Abstract
The purpose of this study was to investigate the mechanism of expression of matrix metalloproteinase-13 (MMP-13) induced by nitric oxide (NO). Human chondrocytes (HCs) were stimulated with a NO donor (MAHMA-NONOate), then mitogen-activated protein kinases’ (MAPKs) and nuclear factor κB’ (NF-κB) activations and MMP-13′ expression were assayed by Western blot analysis. Additionally, the intracellular signalling of NO was investigated using the inhibitors of MAPKs and NF-κB. NO-induced MMP-13 expression was not suppressed by extracellular signal-regulated kinase (ERK) inhibitor (PD98059) or inhibitors of p38 kinase (SB203580), but was inhibited by a c-jun terminal kinase (JNK) inhibitor (SP600125) and inhibitors of NF-κB (SN-50). Additionally, SP600125 treatment reduced NF-κB activation, but SN-50 treatment did not significantly affect JNK activation. These results suggest that NO induces MMP-13 expression by JNK and NF-κB activation in HCs.
Introduction
Articular cartilage is composed of chondrocytes that produce an extracellular matrix composed primarily of type II collagen (CII) fibrils and the proteoglycan aggrecan [19].
Loss of collagen with associated fibrillation of the cartilage is a prominent characteristic of osteoarthritis (OA). In severe disease, the integrity of cartilage can be seriously compromised, with lesions extending to the bone. A positive correlation exists between matrix metalloproteinases (MMPs) activity in OA cartilage and the severity of lesions [12]. MMPs are a family of zinc-containing, calcium-dependent neutral proteases that share a common domain structure. OA chondrocytes express MMP-13, which cleaves CII more efficiently than MMP-1 and MMP-8 do [13].
Moreover, MMP-13 expression co-localises with CII degradation in active OA lesions, implying that the enzyme plays a pivotal role in cartilage degradation in this disease [13]. Additionally, inhibition of MMP-13 in cartilage explant models greatly reduces CII destruction, and overexpression of MMP-13 by articular chondrocytes in a transgenic mouse model results in cartilage destruction that strongly resembles OA [15]. MMP-13 is, therefore, believed to mediate CII degradation in OA.
OA chondrocytes and the adjacent synovial tissue secrete the inflammatory cytokine interleukin-1β (IL-1β), which induces both nitric oxide (NO) synthase activity and the expression of MMPs in chondrocytes [10]. The NO donor S-nitroso-N-acetyl-D,L-penicillamine (SNAP) also induces expression of MMP-13 in a dose-dependent fashion [14, 21]. These data provide evidence that NO plays a regulatory role in the expression of MMPs in articular chondrocytes and cartilage.
However, the mechanism whereby NO modulates the expression of MMP-13 has not been determined. The human MMP-13 promoter region contains an activator protein-1 (AP-1) responsive element, which has been shown to be essential for correct expression of the gene in all cell types [1]. Karin et al. found that c-Jun N-terminal kinases (JNKs) phosphorylate and activate the AP-1 family member c-Jun [5, 9], which dimerises with c-Fos to drive transcription of multiple MMP genes. Pfeilschifter et al. found that NO induced c-Jun phosphorylation in rat glomerular mesangial cells and primary cultures of bovine glomerular endothelial cells [16]. Therefore, we postulate that JNK is the main target for NO which may be critical for the NO-induced expression of MMP-13.
To test this hypothesis, we investigated the role of an exogenous NO donor in the modulation of expression of MMP-13 in human chondrocytes (HCs). Furthermore, the intracellular signalling of NO was investigated using the inhibitors of mitogen-activated protein kinases’ (MAPKs) and nuclear factor κB (NF-κB).
