Abstract
Purpose
Infections associated with orthopaedic implants remain a serious complication. The main objective in acute infection control is component retention, whereas this option is usually not considered for chronic infections.
Methods
This multi-centre prospective, non-randomised observational study investigated one possible treatment option for implant retention in combination with negative pressure wound therapy with instillation (NPWTi). Thirty-two patients with an infected orthopaedic implant were analysed. Twenty-two patients had an acute infection (< 8 weeks after implantation) and ten patients had a chronic infection (> 8 weeks and < 36 weeks after implant placement). Polyhexanide was used as the instillation solution in 31 of the 32 cases.
Results
Nineteen patients (86.4%) with an acute infection and eight patients (80%) with a chronic infection retained their implant at 4–6 months follow-up after treatment.
Conclusions
Our study showed that NPWTi can be used as adjunctive therapy for salvage of acutely infected orthopaedic implants and may even be considered for early chronically infected implants.
Introduction
Infections associated with orthopaedic implants (OI) such as hip and knee prostheses often results in serious disability of the patients. The number of total hip and knee replacements has increased over the years, leading to an overall increased number of associated infections. The incidence of infected primary total hip arthroplasty (THA) or total knee arthroplasty (TKA) is 1.5–2.5%. Revision THA or TKA carries a respective infection risk of 3.2–5.6% and up to 15% in mega-implants [12]. The estimated cost of treating an infected arthroplasty is over US $50,000 per episode [1, 17]. The major clinical objective in treating an infected OI without signs of loosening is eradication of the infection with retention of the implant since exchange revision surgery may be associated with serious complications, such as bone loss, impaired function of the joint and restricted patient mobility. In most cases, infections associated with OIs are difficult to manage and require a longer period of systemic antibiotic therapy and often-repeated surgical procedures [15, 23, 26].
The four commonly used surgical interventions for treatment of an infected joint prosthesis are (1) debridement plus retention of the prosthesis (with or without temporary use of antibiotic-loaded bead chains) [2, 14], (2) one-stage replacement [22], (3) two-stage replacement [21, 28] and (4) removal of the infected implant without replacement [15].
Surgical debridement in combination with systemic antibiotic therapy is the preferred approach because this option allows for retention of the prosthesis and can be successful in patients with early postoperative or early diagnosed late haematogenous infection of well-fixed THA/TKA [18]. Surgeons have reported success rates between 30 and 80% with surgical debridement and retention of the implant [7]. A variation on the method of debridement plus retention of the prosthesis is suction irrigation drainage, which has a reported success rate of up to 60% [19].
The one-stage replacement approach is more likely to be successful when the microorganism in the wound is known and the implant usually has to be inserted with cement.
The two-stage replacement approach is the most commonly used therapy for chronic prosthetic joint infection. It involves removal of the infected implant, placement of an antimicrobial carrier and placement of a new joint prosthesis after at least 6 weeks. This approach is believed to result in infection eradication in up to 90% of cases, thus preserving the function of the joint [3, 11, 20]. However, the two-stage procedure is expensive and may result in large skeletal defects, increased hospitalisations and costs, severe functional impairment with delayed mobility, and sometimes death [18].
Removal of the infected implant without replacement [15] is directly associated with significantly impaired function of the extremity. This option is only used when no other treatment options are available.
The treatment of wound infections by combining negative pressure wound therapy with instillation (NPWTi) was first described by Fleischmann et al. in 1998 [10]. In 2009, Timmers et al. reported successful results from the first use of NPWTi to treat post-traumatic osteomyelitis [25]. Our study was designed as a next step to collect clinical outcome information on patients with infected OIs that were treated with NPWTi using V.A.C. Instill® Wound Therapy (KCI USA, Inc., San Antonio, TX, USA).
Methods
This study was designed as a prospective, multi-centre, single-arm, post-market, observational study. The study protocol was reviewed and approved by the applicable Ethics Committees, and informed consent was obtained for all patients. The main inclusion criteria were infection of an OI (knee, hip, other osteosynthesis material) occurring within 8 weeks after implant or infection older than 8 weeks of “stable” OI if NPWTi could be used as first-line adjunct treatment after adequate debridement. The main exclusion criteria were multiple organ failure or high risk for developing multiple organ failure, fistulas in the area of the wound, malignancy in the wound and/or medication affecting coagulation.
