Abstract
Purpose
There is still debate over whether vertebroplasty (VP) or kyphoplasty (KP) is superior for the treatment of osteoporosis vertebral compression fractures (VCFs). We performed a systematic review and meta-analysis of randomised and non-randomised controlled trials comparing VP with KP to reach a relatively conclusive answer.
Methods
We searched computerised databases comparing efficacy and safety of VP and KP in osteoporotic fractures. These trials reported pain relief (Visual Analogue Scale), disability (Oswestry disability score) and complications (i.e., cement leakage, incident fractures) as the primary outcome.
Results
Eight studies involving 848 patients were identified. The outcome showed that VP is more effective in the short-term (no more than seven days) pain relief. Kyphoplasty had a superior capability for intermediate-term (around three months) functional improvement. As for long-term pain relief and functional improvement, there is no significant difference between these two interventions. Consistently, both interventions have similar risk for subsequent fracture and cement leakage.
Conclusion
Thus considering the higher cost of the KP procedure, we recommend VP over KP for the treatment of osteoporotic VCFs.
Introduction
Vertebral compression fractures (VCFs) constitute a major health care problem of osteoporosis worldwide. Due to the increasing ageing of the population there has been a constant rise in osteoporotic VCFs during the last decade [1]. It is estimated that 750,000 new vertebral fractures occur in the United States each year. Although only about one-third of these fractures become symptomatic, they can result in height loss, spinal deformity, acute and chronic pain, restriction of thoracic and abdominal contents, impaired mobility and disability.
Different approaches for the management of painful osteoporotic VCFs are currently available. Standard medical therapy includes bed rest, analgesia, bracing, external fixation, rehabilitation and a combination of these treatments [2]. However, there are still limitations of these methods. Long-term bed rest often leads to further demineralisation and may predispose the patients to future VCFs. As for medication of anti-inflammatory drugs and certain types of analgesics, it may be difficult for patients, especially the older ones, to tolerate the side effects. And medical management does not reverse kyphotic deformity. The surgical treatment for VCFs, refractory to medical therapy, involves surgical stabilisation via dorsal instrumentation [3]. However, because of the poor quality of osteoporotic bone, surgical fixation often fails and open surgical treatment is reserved to the rare cases of neurological deterioration or to the more frequent cases of persistent intractable pain. What’s more, such treatments are only partially effective in relieving symptoms. About one-third of patients have been reported to suffer from persistent pain and progressive functional limitation [4].
As we know, percutaneous vertebroplasty (VP) was first performed to treat patients with extreme pain caused by a haemangioma [5]. Kyphoplasty (KP) as a modified procedure of VP which aims at restoration of the vertebral body height. Both have become widely accepted and routine procedures for the management of pain associated with osteoporotic VCFs. Recently, two RCTs comparing KP or VP versus conservative treatments confirmed that immediate pain relief can be achieved with the help of both of these two minimally invasive treatments [6, 7]. Consistently, systemic reviews have indicated that both VP and KP were effective and safe medical interventions to control the pain of osteoporotic VCFs [8–10].
However, which of these interventions provides a better outcome than the other and whether the long-term outcomes are as favourable as the short-term outcomes remains unclear [11]. Although several systemic reviews have been performed to compare the efficacy and safety of VP versus KP in patients with symptomatic VCFs from osteoporosis, almost all of them only reported case series studies examining VP and KP for VCF as a result there is little literature directly comparing VP and KP [12–15]. There is only one systematic review to date by Taylor et al. reporting one article directly comparing these two interventions for treatment of VCFs from myeloma and other malignancies [15].
Recently, several studies directly comparing VP and KP, both randomised controlled trials (RCTs) and non-randomised controlled trials (NRTs), have been published. We therefore performed this meta-analysis to check if either of these two minimally invasive operations is superior to the other.
Materials and method
We conducted a computerised search of the electronic databases OVID MEDLINE, PubMed MEDLINE, and ISI web of knowledge MEDLINE. Searches were conducted from 1983 onwards until October 2010. Search terms were selected in order to maximise both the search sensitivity and specificity. Additionally, a manual search of peer-reviewed English documents was performed by cross-checking the bibliographies of selected articles. If multiple studies of the same patient population were identified, we only included the published report with the largest sample size. We did not seek unpublished investigations.
Inclusion criteria
Studies were included in this review if they met the following criteria:
Experimental studies (i.e., randomised or quasi-randomised trials) or observational studies (i.e., cohort or case-control studies) comparing balloon KP versus VP for treatment of patients with VCFs of osteoporotic a etiology.
Report of at least one of the following outcomes: efficacy, pain relief (visual analogue scale) or disability (Oswestry disability score); safety, cement leakage or incident fractures.
The two investigators (Shiliang Han and Shuanglin Wan) independently selected the documents according to the criteria described above. Consensus was reached by negotiation.