Materials and methods
Materials
HCs and chondrocytes growth medium were purchased from Cell Applications, Inc. (San Diego, CA). They were cryopreserved at the second passage and used at the third passage. The rabbit anti-phospho-p38 kinase polyclonal antibody, mouse anti-phospho-extracellular-signal regulated kinase 1/2 (ERK1/2) monoclonal antibody, rabbit anti-phospho-JNK polyclonal antibody, rabbit anti-phospho-inhibitor of kappaB (IκB)-α polyclonal antibody, rabbit anti-phospho-inhibitor κB kinase (IKK) α/β polyclonal antibody, mouse anti-MMP-13 polyclonal antibodies, as well as anti-mouse and anti-rabbit IgG horseradish peroxidase (HRP)-conjugated antibody were purchased from Cell Signaling Technology (Beverly, MA). Autoradiography film was from Kodak (Eastman Kodak, Rochester, NY, USA). Polyvinylidine difluoride (PVDF) protein transfer membranes were from Millipore (Millipore, Iberica, Madrid, Spain), and the enhanced chemiluminescence (ECL) detecting immunoblot system was from Amersham-Pharmacia (Buckinghamshire, UK). The NO donor, NOC-9, 6-(2-Hydroxy-1-methyl-2-nitrosohydrazino)-N-methyl-1-hexanamine (MAHMA-NONOate), was from Sigma, St. Louis, USA. All cell culture nutrients were from Gibco-BRL, Basel, Switzerland. The p38 kinase inhibitor (SB203580), NF-κB inhibitor (SN-50), the ERK1/2 inhibitors (PD98059), and the JNK inhibitor (SP600125) were purchased from Calbiochem (La Jolla, CA).
Cell culture
HCs were grown in chondrocytes growth medium supplemented with 100 units/ml penicillin G and 100 µg/ml streptomycin in dishes at 37°C in humidified 95% air and 5% CO2. Before stimulation, the medium was replaced with fresh DMEM supplemented with 100 units/ml penicillin G and 100 µg/ml streptomycin and the cells were incubated for 24 h, then the cells were treated with MAHMA-NONOate. For the inhibition assay, the medium was again replaced with a test compound (SB203580, SP600125, PD98059 or SN-50) originally dissolved in DMSO. Then, one hour later, cells were treated with MAHMA-NONOate.
Western blot analysis
HCs in dishes without serum were stimulated as indicated, then the cells were disrupted in protein lysis buffer (50 mmol/L Tris-HCl), 1 mmol/L ethylenediaminetetraacetic acid (EDTA), 1 mmol/L ethylene glycol-bis(beta-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 0.2 µg/mL phenylmethylsulfonyl fluoride (PMSF), and 2 µg/mL of the protease inhibitors (leupeptin, pepstatin, antipain, and antitrypsin). The homogenate was briefly centrifuged and supernatants were collected. Samples were quantified and stored for subsequent analysis.
Homogenates were fractionated by sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to PVDF membranes. Six kinds of protein expression were detected by incubating the membranes with the appropriate antibodies. Membranes were washed and incubated with conjugated horseradish peroxidase-anti-rabbit secondary antibodies, and developed with the ECL, according to the manufacturer’s instructions. We processed the strips from Western blots with Adobe Photoshop software, then measured their gray values with Bandscan analysis software to quantitate the expressions of proteins detected.
Statistical analysis
All experiments were repeated at least twice, and similar results were obtained in the repeated experiments. Statistical analysis was performed by one-way ANOVA. Data are expressed as the mean ± standard deviation (SD). P < 0.05 was considered significant.
Results
Western blot analysis with specific anti-MMP-13 antibodies demonstrated the biphasic concentration dependency of MMP-13 expression by MAHMA-NONOate in HCs with a peak at a concentration of 100 µM (Fig. 1).
Fig. 1.
MAHMA-NONOate-induced MMP-13 expression. Cells were stimulated with various concentrations of MAHMA-NONOate for 24 h and the levels of MMP-13 were determined by Western blot analysis. Points and bars are the means and standard deviations (SD), respectively, of the values obtained from three independent experiments performed in duplicate. *P < 0.05, significantly different from the control cultures
Because NF-κB activation requires phosphorylation of IκB-α and IKKα/β protein, we examined whether NO induced NF-kB activation by IκB-α and IKKα/β phosphorylation. Western blot analysis showed the concentration dependency of MAPKs’ and NF-κB’ phosphorylation by MAHMA-NONOate with a plateau at a concentration of 100 µM (Fig. 2).
