Abstract
The wide surgical tumour resection is the only effective treatment in chondrosarcoma. However, a major problem remains the high rate of local recurrences and metastases due to the lack of adjuvant therapies. In this study the cytotoxic effect of the bisphosphonate clodronate (0.1–1000 μM) and zoledronate (0.1–1000 μM) in different concentrations on two chondrosarcoma cell lines (HTB-94 and CAL-78) has been investigated. After an incubation period of 48, 72 and 96 hours the chondrosarcoma cell viability was measured as the MTT-proliferation rate. In concentrations of >1 μm zoledronate the cell activity was reduced by up to 95% for the CAL-78 cells. Further, zoledronate has been more effective in lower concentrations than clodronate in the reduction of cell viability for both cell lines. However, clodronate showed significant cytotoxic effects in high concentrations and after longer incubation periods. Further research is necessary, but in the light of these results bisphosphonates may also play a role in the treatment of chondrosarcomas.
Introduction
The chondrosarcoma is the second most common primary malignant bone tumour. These tumours appear mainly in elderly patients. In contrast to other primary malignant bone tumours such as osteosarcoma or Ewing’s sarcoma, no effective adjuvant chemotherapy exists. The wide tumour resection according to Enneking is still the only effective treatment for these tumours [1]. Therefore, particularly for the group of high grade chondrosarcoma, high rates of local recurrence (>30%) and metastasis (>40%) are reported. This fact worsens the prognosis of these patients. Five-year overall survival rates of less than 50% are common in high grade chondrosarcoma [1, 2]. To improve the prognosis of these patients new therapy methods are needed. Bisphosphonates are an important class of drugs with a broad spectrum of clinical use in patients suffering from different solid tumors such as breast and lung cancer or multiple myeloma. In these patients bisphosphonates have been successfully used in the treatment of hypercalcemia and to reduce skeletal-related events [3–6].
The effects of bisphosphonates are related to different cellular and molecular modes of action. The non amino bisphosphonates, lacking a nitrogen atom in the R2 side chain (e.g. clodronate), are metabolically incorporated into non-hydrolysable analoges of the ATP, thereby inhibiting the ATP-dependant enzymes. Bisphosphonates containing a nitrogen atom within the R2 side chain act by inhibiting the farnesyldiphosphate synthase, an important enzyme in the mevalonate pathway. Inhibition of this enzyme prevents the synthesis of its metabolic products which are required for the prenylation of small GTPases. Besides the inhibitory effects on osteoclasts and antiangiogenetic effects there is recent evidence that bisphosphonates have a direct cytotoxic effect on different tumour cells [7–9].
In this study we use an in vitro approach to investigate the effect of two bisphosphonates (clodronate, zoledronate), widely used in clinical oncology, on the chondrosarcoma cell lines HTB-94 and CAL-78.
Material and methods
Reagents
The biphosphonates clodronate (dichloromythelene bisphosphonate) and zoledronate were used. Stock solutions of biphosphonates and 3-aminopropyl phosphonate were prepared in phosphate buffered saline (PBS), adjusted to pH 7.4 and sterilised by filtration.
Other chemicals and reagents were of analytic grade.
Cell lines and culture maintenance
The chondrosarcoma cell line used, CAL-78, is a cell line from the recurrence of a high grade (grade III) chondrosarcoma from a 76-year-old man.
The HTB-94 chondrosarcoma cell line derives from a human grade II chondrosarcoma of the humerus.
The cells were cultured in RPMI 1640 medium supplement with 2 mM l-glutamine, 100 U/ml penicillin G, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin B and 10% foetal calf serum in a humidified atmosphere of 5% CO2 at 37°C.
Cell viability assay
The MTT assay was used to measure the cytotoxicity. Cells were grown in 96-well tissue culture plates coated with collagen. In each well 5,000 cells were seeded in 100 μl RPMI 1640 medium. Cells were allowed to attach to the collagen matrix and to resume exponential growth before 100 μl of complete cell culture medium containing the drugs at different concentrations was added. Five concentrations of each bisphosphonate ranging from 0.1 to 1000 μM were tested. After 48, 72 and 96 hours, 20 μl of the MTT reagent (5 mg/ml MTT dissolved in phosphate buffered saline, pH 7.4) was added and incubated for another three hours. Metabolically active cells cleaved the yellow tetrazolium salt to a purple formazan dye. The formazan crystals were dissolved and the solution was quantified spectrophotometrically at a wavelength of 560 nm using a microplate reader. Four replicates were performed for each passageway.