Assessment of risk of bias
We applied the “assessing risk of bias” table recommended in the Cochrane handbook 5.0.2 to assess the risk of bias of the articles included. There were several reasons for using this method. First, the key consideration in a Cochrane meta-analysis is the extent to which results of included studies should be believed. No such tools for assessment of methodological quality of the document can target this question squarely except for the “assessing risk of bias” table. Second, both randomised and non-randomised studies can be assessed for risk of bias by this method. We avoided assessing the quality of the studies in two different ways for randomised and non-randomised studies respectively.
The two investigators (Shiliang Han and Shuanglin Wan) assessed studies for “risk of bias” in strict accordance with the introduction of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0.
Data extraction
First, two investigators (Shiliang Han and Shuanglin Wan) independently tabulated the data. A double-check procedure was performed to make sure of the accuracy of the data extracted. Then, a manager inputted the extracted data into a spreadsheet. The following information was abstracted from the studies: the first author, publishing year, study design, sample volume, patients lost to follow-up, baseline demographic characteristic of patients and any possible data on efficacy (i.e., visual analog scale, Oswestry score) and safety (i.e., cement leakage and incident fractures).
Data analysis
The following methods were conducted and reported in strict accordance with the “2009 Updated Method Guidelines for Systematic Reviews in the Cochrane Back Review Group” [16] and “Cochrane Handbook for Systematic Reviews of Interventions 5.0.2”.
For dichotomous data, we calculated the crude RR (relative risks) and 95% CI for each study whenever possible. And the meta-analysis was performed on crude data extracted from the text. For continuous outcomes, we pooled data by employing weighted mean differences (WMD) of the final value across groups to check which one was superior to the other.
In instances in which a standard error for the final value outcome was not reported, we calculated the standard error of mean differences across groups by converting the p value to a z-score and solving for the standard error with the formula: z = mean difference/standard error [17]. In instances in which a study did not report the outcome at the exact time supposed, we took the one closest to the time examined for pooling [16].
Prior to analysing the data, Q statistics test was employed for the assessment of heterogeneity. The fixed-effects model (inverse-variance model) was used when the effects were assumed to be homogenous (p > 0.05), while the random-effects model (inverse-variance model) was used when they were heterogeneous (p < 0.05). Subgroup analysis was performed according to the study design. Sensitivity analysis was performed by excluding the study with obvious methodological heterogeneity.
Publication bias was assessed with funnel plots, in which the outcome (e.g. intervention effect) is plotted on the vertical axis and the covariate (e.g. the standard error of the logarithm of intervention effect) is plotted on the horizontal axis. Bias is revealed if the plots are asymmetrical about the pooled RR, whereas a plot resembling a symmetric funnel shows that no bias is present.
In addition, all statistical tests for this meta-analysis were performed with STATA Version 11.0. However, “risk of bias” assessment was realised by employing the Review Manager Version 5.0.
Results
Identification and selection of studies
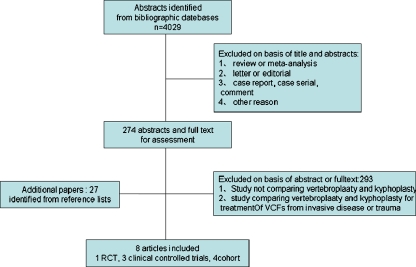
A total of 4,029 citations (1271 from OVID MEDLINE, 1822 from Pubmed MEDLINE, 936 from ISI web of knowledge MEDLINE) were obtained from searches of the various electronic bibliographies. A further 29 papers were obtained from the citation list of included studies. All the documents were selected strictly according to the criteria described. Ten articles comparing VP and KP were identified. One article was excluded because they employed these two interventions for treatment of VCFs due to either osteoporosis or trauma [18]. The other article was excluded because they compared the effect of these interventions for malignant cancer [19]. The study selection process and reasons for exclusions are summarised in Fig. 1.
Fig. 1.
Flow chart summarised the selection process of trials
Study characteristics and quality
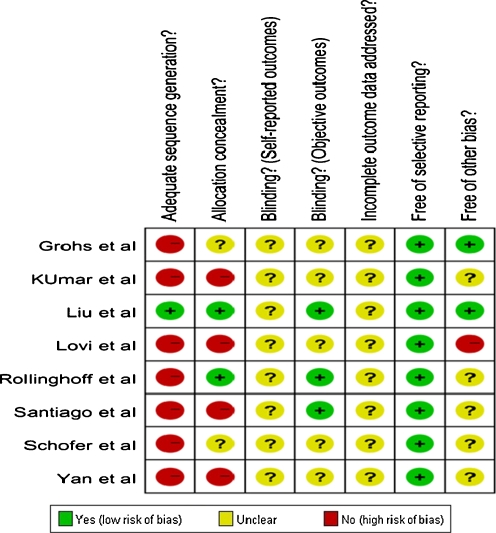
Eight articles directly comparing KP and VP were included in this meta-analysis: one randomised controlled trial [20], three clinical controlled trials [21–23] and four cohorts [24–27] (three prospective cohorts [24–26] and one retrospective cohort [27]). These studies were evaluated with the help of the “assessing risk of bias” table and the exact outcome was summarised in Fig. 2.