Fig. 2.
MAHMA-NONOate-induced MAPKs and NF-κB activations. Cells were stimulated with various concentrations of MAHMA-NONOate for 30 min and the levels of pp38, pERK, pJNK, pIκB-α and pIKKα/β were determined by Western blot analysis with five kinds of antibodies respectively. Points and bars are the means and standard deviations (SD), respectively, of the values obtained from three independent experiments performed in duplicate. *P < 0.05, significantly different from the control cultures
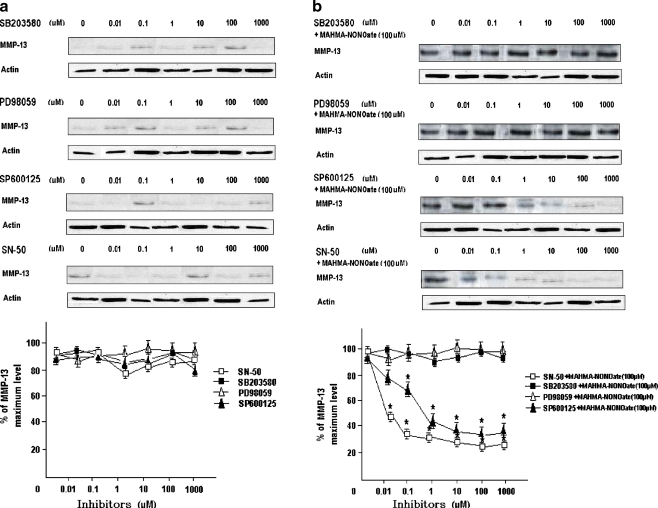
In addition, the inhibition assays using the inhibitors of MAPKs and NF-κB showed that every inhibitor itself had no effect on the expression of MMP-13 (Fig. 3a), and that all inhibitors did not inhibit NO-induced expression of MMP-13. For example, SP600125 and SN-50 both generated concentration-dependent inhibiting roles to NO-induced expression of MMP-13 with a plateau at a concentration of 10 µM and 0.1 µM, respectively, but PD98059 and SB203580 did not (Fig. 3b).
Fig. 3.
The inhibition of MMP-13 expression using the inhibitors of MAPKs and NF-κB. Cells were stimulated with various concentrations of SB203580, PD98059, SP600125 or SN-50 for 1 h respectively, and the levels of MMP-13 were determined by Western blot analysis (a). Cells were stimulated with various concentrations of SB203580, PD98059, SP600125 or SN-50 for 1 h respectively, then treated with 100 µM MAHMA-NONOate for 24 h and the levels of MMP-13 were determined by Western blot analysis (b). Points and bars are the means and standard deviations (SD), respectively, of the values obtained from three independent experiments performed in duplicate. *P < 0.05, significantly different from the control cultures
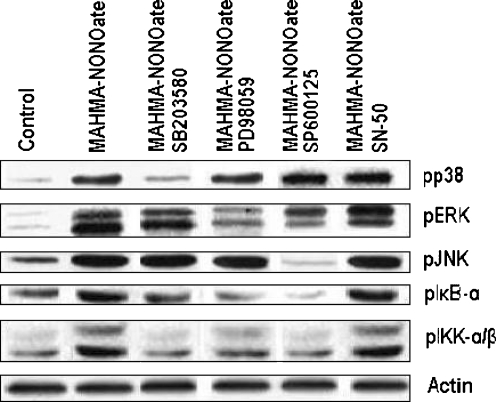
Since we found that NO induced activation of JNK and NF-κB, and that SP600125 (10 µM) and SN-50 (0.1 µM) strongly attenuated NO-induced expression of MMP-13, we next investigated whether a functional relationship exists between the activations of JNK and NF-κB in this context. In HCs, NO treatment caused activation of NF-κB, as demonstrated by IκB-α or IKKα/β phosphorylation analysis (Fig. 2), but the phosphorylations of IκB-α or IKKα/β were inhibited by SP600125 (Fig. 4). The latter experiments revealed that inhibition of JNK activation by treatment with SP600125 reduced NO-induced NF-κB activation levels to about 25% of the levels seen in NO-treated control levels (Fig. 4). In contrast, inhibition of NF-κB activation by treatment with SN-50 did not significantly affect NO-induced JNK activation (Fig. 4).