The primary efficacy endpoint was percentage of implant retention without infection at follow-up (4–6 months after start of treatment with NPWTi) for patients with an acutely infected OI. Acute infection was defined as infection occurring within 8 weeks of implant placement, whereas chronic infection was defined as infection occurring any time after 8 weeks of implant placement. The secondary endpoints were percentage of implant retention for patients with a chronic infection, incidence of treatment-related adverse events (AEs) and incidence of infection recurrence. Additional criteria that were evaluated included duration of NPWTi, duration of clinical signs of infection and length of hospital stay. Safety assessments were conducted throughout the observational study.
NPWTi allows for a combination of NPWT with timed, intermittent delivery of topical solutions. The NPWTi therapy unit has an additional instillation tube that connects the dressing with an infusion bag containing the solution. Separate vacuum and instillation clamps open and close at set intervals, allowing for automated delivery of the solution (instillation phase) followed by a hold time for penetration and incubation (hold phase), and resumption of negative pressure to extract the solution from the wound bed and dressing and to apply NPWT to the wound (vacuum phase). The three phases together are called a cycle.
In this study, treatment with NPWTi varied on a patient-by-patient basis. The decision regarding which agent or solution to use was at the discretion of the treating surgeon as long as the solution used was compatible with the dressings and NPWTi system.
The sample size was based on clinical and practical considerations and not on formal statistical power calculations. Patients who had no follow-up information, were withdrawn by an investigator or received “off-label” treatment (which did not follow the manufacturer’s guidelines for appropriate use of NPWTi) were omitted from the analysis.
Continuous patient characteristics were summarised using descriptive statistics (n, mean, median and standard deviation). Categorical patient characteristics were summarised as proportions. Statistical comparisons for continuous data were conducted using the Wilcoxon rank sum test, which is based on ranks of the data and was used to compare location parameters (such as the median between OI retained groups vs OI not-retained groups) without the assumption of normally distributed data. Statistical comparisons for categorical data or proportions were conducted using either Fisher’s exact test or a binomial test, based on the 5% significance level. All statistical analyses were performed using SAS® version 9.1.3.
Results
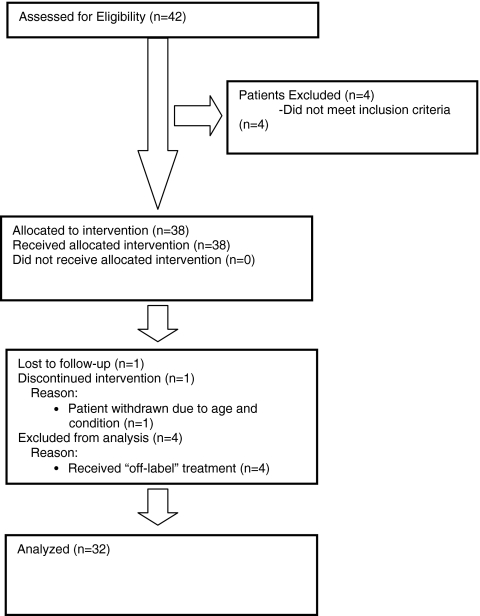
Forty-two patients from eight centres in Germany, The Netherlands and the UK were enrolled in the study (Fig. 1). Four patients did not meet the inclusion criteria (OI explanted prior to NPWTi) and were excluded from the analysis because retention of the OI at follow-up could not be analysed. One patient was lost to follow-up, one patient was discontinued by the investigator and four patients received off-label treatment and were omitted from the analysis. This left 32 patients from which data were analysed. Of 32 patients, 20 (62.5%) had an infected hip implant, 10 (31.3%) an infected knee implant and 2 (6.2%) infected osteosynthesis material (acetabulum fixation device, metal plate upper arm). Of 32 patients, 22 (68.7%) had an acute infection and 10 (31.3%) a chronic infection. Table 1 shows an overview of the patient characteristics.
Fig. 1.
CONSORT Statement 2010 flow diagram
Table 1.
