Abstract
Apelin is a recently discovered hormone secreted by adipocytes. The aim of this study, therefore, was to evaluate the distribution of apelin in paired serum and synovial fluid (SF) of osteoarthritis (OA) patients, as compared to that in healthy controls, and to characterise the expression profile of apelin and its cognate receptor APJ in human chondrocytes. Apelin levels in serum and SF were analysed by enzyme-linked immunosorbent assay (ELISA). Expression of apelin and APJ in human chondrocytes was determined by real-time quantitative polymerase chain reaction (PCR). Apelin was found to be present in OA SF, and concentrations were positively correlated with the severity of OA. OA serum exhibited significantly elevated levels of apelin (2.18 ± 0.22 ng/ml) as compared to normal serum (1.31 ± 0.12 ng/ml) (p < 0.05), and serum apelin levels exceeded those in paired SF (p < 0.001). The apelin and APJ transcripts were identified in chondrocytes, and levels were significantly higher in OA cartilage than in healthy donors. These findings suggest that apelin may contribute to the onset and/or progression of OA, and may provide new insights into the pathophysiology of OA.
Introduction
A newly recognised member of the adipose-secreted cytokine family, apelin, was identified in 1998 as an endogenous ligand of the human orphan G protein-coupled receptor (GPCR) APJ. Apelin is initially secreted as a pre-propeptide consisting of 77 amino acid residues, which is then cleaved into various active forms. Named according to their final lengths (apelin-13, -16, -17, -19 and -36), the shorter forms exhibit more potent functionality [1]. Apelin function is mediated by binding to its specific APJ receptor, a 7-transmembrane GPCR [2]. The apelin-APJ conjugate is widely distributed throughout human tissues, including adipose, brain, liver, lung, and heart, and its expression profile is similar to that of the angiotensin II (Ang II)–angiotensin II type 1 (AT1) receptor complex [3]. In adipose tissue, the apelin/APJ signaling pathway was demonstrated to play a key role in the development of the functional vascular network [4]. As to the cardiovascular system, apelin could protect the heart against ischemia reperfusion injury and induce nitric oxide-dependent arterial vasodilation [5].
Recent evidence suggests that apelin can act as a proinflammatory adipocyte-derived factor that participates in vascular wall inflammation [6]. Apelin infusion significantly decreases the mean messenger RNA (mRNA) levels of monocyte chemattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1, interleukin (IL)-6 and tumour necrosis factor (TNF)-α in aneurysmal aorta. Moreover, apelin treatment of cultured macrophages induced a marked reduction in MCP-1 and TNF-α mRNA levels [6]. Recently, it was reported that exposure of lipopolysaccharide (LPS), IL-6 or interferon gamma (IFN-γ) to rodents led to increased enteric apelin expression [7]. Daviaud et al. demonstrated that TNF-α can act as a direct regulator of apelin expression in both human and mouse adipose tissue, and that injection of TNF-α in mice resulted in increased apelin expression in adipose tissue and blood plasma [8]. Taken together, these findings suggest that apelin may be a proinflammatory adipokine and may play a mediating role or represent a potential link between obesity and inflammation. Recently, it has been demonstrated that apelin can act as a regulator of bone growth by inducing osteoblast proliferation and inhibiting its apoptosis [9–11]. Until our study, nothing was known about the expression of apelin in osteoarthritic patients.
In this study, we sought to investigate the expression profile of apelin in SF and serum obtained from OA patients, and to characterise the particular levels of apelin in adult human females and males. In addition, we examined the relationship between SF apelin levels and the severity of OA, and analysed the gene expression of apelin and APJ in articular cartilage between patients with OA and donors.
