Abstract
Patients with multiple myeloma are often treated surgically as though they have bone metastases. Due to major differences in oncological therapy and comparatively long survival times these patients should be considered separately. Seventy-five multiple myeloma patients were treated surgically (83 interventions) for skeletal complications of the disease. Location and dissemination, symptoms, method of surgery, complications, recurrence and survival time were evaluated retrospectively. Most of the lesions were in the axial skeleton or the proximal extremities apart from one distal lesion of the fibula, and most surgery was performed in the spine (35 patients). The mean follow-up of patients was 5.4 years (range 1–25 years). Survival proved to be very favourable (37% at five years). Patients with a single bone lesion, a negative bone marrow biopsy, no paraproteinaemia in serum or a Salmon-Durie-stage I had a better survival probability. Surgical treatment in patients with multiple myeloma was mostly limited to a palliative approach but survival time was better (37% at five years) than in patients with metastatic bone disease which has to be considered in their surgical treatment.
Introduction
Multiple myeloma induces osteolysis and shifts the normal balance of bone formation toward bone resorption [1]. As a result, focal lytic lesions, pathological fractures and bone pain are common clinical manifestations in multiple myeloma patients. These distinct clinical features are major causes of morbidity and mortality [2]. Whereas disseminated disease with multiple osteolytic lesions is seen in most patients, a solitary lesion may be present in 5–10% of cases [3, 4].
Surgery has a supportive role in the management of myeloma and is performed with the intention to stabilise an impending or existing pathological fracture in cases of therapy-refractory pain, neurological complications, or spinal instability [5–7]. Although patients with bone metastases have been reported to be at an increased risk for skeletal complications, there is limited data on the correlation between bone lesions and survival in patients with multiple myeloma [8]. Because the survival of patients surgically treated for multiple myeloma of the bone exceeds that of patients with metastatic disease, it is important to fully understand the effects of surgery on these patients [9].
Therefore, we retrospectively evaluated the prognostic factors of 75 consecutive patients treated surgically for multiple myeloma.
Patients and methods
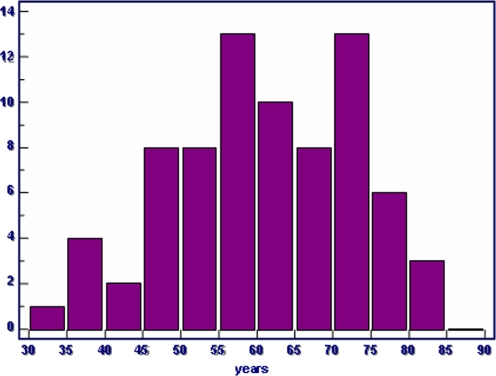
Between 1980 and 2005, 75 patients with multiple myeloma were treated surgically because of skeletal manifestations. Indications for surgery were a pathological or impending fracture or a neurological impairment due to a spinal lesion. The patients’ age, gender, symptoms, location and treatment of the skeletal lesion, the grade of dissemination of the disease, complications, and survival were analysed. The mean age of all patients combined (42 men and 33 women) was 60 years (range 31–85 years) (Fig. 1); mean age of the men was 57 years whereas the women were 65 years old.
Fig. 1.
Age distribution of 75 patients surgically treated for skeletal lesions of multiple myeloma
Following the distribution of haematopoietic bone marrow most of the lesions were located in the axial skeleton or the proximal extremities. The most common location of bone lesions was the spine (n = 8 cervical, n = 16 thoracic and n = 21 lumbar spine), the femur (n = 11) and the humerus (n = 10). Nine patients had a lesion of the trunk (other than the spine) and six patients a lesion of the pelvis. One patient had a lesion of the fibula.
The mean duration of symptoms was 7.5 months (range 0–122; median three months). In addition to pain, present in all patients, 48 (64%) reported pathological fracture and 23 (31%) suffered from neurological impairment.
Forty-one of the patients had a positive bone marrow biopsy, 11 a negative biopsy and in 23 patients the bone marrow biopsy result was unknown. Thirty-six had a Bence-Jones proteinuria, 31 had no proteinuria and in eight cases the status was unknown. In 57 cases we found a paraproteinaemia in serum.
Eleven of the patients had a Salmon-Durie stage of IA, one of IB, 30 of IIA, 11 of IIB, 15 of IIIA, six of IIIB and in 12 cases the Salmon-Durie stage was not calculated.