Fig. 4.
The inhibition of MAPKs and NF-κB activation using the inhibitors of MAPKs or NF-κB. Cells were stimulated with 10 µM SB203580, 10 µM PD98059, 10 µM SP600125 or 0.1 µM SN-50 for 1 h respectively, then treated with 100 µM MAHMA-NONOate for 30 min and the levels of pp38, pERK, pJNK, pIκB-α and pIKKα/β were determined by Western blot analysis with five kinds of antibodies respectively
Taken together, these results indicate that JNK and NF-κB might mediate MMP-13 expression in HCs, and that JNK phosphorylation induced by NO might have some effects on activation of NF-κB.
Discussion
Expression of MMP-13 is low in normal cells, and these low levels allow healthy connective tissue remodelling. In pathological conditions, however, the level of MMP-13 expression increases considerably, resulting in aberrant connective tissue destruction.
In OA, the mechanical insult causes cytokine expression by articular chondrocytes, with subsequent MMP-13 expression [18]. IL-1β and TNF-α elicit a series of shared phosphorylation events within the cells that facilitate transcriptional induction of MMP-13. One group of proteins that mediate some of these phosphorylation events is the MAPK group [3]. The MAPK family of serine/threonine kinases consists of the JNK, ERK and the p38 kinase. The JNK are activated in response to inflammatory cytokines, osmotic stress and apoptotic signals [2]. These stimuli first activate a group of protein kinases (MAPK kinase kinases [MAPKKKs]) that phosphorylate other kinases (MAPK kinases [MAPKKs]), which in turn are responsible for phosphorylation and activation of MAPK. Upon activation by MAPKKs, MAPKs translocate to the nucleus to phosphorylate and activate various transcription factors.
Of particular relevance to MMPs transcription, JNKs phosphorylate and activate the activating protein-1 (AP-1) family member c-Jun [5, 9], which dimerises with c-Fos to drive transcription of multiple MMP genes. Another major cytokine-induced signaling pathway involves translocation of NF-κB family members from the cytoplasm to the nucleus. The MAPK and NF-κB pathways are coordinately activated by IL-1β, and are central pathways in OA pathogenesis.
Sasaki et al. reported that NO mediates IL-1-induced gene expression of MMPs in cultured rabbit articular chondrocytes [17]. Our results also found that IL-1β at 1 ng/ml induced high levels of NO from HCs. NO is a double-edged sword. Synthesised in appropriate amounts it exerts important physiological functions. However, when produced in excessive amounts by the inducible NO synthase, it may contribute to the pathogenesis of a number of severe diseases. The exact mechanisms by which NO exerts its damaging effects are not yet fully understood. NO at high concentrations of 1 mM, reported as an important inducer of apoptosis, plays a considerable role in the pathogenetic mechanisms of articular diseases. The p38 MAPK signal transduction pathway is critical to NO-induced chondrocyte apoptosis, and p38 plays a role by way of stimulating NF-κB, p53 and caspase-3 activation [6, 7, 20].