Materials and methods
Patients and samples
SF and serum were obtained from 30 OA patients (19 females with mean age 70.2 ± 2.91 years, mean BMI 26.2 ± 0.61 kg/m2; 11 males with mean age 66.9 ± 3.29 years, mean BMI 25.2 ± 1.33 kg/m2; with no statistical differences between the females and the males) according to criteria set forth by the American College of Rheumatology [12]. Knee radiograph results were extracted from hospital records for 19 of the OA patients. Normal serum samples were obtained from 14 healthy age-matched individuals. Collected serum was centrifuged at 2000 × g for 15 min and SF was centrifuged at 8000 × g for 15 min, and then stored at −80°C. The personal and clinical characteristics of the patient and control cohorts are summarised in Table 1.
Table 1.
Characteristics of patients with osteoarthritis and control group
| Characteristics | OA (n = 30) | Controls (n = 14) | p1 | p2 |
|---|---|---|---|---|
| Age (years) | 69 ± 2.2 | 61.6 ± 3.4 | NS | |
| Sex (F/M) | 19/11 | 9/5 | ||
| BMI (kg/m2) | 25.8 ± 0.64 | 24.7 ± 0.52 | NS | |
| S-apelin (ng/ml) | 2.18 ± 0.22 | 1.31 ± 0.12 | <0.01 | |
| SF-apelin (ng/ml) | 0.35 ± 0.03 | NM | <0.01 |
OA osteoarthritis, F female, M male, BMI body mass index, S serum, SF synovial fluid, NM not measured
p1 refers to OA vs. controls
p2 refers to OA SF- apelin vs. OA S- apelin
Specimens from OA-affected cartilage tissue were obtained from six patients (mean age 63.7 ± 2.8 years; mean BMI 26.8 ± 0.6 kg/m2; 1:1 male to female ratio) from the femoral condyles and tibial plateaus during total knee replacement surgery. Normal knee joint cartilage was obtained from six otherwise healthy fracture patients who had suffered serious accidents that necessitated amputation (mean age 59.3 ± 2.4 years; mean BMI 25.1 ± 0.7 kg/m2; 1:2 male to female ratio). All patients were treated at the Second Hospital of Medical College, Zhejiang University. The study was approved by the local ethics committee and written informed consent was obtained from each participant.
Apelin protein measurement by ELISA
Apelin concentrations in plasma and SF were measured by using a commercial enzyme-linked immunoassay kit (Phoenix peptides, Burlingame, CA, USA), according to the manufacturer’s instructions. The minimal detectable concentration was 0.07 ng/ml. The ELISA kit has a reported 100% cross-reactivity with human apelin-12, apelin-13 and apelin-36. Before testing, SF samples were pre-treated with 1 mg/ml of hyaluronidase for 45 min at 37°C.
Apelin and APJ mRNA expression measurement by real-time quantitative PCR
OA cartilage samples were pulverised in liquid nitrogen, and total RNA was isolated using the Trizol reagent (Sigma-Aldrich, St. Louis, MO, USA), according to the manufacturer’s instructions. After a treatment period of 20 min at 37°C with 1Uof DNase I (Sigma-Aldrich) to prevent genomic DNA contamination, 1 μg of total RNA was reverse transcribed using 10 pmol random hexanucleotide primers (Promega, USA), 0.5 mM dNTPs and 200U of Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI, USA) at 37°C for 1 h. The reaction was terminated by incubation at 70°C for 10 min. The quantification of gene-expression levels for apelin and APJ were carried out by real-time quantitative PCR on an iCycler system (Bio-Rad, Laboratories, Hercules, CA, USA). iQ™ SYBR Green supermix PCR kit (Bio-Rad) was used for real-time monitoring of amplification (5 ng of template cDNA; 45 cycles: 95°C/15 s, 60°C/15 s) with appropriate primers (Table 2). A parallel amplification of oyster GAPDH transcript (NM_002046) was carried out (forward primer: 5′-CTG CTC CTC CTG TTC GAC AGT-3′; reverse primer: 5′-CCG TTG ACT CCG ACC TTC AC-3′) to normalise the expression data of the target transcripts. The relative expression levels of targeted genes were calculated for 100 copies of the 18 s housekeeping gene according to the formula: 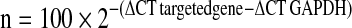
Table 2.