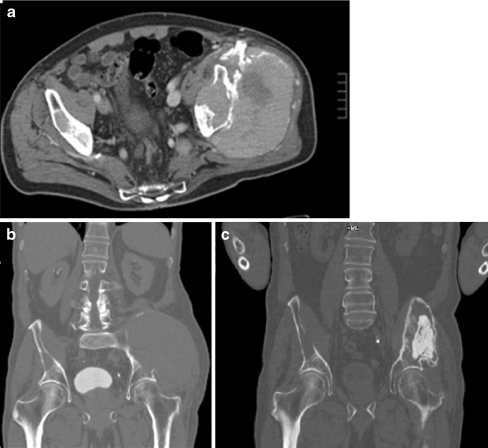
Overall 83 operations were performed. In 14 cases only an incisional biopsy was done. In patients with involvement of the spine, four were treated by decompression alone and 27 by decompression including instrumentation and six by vertebro-/kyphoplasty. In nine patients an endoprosthesis was implanted. In cases not including major parts of the joint, a cement augmented osteosynthesis was done (20 patients). These included plate fixations or intramedullary devices (nails). In one case with a large lesion of the pelvis only, cementation was performed after a preoperative chemo- and radiation therapy (Fig. 2). Two patients had resection of the lesion only without stabilisation.
Fig. 2.
a, b Large lesion of the pelvis secondary to multiple myeloma, preoperatively treated with chemo- and radiation therapy to eliminate the soft tissue tumour at the time of surgery. c Stabilisation of the bone lesion through cementation
Sixty-six patients received radiation therapy, nine patients before surgery and eight before and after surgery. Fifty-eight patients had chemotherapy; 11 of these preoperatively and 14 of these pre- and postoperatively.
Statistical analyses were performed using the Cox regression for multivariate analysis, Kaplan-Meier life table analyses and the log rank test for univariate analysis. We used the MedCalc® Software (MedCalc, Mariakerke, Belgium).
Results
Follow-up was performed between one and 25 years after surgery (average 5.4 years).
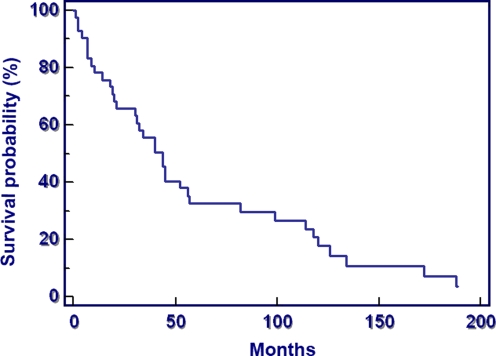
Survival proved to be very favourable (37% at five years, median survival 4.7 years); 81.7% of the patients survived longer than one year after surgical treatment of a skeletal lesion (Fig. 3).
Fig. 3.
Survival probability after surgical therapy of 75 patients with a bone lesion secondary to multiple myeloma
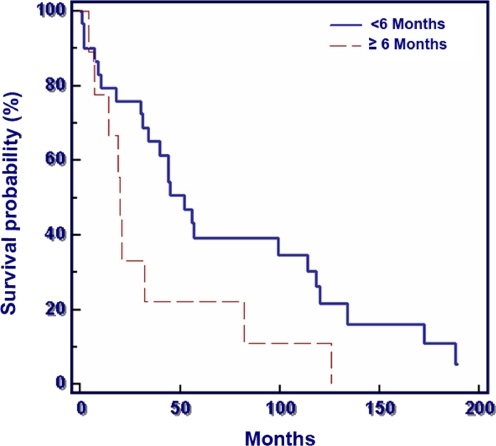
Duration of symptoms (<6 months versus ≥6 months) had no significant influence on survival (p = 0.053), but there was a clear trend towards a poorer survival in the patients with a longer duration of symptoms (Fig. 4).
Fig. 4.
Duration of symptoms of 75 patients surgically treated for bone lesions secondary to multiple myeloma (p = 0.053)
Median survival in the patients with duration of symptoms of less than six months was 4.9 years, and in the group with duration of more than six months it was 2.2 years. The survival probabilities at one year were 77% (symptoms ≥6 months) and 87% (symptoms <6 months).
The main predicting factors for the survival probability are shown in Table 1.
Table 1.
