Abstract
The main goal of total disc replacement (TDR) is to preserve motion. Despite reports of good clinical outcomes, various degrees of heterotopic ossification after TDR have been reported. The purpose of this study was to investigate the prevalence and its clinical relevance of heterotopic ossification. We evaluated 65 consecutive patients (82 segments) with mean follow-up duration of 45 months (range, 12–88 months). Two kinds of prosthesis, ProDisc® for 75 segments (91.5%) and CHARITETM for seven segments (8.5%), were used. Patients with heterotopic ossification were compared with those without heterotopic ossification with regard to segmental flexion–extension ROM, VAS and ODI. We analysed the occurrence site by nine zones. Heterotopic ossification was detected in 25 out of 82 segments (30.5%) at a mean follow-up of 17 months. According to McAfee’s classification, there was Class-I heterotopic ossification in eight segments (9.8%), Class-II in 12 segments (14.6%), and Class-III in five segments (6.1%). There was no Class-IV heterotopic ossification. There were no significant differences in the segmental ROM, VAS and ODI between the patients with Class-I or Class-II heterotopic ossification and those without heterotopic ossification The segmental ROM in the patients with Class-III heterotopic ossification was significantly decreased compared with the patients without heterotopic ossification (p = 0.018). But VAS and ODI were not significantly different compared with those of patients with no heterotopic ossification. Most heterotopic ossification (82.5%) was detected in the anterior and posterior aspects. In conclusion, most of the heterotopic ossification (Classes I and II) did not affect segmental ROM and clinical outcomes such as pain or function. In Class-III heterotopic ossification segmental ROM was decreased, but it did not affect clinical outcomes.
Introduction
The current gold standard for surgical treatment of lumbar degenerative disc disease (DDD) is fusion surgery. This technique has shown relatively satisfying clinical results by eliminating the abnormal motion and instability at the degenerated levels, thereby reducing low back pain. However, it destroys the normal spinal biomechanics and kinematics which may accelerate the degeneration of the adjacent segments [19]. It also has complications associated with harvesting bone graft [19].
The main goal of lumbar total disc replacement (TDR) is pain relief while maintaining or restoring range of motion (ROM) to overcome the complications of fusion surgery. So far, many kinds of TDR products have been commercially used thanks to the improvement of implant design and materials. TDR was considered as the theoretically ideal method for treating DDD in that it might preserve the normal motion of the intervertebral segment, it might not damage posterior paraspinal muscles, and it might not cause adjacent segment problems following interbody fusion. Most studies have reported favourable results after TDR [1, 2, 14, 15, 18]. However, some complications after TDR have been noted including heterotopic ossification, which may negatively affect clinical outcomes [2, 4, 9, 16]. If motion preservation is hindered by heterotopic ossification, the main goal of TDR can not be achieved, and better clinical outcomes may not be realised because the higher postoperative ROM was associated with better clinical outcomes [5, 10]. It was not uncommon for us to find heterotopic ossification during the follow-up after lumbar TDR.
The purposes of this study was to investigate the prevalence of heterotopic ossification and whether heterotopic ossification really hinders the ROM and affects the clinical results, such as pain relief and disability.
Materials and methods
We evaluated 65 consecutive patients who underwent lumbar TDR by two orthopaedic spine surgeons and had been followed for a minimum of one year. The total number of treated segments were 82 including 17 patients who underwent bisegmental TDR (Table 1). The patients comprised 24 men (36.9%) and 41 women (63.1%). The mean age at the time of operation was 43.8 years (range, 23–64) and the mean duration of follow-up was 45 months (range, 12–88). We used two kinds of prostheses, ProDisc® for 75 segments (91.5%) and CHARITETM for seven segments (8.5%).
Table 1.