Patient characteristics
| Variable | n | % |
|---|---|---|
| Sex | ||
| Male | 18 | 56.3 |
| Female | 14 | 43.7 |
| Risk factors | ||
| Smoking | 8 | 25 |
| Diabetes mellitus | 6 | 18.8 |
| Race | ||
| Caucasian | 30 | 93.8 |
| Other | 2 | 6.2 |
| OIs | ||
| THA | 20 | 62.5 |
| TKA | 10 | 31.3 |
| Other implants | 2 | 6.2 |
| Infection | ||
| Acute | 22 | 68.7 |
| Chronic | 10 | 31.3 |
| Median | Range | |
| Age (years) | 60 | 34–88 |
| BMI | 28 | 17–47 |
BMI body mass index
Treatment consisted of surgical debridement of the wound in combination with lavage, systemic antibiotic therapy and NPWTi. In 25 of 32 (78.1%) cases, debridement of the wound was documented, and in all cases (100%) lavage was performed (jet lavage (4/32, 12.5%) with polyhexamethylene biguanide 0.04% solution (polyhexanide or PHMB, B. Braun Melsungen AG, Melsungen, Germany) (16/32, 50.0%), Ringer’s solution (1/32, 3.1%), povidone-iodine (9/32, 28.1%) or octenidine dihydrochloride (Octenisept, Schülke & Mayr, Norderstedt, Germany) (2/32, 6.2%).
Prior to NPWTi, all wounds (39/39, 100%) were clinically infected. Infection was defined as: presence of at least one of the following: positive culture, abnormally elevated C-reactive protein/white blood cell count and in addition clinical signs, such as exudating wound, redness, swelling or pain. Positive cultures were obtained from 28 of 32 patients (87.5%) (Table 2). Typical microorganisms were present prior to NPWTi, and 8 of 32 cases had more than one type of microorganism present (Table 2). No multi-resistant microorganisms were discovered. Systemic antibiotic treatment depended on the microorganism(s) present in the wound and was administered per institutional standards. Generally, antibiotic treatment was given for 6 weeks after termination of NPWTi.
Table 2.
Microorganisms present in wound prior to NPWTi
| No. of patients with polymicrobial wounds | No. of patients | No. of wounds with microorganism types present | ||||
| No microorganisms present in the wound | 4 | 0 | ||||
| One microorganism in the wound | 20 | 20 | ||||
| Two microorganisms in the wound | 6 | 12 | ||||
| Three microorganisms in the wound | 1 | 3 | ||||
| Four microorganisms in the wound | 1 | 4 | ||||
| Total | 32 | 39 | ||||
| Bacterial types | Microorganisms present prior to NPWTi (n = 39) | |||||
| Not retained | Retained | Overall (not retained + retained) | ||||
| n | % | n | % | n | % | |
| Enterobacter cloacae | 1 | 2.6 | – | – | 1 | 2.6 |
| Staphylococci (aureus, epidermidis) | 4 | 10.3 | 14 | 35.9 | 18 | 46.2 |
| Streptococci | 1 | 2.6 | 5 | 12.8 | 6 | 15.4 |
| Anaerobic Gram-negative rod | – | – | 1 | 2.6 | 1 | 2.6 |
| Escherichia coli | – | – | 1 | 2.6 | 1 | 2.6 |
| Enterococci | – | – | 7 | 17.9 | 7 | 17.9 |
| Gram-positive cocci | – | – | 1 | 2.6 | 1 | 2.6 |
| Negative bacterial strain | – | – | 1 | 2.6 | 1 | 2.6 |
| Pseudomonas aeruginosa | – | – | 2 | 5.1 | 2 | 5.1 |
| Not available | – | – | 1 | 2.6 | 1 | 2.6 |
Thirty-two patients had a mean of 11.8 days (median 6.0 days, range 1.0–109.0 days) between diagnosis of infection and start of NPWTi. The results showed that the time interval had no influence on implant retention (p = 0.382).
The analysis of the primary endpoint, implant retention at follow-up, is summarised in Table 3. The mean time of follow-up was 176 days (median 164 days, range 57–490 days). Overall findings showed that 27 of 32 patients (84.4%) retained their implant (Table 3): 19 of 22 patients (86.4%) with an acute infection (< 8 weeks) and 8 of 10 patients (80%) with a chronic infection (>8 weeks and <36 weeks). Statistical comparisons between the groups (retained vs not-retained OIs) showed no statistical significance in sex, risk factors, race, OIs and acute or chronic infection (p > 0.05).