Statistical analysis
The statistical analysis was performed with the SPSS program (SPSS Inc., Chicago, IL, USA). For calculation of the data the Wilcoxon test was used.
Results
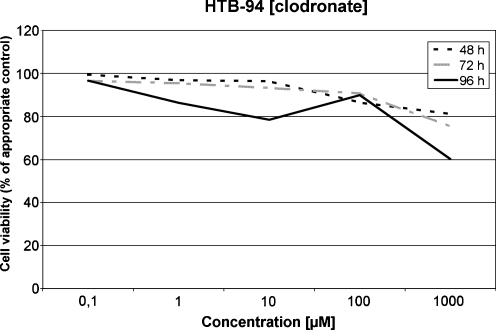
Sensitivity of HTB-94 to clodronate
After incubation periods of 48, 72 and 96 hours only slight effects on the tumour cells were observed in lower concentrations. In high concentrations of 1000 μM at incubation periods of 72 and 96 hours, a reduction of the cell viability to 75.35% and 60.19%, respectively, compared to the untreated control cultures was observed. These reductions were statistically highly significant (p < 0.01). In lower concentrations the effect of clodronate on the HTB-94 cells was less pronounced (Fig. 1).
Fig. 1.
Sensitivity of HTB-94 to clodronate
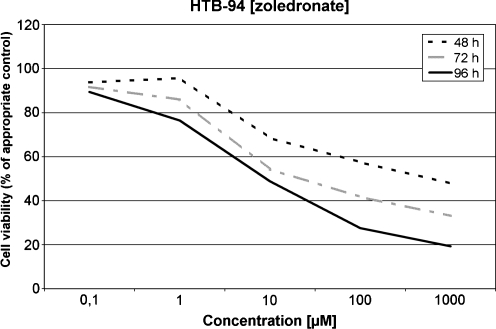
Sensitivity of HTB-94 to zoledronate
In this series the effect of zoledronate on the grade II chondrosarcoma cells was observed. In contrast to clodronate, the zoledronate led to a much more pronounced reduction of the cell viability compared to the untreated control. In concentrations of 10 and 100 μM after incubation periods of 48, 72 and 96 hours, a statistically significant reduction of the viable cells compared to the control was observed. In concentrations of 100 and 1000 μM even after 48 hours of incubation the cell viability was statistically reduced (p < 0.01) to 57.5% and 47.9%, respectively (Fig. 2).
Fig. 2.
Sensitivity of HTB-94 to zoledronate
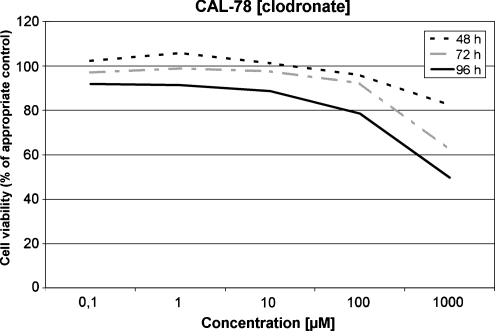
Sensitivity of CAL-78 to clodronate
The G III chondrosarcoma cells showed a comparable reaction to clodronate as the HTB-94 cells. After an incubation period of 48 hours a significant reduction of the chondrosarcoma cell proliferation activity was observed at 1000 μM only. Statistically significant reductions (p < 0.05) of the cell viability (38.9% and 51.4%) were observed after 72 hours (1000 μM) and 96 h (1000 μM), respectively. After an incubation period of 48 hours and in concentrations of and below 10 μM at any incubation period, only slight effects were seen (Fig. 3).
Fig. 3.
Sensitivity of CAL-72 to clodronate
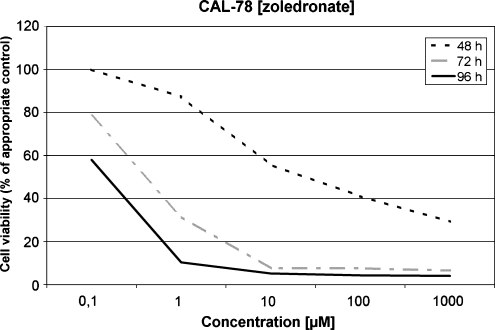
Sensitivity of CAL-78 to zoledronate
For the CAL-78 cells the cytotoxic effect of zoledronate was much more pronounced in lower concentrations and after a shorter incubation period compared to clodronate. After an incubation period of just 48 h with a concentration of only 10 μM the cell viability was reduced to 51% compared to the untreated controls. After 72 hours of incubation this value was achieved even in concentrations of 1 μM of zoledronate. In higher concentrations (100 and 1000 μM) a cell viability reduction of more than 40% was detected even after 48 hours. After 96 hours the chondrosarcoma cell viability was reduced to less than 10% compared to the untreated control group for concentrations of 1–1000 μM (Fig. 4). Even in a concentration of 0.1 μM the cell viability was reduced to 58% compared to the control after 96 hours. Furthermore, all values after an incubation period of 96 hours in any concentration were statistically significant (p < 0.05) or highly significant (p < 0.01).