Operated segments and heterotopic ossification (HO) categorised by level
| Operation level | One-level operation | Two-level operation | Total | |||
|---|---|---|---|---|---|---|
| L3-4 | L4-5 | L5-S1 | L3-4, 4-5 | L4-5,5-S1 | ||
| Operated patient(s) | 1 | 29 | 18 | 2 | 15 | 65 |
| Operated segment(s) | 1 | 29 | 18 | 4 | 30 | 82 |
| Patients with HO | 0 | 9 | 6 | 1 | 8a | 24 |
| Segments with HO | 0 | 9 | 6 | 1 | 9a | 25 |
a One patient had heterotopic ossification in both L4-5 and L5-S1
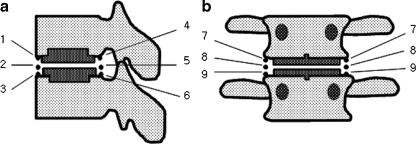
Anteroposterior, lateral, and lateral flexion and extension plain radiographs were taken preoperatively, immediately postoperatively as well as at one and six weeks, and three, six, and 12 months after surgery and every six months thereafter. The radiographs at the final follow-up were used to evaluate heterotopic ossification and segmental flexion–extension ROM. Segmental flexion–extension ROM was defined as the difference between two segmental angles in flexion and extension. We classified heterotopic ossification according to McAfee’s classification of heterotopic ossification [15]. The location of heterotopic ossification was also evaluated by dividing the peripheral area of implant into nine zones (Fig. 1). Because some of the heterotopic ossification did not occur within a single point, we counted all zones covered by the area of heterotopic ossification. All radiological measurements were repeated twice at an interval of two months by a fellowship-trained spine surgeon. To calculate the intra-observer reliability, Kappa statistics were used. According to the standard set by Landis and Koch, kappa values were analysed (<0.00, poor; 0.00–0.20, slight; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; 0.81–1.00, almost perfect agreement) [12]. Clinical outcomes were measured using the VAS (visual analog scale) and the ODI (Oswestry disability index) scores.
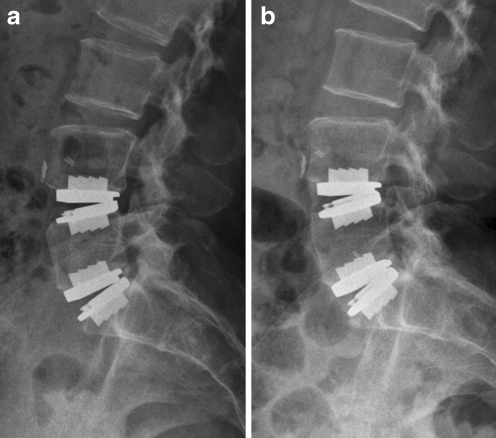
Fig. 1.
Lateral radiograph of female patient who underwent bisegmental total disc replacement (TDR). The ROM of L4-5 was 15.0° preoperatively, 13.7° at 3.5 years after surgery and 3.3° at final follow-up. a Follow-up radiograph 3.5 years after surgery. b Final follow-up radiograph 5 years after surgery. Note Class-III heterotopic ossification in anterior aspect of implanted L4-5 (zones 1,2,3)
Patients with heterotopic ossification were compared with those without heterotopic ossification with regard to segmental flexion–extension ROM, VAS and ODI. Statistical analysis was performed using Mann-Whitney U test with significance set at less than 0.05.
Results
In the 25 segments (25/82, 30.5%), heterotopic ossification was first detected at the mean follow-up of 17 months (range, 3–55) (Table 1), mostly within 24 months after operation. According to McAfee’s classification, there was Class-I heterotopic ossification in eight segments (9.8%), Class-II in 12 segments (14.6%), and Class-III in five segments (6.1%). Class-IV heterotopic ossification was not developed (Table 2). The average segmental flexion–extension ROM, VAS, and ODI scores in each class of heterotopic ossification are shown in Table 2. The average segmental ROM, VAS, and ODI in patients without heterotopic ossification were 11.4°, 2.9, and 17.6, respectively. The average segmental ROM, VAS, and ODI in patients with heterotopic ossification was 11.6°, 3.3, and 20.0, respectively.
Table 2.
Segmental ROM, VAS, and ODI by each class of heterotopic ossification
| McAfee classification | Segments (%) | Segmental ROM (range) | VAS (range) | ODI (range) |
|---|---|---|---|---|
| 0 | 57 (69.5 %) | 11.4° (7–21) | 2.9 (0–6) | 17.6 (2–54) |
| I | 8 (9.8 %) | 15.4° (10–19) | 4.6 (1–7) | 22.6 (12–36) |
| II | 12 (14.6 %) | 11.6° (8–22) | 2.1 (0–4) | 16.9 (2–40) |
| III | 5 (6.1 %) | 5.5° (4–8) | 4.0 (2–6) | 23.3 (6–52) |
| IV | 0 | - | - | - |
ROM range of motion, VAS visual analog scale, ODI Oswestry disability index
While there was no significant difference in the segmental ROM between the patients with Class-I or Class-II heterotopic ossification and those without heterotopic ossification, the segmental ROM in the patients with Class-III heterotopic ossification was significantly decreased as compared to the patients without heterotopic ossification (p = 0.048) (Fig. 1). In the VAS and ODI there was no significant difference between the patients with heterotopic ossification and those without heterotopic ossification (ODI: p = 0.586, 0.132, 0.320; VAS: p = 0.640, 0.898, 0.365, respectively, for Classes I, II, and III).