Table 3.
Retention of acutely and chronically infected OIs
| n = 32 | Acutely infected OI (n = 22) | Chronically infected OI (n = 10) | ||
|---|---|---|---|---|
| Retained | Not retained | Retained | Not retained | |
| Knees | 3/3 (100%) | 0/3 (0%) | 5/7 (71.4%) | 2/7 (28.6%) |
| Hips | 14/17 (82.4%) | 3/17 (17.6%) | 3/3 (100%) | 0/3 (0%) |
| Osteosynthesis material | 2/2 (100%) | 0/2 (0%) | – | – |
| Total | 19/22 (86.4%) | 3/22 (13.6%) | 8/10 (80%) | 2/10 (20%) |
| Published retention dataa | 65% [4, 5, 15, 19] | 35% [4, 5, 15, 19] | 30% [4, 7, 18] | 70% [4, 7, 18] |
aAverage of published results for patients with infected OIs who were treated without NPWTi
For acutely infected OIs, assuming an average retention rate of 65% based on published data [4, 5, 15, 19], there was a significant difference between patients who retained their implant versus those patients who did not retain their implant (i.e. 86.4 and 13.6%, respectively, p = 0.036). Likewise, for chronically infected OIs, assuming an average retention rate of 30% [4, 7, 18], there was a significant difference between patients who retained their implant versus those patients who did not retain their implant (i.e. 80 vs 20%, respectively, p = 0.001). When considering overall implant retention (acutely and chronically infected OIs), and assuming an average retention rate of 50% [4, 5, 7, 15, 18, 19], a significant difference was detected between patients who retained their implant versus patients who did not retain their implant (84.4 vs 15.6%, respectively, with p < 0.001).
The mean duration of NPWTi was 16.3 days (median 15.0 days, range 9–46, p = 0.486). Reasons to discontinue treatment were local negative bacterial culture (25/32, 78.1%) as per institutional procedure and clinical judgment of the surgeon (6/32, 18.7%). One case was not documented. PHMB was used in 31 of 32 (96.9%) cases (concentrations 0.04–0.2%); in 1 case (3.1%), saline was used. The mean NPWTi negative pressure setting was 138.3 mmHg (median 125 mmHg, range 125–200 mmHg). Instillation time was in all cases <1 min. The mean hold time was 19 min (median 20.0, range 5–30 min), and the mean vacuum time was 70.3 min (median 60.0, range: 30–270 min). A mean of 16.5 cycles (instillation + hold + vacuum) per day were applied (median 18.0 cycles, range 5–40 cycles per day).
Recurrence of infection was monitored by the investigator or general practitioner through regular wound checks as per institutional standard. In cases of wound problems such as pain, swelling, redness, discharge or systemic signs of infection patients were required to return to the hospital for clinical control to confirm recurrence of infection. Infection eradication was reported in 24 of 32 patients (75%). In 6 of 32 patients (18.8%) recurrence of infection was reported and 2 of 32 patients (6.2%) had an ongoing infection. In 3 of 32 patients (9.4%), where recurrence of infection was diagnosed, the surgeons decided to perform a second treatment with NPWTi and were thereafter able to eradicate the infection and retain the implant.
Thirty patients (93.8%) had a mean duration of clinical signs of infection of 27.3 days (median 20.5 days, range 10–125 days). Duration of clinical signs of infection was defined as the date that the infection was diagnosed until surgical closure.
For all 32 patients evaluated, the mean number of dressing changes was 3.5 (median 4.0 dressing changes, range 1–8 dressing changes). The mean hospital stay was 39.5 days (median 35 days, range 12–97 days).
The influence of known major risk factors was not significant. Four of six patients with diabetes were able to retain their implant (p = 0.228), as did all eight patients who were smokers or had a history of smoking (p = 1.0).
Safety analyses were described by the secondary endpoints: incidence of treatment-related complications and device complaints. Of 32 patients, 12 (37.5%) experienced a total of 17 AEs; however, none of the AEs were treatment or device related. One patient (3.1%) died prior to follow-up due to age and condition. In two instances, device problems were reported (not able to reset alarm), and the systems had to be replaced.
