Abstract
Purpose
This review was written to analyse the potential role of the cholinergic anti-inflammatory pathway in smoking-induced impairment of the bone healing process.
Methods
Literature in PubMed was reviewed by entering the following keywords “smoking AND bone healing”, “cholinergic anti-inflammatory pathway AND tumour necrosis factor”, “tumour necrosis factor AND bone healing”. All the related papers were recruited and carefully selected according to the content of this paper.
Results
Literature review indicated that tumour necrosis factor alpha (TNF-α) plays a pivotal role in the fracture healing process. In brief, TNF-α may accelerate the endochondral ossification process by increasing matrix metalloproteinases (MMPs) level, chondrocyte apoptosis, as well as osteoclast formation, therefore reducing the cartilaginous stage leading to the acceleration of fracture healing. Nicotine is the main effective ingredient of tobacco, which has been found to inhibit the secretion of TNF-α through activation of the cholinergic anti-inflammatory pathway.
Conclusions
It is reasonable to believe that the nicotine in tobacco at least partly contributes to the delayed fracture healing by inhibiting TNF-α secretion through the activation of the cholinergic anti-inflammatory pathway. An in-depth study of this issue will contribute to the clinical treatment of nonunion, as well as the development of new therapies to accelerate bone healing.
Introduction
Much has been reported on the adverse effects of cigarette smoke on wound healing. There is also some evidence that bone heals more slowly in smokers than in nonsmokers [13]. While it is still unclear how smoking impairs bone healing, based on the review of the current research on nicotine, the following potential pathway may explain the mechanism behind the phenomenon.
Tumour necrosis factor alpha (TNF-α) plays important role in bone healing
In addition to being an important proinflammatory factor, TNF-α is also an essential part of the innate immune response to injury, which contributes to the normal regulatory processes of bone resorption. The role of TNF-α during fracture healing was examined in wild-type and TNF-α receptor (p55(−/−)/p75(−/−))-deficient mice [10]. Histomorphometric measurements of the cartilage and bone as well as apoptotic cell counts in hypertrophic cartilage were detected which showed that endochondral tissue resorption was delayed two to three weeks in the TNF-α receptor knockout mice, indicating TNF-α mediated chondrocyte apoptosis as well as the expression of proresorptive cytokines which control endochondral tissue remodelling by osteoclasts. Similar results have been proven by other research groups [1, 16]. Furthermore, the induction of osteoprogenitor cell recruitment or osteogenic cell activation of TNF-α in the context of intramembranous bone formation was found by the same research group [9]. Research on the effects of TNF-α on cartilage cells indicated that TNF-α may alter the expression of a complex array of genes within murine chondrocytes that contribute to the destruction of joint surfaces, independent of its actions on synovial and immune cells [5]. Furthermore, studies on diabetes models also have shown that TNF-α may increase the expression of resorptive factors in chondrocytes through a process that involves activation of FOXO1 and TNF-α dysregulation and leads to enhanced osteoclast formation and accelerated loss of cartilage [2]. In addition, TNF-α appears to regulate, in part, the expression of three key proteolytic enzymes, i.e. matrix metalloproteinase 9 (MMP9), MMP13 and MMP14, that are known to be crucial to the progression of vascularisation and turnover of mineralised cartilage [12, 14]. Therefore, TNF-α signalling in healing fractures appears to coordinate the expression of specific regulators of endothelial cell survival and metalloproteolytic enzymes and is essential in the transition and progression of the endochondral phase of fracture repair. Our study examined the potential role of TNF-α as a regulator of MMPs in the retinal neovascularisation process which demonstrated an increased production of MMPs stimulated by TNF-α, enhancing the production of specific members of the MMP family [15]. These results suggested that TNF-α was able to enhance the expression of the MMP family in a wide range of types of cells. Another study showed that TNF-α could up-regulate the expression of intercellular adhesion molecule1 (ICAM-1) in mesenchymal stem cells (MSCs) and enhance the cells' migration ability, and the p38 signalling pathway might be involved in the TNF-α-induced migration ability for a role in wound repair and regeneration [8]. Therefore, it seems that TNF-α was an essential cytokine for fracture healing and proper concentrations of TNF-α ensure the normal bone healing process.