The region of ossification was evaluated by dividing it into nine zones (Fig. 2). On the lateral view, there were 20 heterotopic ossifications (42.5%) in the anterior aspect of the treated segment and 19 heterotopic ossifications (40.4%) in the posterior aspect. On the anteroposterior view, there were eight heterotopic ossifications (17.1%) in the lateral side of the treated segment (Table 3).
Fig. 2.
Heterotopic ossifications following lumbar total disc replacement were divided into nine areas as drawings. a Lateral view. b Anteroposterior view
Table 3.
Total segments with heterotopic ossification (HO) classified in nine zones
| Segments with HO | Zone | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| Number of segments | 8 | 3 | 9 | 11 | 4 | 4 | 2 | 3 | 3 | 47 |
| % of total | 17.0 | 6.4 | 19.1 | 23.4 | 8.5 | 8.5 | 4.3 | 6.4 | 6.4 | 100 |
In detecting the development of heterotopic ossification, the intra-observer kappa value was 0.831, representing almost perfect agreement with 92.7% concordance rate. The intra-observer kappa values for determination of class and occurrence site were 0.790 and 0.776, representing substantial agreement with 87.0% and 82.6% concordance rates.
The class of some heterotopic ossification had changed during follow-up. In two cases, Class-I heterotopic ossification had proceeded to Class-II heterotopic ossification after six months. Three cases had shown just increased radio-opacity without the change of class within six to 12 months follow-up.
There was one patient who developed heterotopic ossification with subsidence of the prosthesis at L5-S1 after bisegmental TDR at L4-L5 and L5-S1.
Discussion
Heterotopic ossification is a well-known phenomenon after total hip arthroplasty [3, 21]. The reported prevalence rates have varied from 0.6% to 61%. With increased use of total disc replacement in the spine field there has been a growing concern about heterotopic ossification following TDR. In 2003, McAfee et al. classified heterotopic ossification into five classes based on Brooker’s classification of heterotopic ossification after total hip arthroplasty [3, 15]. Several studies have been conducted to identify this problem especially in the field of total cervical disc replacement. Christoph et al. evaluated the rate of heterotopic ossification and its clinical relevance in 54 patients, with 77 implanted levels. They found only 33.8% of the patients did not show any sign of heterotopic ossification, and the rate of spontaneous fusion after total cervical disc replacement was unexpectedly high [16].
In the lumbar spine, there have been a few reports to address heterotopic ossification. Tortolani et al. investigated 276 patients treated with lumbar TDR using CHARITETM [22]. They reported the prevalence of heterotopic ossification was 4.3% at two-year follow-up and no difference in the ROM or the clinical outcomes was found between the patients with heterotopic ossification and those without heterotopic ossification [22]. Its incidence after lumbar TDR has been described as 1.4–83%, mostly between 10 and 15% [6, 9, 13, 20, 23].
In this study, there was a relatively high rate of heterotopic ossification (25 out of 82 segments, 30.5%) compared with previous reports. We think the reason for this is the meticulous search for any small ossification according to McAfee’s classification.
Tortolani et al. detected 11 of 12 cases with heterotopic ossification within the first three months after surgery [22]. They stated that if heterotopic ossification was not present within six months after surgery, it was unlikely to appear. Lemaire et al., on the other hand, reported heterotopic ossification detected in 3% with a minimum of ten years follow-up, and all of the heterotopic ossification appeared after the fifth year postoperatively [13]. In this study heterotopic ossification was detected at average 17 months after surgery, most (76%, 19 out of 25 cases) of which was detected within 24 months postoperatively. Late onset heterotopic ossification was also detected (Fig. 1). Four segments with heterotopic ossification were detected after more than three years postoperatively. We also found that heterotopic ossification can progress during follow-up. Therefore longer follow-up is necessary for detection of late occurrences. The occurrence rate of 30.5% in this study might be an underestimation of real incidence of late onset heterotopic ossification.
Only high degree heterotopic ossification (Class-III) was associated with subsequent loss of movement at the implanted segment. The low grade heterotopic ossification (Class-I or Class-II) was not associated with loss of ROM. It was unclear whether preservation of ROM results in a better clinical outcome. The clinical outcomes showed no difference between the patients with heterotopic ossification and those without heterotopic ossification and even high degree heterotopic ossification (Class-III) did not show any difference in clinical outcome. These results were in close agreement with previous studies [20, 22]. Putzier et al. reported that patients with heterotopic ossification with spontaneous fusion showed better clinical results [19]. Further follow-up is required to prove the correlation of ROM with clinical results.