Discussion
This post-market, non-randomised, observational study sought to provide insight into disease course and treatment outcomes of patients with infected OIs who were treated with NPWTi. NPWTi provides an automated, repetitive lavage of the infected area. Historical data from the clinical literature indicate an average retention rate of 65% [4, 5, 15, 19] in patients with an acutely infected OI not treated with NPWTi. The results from this observational study seem to confirm or exceed retention rates reported in the literature [8, 16] and suggest that NPWTi, in combination with debridement and systemic antibiotic therapy, is an effective adjunctive therapy in managing acutely infected OIs. In this study, negative pressure settings of 125–200 mmHg were applied. Timmers et al. showed in their experimental randomised study that negative pressure settings higher than 125 mmHg (international standard) can be favourable to promote perfusion and reduce the risk of recurrence of infection [24].
This treatment modality also seems to be effective for treatment of chronically infected OIs. Retention rates of approximately 30% have been published [4, 7, 18], whereas in this study in 80% of the cases the OIs were retained. One reason could be that in this study all patients were treated within 36 weeks of implant placement, which is a relatively early chronic infection.
Retention of chronically infected knee implants is generally associated with a poor outcome [4], due to problems with the critical soft tissue coverage. It was interesting to note that five of seven (71.4%) chronically infected knee implants were retained with the use of NPWTi as adjunctive therapy. While this sample is much too small to draw any conclusions, the results may warrant further exploration.
This observational study has two limitations. The first limitation is that this was a single-arm, prospective, non-randomised, observational study with no control group. Comparison to historical data is often difficult since different criteria are being used and no simple one-to-one comparison is possible. On the other hand, this study was observational, and the cases reflect the “real-life” situations found in the participating centres. Thus, these findings may be valuable for other centres to compare their results to the results of this observational study [6].
The second limitation is the short follow-up period (4–6 months after treatment). Clinical experience shows that recurrence of infection in acute wounds usually occurs within this time frame. A longer follow-up period is required to confirm retention of the OI over the long term.
Results of treatment of infected OIs with NPWTi are similar and independent of the type of implant. However, NPWTi is an adjunctive therapy and must be used in combination with mechanical debridement and systemic antibiotic therapy when used for treatment of infected OI. Microorganisms that are usually difficult to eradicate with retained implants, such as Enterococci and Staphylococcus aureus/epidermidis, could be effectively treated with this treatment modality [9].
All centres used the same antiseptic instillation solution (PHMB) [13], except for one patient treated with saline. More research is required with different concentrations and other solutions, such as saline, antibiotics, etc., to find the optimal solution for managing infection and allowing for OI retention [27].
The advantages of NPWTi are that it is relatively easy to use, has minimal side effects and is associated with relatively short therapy duration and length of hospital stay.
Conclusion
The results from this observational study are promising and suggest that NPWTi with PHMB may be effective as an adjunctive therapy to manage infected OIs by removing infectious materials and helping to retain the implant, independent of the type of infection (i.e. acute or chronic) or microorganism. The results exceed, or are similar to, what has been reported in the literature without the use of NPWTi.
The automated instillation therapy and the high percentage of prosthesis retention in combination with a relatively short therapy duration and length of hospital stay may contribute to reducing overall treatment time and costs.
Further research is required to study the mechanism of action of NPWTi, investigate the optimal treatment regimen and instillation fluid, and collect long-term follow-up information in a larger patient population. The findings presented here, however, show promising results for the management and retention of infected OIs treated with NPWTi as part of the treatment regimen.
Acknowledgements
This observational study was supported by Kinetic Concepts, Inc. (San Antonio, TX, USA). The setup of the study and evaluation of the data were performed by the individual members of the Steering Committee.
