Abstract
Failure to diagnose injury to the posterolateral structures has been found to increase the forces experienced by the anterior cruciate ligament (ACL) and ACL grafts which may cause their subsequent failure. An isolated injury to the popliteus complex (PC) consisting of the popliteus tendon and popliteofibular ligament is not uncommon. Therefore, the purpose of this study was to discover if an isolated injury to the PC can significantly affect the forces experienced by the ACL graft under external loading conditions. We hypothesised that, under external tibial torque, the ACL graft will experience a significant increase in force, in knees with PC injury compared to the intact PC condition. Under varus tibial torque (10 N m), we observed minimal changes in the varus tibial rotation due to isolated sectioning of the PC in an ACL reconstructed knee (P > 0.05). Consequently, no significant increase in the ACL graft force was observed under varus tibial torque. In contrast, sectioning the PC resulted in a significant increase in the external tibial rotation compared to the intact PC knee condition under the external rotational tibial torque (5 N m) at all flexion angles (P < 0.05). These changes in kinematics under external tibial torque were manifested as elevated ACL graft forces at all selected flexion angles (P < 0.05). Prompt diagnosis of isolated PC injury and its treatment are warranted to prevent potential failure of ACL reconstruction.
Introduction
As a unit, the posterolateral structures (PLS) are known to primarily resist tibial varus and external rotations and posterior translation [1–3]. Injury to the PLS is manifested as posterolateral rotational instability. Patients with these injuries often complain about their knee “giving way backward” and report difficulty in stair accent and descent [4]. While isolated injuries to the PLS are less common (28%) [5], about 29–89% of the patients injure their PLS in conjunction with the anterior cruciate ligament (ACL) or posterior cruciate ligament (PCL) or both [4, 6–9]. Failure to diagnose the injury to the PLS has been found to increase the forces experienced by the ACL and ACL grafts and lead to their subsequent failure [10, 11]. Hence, recently more rigorous examination for posterolateral injuries and their treatment has been emphasised.
Load sharing patterns of intact ACL and PCL in knees with uninjured and combined injury of lateral collateral ligament (LCL) and PLS were investigated in a cadaver model under various external loading conditions [10]. This study demonstrated that sectioning of LCL and PLS increased the forces in the ACL under both varus and internal tibial torques. Further, LaPrade et al. [11] studied the effect of grade III injuries to the PLS [sequential sectioning of LCL, popliteofibular ligament (PFL) and popliteus tendon (PT)] on the force experienced by the ACL grafts. They found that sectioning the LCL increased the forces in the ACL graft both under varus and combined varus and internal torques. Additional sectioning of the PFL and PT further elevated the ACL graft forces under the two loading conditions. However, PLS function as a unit with complex interactions between the individual structures [2, 3]. Therefore, the sequence of sectioning the PLS can mask critical roles of some individual structures.
An isolated injury to the popliteus complex (PC) consisting of the PT and PFL is not uncommon [12–16]. In a prospective study by LaPrade et al. [5] it has been reported that only 23% of the posterolateral corner injured patients had an LCL injury. Despite their common incidence, it is believed that PC injuries are less commonly diagnosed [16]. Sectioning studies of the ligamentous structures of the posterolateral corner identified that the stabilising function of the PC is to resist external tibial rotation and posterior tibial translation [2, 17, 18]. External tibial rotation due to the external tibial torque, generated during pivoting or twisting activities, in a PC injured knee could potentially cause ACL graft impingement against the intercondylar notch and hence lead to an elevated risk of its failure. Therefore, the purpose of this study was to evaluate, by using a robotic testing system, whether an isolated injury to the PC can significantly affect the forces experienced by the ACL graft under external loading conditions. We hypothesised that, under external tibial torque, the ACL graft will experience a significant increase in force, in knees with PC injury compared to the intact PC condition.