Fig. 2.
Risk of bias assessment of included studies
All of these articles were aimed at assessing efficacy and safety of VP and KP for treatment of osteoporotic VCFs, although not all of them reported the method for diagnosis of osteoporosis. Altogether, 848 patients were involved in this review. The sample sizes of the trials ranged from 51 to 244. The mean duration between injury and surgery varied greatly across the studies. VP or KP were the only procedures recommended to patients. PMMA was the only type of cement for the treatment of VCFs, although the volume used was not included in most of the articles. The demographic characteristic of the studies without significant difference between these two groups is summarised in Table 1.
Table 1.
Description of the studies included in the meta-analysis
| Author, year, country | Study design | SS | Age (years): VP/KP | Gender (M/F) | Volume of cement injected | Length of follow-up | Lost to follow-up | |
|---|---|---|---|---|---|---|---|---|
| VP | KP | |||||||
| Liu et al., 2009,Taiwan | RCT | 100 | 74.3 ± 6.4/72.3 ± 7.6 | 23/77 | 4.91 ± 0.65 | 5.56 ± 0.62 | 6 months | NR |
| Lovi et al., 2009,Italy | Prospective cohort | 164 | 67.6 (53–95)/67.6 (53–95) | 56/98 | 2.5 | 3.2 | 33 months | 10 |
| Röllinghoff et al., 2009, USA | CCT | 90 | 68.9 ± 10.4/68.9 ± 10.4 | 1/4.3 | NR | NR | 12 months | 10 |
| Grohs et al., 2009, Austria | CCT | 51 | 70 (64–77)/70 (65–74) | 12/39 | NR | NR | 24 months | NR |
| Kumar et al., 2010, Canada | Prospective cohort | 52 | 78 (57–94)/73 (52–89) | 16/36 | 3.2 (1.0-7.0) | 1.8 (0.75-5.0) | 42.2 months | 6 |
| Schofer et al., 2009, Germany | CCT | 60 | 73.8 ± 6.4/72.5 ± 5.7 | 14/46 | 4.9 ± 1.2 | >12 months | >12 months | 11 |
| Santiago et al., 2010, Spain | Prospective cohort | 60 | 73.0 ± 1.5/65.9 ± 1.9 | 14/46 | NR | NR | 12 months | NR |
| Yan et al., 2010,China | retrospective cohort | 244 | 77.2 ± 10.3/76.9 ± 11.5 | 80/112 | NR | NR | >12 months | 52 |
SS sample size, M/F male/female, RCT randomised controlled trial, CCT clinical controlled trial
In particular, there were two studies needing special attention. One reported the number of vertebra being treated but not the exact allocation of patients [21]. While in the other study more than 20% of the patients were lost to follow-up [27].
Visual analogue scales (VAS)
The pain intensity measured by VAS pain score was extracted and summarised as short-term (no more than seven days), mediate-term (closest to six months) and long-term (closest to one year) follow-ups. And then we pooled mean differences across the group.
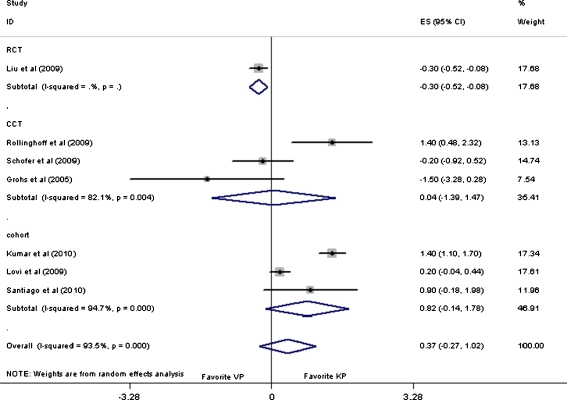
The overall pooled WMD on VAS pain score was 0.37 (95% CI: −0.27 to 1.02, p = 0.25) at short-term follow-up. Subgroup analysis of CCTs and cohorts showed that there was no significant difference across the VP and KP groups. However, subgroup analysis of a randomised controlled trial (RCT) [20] showed that VP was more effective than KP with the pooled WMD value of −0.30 (95% CI: −0.52 to −0.08, p = 0.01) as shown in Fig. 3.
Fig. 3.