Nicotine may inhibit the secretion of TNF-α
It has been widely accepted that nicotine is one of the main effective ingredients that contributes to the harmful effect of smoking, and is also an agonist of the nicotinic receptor. Recently, a new anti-inflammatory pathway called the “cholinergic anti-inflammatory pathway” was discovered [18]. The research showed that the α7 nicotinic acetylcholine receptor (α7 nAChR) was the most important therapeutic target for inflammation [22] which is mainly expressed on the membrane of immune cells, including monocytes, macrophages, T and B lymphocytes, dendritic cells [6], and fibroblast-like synoviocytes (FLS) [20]. In brief, α7nAChR exerts its effects on immune cells by combining nicotinic agonists (nicotine, acetylcholine, etc.) via the triggering of various signalling mechanisms that probably interact with each other to achieve the anti-inflammatory effect, among which the JaK2–STAT3 and NF-κB signalling pathways were included [7, 21]. More experiments have confirmed the anti-inflammatory effect of α7nAChR using different models of inflammation [11, 17, 19].
Viewpoints
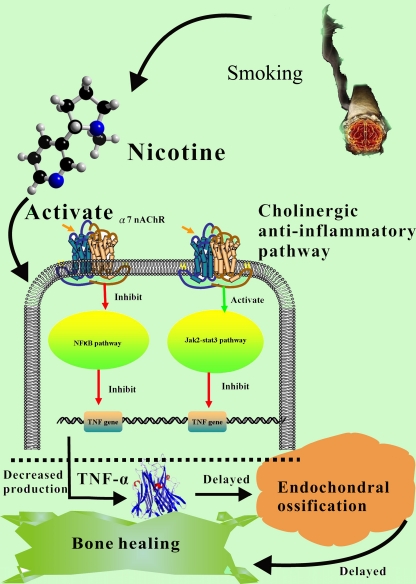
Based on the above-mentioned literature review and analysis, it is reasonable to believe that the nicotine in tobacco may at least partly contribute to the delayed healing of fracture by inhibiting TNF-α expression through the activation of the cholinergic anti-inflammatory pathway. In detail, the nicotine released from smoking may combine with the α7 nAChR and activate the anti-inflammatory pathways; the JaK2–STAT3 signalling pathway will be activated and the NF-κB signalling pathway will be inhibited consequently. As a result, the production of TNF-α will be greatly reduced which may result in prolonged soft callus stage and less MSCs migration in the bone healing process (Fig. 1).
Fig. 1.
The diagrammatic description of the viewpoints in this paper. α7nAChR α7 nicotinic acetylcholine receptor, TNF-α tumour necrosis factor α
However, we should note that many other elements in smoking may also contribute to the delayed bone healing process. It is well known that more than 4,700 chemical compounds, including 43 cancer-causing substances, have been isolated in cigarette smoke, and nicotine and carbon monoxide (CO) are the elements of cigarette smoke associated with impairment of the healing process [3]. It has been reported that CO may bind to haemoglobin in the pulmonary capillaries to form the stable compound carboxyhaemoglobin, resulting in reduced oxygen-carrying capacity of blood and tissue hypoxia which may lead to the impaired bone healing process as well [4].
Prospect
In this paper, based on the current literature review, a reasonable explanation for the mechanism of smoking delayed fracture healing is illustrated. From our point of view, although many elements (CO, etc.) may contribute to the smoking induced impairment of the bone healing process, the potential role of nicotine and the cholinergic anti-inflammatory pathway are of great importance. From another point of view, this will be helpful to accelerate fracture healing. An appropriate level of TNF-α may facilitate endochondral ossification, which will inevitably accelerate the fracture healing process. In-depth research (in vitro, in vivo experimental evidence) on this subject will be more constructive and meaningful.
Acknowledgement
This research was supported by the National Natural Science Foundation of China (support number: 81000786).
