris during intramedullary reaming. An experimental study on sheep femurs. Acta Orthop Belg. 2000;66(4):337–340. [PubMed] [Google Scholar]
- 36.Forster MC, Bruce AS, Aster AS. Should the tibia be reamed when nailing? Injury. 2005;36(3):439–444. doi: 10.1016/j.injury.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 37.Bianco P, Riminucci M, Gronthos S, et al. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 38.Kolf CM, Cho E, Tuan RS. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther. 2007;9:204. doi: 10.1186/ar2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruder SP, Fink DJ, Caplan AI. Mesencymal stem cells in bone development, bone repair and skeletal regeneration therapy. J Cell Biochem. 1994;56:283–294. doi: 10.1002/jcb.240560303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruder SP, Kurth AA, Shea M, et al. Bone regeneration by implantation of purified culture-expanded human mesenchymal stem cells. J Orthop Res. 1998;16:155–162. doi: 10.1002/jor.1100160202. [DOI] [PubMed] [Google Scholar]
- 41.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 42.Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 43.Castro-Malaspina H, Gay RE, Resnick G, et al. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56:289–301. [PubMed] [Google Scholar]
- 44.Jones EA, Kinsey SE, English A, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46(12):3349–3360. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 45.Wakitani S, Goto T, Pineda SJ, et al. Mesenchymal cell based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 46.Banfi A, Muraglia A, Dozin B, et al. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 2000;28:707–715. doi: 10.1016/S0301-472X(00)00160-0. [DOI] [PubMed] [Google Scholar]
- 47.Jones EA, English A, Kinsey SE, et al. Optimization of a flow cytometry-based protocol for detection and phenotypic characterization of multipotent mesenchymal stromal cells from human bone marrow. Cytometry B Clin Cytom. 2006;15;70(6):391–399. doi: 10.1002/cyto.b.20118. [DOI] [PubMed] [Google Scholar]
- 48.Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatol Oxf. 2008;47(2):126–131. doi: 10.1093/rheumatology/kem206. [DOI] [PubMed] [Google Scholar]
- 49.Ebraheim NA, Elgafy H, Xu R. Bone-graft harvesting from iliac and fibular donor sites: techniques and complications. J Am Acad Orthop Surg. 2001;9:210–218. doi: 10.5435/00124635-200105000-00007. [DOI] [PubMed] [Google Scholar]