Forest plot: mean difference in VAS and 95% CI for short-term follow-ups
As for mid- and long-term follow-up, outcomes revealed statistically no significant difference between these two interventions, with the overall pooled WMD value of 0.05 (95% CI: −0.30 to 0.40, p = 0.77) and 0.99( 95% CI: −0.01 to 1.98, p = 0.05), respectively. Furthermore, the outcome was relatively stable when subgroup analysis was performed according to the study design. No statistically significant difference was defined either, except for the subgroup analysis of cohort studies for long-term results with the pooled WMD of 1.39 (95% CI: 0.10–2.67) as summarised in Table 2.
Table 2.
The pooled outcome for VAS pain score
| Outcome | VAS (short-term follow-up) | VAS (mediate-term follow-up) | VAS (long-term follow-up) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | WMD (95% CI) | Heterogeneity | n | WMD (95% CI) | Heterogeneity | n | WMD (95% CI) | Heterogeneity | |
| All studies | 5 | 0.37 (−0.27 to 1.01) | p = 0.00 | 5 | 0.05 (−0.30 to 0.40) | p = 0.05 | 7 | 0.99 (−0.01 to 1.98) | p = 0.00 |
| RCT only | 1 | -0.30 (−0.52 to −0.08) | NA | 1 | 0.00 (−0.24 to 0.24) | NA | 0 | NA | NA |
| CCT only | 2 | 0.05 (−1.39 to 1.48) | p = 0.00 | 1 | −1.40 (−3.07 to 0.27) | NA | 3 | −0.03 (−0.92 to 0.87) | p = 0.17 |
| Cohort only | 2 | 0.82 (−0.14 to 1.78) | p = 0.00 | 3 | 0.23 (−0.35 to 0.82) | p = 0.04 | 4 | 1.39 (0.10 to 2.67) | p = 0.00 |
WMD weighted mean difference, CI confidence interval, NA not applicable
short-term follow-up: no more than 7 days; mediate-term follow-up: close to 6 months; long-term follow-up: close to 1 year
Oswestry disability score (ODI)
Disability measured by ODI was extracted and summarised as intermediate-term (closest to three months) and long-term (closest to one year). And then we pooled the data across the group.
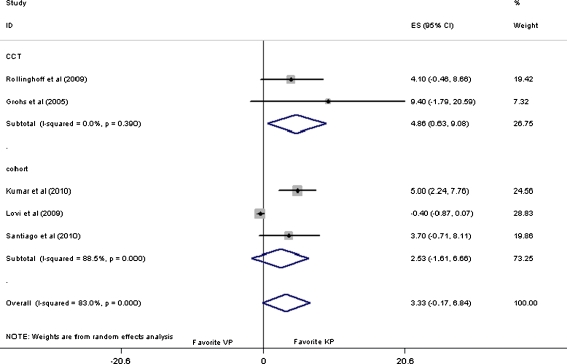
As for intermediate-term follow-up, an overall pooled WMD value of 3.33 (95% CI: −0.17 to 6.84, p = 0.06) was obtained, indicating that there is no difference between VP and KP for the functional improvement of patients with osteoporotic VCFs. Subgroup analysis of the cohorts still showed no difference [24–26]. However, with a pooled WMD value of 4.86 (95% CI: 0.63 to 9.08, p = 0.02) for subgroup analysis of the CCTs [21, 22, 26], it was manifested that KP had a superior capability for intermediate-term functional improvement to VP as shown in Fig. 4.
Fig. 4.
Forest plot: mean difference in ODI and 95% CI for intermediate-term follow-ups
As for long-term follow-up, with an overall pooled mean difference value of (95% CI: 0.35–7.91, p = 0.03), it appeared that KP had a superior capability for the functional improvement to the VP. However, with pooled mean difference values of 2.69 (95% CI: −2.28 to 7.6, p = 0.29) and 4.81 (95% CI: −0.19 to 9.81), respectively, for subgroup analysis of the CCTs [21, 22, 26] and cohorts [24–26], there appeared to be no difference across VP and KP for long-term functional improvement of patients with osteoporosis VCFs as summarised in Table 3.
Table 3.
The pooled outcome for Oswestry disability score
| Outcome | VAS (mediate-term follow-up) | VAS (long-term follow-up) | ||||
|---|---|---|---|---|---|---|
| n | WMD (95% CI) | Heterogeneity | n | WMD (95% CI) | Heterogeneity | |
| All studies | 5 | 3.33 (−0.17 to 6.8) | p = 0.00 | 5 | 4.13 (0.35 to 7.91) | p = 0.00 |
| CCT only | 2 | 4.86 (0.63 to 9.08) | p = 0.39 | 2 | 2.69 (−2.28 to 7.67) | p = 0.27 |
| Cohort only | 3 | 2.53 (−1.61 to 6.66) | p = 0.00 | 3 | 4.81 (−0.19 to 9.81) | p = 0.00 |
WMD weighted mean difference, CI confidence interval, NA not applicable
Intermediate-term follow-up: close to 3 months; long-term follow-up: close to 1 year
Complications
As cement leakage and subsequent VCFs were the only complications included in the article, safety assessment for these two interventions was realised by extracting and pooling relative data.