The main predicting factors for the survival probability in patients with multiple myeloma
| Factor | Definition | p-value |
|---|---|---|
| Bone lesion (n = 75) | Single versus multiple | 0.04 |
| Bone marrow biopsy (n = 52) | Negative versus positive | 0.0007 |
| Radiation (n = 66) | Post- versus preoperative | 0.02 |
| Salmon-Durie stage (n = 63) | Stage I versus II and III | 0.04 |
| Paraproteinaemia in serum (n = 70) | Yes versus no | 0.03 |
Patients with a single bone lesion proved to have a better median survival of 9.5 years compared to 3.7 years in patients with multiple bone lesions (p = 0.04).
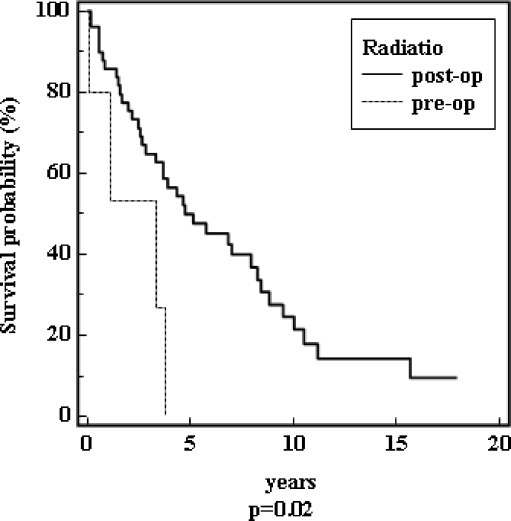
Patients with a positive bone marrow biopsy had a median survival time of 3.8 years in contrast to ten years in patients with a negative bone marrow biopsy (p = 0.007). Patients with a postoperative radiation had a better survival prognosis than patients with preoperative radiation (Fig. 5). The median survival for patients with Salmon Durie stages II and III was nearly identical (stage II was 3.66 years; stage III was 3.33 years) in contrast to patients with stage I (9.5 years, p = 0.004). In patients without paraproteinaemia the median survival was 8.8 years, and in patients with paraproteinaemia it was 3.3 years (p = 0.03).
Fig. 5.
Survival of the patients depending on a pre- or postoperative radiation (n = 66)
The location of the bone lesion, whether a fracture was present or not and the patient’s age and gender did not influence the prognosis (p > 0.05).
Postoperative complications were deep vein thrombosis in one patient, respiratory insufficiency in four cases and cardiovascular insufficiency in two cases. Two patients developed a septicaemia in the postoperative period. Two patients needed revision because of a deep wound infection. One patient had a transient bowel atonia. One patient suffered a pleural effusion. One patient developed a haemothorax as a consequence of postoperative bleeding, while five patients showed severe bleeding during surgery and three patients postoperatively. Two patients had vascular injuries at surgery and another five had progressive neurological impairment with paraplegia including atonia of the bladder and the rectum in two cases. Another five patients showed prolonged wound healing.
Discussion
Patients with multiple myeloma are treated in many centres in the same way as patients having metastatic disease [8, 10–14]. Due to major differences in oncological therapy, for example, bone marrow transplantation [15–17] and comparatively long survival times [9, 18], this group of patients should be considered as a separate entity. Ultimately, surgical risks must be weighed against life expectancy and quality of life to justify surgical intervention.
Thus, we evaluated multiple variables as predicting factors.
Whereas 60% of the patients suffered from skeletal pain in the 1960s and this symptom decreased to 40% in the 1990s [19], in our group the duration of symptoms until surgical intervention was long. The suspected diagnosis of multiple myeloma is mainly based on immuno-electrophoretical examination. This was positive for 57 (76%) in the serum and 36 (48%) in the urine. We could confirm that serological investigations, such as immune electrophoretic measurements, can lead to preoperative diagnosis and are important for postoperative prognosis [20]. As the diagnosis of multiple myeloma can be confirmed easily with an immuno-electrophoretical examination it should be performed in every patient with prolonged non-specific musculo-skeletal symptoms. Only with this setting a long duration of symptoms with a probably worse prognosis can be avoided. Even if there were no statistical difference in the prognosis between the patients with a longer duration of symptoms, there was a clear trend towards a worse prognosis (p = 0.053).
Saad et al. reported, in a study of 3,049 subjects that patients with multiple myeloma had the highest rate of fractures (43%), compared to all patients with malignant bone disease, with an associated increased risk of death, which could not be confirmed on survival by site (p > 0.05) [8]. Although 64% of our patients showed a pathological fracture at the time of surgical treatment, we could not find a decreased survival probability (p > 0.05).