We would like to thank the following hospitals and their local investigators who have contributed data to this study: Willy Izbikci, M.D., Evangelisches Krankenhaus Muelheim a.d. Ruhr, Germany; Martin Fischer, M.D., Evangelisches Krankenhaus, Muelheim a.d. Ruhr, Germany; Louk van Doorn, Leiderdorp Hospital, Leiderdorp, The Netherlands; Markus Schmoeger, M.D., Rhoen-Saale Klinik, Bad Neustadt, Germany; Wolfgang Huber, M.D., Zollernalb Klinikum, Balingen, Germany; Emese Scholl, M.D., Zollernalb Klinikum, Balingen, Germany; Rhidian Morgan-Jones, M.D., University Hospital Cardiff, Cardiff, UK; Paul Zirker, University Hospital Cardiff, Cardiff, UK; Kay Schendel, M.D., University Hospital Halle-Wittenberg, Halle a.d. Saale, Germany.
We also thank Herman Chavarria, M.S. and David Flores for their statistical analyses and Laura McMorris, Ph.D. for her editorial assistance.
Conflict of interest Burkhard Lehner, M.D. and Gerrolt N. Jukema, M.D., Ph.D. were appointed as consultants to this observational study and are members of the study Steering Committee. Wim Fleischmann, M.D. is the patent holder of the V.A.C. Instill® system and member of the study Steering Committee.
References
- 1.Berbari EF, Hanssen AD, Duffy MC, Steckelberg JM, Ilstrup DM, Harmsen WS, Osmon DR. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis. 1998;27(5):1247–1254. doi: 10.1086/514991. [DOI] [PubMed] [Google Scholar]
- 2.Brandt CM, Sistrunk WW, Duffy MC, Hanssen AD, Steckelberg JM, Ilstrup DM, Osmon DR. Staphylococcus aureus prosthetic joint infection treated with debridement and prosthesis retention. Clin Infect Dis. 1997;24(5):914–919. doi: 10.1093/clinids/24.5.914. [DOI] [PubMed] [Google Scholar]
- 3.Buchholz HW, Elson RA, Engelbrecht E, Lodenkämper H, Röttger J, Siegel A. Management of deep infection of total hip replacement. J Bone Joint Surg Br. 1981;63-B(3):342–353. doi: 10.1302/0301-620X.63B3.7021561. [DOI] [PubMed] [Google Scholar]
- 4.Chiu FY, Chen CM. Surgical débridement and parenteral antibiotics in infected revision total knee arthroplasty. Clin Orthop Relat Res. 2007;461:130–135. doi: 10.1097/BLO.0b013e318063e7f3. [DOI] [PubMed] [Google Scholar]
- 5.Cobo J, Miguel LG, Euba G, Rodríguez D, García-Lechuz JM, Riera M, Falgueras L, Palomino J, Benito N, Toro MD, Pigrau C, Ariza J. Early prosthetic joint infection: outcomes with debridement and implant retention followed by antibiotic therapy. Clin Microbiol Infect. 2010 doi: 10.1111/j.1469-0691.2010.03333.x. [DOI] [PubMed] [Google Scholar]
- 6.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crockarell JR, Hanssen AD, Osmon DR, Morrey BF. Treatment of infection with débridement and retention of the components following hip arthroplasty. J Bone Joint Surg Am. 1998;80:1306–1313. doi: 10.2106/00004623-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Deirmengian C, Greenbaum J, Stern J, Braffman M, Lotke PA, Booth RE, Jr, Lonner JH. Open debridement of acute gram-positive infections after total knee arthroplasty. Clin Orthop Relat Res. 2003;416:129–134. doi: 10.1097/01.blo.0000092996.90435.35. [DOI] [PubMed] [Google Scholar]
- 9.Pozo JL, Patel R. Infection associated with prosthetic joints. N Engl J Med. 2009;361:787–794. doi: 10.1056/NEJMcp0905029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleischmann W, Russ M, Westhauser A, Stampehl M. Vacuum sealing as carrier system for controlled local drug administration in wound infection. Unfallchirurg. 1998;101(8):649–654. doi: 10.1007/s001130050318. [DOI] [PubMed] [Google Scholar]
- 11.Haddad FS, Muirhead-Allwood SK, Manktelow AR, Bacarese-Hamilton I. Two-stage uncemented revision hip arthroplasty for infection. J Bone Joint Surg Br. 2000;82(5):689–694. doi: 10.1302/0301-620X.82B5.9668. [DOI] [PubMed] [Google Scholar]