Fig. 4.
Sensitivity of CAL-72 to zoledronate
Discussion
Bisphopshonates are used successfully in the treatment of patients suffering from different malignant metastatic diseases to reduce skeletal-related complications of bone metastases [10].
In addition, there is evidence that bisphosphonates have direct antitumour effects on diverse tumour cells. In in vitro and in vivo studies these effects of bisphosphonates on multiple myeloma cells, prostate cancer, breast cancer or lung cancer cells have been proven [3, 11–13]. In most of these studies the bisphosphonates act by inducing apoptosis and/or cytostasis in the tumour cells. Additionally, in vitro data suggest that bisphosphonates inhibit angiogenesis in tumour cells, and cell migration and cell invasion in breast cancer and prostate cancer cells [14–17].
Nevertheless, little information exists about the effect of bisphosphonates on primary bone tumours. Sonnemann et al. reported on the cytotoxic effects of clodronate and pamidronate on osteosarcoma and further on Ewing’s sarcoma cells [18, 19]. In these studies they reported a dose-dependent reduction of the sarcoma cell viability with increased concentrations of clodronate (50–1000 μM) and pamidronate (50–1000 μM). Though, the N-bisphosphonate pamidronate was significantly more effective than the non-amino-bisphosphonate clodronate. These results were comparable to the results achieved in our study. In this study the non-amino bisphosphonate clodronate showed significant effects after a long incubation period (>72 h) and in high concentrations (100–1000 μM) on the chondrosarcoma cells only, whereas the amino-bisphosphonate zoledronate showed a significant cell viability reduction even after short incubation periods and in low concentrations (0.1–10 μM) for both cell lines. Interestingly, the cytotoxic effect was much more pronounced for both the clodronate and the zoledronat on the grade III cells. We attribute this to the fact that the high grade tumour cells have a higher proliferation activity than the grade II cells and therefore are more sensitive to the bisphosphonate cytotoxic effect. This is in concurrence with the results of Sonnemann et al. Their results with the high grade osteosarcoma and the highly proliferative Ewing’s sarcoma are more comparable to the results of the CAL-74 cells than to the HTB-94 cells. However, in particular, zoledronat showed a significant cytotoxic effect even on the less proliferative grade II cells HTB-94. Therefore, we think that clinically, even in intermediate grade chondrosarcoma, bisphosphonates may be helpful in the prevention of local recurrences or metastases in view of the observed cytotoxic effect.
Regarding the fact that the amino-bisphosphonate zoledronat was more effective than the non-amino-bisphosphonate clodronate, one should consider that in various clinical studies of the role of oral therapy in cancer patients, both kinds of bisphosphonates led to significant effects with regard to the reduction of skeletal related events (e.g. pathological fractures, bone pain) [20–22]. Diehl et al., for example, reported of 157 patients with primary breast cancer and tumour cells in the bone marrow at presentation, treated with the non-amino bisphosphonate clodronate. These patients received daily 1600 mg clodronate orally. Compared to an untreated control group, this group developed a significantly (p < 0.001) reduced number of distant bone metastases (total 21 vs. 42). Furthermore, significantly less patients died in the clodronate group (6 vs. 22 deaths, p > 0.001) over the study period [20]. This in vivo effect of clodronate might be explained by a continuous accumulation of the drug in the bone over the whole study period.
However, there are no published clinical data to date on the use of biphosphonates in the treatment of primary bone tumours.