Primers of targeted genes
Statistical analyses
All data have been expressed as mean ± standard deviation (SD). Statistical analyses were performed with statistical software package SPSS version 12.0 for Windows (SPSS, Inc., Chicago, IL, USA). Statistical significance between matched pairs of SF and serum was determined using the paired samples t-test. Differences between patients with OA and healthy controls, female and male were analysed by one-way ANOVA test. The Spearmen correlation coefficient was used to examine the relationship between SF apelin levels and severity of OA. P values less than 0.05 were considered significant.
Results
Apelin expression in OA patients and healthy, non-OA individuals
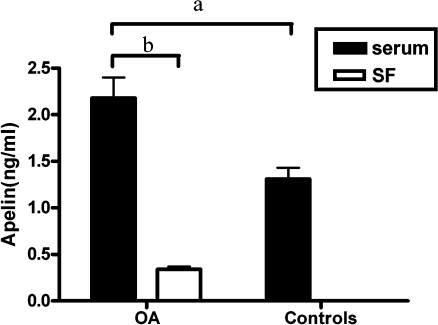
OA serum exhibited significantly elevated levels of apelin (2.18 ± 0.22 ng/ml, n = 30), as compared with non-OA serum (1.31 ± 0.12 ng/ml, n = 14). Apelin levels in SF from OA patients were 0.35 ± 0.03 ng/ml (Fig. 1).
Fig. 1.
Apelin levels in serum and synovial fluid (SF). Serum apelin was significantly higher in osteoarthritis (OA) patients than in controls (for a, p < 0.05). Apelin levels in synovial fluid were lower than in paired serum samples in OA patients (for b, p < 0.001)
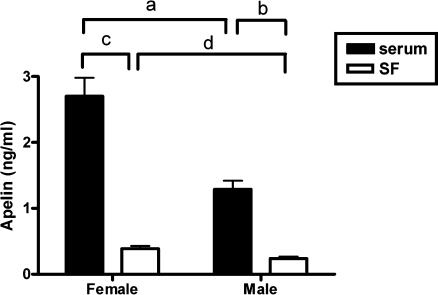
In the OA group, both serum and SF apelin levels were found to be significantly higher in the female sub-group than those in the male sub-group (Fig. 2). The serum apelin concentration was 2.70 ± 0.28 in females (n = 19) and 1.29 ± 0.13 ng/ml in males (n = 11) (p < 0.01), while SF apelin concentration was found to be 0.39 ± 0.04 in the females and 0.27 ± 0.03 ng/ml in the males (p < 0.05).
Fig. 2.
Apelin levels in paired samples of serum and synovial fluid (SF) obtained from female and male patients with osteoarthritis (OA). SF and serum levels of apelin in the female group were found to be significantly higher than that in the male group (for a, p < 0.01; for d, p < 0.05). Serum apelin was significantly higher than SF in both the female and male groups (for b, p < 0.001; for c, p < 0.001)
Relationship between SF apelin levels and severity of OA
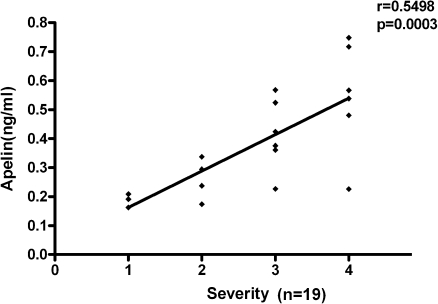
We also studied whether a relationship existed between OA severity and SF apelin levels. OA severity was evaluated by two independent orthopaedic surgeons in accordance with the Kellgren–Lawrence criteria [13]. The assessments were carried out blinded. A significantly positive correlation between SF apelin levels and the clinical severity of OA was observed (r = 0.5498, p = 0.0003; Fig. 3).