Because we determined the expression of several kinds of proteins with Western blot, MAHMA-NONOate treatment durations were different. For MMP-13, IL-1 or TNF treatment causes an increase in the human chondrosarcoma cell line SW1353 after 20–24 h [8]. Similarly, NO donor treatment also causes an increase in human and bovine chondrocytes after 24 h [14] and after 16 h [21]. Our preliminary experiment showed that MAHMA-NONOate treatment also causes an increase in MMP-13 expression in human chondrocytes at the third passage within 16–48 h with a peak at 24 h. So we set 24 h as MAHMA-NONOate treatment durations for the determination of MMP-13. For MAPKs or NF-κB, MAHMA-NONOate treatment causes a very rapid and maximal increase in MAPK or NF-κB activation in rat renal mesangial cells after 10 min. This activation of MAPK or NF-κB returns to basal level after 60 min of stimulation [4, 16]. Although MAHMA-NONOate treatment also causes a maximal increase in MAPK or NF-κB activation in primary cultures of bovine articular chondrocytes after 15 min [11], our preliminary experiment showed that MAHMA-NONOate treatment also causes an increase in MAPK or NF-κB activation in human chondrocytes at the third passage within 10–60 min with a peak at 30 min, perhaps because of slightly lower cell viability. So we set 30 min as MAHMA-NONOate treatment durations for the determination of MAPK or NF-κB.
Our study shows that NO increased expression of MMP-13. Figure 1 shows the biphasic concentration dependency of MMP-13 expression by MAHMA-NONOate in HCs with a peak at a concentration of 100 µM, perhaps because higher concentrations than 100 µM of MAHMA-NONOate can induce apoptosis of the chondrocytes. Maybe MAPKs or NF-κB mediate NO-induced apoptosis of the chondrocytes, because Fig. 2 shows MAHMA-NONOate activates p38 kinase, ERK1/2, JNK, IκB-α and IKKα/β in a dose-dependent fashion from HCs, even in higher concentrations than 100 µM.
In addition, MAHMA-NONOate could induce MMP-13 expression within the concentration from 0.5 µM to 100 µM with a peak at 100 µM (Fig. 1), so we chose 100 µM to highlight the inhibiting effects in the inhibiting experiments (Fig. 3). SP600125 treatment reduced NO-induced NF-κB activation levels (Fig. 4), but inhibition of NF-κB activation by treatment with SN-50 did not significantly affect NO-induced JNK activation (Fig. 4). Taken together, these results indicate that JNK and NF-κB might mediate MMP-13 expression in HCs, and that JNK phosphorylation induced by NO might have some effects on activation of NF-κB by an unknown pathway in HCs.
Acknowledgements
This work was supported by Beijing Institute of Tropical Diseases. We are grateful to Bing-qiang Wang, Gao-Chao Zhao, Shu-lei Zhao, and Zhe Xu, PhD for assistance.
Conflict of interest None.
Contributor Information
Ai Guo, Email: phdyang45@gmail.com.
Jun-Chao Gu, Email: linye.yang@yahoo.com.cn.
References
- 1.Balbin M, Pendas AM, Uria JA, Jiménez MG, Freije JP, López-Otín C. Expression and regulation of collagenase-3 (MMP-13) in human malignant tumors. APMIS. 1999;107:45–53. doi: 10.1111/j.1699-0463.1999.tb01525.x. [DOI] [PubMed] [Google Scholar]
- 2.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/S0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 3.Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–218. doi: 10.1016/S0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- 4.Huwiler A, Pfeilschifter J. Nitric oxide stimulates the stress-activated protein kinases p38 in rat renal mesangial cells. J Exp Biol. 1999;202:655–660. doi: 10.1242/jeb.202.6.655. [DOI] [PubMed] [Google Scholar]
- 5.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 6.Kim SJ, Hwang SG, Shin DY, Kang SS, Chun JS. p38 kinase regulates nitric oxide-induced apoptosis of articular chondrocytes by accumulating p53 via NFkappa B-dependent transcription and stabilization by serine 15 phosphorylation. J Biol Chem. 2002;277(36):33501–33508. doi: 10.1074/jbc.M202862200. [DOI] [PubMed] [Google Scholar]