Therefore, the aim of this study was to confirm the option of a treatment alternative in the therapy of chondrosarcoma. However, to determine the human in vivo cytotoxic potential of bisphosphonates on chondrosarcoma it is important to learn which concentrations can be reached in the bone. In the literature this question has not been sufficiently answered yet. As is known from bishosphonates pharmacology, once incorporated in the blood stream the bisphosphonates are rapidly cleared from the circulation and accumulated in the bone. Bisphosphonate concentrations of several hundred μM in human bone are estimated, but there are few studies showing the bisphosphonate concentration in the resorbtion lacunas. Sato et al., for example, showed a concentration of 300 μM of alendronate in the resorbtion lacunas in rat bones. Transferred to human bone they estimated alendronate concentrations range between 100 and 1000 μM [23]. Similar concentrations should be estimated for other bisphosphonates as well. In our study, even the clodronate showed significant cytotoxic effects on the chondrosarcoma cells in these concentrations, though much less than the zoledronate. Despite the bisphosphonate concentration reached in the bone, an even more important question will be which concentration could be reached in vivo in the tumour itself. Most malignant bone tumours induce an osteolytic destruction of the surrounding bone triggered by an activation of the osteoclasts through cell mediators produced by the tumour cells [24–27]. Therefore, it could be assumed that bisphosphonates previously deposited in the bone or incorporated in the osteoclasts should lead to effective bisphosphonate concentrations in the tumour and its environs.
As regards this lack of evidence, further in vivo studies are necessary to investigate whether sufficient bisphosphonate concentrations can be achieved in the bone and the tumour itself and to confirm the in vivo effect of the bisphosphonate on the chondrosarcoma concerning the cytotoxic properties shown in this study. On this issue, Gouin et al. [28] confirmed a positive effect of zoledronate in chondrosarcoma bearing rats. They observed a prolonged overall survival probability in a curative setting and a delay of local recurrences and a smaller tumour volume in a palliative setting in animals treated with zoledronate compared to an untreated control group. However, they did not measure the biphosphonate concentrations reached in the bone or the tumours themselves.
Conclusion
In conclusion, our study provides strong evidence that bisphosphonates used in a dose-dependent manner in vitro directly affect the growth of the two chondrosarcoma cell lines. However, more research and further preclinical studies are necessary to confirm the potential clinical use of bisphosphonates on chondrosarcoma in humans.
References
- 1.Rozeman LB, Cleton-Jansen AM, Hogendoorn PCW. Pathology of primary mailgnant bone and cartilage tumours. Int Orthop. 2006;30:437–444. doi: 10.1007/s00264-006-0212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiorenza F, Abudu A, Grimer RJ, Carter SR, Tillman R, Ayoub K, et al. Risk factors for survival and local control in chondrosarcoma of bone. J Bone Joint Surg Br. 2002;84(1):93–99. doi: 10.1302/0301-620X.84B1.11942. [DOI] [PubMed] [Google Scholar]
- 3.Brown JE, Neville-Webbe H, Coleman RE. The role of bisphosphonates in breast and prostate cancers. Endocr Relat Cancer. 2004;11(2):207–224. doi: 10.1677/erc.0.0110207. [DOI] [PubMed] [Google Scholar]
- 4.Coleman RE. Efficacy of zoledronic acid and pamidronate in breast cancer patients: a comparative analysis of randomized phase III trials. Am J Clin Oncol. 2002;25(6 Suppl 1):25–31. doi: 10.1097/00000421-200212001-00005. [DOI] [PubMed] [Google Scholar]
- 5.Berenson JR, Lipton A. Bisphosphonates in the treatment of malignant bone disease. Annu Rev Med. 1999;50:237–248. doi: 10.1146/annurev.med.50.1.237. [DOI] [PubMed] [Google Scholar]
- 6.Rosen L, Harland SJ, Oosterlinck W (2002) Broad clinical activity of zoledronic acid in osteolytic to osteoblastic bone lesions in patients with a broad range of solid tumors. Am J Clin Oncol 25(6 Suppl 1):19–24 [DOI] [PubMed]