Fig. 3.
Relationship between synovial fluid (SF) apelin levels and the severity of osteoarthritis (OA)
Differential mRNA expression of apelin and APJ in cartilage
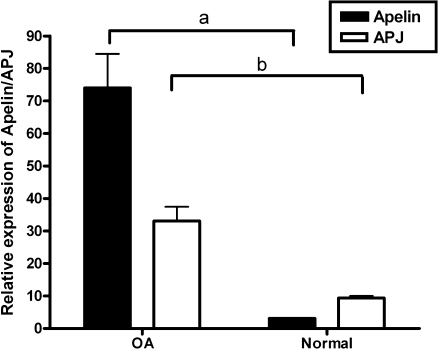
To assess the mRNA levels of apelin and APJ, we compared the corresponding transcripts in OA cartilage and normal cartilage by real-time quantitative PCR (Fig. 4). Both apelin and APJ mRNAs were detected in normal cartilage and at remarkably increased levels in the OA group. OA articular cartilage exhibited a significant 25-fold increase in apelin expression and a three-fold increase in APJ expression, as compared with normal articular cartilage.
Fig. 4.
mRNA expression of apelin and APJ by real-time quantitative polymerase chain reaction (PCR) in osteoarthritis (OA) articular cartilage and normal articular cartilage. Both apelin and APJ mRNA expression were increased in the OA group compared to the normal group (for a, p < 0.001; for b, p < 0.01)
Discussion
Previous studies have shown that adipose tissue produces many metabolically important factors, including cytokines IL-1 and TNF-α and the adipokines leptin, adiponectin, resistin, and visfatin [14]. Data associating these adipokines with various joint disorders have indicated that they may also have an active role in the pathogenesis of OA [15–17]. However, there are no detailed investigations on the expression and effects of apelin and APJ in chondrocytes. Our study is the first to demonstrate: (1) the presence of apelin in SF obtained from patients with OA; (2) positive correlations between SF apelin levels and the clinical severity of OA; (3) increased serum apelin in OA patients, as compared to healthy, non-OA individuals; and (4) the increased expression levels of apelin and APJ mRNA in OA cartilage.
In this study, we first detected apelin in SF. And the mean levels of apelin in SF obtained from OA patients were lower than in the paired serum. This finding suggested that circulating levels of apelin did not accurately represent the situation in the joint. The joint cavity is a physically isolated space where adipokines act within special regulatory pathways that differentially affect the various systemic tissues. This phenomenon is consistent with findings from recent studies, in which differential distribution of adipokines was identified between serum and synovial fluid in patients suffering from osteoarthritis [18, 19]. Previous studies have also indicated that various tissues, like synovium, infrapatellar fat pad, meniscus, osteophyte, cartilage and bone obtained from OA-affected joints were able to synthesise the adipokines leptin and adiponectin [19].
In our cohort, a significant positive correlation between SF apelin levels and the clinical severity of OA indicated that apelin might play a key role in the local environment and induce the degeneration of cartilage in OA conditions.
Our data also indicated that apelin concentrations were markedly higher in OA serum than that of healthy controls. Otero et al. had investigated plasma levels of leptin, adiponectin and visfatin and found that each was highly expressed in patients with rheumatoid arthritis. Their results showed that adipokine up-expression in plasma may be responsible for a chronic subclinical presentation of a proinflammatory state in arthritis patients [20]. Though OA has long been considered as a non-inflammatory disease, distinguishable from ‘inflammatory arthritis’ states such as rheumatoid arthritis, inflammation has become increasingly recognised as a contributing factor in the pathophysiology of OA [21]. We postulate, therefore, that high apelin serum levels in OA patients may represent a chronic proinflammatory state.