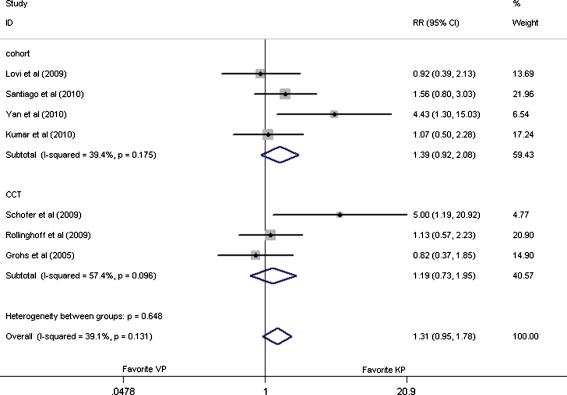
In the seven studies providing cement leakage data, 748 patients were involved in this assessment [21–27]. And there were no significant difference between these two interventions, with the pooled RR values of 1.31 (95% CI: 0.95–1.78, p = 0.13), 1.19 (95% CI: 0.73–31.95, p = 0.10), and 1.39 (95% CI: 0.92–2.08, p = 0.19), respectively, for overall analysis and subgroup analysis of CCTs [21–23] and cohort [24–27] analysis as shown in Fig. 5.
Fig. 5.
Forest plot: relative risks in cement leakage and 95% CI
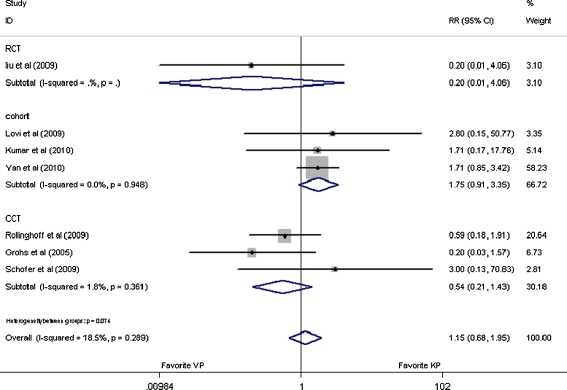
In the seven studies providing subsequent fracture, 788 patients were involved in this assessment. With the pooled RR values of 1.15 (95% CI: 0.68–1.95, p = 0.61), 0.20 (95% CI: 0.01–4.06, p = 0.30), 0.54 (95% CI: 0.21–1.43, p = 0.22), and 1.75 (95% CI: 0.91 to 3.35, p = 0.09), respectively, for overall analysis and subgroup analysis of RCT [20], CCT [21–23] and cohort [24–27] analysis, it was indicated that these two interventions had similar risk for subsequent fracture as shown in Fig. 6.
Fig. 6.
Forest plot: relative risks in subsequent fracture and 95% CI
Sensitivity analysis was performed by excluding the study which only reported the number of vertebra being treated but not the exact allocation of patients [21]. With the overall pooled outcome value of 1.12 (95% CI: 0.54–2.66, p = 0.66) and 1.47 (95% CI: 0.88–2.43, p = 0.14) for subsequent fracture and cement leakage, respectively, it appeared that there were no significant differences between these two methods. Further subsequent analysis according to study design showed that the outcome was relatively stable.
Publication bias
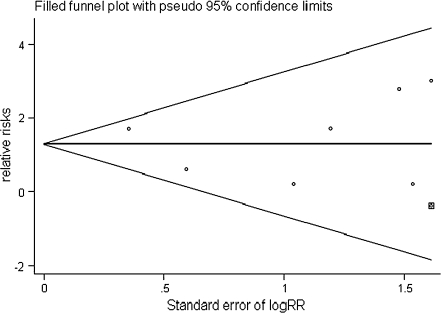
Figure 7 shows a funnel plot for studies reporting the RRs of subsequent fracture as a measure of treatment effect. The plot is symmetrical, and all studies fall within the 95% CI axis for a given standard error. There may be few studies missing from the search strategy, and so there is minimal evidence of publication bias.
Fig. 7.
Funnel plot for the outcome of subsequent vertebral compression fractures
Discussion
To our knowledge, this is the first quantitative comparative meta-analysis of studies directly comparing kyphoplasty and vertebroplasty for the treatment of osteoporotic VCFs. Ultimately, eight studies in the literature—one randomised controlled trial [20], three clinical controlled trials [21–23] and four cohorts [24–27] (three prospective cohorts [24–26] and one retrospective cohort [27])—were included in our systematic review. In order to assess the efficacy and safety of VP and KP, we extracted relative data as much as possible and we pooled the outcome whenever possible.