References
- 1.Aizawa T, Kon T, Einhorn TA, Gerstenfeld LC. Induction of apoptosis in chondrocytes by tumor necrosis factor-alpha. J Orthop Res. 2001;19:785–796. doi: 10.1016/S0736-0266(00)00078-4. [DOI] [PubMed] [Google Scholar]
- 2.Alblowi J, Kayal RA, Siqueria M, McKenzie E, Krothapalli N, McLean J, Conn J, Nikolajczyk B, Einhorn TA, Gerstenfeld L, Graves DT. High levels of tumor necrosis factor-alpha contribute to accelerated loss of cartilage in diabetic fracture healing. Am J Pathol. 2009;175:1574–1585. doi: 10.2353/ajpath.2009.090148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartecchi CE, MacKenzie TD, Schrier RW. The global tobacco epidemic. Sci Am. 1995;272:44–51. doi: 10.1038/scientificamerican0595-44. [DOI] [PubMed] [Google Scholar]
- 4.Birnstingl MA, Brinson K, Chakrabarti BK. The effect of short-term exposure to carbon monoxide on platelet stickiness. Br J Surg. 1971;58:837–839. doi: 10.1002/bjs.1800581110. [DOI] [PubMed] [Google Scholar]
- 5.Cho TJ, Lehmann W, Edgar C, Sadeghi C, Hou A, Einhorn TA, Gerstenfeld LC. Tumor necrosis factor alpha activation of the apoptotic cascade in murine articular chondrocytes is associated with the induction of metalloproteinases and specific pro-resorptive factors. Arthritis Rheum. 2003;48:2845–2854. doi: 10.1002/art.11390. [DOI] [PubMed] [Google Scholar]
- 6.Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonge WJ, Zanden EP, The FO, Bijlsma MF, Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 8.Fu X, Han B, Cai S, Lei Y, Sun T, Sheng Z. Migration of bone marrow-derived mesenchymal stem cells induced by tumor necrosis factor-alpha and its possible role in wound healing. Wound Repair Regen. 2009;17:185–191. doi: 10.1111/j.1524-475X.2009.00454.x. [DOI] [PubMed] [Google Scholar]
- 9.Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Cruceta J, Graves BD, Einhorn TA. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-alpha signaling. Cells Tissues Organs. 2001;169:285–294. doi: 10.1159/000047893. [DOI] [PubMed] [Google Scholar]
- 10.Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Tsay A, Fitch J, Barnes GL, Graves DT, Einhorn TA. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res. 2003;18:1584–1592. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- 11.Giebelen IA, Westerloo DJ, LaRosa GJ, Vos AF, Poll T. Local stimulation of alpha7 cholinergic receptors inhibits LPS-induced TNF-alpha release in the mouse lung. Shock. 2007;28:700–703. doi: 10.1097/shk.0b013e318054dd89. [DOI] [PubMed] [Google Scholar]
- 12.Kosaki N, Takaishi H, Kamekura S, Kimura T, Okada Y, Minqi L, Amizuka N, Chung UI, Nakamura K, Kawaguchi H, Toyama Y, D'Armiento J. Impaired bone fracture healing in matrix metalloproteinase-13 deficient mice. Biochem Biophys Res Commun. 2007;354:846–851. doi: 10.1016/j.bbrc.2006.12.234. [DOI] [PubMed] [Google Scholar]
- 13.Krannitz KW, Fong HW, Fallat LM, Kish J. The effect of cigarette smoking on radiographic bone healing after elective foot surgery. J Foot Ankle Surg. 2009;48:525–527. doi: 10.1053/j.jfas.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Lehmann W, Edgar CM, Wang K, Cho TJ, Barnes GL, Kakar S, Graves DT, Rueger JM, Gerstenfeld LC, Einhorn TA. Tumor necrosis factor alpha (TNF-alpha) coordinately regulates the expression of specific matrix metalloproteinases (MMPS) and angiogenic factors during fracture healing. Bone. 2005;36:300–310. doi: 10.1016/j.bone.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Majka S, McGuire PG, Das A. Regulation of matrix metalloproteinase expression by tumor necrosis factor in a murine model of retinal neovascularization. Invest Ophthalmol Vis Sci. 2002;43:260–266. [PubMed] [Google Scholar]
- 16.Polzer K, Schett G, Zwerina J. The lonely death: chondrocyte apoptosis in TNF-induced arthritis. Autoimmunity. 2007;40:333–336. doi: 10.1080/08916930701356721. [DOI] [PubMed] [Google Scholar]
- 17.The FO, Boeckxstaens GE, Snoek SA, Cash JL, Bennink R, Larosa GJ, Wijngaard RM, Greaves DR, Jonge WJ. Activation of the cholinergic anti-inflammatory pathway ameliorates postoperative ileus in mice. Gastroenterology. 2007;133:1219–1228. doi: 10.1053/j.gastro.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 19.Maanen MA, Lebre MC, Poll T, LaRosa GJ, Elbaum D, Vervoordeldonk MJ, Tak PP. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 2009;60:114–122. doi: 10.1002/art.24177. [DOI] [PubMed] [Google Scholar]
- 20.Waldburger JM, Boyle DL, Pavlov VA, Tracey KJ, Firestein GS. Acetylcholine regulation of synoviocyte cytokine expression by the alpha7 nicotinic receptor. Arthritis Rheum. 2008;58:3439–3449. doi: 10.1002/art.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walker JG, Smith MD. The Jak-STAT pathway in rheumatoid arthritis. J Rheumatol. 2005;32:1650–1653. [PubMed] [Google Scholar]
- 22.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, Al-Abed Y, Czura CJ, Tracey KJ. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]