- 7.Kim SJ, Ju JW, Oh CD, Yoon YM, Song WK, Kim JH, Yoo YJ, Bang OS, Kang SS, Chun JS. ERK-1/2 and p38 kinase oppositely regulate nitric oxide-induced apoptosis of chondrocytes in association with p53, caspase-3, and differentiation status. J Biol Chem. 2002;277(2):1332–1339. doi: 10.1074/jbc.M107231200. [DOI] [PubMed] [Google Scholar]
- 8.Konda VR, Desai A, Darland G, Bland JS, Tripp ML. Rho iso-alpha acids from hops inhibit the GSK-3/NF-kappaB pathway and reduce inflammatory markers associated with bone and cartilage degradation. J Inflamm. 2009;6:26. doi: 10.1186/1476-9255-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leppa S, Saffrich R, Ansorge W, Bohmann D. Differential regulation of c-Jun by ERK and JNK during PC12 cell differentiation. Embo J. 1998;17:4404–4413. doi: 10.1093/emboj/17.15.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lianxu C, Hongti J, Changlong Y. NF-kappaBp65-specific siRNA inhibits expression of genes of COX-2, NOS-2 and MMP-9 in rat IL-1beta-induced and TNF-alpha-induced chondrocytes. Osteoarthr Cartil. 2006;14:367–376. doi: 10.1016/j.joca.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Lo Y, Wong J, Cruz T. Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases. J Biol Chem. 1996;271:15703–15707. doi: 10.1074/jbc.271.36.21906. [DOI] [PubMed] [Google Scholar]
- 12.Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kB. Arthritis Rheum. 2000;43:801–811. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE. Cloning, expression and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murrell GA, Jang D, Williams RJ. Nitric oxide activates metalloprotease enzymes in articular cartilage. Biochem Biophys Res Commun. 1995;206:15–21. doi: 10.1006/bbrc.1995.1003. [DOI] [PubMed] [Google Scholar]
- 15.Neuhold LA, Killar L, Zhao W, Sung ML, Warner L, Kulik J, Turner J, Wu W, Billinghurst C, Meijers T, Poole AR, Babij P, DeGennaro LJ. Postnatal expression in hyaline cartilage of constitutively active human collagenase-3 (MMP-13) induces osteoarthritis in mice. J Clin Invest. 2001;107:35–44. doi: 10.1172/JCI10564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfeilschifter J, Huwiler A. Nitric oxide stimulates stress-activated protein kinases in glomerular endothelial and mesangial cells. FEBS Lett. 1996;396:67–70. doi: 10.1016/0014-5793(96)01070-8. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki K, Hattori T, Fujisawa T, Takahashi K, Inoue H, Takigawa M. Nitric oxide mediates interleukin-1-induced gene expression of matrix metalloproteinases and basic fibroblast growth factor in cultured rabbit articular chondrocytes. J Biochem. 1998;123:431–439. doi: 10.1093/oxfordjournals.jbchem.a021955. [DOI] [PubMed] [Google Scholar]
- 18.Shlopov BV, Lie WR, Mainardi CL, Cole AA, Chubinskaya S, Hasty KA. Osteoarthritic lesions: involvement of three different collagenases. Arthritis Rheum. 1997;40:2065–2074. doi: 10.1002/art.1780401120. [DOI] [PubMed] [Google Scholar]
- 19.Sondergaard BC, Wulf H, Henriksen K, Schaller S, Oestergaard S, Qvist P, Tankó LB, Bagger YZ, Christiansen C, Karsdal MA. Calcitonin directly attenuates collagen type II degradation by inhibition of matrix metalloproteinase expression and activity in articular chondrocytes. Osteoarthr Cartil. 2006;14:759–768. doi: 10.1016/j.joca.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Wang Z, Chen J, Wu J. Apoptosis induced by NO via phosphorylation of p38 MAPK that stimulates NF-kappaB, p53 and caspase-3 activation in rabbit articular chondrocytes. Cell Biol Int. 2007;31(9):1027–1035. doi: 10.1016/j.cellbi.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Zaragoza C, Balbín M, López-Otín C, lamas S. Nitric oxide regulates matrix metalloprotease-13 expression and activity in endothelium. Kidney Int. 2002;61:804–808. doi: 10.1046/j.1523-1755.2002.00224.x. [DOI] [PubMed] [Google Scholar]