First, we took the outcome of VAS pain score and ODI for efficacy assessment. As for VAS, overall outcome showed that there was no significant difference across these two interventions, with the pooled WMD of 0.37 (95% CI: −0.27 to 1.02, p = 0.25), 0.05 (95% CI: −0.30 to 0.40, p = 0.77) and 0.99 (95% CI: −0.01 to 1.98, p = 0.05), respectively, for short-term, mediate-term and long-term follow-ups. As a result of the weakness of the study design of the observational study, results of experimental studies such as RCT and CCT are more credible, as shown in the “risk of bias” table (Fig. 2). And after subgroup analysis of outcome of RCT or CCT, it was shown that VP is more effective for short-term pain relief of patients with the pooled outcome of −0.30 (95% CI: −0.52 to −0.08, p = 0.01) [20]. As for mid- and long-term follow-ups, no significant difference across these two interventions was identified.
A similar trend was also found when assessing for ODI. With the pooled outcome of 4.86 (95% CI: 0.63–9.08, p = 0.02) for subgroup analysis of the outcomes of intermediate-term follow-ups of the CCTs [21, 22, 26], KP appears more effective for intermediate-term functional improvement. As for long-term follow-ups, no significant difference between these two interventions was identified either.
As for safety assessment, we just analysed the most common complications, namely, subsequent VCF and cement leakage, for they were the only complications reported in studies. Again the outcomes show that there was no significant difference between these two interventions. What’s more, subgroup analysis and sensitive analysis did not detect any differences either.
As a meta-analysis for randomised and non-randomised studies, there are several limitations to our studies. First, almost all of the studies included in this meta-analysis are NRTs, such as clinical controlled trial, prospective or retrospective cohort and as a result of study design limitations, these studies were more likely to suffer from various kinds of bias. Furthermore, confounding factors which were balanced by randomisation in RCTs often disturbed the observation of effect of the intervention in NRTs. Thus it was not surprising that almost all of them were at relatively high risk when we assessed the risk of bias for the studies included (Fig. 2).
Second, heterogeneity was statistically significant across several outcomes. Although we employed the subgroup and sensitivity analysis according to the study designs, the heterogeneity can be only partially resolved. Potential explanations for such heterogeneity might be that it is more likely to encounter greater heterogeneity in a systematic review of NRTs than that of RCTs. This is due to the increased potential for variation in the way in which confounding is considered in the analysis and greater risk of all kinds of biases through poor design of NRTs. However, there is still no way for controlling for these biases in the analysis of primary studies and no established method for assessing how these biases affect primary studies.
Third, no authors were contacted for further information. We extracted the data either directly from the article or by extrapolation. Taking the “effect size”, for instance, although the method of “change from baseline” is more credible, we took the final outcome as “effect size” while rejecting the method of “change from baseline”. Because all documents have given standard deviations for the baseline and the final measures but not the standard deviation for the mean difference of the pre- and post-operative, it is impossible for us to calculate the standard deviation of mean difference with the data extracted from the text. However, if we chose the final outcome as the “effect size”, it would be expected to give a relatively wider confidence interval especially for repeated measurement of the outcome on the same patient. It is therefore necessary for us to interpret the outcome carefully.
Forth, the mean duration between injury and surgery varied greatly between the studies included. There were studies aimed to clarify whether VP and KP had additional value for patients with acute or fresh vertebral compression fractures [21–23]. There were also studies assessing the treatment value of these two minimally invasive operations for chronic VCFs (more than three months). It is well known that the natural history for spontaneous pain reduction is three months [28]. When the duration of fracture is as long as the natural healing time, it is difficult for us to distinguish the effect of intervention from the natural resolution. A plausible explanation might be that these interventions can also be recommended to patients, refractory to medical therapy.
Finally, both of these two interventions can restore vertebral body height and kyphotic wedge angle [29]. However, KP involves the use of an inflatable bone tamp which can be introduced into the vertebral body and help restore vertebral height by forming a space into which acrylic cement can be injected [30, 31]. Theoretically, KP is more effective for vertebral height restoration. Nevertheless, the literature included in this meta-analysis only reported limited information on kyphotic restoration. It is therefore impossible for us to assess the radiological result of these two minimally invasive surgeries.
In summary, we believe that this meta-analysis, comparing VP versus KP for treatment of osteoporotic VCFs, offers useful conclusions and shows that both interventions have similar risk for subsequent fracture and cement leakage. VP is more effective for short-term (no more than seven days) pain relief. KP had a superior capability for intermediate-term (close to three months) functional improvement to VP. As for long-term pain relief and functional improvement, there is no significant difference between these two interventions. Thus, considering the higher cost of the KP procedure, we recommend VP over KP for the treatment of osteoporotic VCFs.
Acknowledgements
The intent of this statement is to display our idea on osteoporotic vertebral compression fracture. All the authors state that there are no outside grants in support of this research this work. There is no financial and personal relationships with other people or organisations that could inappropriately influence (bias) our work.