Furthermore, our study has revealed that there was a gender component associated with plasma apelin levels in OA patients. Females were found to have considerably higher circulating apelin levels than did men (2.70 ± 0.28 vs. 1.29 ± 0.13 ng/ml). This sex-based differential distribution has never been reported before. Other adipokines, including leptin and adiponectin, are known to exhibit higher serum levels in females than in males [19]. It is interesting, then, to consider that females are well recognised as particularly susceptible to OA [19]. Evidence-based medicine has shown that OA prevalence and incidence are generally higher in females [22, 23]. Taken together, these findings suggest that the presence of apelin may contribute to differences in the incidence of OA in males and females.
The effect of apelin/APJ on human chondrocytes remains to be completely understood. From our research, it was shown that apelin and APJ gene expression in chondrocytes was significantly higher in OA cartilage than in healthy donors (p < 0.001). This result suggests that the apelin/APJ system may be involved in the pathophysiology of OA. Up to now, little effort has been devoted to assessing the effects of apelin on the osteoblasts. It has been reported that apelin can induce bone metabolism by promoting osteoblast proliferation, and this effect can be abolished by suppression of APJ with small-interfering RNA (siRNA) or by blocking the APJ/Akt pathway [9]. These investigators also found that apelin was able to suppress serum deprivation-induced apoptosis in human osteoblasts via the APJ/Akt signaling pathway [10, 11]. Interestingly, both human chondrocytes and osteoblasts are derived from mesenchymal precursors and may share common signal transduction; therefore, it may not be very surprising that we found a similar response of chondrocytes to apelin. Still, the exact mechanism of apelin in the development of arthritis has not been established and warrants further investigation.
In conclusion, this study provides the first evidence that human chondrocytes express apelin and APJ, and apelin can be detected in SF of OA patients. We also found that OA patients had an increased serum apelin concentration, as compared to healthy, non-OA individuals. Furthermore, OA cartilage expressed high levels of apelin and APJ mRNA. Taken together, the results of this study provide evidence for a potential key role of apelin in the pathophysiology of OA. Clearly, the relationship between obesity and osteoarthritis is complex. Future research efforts should pay particular attention to adipokines, especially apelin.
Acknowledgments
The authors thank Jun Liu and Wei-guo Yang for providing technical assistance. This work was supported by the Health Bureau of Zhejiang Province (2006A055).
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, Kawamata Y, Fukusumi S, Hinuma S, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251(2):471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 2.O'Dowd BF, Heiber M, Chan A, Heng HH, Tsui LC, Kennedy JL, Shi X, Petronis A, George SR, Nguyen T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene. 1993;136(1–2):355–360. doi: 10.1016/0378-1119(93)90495-O. [DOI] [PubMed] [Google Scholar]
- 3.Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, Osmond DH, George SR, O'Dowd BF. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74(1):34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 4.Kunduzova O, Alet N, Delesque-Touchard N, Millet L, Castan-Laurell I, Muller C, Dray C, Schaeffer P, Herault JP, Savi P, Bono F, Valet P. Apelin/APJ signaling system: A potential link between adipose tissue and endothelial angiogenic processes. FASEB J. 2008;22(12):4146–4153. doi: 10.1096/fj.07-104018. [DOI] [PubMed] [Google Scholar]