The aetiology of heterotopic ossification is still unknown. It has been reported that factors associated with heterotopic ossification include perioperative bleeding in the vicinity of implant, especially at the keel cut site, rough tissue dissection, underlying diffuse idiopathic skeletal hyperostosis (DISH) and annular repair after implantation of the prosthesis [7, 8, 11, 22]. The perioperative administration of nonsteroidal anti-inflammatory drugs (NSAIDs) is recognised as important prophylactic treatment for patients undergoing total disc replacement [7, 17]. Therefore, meticulous haemostasis, gentle muscle dissection and the use of NSAID are generally recommended.
With regard to location, we observed most cases (82.9%) of heterotopic ossification on the anterior or posterior side of the implanted level. Only 17.1% of heterotopic ossification developed in the lateral side of the treated level. One possible reason for this finding is that the lumbar spine is more responsible for flexion–extension motion than lateral bending especially in the L4-5, L5-S1 level. The other reason is there is a possibility of underestimating heterotopic ossification developing on the lateral side. On simple anteroposterior radiographs the disc space may not be parallel to the beam of the X-ray. Especially at the L4-5, L5-S1 spinal level the disc space is inclined anteriorly in various degrees. Because the rectangular shape of the spinal body could not be obtained with these radiographs, it was hard to detect heterotopic ossification on the lateral side of the disc space. Ferguson view or CT may be helpful for more precise evaluation.
The first limitation of this study was relatively short follow-up period in relation to late onset heterotopic ossification. Secondly, we did not assess the effects of heterotopic ossification on lateral bending radiographs. Thirdly, there were insufficient cases to provide statistical power. We could not compare the incidence between two different prosthesis (ProDisc® and CHARITETM) because CHARITETM was implanted in only seven levels of which there was just one case with heterotopic ossification.
In conclusion, we detected heterotopic ossification after lumbar total disc replacement in 30.5% (25 segments out of 82 operated segments) at average follow-up of 17 months. Most of the heterotopic ossification (Classes I and II) did not affect segmental ROM and clinical outcomes such as pain or function. In Class-III heterotopic ossification segmental ROM was decreased, but it did not affect clinical outcomes. Further research is warranted to identify the potential risk factors and long-term clinical impact of heterotopic ossification.
References
- 1.YJ BR, Naveiva R, Fenk-Mayer A, Husted DS, Shah RV, Emerson JW. Lumbar total disc arthroplasty in patients older than 60 years of age: a prospective study of the ProDisc prosthesis with 2-year minimum follow-up period. J Neurosurg Spine. 2006;4:85–90. doi: 10.3171/spi.2006.4.2.85. [DOI] [PubMed] [Google Scholar]
- 2.Blumenthal S, McAfee PC, Guyer RD, Hochschuler SH, Geisler FH, Holt RT, Garcia R, Jr, Regan JJ, Ohnmeiss DD. A prospective, randomized, multicenter Food and Drug Administration investigational device exemptions study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine. 2005;30:1565–1575. doi: 10.1097/01.brs.0000170587.32676.0e. [DOI] [PubMed] [Google Scholar]
- 3.Brooker AF, Bowerman JW, Robinson RA, Riley LH., Jr Ectopic ossification following total hip replacement Incidence and a method of classification. J Bone Joint Surg Am. 1973;55:1629–1632. [PubMed] [Google Scholar]
- 4.Buttner-Janz K, Schellnack K. Intervertebral disk endoprosthesis—development and current status. Beitr Orthop Traumatol. 1990;37:137–147. [PubMed] [Google Scholar]
- 5.Chung SS, Lee CS, Kang CS. Lumbar total disc replacement using ProDisc II: a prospective study with a 2-year minimum follow-up. J Spinal Disord Tech. 2006;19:411–415. doi: 10.1097/00024720-200608000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Cinotti G, David T, Postacchini F. Results of disc prosthesis after a minimum follow-up period of 2 years. Spine. 1996;21:995–1000. doi: 10.1097/00007632-199604150-00015. [DOI] [PubMed] [Google Scholar]