However, the most important positive predicting factors were a negative bone marrow biopsy and no paraproteinaemia in serum and the absence of further lesions. Furthermore, a Salmon-Durie stage I was associated with an improved the prognosis. In the very few cases where a single lesion was resected mainly by reconstruction with an endoprosthesis, a longer survival was observed. This was probably due to the early stage of the disease in these patients. All of them later developed systemic progression.
The decision for a surgical procedure depended on the clinical status of the patient and survival estimates based on prognostic factors [5]. Therefore, our surgical treatment ranged from biopsy to spinal decompression with stabilisation. Surgical treatment of multiple myeloma is a palliative procedure in the majority of cases, but approximately 40% of the patients survived the next five years. This survival rate is longer than in patients treated surgically with metastatic spread to bone from another primary [21]. Only a small rate of solitary multiple myeloma of the peripheral extremities, such as the fibular lesion reported above, offers the chance of a real cure [9, 22].
In accordance with the literature [22, 23], the most common site of fracture in our analysis was the spine (59%), especially in the lower thoracic or lumbar vertebral bodies. Other common sites of fracture included the femur, pelvis, trunk and humerus. Overall, the peri- and postoperative complication rate in our patients was high, which we attribute on one hand to a progressive disease and on the other to the procedures performed. In particular, patients treated in the initial years without embolisation of the spine showed a worse complication rate.
Conclusion
Whereas surgical treatment in patients with multiple myeloma was limited to a palliative approach, the survival time achieved was more favourable than in any other subgroup of patients with metastatic disease to the bone. This has to be considered in any surgical approach to skeletal lesions.
In many patients the duration of symptoms was long, which could lead to a worse prognosis. Bone marrow biopsy and electrophoresis are important diagnostic features to avoid missed or late diagnosis. Multiple bone lesions, a positive bone marrow biopsy, a Salmon-Durie stage II or III and a paraproteinaemia in serum are factors predicting a poorer survival probability.
Adjuvant radiotherapy is an important method to reduce local recurrence of intralesional surgery, but one has to be aware that radiotherapy has to be used restrictively due to the necessity of viable bone marrow in those patients receiving aggressive chemotherapy.
Acknowledgements
No benefits or funds were received in support of the study.
Conflict of interest statement All authors disclose any financial and personal relationships with other people or organisations that could inappropriately influence (bias) this work.
References
- 1.Mundy GR, Bertolini DR. Bone destruction and hypercalcemia in plasma cell myeloma. Semin Oncol. 1986;13:291–299. [PubMed] [Google Scholar]
- 2.Kyle RA. Multiple myeloma: review of 869 cases. Mayo Clin Proc. 1975;50:29–40. [PubMed] [Google Scholar]
- 3.Bataille R, Sany J. Solitary myeloma: clinical and prognostic features of a review of 114 cases. Cancer. 1981;48:845–851. doi: 10.1002/1097-0142(19810801)48:3<845::AID-CNCR2820480330>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 4.Chak LY, Cox RS, Bostwick DG, Hoppe RT. Solitary plasmacytoma of bone: treatment, progression, and survival. J Clin Oncol. 1987;5:1811–1815. doi: 10.1200/JCO.1987.5.11.1811. [DOI] [PubMed] [Google Scholar]
- 5.Zahlten-Hinguranage A, Goldschmidt H, Cremer FW, Egerer G, Moehler T, Witte D, et al. Preoperative elevation of serum C-reactive protein is predictive for prognosis in myeloma bone disease after surgery. Br J Cancer. 2006;95:782–787. doi: 10.1038/sj.bjc.6603329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonsson B, Sjostrom L, Jonsson H, Jr, Karlstrom G. Surgery for multiple myeloma of the spine. A retrospective analysis of 12 patients. Acta Orthop Scand. 1992;63:192–194. doi: 10.3109/17453679209154821. [DOI] [PubMed] [Google Scholar]