Materials and methods
Eight fresh-frozen cadaveric human knee specimens from six male and two female donors (age range of 47 to 60 years) were tested in this study. Each knee was examined for evidence of previous operations, ligamentous injuries or osteoarthritis using fluoroscopy and manual laxity test. Specimens with any of these conditions were excluded from the study. The experiment began by thawing the specimens (which were frozen at −20°C) for 24 hours at room temperature prior to the testing. After the specimens were completely thawed, they were further prepared for the experiment by truncating the femur and tibia at approximately 25 cm from the joint line, leaving all the soft tissues (skin, knee ligaments, joint capsule and musculature) around the knee intact. The diaphyses of the femur and tibia were exposed by stripping off the surrounding musculature and were then secured in thick-walled aluminum cylinders using bone cement. A bone screw was used to firmly secure the fibula to the tibia in its anatomical position.
The ACL of the intact knee was resected through a small medial arthrotomy under arthroscopic guidance to simulate an ACL deficient knee condition. ACL reconstruction was then performed using arthroscopically assisted techniques by a single surgeon. For the ACL reconstruction, semitendinosus and gracilis tendons were harvested from the specimen and used as the graft material. The harvested grafts were pre-tensioned on a graft preparation board (DePuy Mitek, Raynham, MA, USA) with 20 lb of force for approximately 20 min. First, the tibial tunnel was formed at the centre of the ACL remnant through the anteromedial surface of the tibia at the level of the tibial tubercle using a tibial guide (DePuy Mitek, Raynham, MA, USA) set at 55°. The femoral tunnel was then prepared by first placing a K-wire into the lateral femoral condyle at the 1:30 or 10:30 o’clock position through the anteromedial portal with the knee flexed to 120°. An offset guide was used to place the K-wire such that a 2-mm posterior wall was intact after the femoral tunnel was reamed. With the inserted K-wire as the reference, a femoral tunnel was reamed to the lateral cortex of the distal femur using a 4.5-mm ENDOBUTTON drill (Smith & Nephew Endoscopy, Andover, MA, USA). Further, a 30-mm long femoral socket was then created by a cannulated reamer that matched the prepared graft diameter. The prepared quadruple hamstrings graft was then secured by an ENDOBUTTON CL (Smith & Nephew Endoscopy, Andover, MA, USA) on the lateral cortex of the femur followed by the fixation at the tibial end by a tibial INTRAFIX system (DePuy Mitek, Raynham, MA, USA) while a 40 N axial graft tension was maintained at full extension. After the graft was fixed at both ends, arthrotomy and skin incisions were repaired with sutures.
The specimen was then secured on a robotic testing system comprising a robotic arm and a load cell. The end effector of the robotic arm is capable of 6 df motion and the load cell can measure three components of forces and moments. This system can be operated under both force and displacement control modes to study the knee biomechanics. The operation of the robotic testing system has been detailed in several studies in the literature [19, 20].
The testing process began by determining a passive flexion path of the ACL reconstructed knee from 0 to 120° of flexion in 1° increments of knee flexion. The passive position is described as a position of the knee when all resultant forces and moments at the knee centre were minimal (<5 N and <0.5 N m, respectively). Following the determination of the passive path, each knee was subjected to two different external loading conditions (10 N m varus tibial torque and 5 N m external tibial torque) by the robotic testing system at selected flexion angles of 0, 30, 60 and 90°. The kinematic responses of the ACL reconstructed knee with the PC intact were recorded at each selected flexion angle under both external loading conditions.
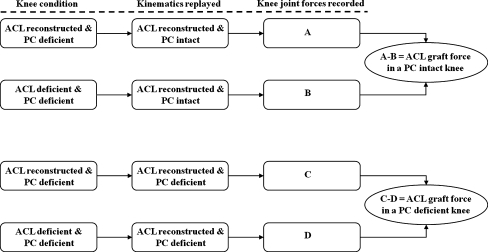
To simulate an isolated PC injury, the PT was resected at its femoral insertion site using a scalpel. The incision that was made to access the PT was then closed by sutures. The kinematics of the ACL reconstructed knee with intact PC were replayed and the resulting knee joint forces (A) were recorded. The ACL reconstructed knee with an injury to the PC was again tested under the two external loading conditions at all selected flexion angles and the kinematic responses, knee joint forces (C), were recorded. The ACL graft was then released and the kinematics of the ACL reconstructed knee with intact PC were replayed to measure the knee joint forces (B). Finally, the kinematics of the ACL reconstructed knee with isolated PC injury were replayed to record the knee joint force (D). The method of superposition [21, 22] was used to calculate the forces experienced by the ACL graft before and after the PC injury. The forces experienced by the ACL graft with the PC intact are calculated as the difference between the forces A and B (Fig. 1). Similarly, the forces experienced by the ACL graft with an injury to the PC are calculated as the difference between the forces C and D (Fig. 1).