- 7.Fransen M, Neal B (2004) Non-steroidal anti-inflammatory drugs for preventing heterotopic bone formation after hip arthroplasty. Cochrane Database Syst Rev CD001160 [DOI] [PubMed]
- 8.Geisler FH. Surgical technique of lumbar artificial disc replacement with the Charite artificial disc. Neurosurgery. 2005;56:46–57. doi: 10.1227/01.NEU.0000153215.60994.D3. [DOI] [PubMed] [Google Scholar]
- 9.Griffith SL, Shelokov AP, Buttner-Janz K, LeMaire JP, Zeegers WS. A multicenter retrospective study of the clinical results of the LINK SB Charite intervertebral prosthesis. The initial European experience. Spine. 1994;19:1842–1849. doi: 10.1097/00007632-199408150-00009. [DOI] [PubMed] [Google Scholar]
- 10.Huang RC, Girardi FP, Cammisa FP, Jr, Lim MR, Tropiano P, Marnay T. Correlation between range of motion and outcome after lumbar total disc replacement: 8.6-year follow-up. Spine. 2005;30:1407–1411. doi: 10.1097/01.brs.0000166528.67425.0e. [DOI] [PubMed] [Google Scholar]
- 11.Kerr EJ, Jawahar A, Kay S, Cavanaugh DA, PD N. Implant design may influence delayed heterotopic ossification after total disk arthroplasty in lumbar spine. Surg Neurol. 2009;72:747–751. doi: 10.1016/j.surneu.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 13.Lemaire JP, Carrier H, Sariali el H, Skalli W, Lavaste F. Clinical and radiological outcomes with the Charite artificial disc: a 10-year minimum follow-up. J Spinal Disord Tech. 2005;18:353–359. doi: 10.1097/01.bsd.0000172361.07479.6b. [DOI] [PubMed] [Google Scholar]
- 14.McAfee PC, Cunningham B, Holsapple G, Adams K, Blumenthal S, Guyer RD, Dmietriev A, Maxwell JH, Regan JJ, Isaza J. A prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of lumbar total disc replacement with the CHARITE artificial disc versus lumbar fusion: part II: evaluation of radiographic outcomes and correlation of surgical technique accuracy with clinical outcomes. Spine. 2005;30:1576–1583. doi: 10.1097/01.brs.0000170561.25636.1c. [DOI] [PubMed] [Google Scholar]
- 15.McAfee PC, Cunningham BW, Devine J, Williams E, Yu-Yahiro J. Classification of heterotopic ossification (HO) in artificial disk replacement. J Spinal Disord Tech. 2003;16:384–389. doi: 10.1097/00024720-200308000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Mehren C, Suchomel P, Grochulla F, Barsa P, Sourkova P, Hradil J, Korge A, Mayer HM. Heterotopic ossification in total cervical artificial disc replacement. Spine (Phila Pa 1976) 2006;31:2802–2806. doi: 10.1097/01.brs.0000245852.70594.d5. [DOI] [PubMed] [Google Scholar]
- 17.Neal B, Rodgers A, Dunn L, Fransen M (2000) Non-steroidal anti-inflammatory drugs for preventing heterotopic bone formation after hip arthroplasty. Cochrane Database Syst Rev CD001160 [DOI] [PubMed]
- 18.Ogon M, Howanietz N, Tuschel A, Chavanne A, Meissner J, Becker S. Implantation of the ProDisc intervertebral disk prosthesis for the lumbar spine. Oper Orthop Traumatol. 2007;19:209–230. doi: 10.1007/s00064-007-1203-9. [DOI] [PubMed] [Google Scholar]
- 19.Putzier M, Funk JF, Schneider SV, Gross C, Tohtz SW, Khodadadyan-Klostermann C, Perka C, Kandziora F. Charite total disc replacement–clinical and radiographical results after an average follow-up of 17 years. Eur Spine J. 2006;15:183–195. doi: 10.1007/s00586-005-1022-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regan JJ. Clinical results of charite lumbar total disc replacement. Orthop Clin North Am. 2005;36:323–340. doi: 10.1016/j.ocl.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Ritter MA, Vaughan RB. Ectopic ossification after total hip arthroplasty. Predisposing factors, frequency, and effect on results. J Bone Joint Surg Am. 1977;59:345–351. [PubMed] [Google Scholar]
- 22.Tortolani PJ, Cunningham BW, Eng M, McAfee PC, Holsapple GA, Adams KA. Prevalence of heterotopic ossification following total disc replacement. A prospective, randomized study of two hundred and seventy-six patients. J Bone Joint Surg Am. 2007;89:82–88. doi: 10.2106/JBJS.F.00432. [DOI] [PubMed] [Google Scholar]
- 23.Ooij A, Oner FC, Verbout AJ. Complications of artificial disc replacement: a report of 27 patients with the SB Charite disc. J Spinal Disord Tech. 2003;16:369–383. doi: 10.1097/00024720-200308000-00009. [DOI] [PubMed] [Google Scholar]