- 12.Hanssen AD, Rand JA. Evaluation and treatment of infection at the site of a total hip or knee arthroplasty. Instr Course Lect. 1999;48:111–122. [PubMed] [Google Scholar]
- 13.Hübner NO, Kramer A. Review on the efficacy, safety and clinical applications of polihexanide, a modern wound antiseptic. Skin Pharmacol Physiol. 2010;23:17–27. doi: 10.1159/000318264. [DOI] [PubMed] [Google Scholar]
- 14.Klemm K. The use of antibiotic-containing bead chains in the treatment of chronic bone infections. Clin Microbiol Infect. 2001;7(1):28–31. doi: 10.1046/j.1469-0691.2001.00186.x. [DOI] [PubMed] [Google Scholar]
- 15.Lehner B, Witte D, Suda AJ, Weiss S. Revision strategy for periprosthetic infection. Orthopade. 2009;38(8):681–688. doi: 10.1007/s00132-009-1434-6. [DOI] [PubMed] [Google Scholar]
- 16.Lehner B (2009) Application of V.A.C. Instill therapy in case of periprosthetic infection in hip arthroplasty. Infection 37(Suppl I-04):13–17
- 17.Lentino JR. Prosthetic joint infections: bane of orthopedists, challenge for infectious disease specialists. Clin Infect Dis. 2003;36(9):1157–1161. doi: 10.1086/374554. [DOI] [PubMed] [Google Scholar]
- 18.Meehan AM, Osmon DR, Duffy MCT, Hanssen AD, Keating MR. Outcome of penicillin-susceptible streptococcal prosthetic joint infection treated with debridement and retention of the prosthesis. Clin Infect Dis. 2003;36(7):845–849. doi: 10.1086/368182. [DOI] [PubMed] [Google Scholar]
- 19.Mella-Schmidt C, Steinbrink K. Value of irrigation-suction drainage in the treatment of early infection of joint implants. Chirurg. 1989;60(11):791–794. [PubMed] [Google Scholar]
- 20.Park SJ, Song EK, Seon JK, Yoon TR, Park GH. Comparison of static and mobile antibiotic-impregnated cement spacers for the treatment of infected total knee arthroplasty. Int Orthop. 2010;34(8):1181–1186. doi: 10.1007/s00264-009-0907-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parvizi J, Zmistowski B, Adeli B. Periprosthetic joint infection: treatment options. Orthopedics. 2010;33(9):659. doi: 10.3928/01477447-20100722-42. [DOI] [PubMed] [Google Scholar]
- 22.Saito S, Tokuhashi Y, Ishii T, Mori S, Hosaka K, Taniguchi S (2010) One- versus two-stage bilateral total hip arthroplasty. Orthopedics 33(8). doi:10.3928/01477447-20100625-07 [DOI] [PubMed]
- 23.Senthi S, Munro JT, Pitto RP. Infection in total hip replacement: meta-analysis. Int Orthop. 2011;35(2):253–260. doi: 10.1007/s00264-010-1144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timmers MS, Cessie S, Banwell P, Jukema GN. The effect of varying degrees of pressure delivered by negative-pressure wound therapy on skin perfusion. Ann Plast Surg. 2005;55:665–671. doi: 10.1097/01.sap.0000187182.90907.3d. [DOI] [PubMed] [Google Scholar]
- 25.Timmers MS, Graafland N, Bernards AT, Nelissen RG, Dissel JT, Jukema GN. Negative pressure wound treatment with polyvinyl alcohol foam and polyhexanide antiseptic solution instillation in posttraumatic osteomyelitis. Wound Repair Regen. 2009;17(2):278–286. doi: 10.1111/j.1524-475X.2009.00458.x. [DOI] [PubMed] [Google Scholar]
- 26.Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. 2002;23(4):183–189. doi: 10.1086/502033. [DOI] [PubMed] [Google Scholar]
- 27.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 28.Zywiel MG, Johnson AJ, Stroh DA, Martin J, Marker DR, Mont MA. Prophylactic oral antibiotics reduce reinfection rates following two-stage revision total knee arthroplasty. Int Orthop. 2011;35(1):37–42. doi: 10.1007/s00264-010-0992-x. [DOI] [PMC free article] [PubMed] [Google Scholar]