- 7.McLain RF, Weinstein JN. Solitary plasmacytomas of the spine: a review of 84 cases. J Spinal Disord. 1989;2:69–74. doi: 10.1097/00002517-198906000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Saad F, Lipton A, Cook R, Chen YM, Smith M, Coleman R. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110:1860–1867. doi: 10.1002/cncr.22991. [DOI] [PubMed] [Google Scholar]
- 9.Durr HR, Kuhne JH, Hagena FW, Moser T, Refior HJ. Surgical treatment for myeloma of the bone. A retrospective analysis of 22 cases. Arch Orthop Trauma Surg. 1997;116:463–469. doi: 10.1007/BF00387578. [DOI] [PubMed] [Google Scholar]
- 10.Muhlbauer M, Pfisterer W, Eyb R, Knosp E. Noncontiguous spinal metastases and plasmocytomas should be operated on through a single posterior midline approach, and circumferential decompression should be performed with individualized reconstruction. Acta Neurochir (Wien) 2000;142:1219–1230. doi: 10.1007/s007010070018. [DOI] [PubMed] [Google Scholar]
- 11.Vieweg U, Meyer B, Schramm J. Tumour surgery of the upper cervical spine—a retrospective study of 13 cases. Acta Neurochir (Wien) 2001;143:217–225. doi: 10.1007/s007010170101. [DOI] [PubMed] [Google Scholar]
- 12.Wangsaturaka P, Asavamongkolkul A, Waikakul S, Phimolsarnti R. The results of surgical management of bone metastasis involving the periacetabular area: Siriraj experience. J Med Assoc Thai. 2007;90:1006–1013. [PubMed] [Google Scholar]
- 13.Kunisada T, Choong PF. Major reconstruction for periacetabular metastasis: early complications and outcome following surgical treatment in 40 hips. Acta Orthop Scand. 2000;71:585–590. doi: 10.1080/000164700317362217. [DOI] [PubMed] [Google Scholar]
- 14.Hentschel SJ, Burton AW, Fourney DR, Rhines LD, Mendel E. Percutaneous vertebroplasty and kyphoplasty performed at a cancer center: refuting proposed contraindications. J Neurosurg Spine. 2005;2:436–440. doi: 10.3171/spi.2005.2.4.0436. [DOI] [PubMed] [Google Scholar]
- 15.Yeh HS, Berenson JR. Treatment for myeloma bone disease. Clin Cancer Res. 2006;12:6279s–6284s. doi: 10.1158/1078-0432.CCR-06-0681. [DOI] [PubMed] [Google Scholar]
- 16.Levy V, Katsahian S, Fermand JP, Mary JY, Chevret S. A meta-analysis on data from 575 patients with multiple myeloma randomly assigned to either high-dose therapy or conventional therapy. Medicine (Baltimore) 2005;84:250–260. doi: 10.1097/01.md.0000173272.71949.a1. [DOI] [PubMed] [Google Scholar]
- 17.Segeren CM, Sonneveld P, Holt B, Vellenga E, Croockewit AJ, Verhoef GE, et al. Overall and event-free survival are not improved by the use of myeloablative therapy following intensified chemotherapy in previously untreated patients with multiple myeloma: a prospective randomized phase 3 study. Blood. 2003;101:2144–2151. doi: 10.1182/blood-2002-03-0889. [DOI] [PubMed] [Google Scholar]
- 18.Velde HJ, Liu X, Chen G, Cakana A, Deraedt W, Bayssas M. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica. 2007;2007(92):1399–1406. doi: 10.3324/haematol.11534. [DOI] [PubMed] [Google Scholar]
- 19.Barlogie B. Plasma cell myeloma. In: Williams WJ, Beutler E, editors. Hematology. 5. New York: McGraw-Hill-Publishing Co.; 1995. pp. 1109–1126. [Google Scholar]
- 20.Pasqualetti P, Casale R, Collacciani A, Colantonio D. Prognostic factors in multiple myeloma: a new staging system based on clinical and morphological features. Eur J Cancer. 1991;27:1123–1126. doi: 10.1016/0277-5379(91)90308-Z. [DOI] [PubMed] [Google Scholar]
- 21.Utzschneider S, Weber P, Fottner A, Wegener B, Jansson V, Durr HR. Prognosis-adapted surgical management of bone metastases. Orthopade. 2009;38:308–315. doi: 10.1007/s00132-008-1374-6. [DOI] [PubMed] [Google Scholar]
- 22.Durr HR, Wegener B, Krodel A, Muller PE, Jansson V, Refior HJ. Multiple myeloma: surgery of the spine: retrospective analysis of 27 patients. Spine. 2002;27:320–324. doi: 10.1097/00007632-200202010-00023. [DOI] [PubMed] [Google Scholar]
- 23.Lecouvet FE, Vande Berg BC, Maldague BE, Michaux L, Laterre E, Michaux JL, et al. Vertebral compression fractures in multiple myeloma. Part I. Distribution and appearance at MR imaging. Radiology. 1997;204:195–199. doi: 10.1148/radiology.204.1.9205246. [DOI] [PubMed] [Google Scholar]