Abbreviation
- VCFs
Vertebral compression fractures
- VP
Vertebroplasty
- KP
Kyphoplasty
- RCTs
Randomised controlled trials
- NRTs
Non-randomised controlled trials
- CCTs
Clinical controlled trials
- VAS
Visual analog scale
- ODI
Oswestry disability score
- RR
Relative risks
- WMD
Weighted mean difference
References
- 1.Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Horm Res. 2000;54(Suppl 1):58–63. doi: 10.1159/000063449. [DOI] [PubMed] [Google Scholar]
- 2.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH, 3rd, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282(14):1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 3.Dickman CA, Fessler RG, MacMillan M, Haid RW. Transpedicular screw-rod fixation of the lumbar spine: operative technique and outcome in 104 cases. J Neurosurg. 1992;77(6):860–870. doi: 10.3171/jns.1992.77.6.0860. [DOI] [PubMed] [Google Scholar]
- 4.Phillips FM. Minimally invasive treatments of osteoporotic vertebral compression fractures. Spine (Phila Pa 1976) 2003;28(15 Suppl):S45–53. doi: 10.1097/01.BRS.0000076898.37566.32. [DOI] [PubMed] [Google Scholar]
- 5.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 6.Klazen CA, Lohle PN, Vries J, Jansen FH, Tielbeek AV, Blonk MC, Venmans A, Rooij WJ, Schoemaker MC, Juttmann JR, Lo TH, Verhaar HJ, Graaf Y, Everdingen KJ, Muller AF, Elgersma OE, Halkema DR, Fransen H, Janssens X, Buskens E, Mali WP. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial. Lancet. 2010;376(9746):1085–1092. doi: 10.1016/S0140-6736(10)60954-3. [DOI] [PubMed] [Google Scholar]
- 7.Wardlaw D, Cummings SR, Meirhaeghe J, Bastian L, Tillman JB, Ranstam J, Eastell R, Shabe P, Talmadge K, Boonen S. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet. 2009;373(9668):1016–1024. doi: 10.1016/S0140-6736(09)60010-6. [DOI] [PubMed] [Google Scholar]
- 8.Taylor RS, Fritzell P, Taylor RJ. Balloon kyphoplasty in the management of vertebral compression fractures: an updated systematic review and meta-analysis. Eur Spine J. 2007;16(8):1085–1100. doi: 10.1007/s00586-007-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ploeg WT, Veldhuizen AG, The B, Sietsma MS. Percutaneous vertebroplasty as a treatment for osteoporotic vertebral compression fractures: a systematic review. Eur Spine J. 2006;15(12):1749–1758. doi: 10.1007/s00586-006-0159-z. [DOI] [PubMed] [Google Scholar]
- 10.Lee MJ, Dumonski M, Cahill P, Stanley T, Park D, Singh K. Percutaneous treatment of vertebral compression fractures: a meta-analysis of complications. Spine Phila Pa 1976. 2009;34(11):1228–1232. doi: 10.1097/BRS.0b013e3181a3c742. [DOI] [PubMed] [Google Scholar]
- 11.Karlsson MK, Hasserius R, Gerdhem P, Obrant KJ, Ohlin A. Vertebroplasty and kyphoplasty: New treatment strategies for fractures in the osteoporotic spine. Acta Orthop. 2005;76(5):620–627. doi: 10.1080/17453670510041682. [DOI] [PubMed] [Google Scholar]
- 12.Hulme PA, Krebs J, Ferguson SJ, Berlemann U. Vertebroplasty and kyphoplasty: a systematic review of 69 clinical studies. Spine Phila Pa 1976. 2006;31(17):1983–2001. doi: 10.1097/01.brs.0000229254.89952.6b. [DOI] [PubMed] [Google Scholar]
- 13.Eck JC, Nachtigall D, Humphreys SC, Hodges SD. Comparison of vertebroplasty and balloon kyphoplasty for treatment of vertebral compression fractures: a meta-analysis of the literature. Spine J. 2008;8(3):488–497. doi: 10.1016/j.spinee.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Gill JB, Kuper M, Chin PC, Zhang Y, Schutt R., Jr Comparing pain reduction following kyphoplasty and vertebroplasty for osteoporotic vertebral compression fractures. Pain Physician. 2007;10(4):583–590. [PubMed] [Google Scholar]
- 15.Taylor RS, Taylor RJ, Fritzell P. Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: a comparative systematic review of efficacy and safety. Spine Phila Pa 1976. 2006;31(23):2747–2755. doi: 10.1097/01.brs.0000244639.71656.7d. [DOI] [PubMed] [Google Scholar]
- 16.Furlan AD, Pennick V, Bombardier C, Tulder M. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine Phila Pa 1976. 2009;34(18):1929–1941. doi: 10.1097/BRS.0b013e3181b1c99f. [DOI] [PubMed] [Google Scholar]