- 7.Vorotnjak M, Boos J, Lanvers-Kaminsky C. In vitro toxicity of bisphosphonates on human neuroblastoma cell lines. Anticancer Drugs. 2004;15(8):795–802. doi: 10.1097/00001813-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Jagdev SP, Coleman RE, Shipman CM, Rostami HA, Croucher PI. The bisphosphonate, zoledronic acid, induces apoptosis of breast cancer cells: evidence for synergy with paclitaxel. Br J Cancer. 2001;84(8):126–134. doi: 10.1054/bjoc.2001.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiraga T, Williams PJ, Mundy GR, Yoneda T. The bisphosphonate ibandronate promotes apoptosis in MDA-MB-231 human breast cancer cells in bone metastases. Cancer Res. 2001;61(11):4418–4424. [PubMed] [Google Scholar]
- 10.Green JR. Bisphosphonates in cancer therapy. Curr Opin Oncol. 2002;14(6):609–615. doi: 10.1097/00001622-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Shipman CM, Rogers MJ, Vanderkerken K, Camp B, Graham R, Russell G, et al. Bisphosphonates—mechanisms of action in multiple myeloma. Acta Oncol. 2000;39(7):829–835. doi: 10.1080/028418600750063587. [DOI] [PubMed] [Google Scholar]
- 12.Senaratne SG, Colston KW. Direct effects of bisphosphonates on breast cancer cells. Breast Cancer Res. 2002;4(1):18–23. doi: 10.1186/bcr412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yano S, Zhang H, Hanibuchi M, Miki T, Goto H, Uehara H, et al. Combined therapy with a new bisphosphonate, minodronate (YM529), and chemotherapy for multiple organ metastases of small cell lung cancer cells in severe combined immunodeficient mice. Clin Cancer Res. 2003;9(14):5380–5385. [PubMed] [Google Scholar]
- 14.Virtanen SS, Vaananen HK, Harkonen PL, Lakkakorpi PT. Alendronate inhibits invasion of PC-3 prostate cancer cells by affecting the mevalonate pathway. Cancer Res. 2000;62(9):2708–2714. [PubMed] [Google Scholar]
- 15.Pluijm G, Vloedgraven H, Beek E, Wee-Pals L, Lowik C, Papapoulos S. Bisphosphonates inhibit the adhesion of breast cancer cells to bone matrices in vitro. J Clin Invest. 1996;98(3):698–705. doi: 10.1172/JCI118841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fournier P, Boissier S, Filleur S, Guglielmi J, Cabon F, Colombel M, et al. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res. 2002;62(22):6538–6544. [PubMed] [Google Scholar]
- 17.Boissier S, Magnetto S, Frappart F, Cuzin B, Ebetino FH, Delmas PD, et al. Bisphosphonates inhibit prostate and breast carcinoma cell adhesion to unmineralized and mineralized bone extracellular matrices. Cancer Res. 1997;57(18):3890–3894. [PubMed] [Google Scholar]
- 18.Sonnemann J, Eckervogt V, Truckenbrod B, Boos J, Winkelmann W, Valen F. The bisphosphonate pamidronate is a potent inhibitor of human osteosarcoma cell growth in vitro. Anticancer Drugs. 2001;12(5):459–465. doi: 10.1097/00001813-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Sonnemann J, Eckervogt V, Truckenbrod B, Boos J, Winkelmann W, Valen F. The bisphosphonate pamidronate is a potent inhibitor of Ewing's sarcoma cell growth in vitro. Anticancer Drugs. 2003;14(9):767–771. doi: 10.1097/00001813-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Diel IJ, Solomayer EF, Costa SD, Gollan C, Goerner R, Wallwiener D, et al. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998;339(6):357–363. doi: 10.1056/NEJM199808063390601. [DOI] [PubMed] [Google Scholar]
- 21.Hortobagyi GN, Theriault RL, Porter L, Blayney D, Lipton A, Sinoff C, et al. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med. 1996;335(24):1785–1791. doi: 10.1056/NEJM199612123352401. [DOI] [PubMed] [Google Scholar]
- 22.Kanis JA, Powles T, Paterson AH, McCloskey EV, Ashley S. Clodronate decreases the frequency of skeletal metastases in women with breast cancer. Bone. 1996;19(6):663–667. doi: 10.1016/S8756-3282(96)00285-2. [DOI] [PubMed] [Google Scholar]
- 23.Sato M, Grasser W, Endo N, Akins R, Simmons H, Thompson DD, et al. Bisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest. 1991;88(6):2095–2105. doi: 10.1172/JCI115539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 25.Mohammad KS, Guise TA (2003) Mechanisms of osteoblastic metastases: role of endothelin-1. Clin Orthop Relat Res (415 Suppl):S67–74 [DOI] [PubMed]
- 26.Guise TA, Mundy GR. Cancer and bone. Endocr Rev. 1998;19(1):18–54. doi: 10.1210/er.19.1.18. [DOI] [PubMed] [Google Scholar]
- 27.Choong PF (2003) The molecular basis of skeletal metastases. Clin Orthop Relat Res (415 Suppl):S19–S31 [DOI] [PubMed]
- 28.Gouin F, Ory B, Rédini F, Heymann D. Zoledronic acid slows down rat primary chondrosarcoma development, recurrent tumor progression after intralesional curretage and increases overall survival. Int J Cancer. 2006;119(5):980–984. doi: 10.1002/ijc.21951. [DOI] [PubMed] [Google Scholar]