- 5.Japp AG, Cruden NL, Amer DA, Li VK, Goudie EB, Johnston NR, Sharma S, Neilson I, Webb DJ, Megson IL, Flapan AD, Newby DE. Vascular effects of apelin in vivo in man. J Am Coll Cardiol. 2008;52(11):908–913. doi: 10.1016/j.jacc.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Leeper NJ, Tedesco MM, Kojima Y, Schultz GM, Kundu RK, Ashley EA, Tsao PS, Dalman RL, Quertermous T. Apelin prevents aortic aneurysm formation by inhibiting macrophage inflammation. Am J Physiol Heart Circ Physiol. 2009;296(5):H1329–H1335. doi: 10.1152/ajpheart.01341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han S, Wang G, Qi X, Englander EW, Greeley GH., Jr Involvement of a stat3 binding site in inflammation-induced enteric apelin expression. Am J Physiol Gastrointest Liver Physiol. 2008;295(5):G1068–G1078. doi: 10.1152/ajpgi.90493.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daviaud D, Boucher J, Gesta S, Dray C, Guigne C, Quilliot D, Ayav A, Ziegler O, Carpene C, Saulnier-Blache JS, Valet P, Castan-Laurell I. TNFalpha up-regulates apelin expression in human and mouse adipose tissue. FASEB J. 2006;20(9):1528–1530. doi: 10.1096/fj.05-5243fje. [DOI] [PubMed] [Google Scholar]
- 9.Xie H, Tang SY, Cui RR, Huang J, Ren XH, Yuan LQ, Lu Y, Yang M, Zhou HD, Wu XP, Luo XH, Liao EY. Apelin and its receptor are expressed in human osteoblasts. Regul Pept. 2006;134(2–3):118–125. doi: 10.1016/j.regpep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Xie H, Yuan LQ, Luo XH, Huang J, Cui RR, Guo LJ, Zhou HD, Wu XP, Liao EY. Apelin suppresses apoptosis of human osteoblasts. Apoptosis. 2007;12(1):247–254. doi: 10.1007/s10495-006-0489-7. [DOI] [PubMed] [Google Scholar]
- 11.Tang SY, Xie H, Yuan LQ, Luo XH, Huang J, Cui RR, Zhou HD, Wu XP, Liao EY. Apelin stimulates proliferation and suppresses apoptosis of mouse osteoblastic cell line MC3T3-E1 via JNK and PI3-K/Akt signaling pathways. Peptides. 2007;28(3):708–718. doi: 10.1016/j.peptides.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 13.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 15.Dumond H, Presle N, Terlain B, Mainard D, Loeuille D, Netter P, Pottie P. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 2003;48(11):3118–3129. doi: 10.1002/art.11303. [DOI] [PubMed] [Google Scholar]
- 16.Chen TH, Chen L, Hsieh MS, Chang CP, Chou DT, Tsai SH. Evidence for a protective role for adiponectin in osteoarthritis. Biochim Biophys Acta. 2006;1762(8):711–718. doi: 10.1016/j.bbadis.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol. 2005;174(9):5789–5795. doi: 10.4049/jimmunol.174.9.5789. [DOI] [PubMed] [Google Scholar]
- 18.Schaffler A, Ehling A, Neumann E, Herfarth H, Tarner I, Scholmerich J, Muller-Ladner U, Gay S. Adipocytokines in synovial fluid. JAMA. 2003;290(13):1709–1710. doi: 10.1001/jama.290.13.1709-c. [DOI] [PubMed] [Google Scholar]
- 19.Presle N, Pottie P, Dumond H, Guillaume C, Lapicque F, Pallu S, Mainard D, Netter P, Terlain B. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production. Osteoarthritis Cartilage. 2006;14(7):690–695. doi: 10.1016/j.joca.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gomez-Reino JJ, Gualillo O. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65(9):1198–1201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ning L, Ishijima M, Kaneko H, Kurihara H, Arikawa-Hirasawa E, Kubota M, Liu L, Xu Z, Futami I, Yusup A, Miyahara K, Xu S, Kaneko K, Kurosawa H. Correlations between both the expression levels of inflammatory mediators and growth factor in medial perimeniscal synovial tissue and the severity of medial knee osteoarthritis. Int Orthop. 2010 doi: 10.1007/s00264-010-1045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13(9):769–781. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura A, Hasegawa M, Kato K, Yamada T, Uchida A, Sudo A. Risk factors for the incidence and progression of radiographic osteoarthritis of the knee among Japanese. Int Orthop. 2010 doi: 10.1007/s00264-010-1073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]