- 17.Bhandari M, Bajammal S, Guyatt GH, Griffith L, Busse JW, Schunemann H, Einhorn TA. Effect of bisphosphonates on periprosthetic bone mineral density after total joint arthroplasty. A meta-analysis. J Bone Joint Surg Am. 2005;87(2):293–301. doi: 10.2106/JBJS.D.01772. [DOI] [PubMed] [Google Scholar]
- 18.Negri P, Tirri T, Paternoster G, Modano P. Treatment of painful osteoporotic or traumatic vertebral compression fractures by percutaneous vertebral augmentation procedures: a nonrandomized comparison between vertebroplasty and kyphoplasty. Clin J Pain. 2007;23(5):425–430. doi: 10.1097/AJP.0b013e31805593be. [DOI] [PubMed] [Google Scholar]
- 19.Fourney DR, Schomer DF, Nader R, Chlan-Fourney J, Suki D, Ahrar K, Rhines LD, Gokaslan ZL. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98(1 Suppl):21–30. doi: 10.3171/spi.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]
- 20.Liu JT, Liao WJ, Tan WC, Lee JK, Liu CH, Chen YH, Lin TB. Balloon kyphoplasty versus vertebroplasty for treatment of osteoporotic vertebral compression fracture: a prospective, comparative, and randomized clinical study. Osteoporos Int. 2010;21(2):359–364. doi: 10.1007/s00198-009-0952-8. [DOI] [PubMed] [Google Scholar]
- 21.Rollinghoff M, Siewe J, Zarghooni K, Sobottke R, Alparslan Y, Eysel P, Delank KS. Effectiveness, security and height restoration on fresh compression fractures—a comparative prospective study of vertebroplasty and kyphoplasty. Minim Invasive Neurosurg. 2009;52(5–6):233–237. doi: 10.1055/s-0029-1243631. [DOI] [PubMed] [Google Scholar]
- 22.Grohs JG, Matzner M, Trieb K, Krepler P. Minimal invasive stabilization of osteoporotic vertebral fractures: a prospective nonrandomized comparison of vertebroplasty and balloon kyphoplasty. J Spinal Disord Tech. 2005;18(3):238–242. [PubMed] [Google Scholar]
- 23.Schofer MD, Efe T, Timmesfeld N, Kortmann HR, Quante M. Comparison of kyphoplasty and vertebroplasty in the treatment of fresh vertebral compression fractures. Arch Orthop Trauma Surg. 2009;129(10):1391–1399. doi: 10.1007/s00402-009-0901-1. [DOI] [PubMed] [Google Scholar]
- 24.Lovi A, Teli M, Ortolina A, Costa F, Fornari M, Brayda-Bruno M. Vertebroplasty and kyphoplasty: complementary techniques for the treatment of painful osteoporotic vertebral compression fractures. A prospective non-randomised study on 154 patients. Eur Spine J. 2009;18(Suppl 1):95–101. doi: 10.1007/s00586-009-0986-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar K, Nguyen R, Bishop S. A comparative analysis of the results of vertebroplasty and kyphoplasty in osteoporotic vertebral compression fractures. Neurosurgery. 2010;67(3 Suppl Operative):171–188. doi: 10.1227/01.NEU.0000380936.00143.11. [DOI] [PubMed] [Google Scholar]
- 26.Santiago FR, Abela AP, Alvarez LG, Osuna RM, Garcia Mdel M. Pain and functional outcome after vertebroplasty and kyphoplasty. A comparative study. Eur J Radiol. 2010;75(2):e108–113. doi: 10.1016/j.ejrad.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Yan D, Duan L, Li J, Soo C, Zhu H, Zhang Z. Comparative study of percutaneous vertebroplasty and kyphoplasty in the treatment of osteoporotic vertebral compression fractures. Arch Orthop Trauma Surg. 2010 doi: 10.1007/s00402-010-1188-y. [DOI] [PubMed] [Google Scholar]
- 28.Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359(9319):1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- 29.McKiernan F, Faciszewski T, Jensen R. Reporting height restoration in vertebral compression fractures. Spine (Phila Pa 1976) 2003;28((22):2517–2521. doi: 10.1097/01.BRS.0000092424.29886.C9. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman IH, Dudeney S, Reinhardt MK, Bell G. Initial outcome and efficacy of "kyphoplasty" in the treatment of painful osteoporotic vertebral compression fractures. Spine (Phila Pa 1976) 2001;26(14):1631–1638. doi: 10.1097/00007632-200107150-00026. [DOI] [PubMed] [Google Scholar]
- 31.Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine (Phila Pa 1976) 2001;26(14):1511–1515. doi: 10.1097/00007632-200107150-00002. [DOI] [PubMed] [Google Scholar]