Fig. 1.
Flowchart of the process for measuring the ACL graft forces before and after PC injury
In this study, each knee was tested twice before and after injury to the PC which facilities a within-subjects statistical analysis of knee kinematics and the ACL graft forces. The paired t test was used to detect statistically significant differences in the kinematic responses of the knee and the forces experienced by the ACL graft under the two knee conditions at each of the selected flexion angles. Further, a one-way repeated measures analysis of variance (ANOVA) was used to study the effect of the flexion angle on the kinematics and the ACL graft forces. Differences were considered statistically significant at P < 0.05.
Results
Varus tibial torque (10 N m)
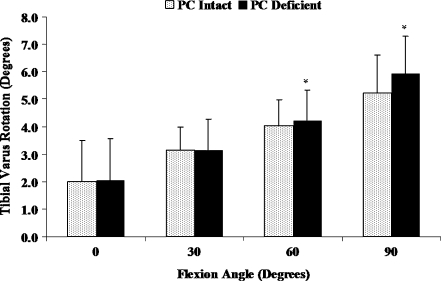
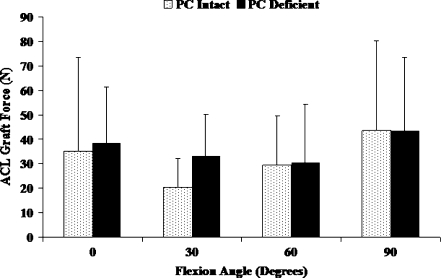
Isolated sectioning of the PC in an ACL reconstructed knee did not significantly affect the varus tibial rotation between 0 and 30° of flexion (Fig. 2, P > 0.05). Although the varus tibial rotations were significantly increased following the isolated sectioning of the PC at 60 and 90° of flexion (P < 0.05), the maximum increase in varus tibial rotation which occurred at 90° of flexion was 0.7 ± 0.5°. Varus tibial torque induced a significant increase in coupled external tibial rotation between 60 and 90° of flexion due to isolated sectioning of the PC (Table 1, P < 0.05). No significant changes in the anterior-posterior and medial-lateral translations were observed following isolated sectioning of the PC (Table 1). Under varus tibial torque, isolated sectioning of the PC did not significantly increase the ACL graft forces at any of the selected flexion angles (Fig. 3, P > 0.05).
Fig. 2.
Varus tibial rotations of PC intact and PC deficient knee under varus tibial torque. *Significant difference compared to the intact PC knee condition (P < 0.05)
Table 1.
Kinematics (average ± standard deviation) of tibia with respect to femur under varus tibial torque (10 N m)
| (-)Anterior/(+)posterior (mm) | (-)Medial/(+)lateral (mm) | (-)Internal/(+)external (°) | ||||
|---|---|---|---|---|---|---|
| Intact PC | PC deficient | Intact PC | PC deficient | Intact PC | PC deficient | |
| 0° | −0.8 ± 2.1 | −1.4 ± 2.0* | 1.3 ± 1.5 | 1.3 ± 1.5 | 3.7 ± 4.7 | 4.3 ± 4.6 |
| 30° | −1.2 ± 2.3 | −1.5 ± 2.7 | 2.1 ± 1.4 | 2.1 ± 1.6 | 4.1 ± 5.8 | 4.9 ± 6.1 |
| 60° | −1.9 ± 1.3 | −1.9 ± 1.6 | 2.4 ± 1.3 | 2.6 ± 1.3 | 3.9 ± 4.1 | 5.9 ± 5.4* |
| 90° | −2.3 ± 1.7 | −1.9 ± 2.0 | 2.3 ± 1.4 | 2.8 ± 1.3 | 4.5 ± 4.1 | 7.2 ± 4.3* |
*Significant difference compared to the intact PC knee condition (P < 0.05)
Fig. 3.
ACL graft forces in PC intact and PC deficient knee under varus tibial torque
External tibial torque (5 N m)
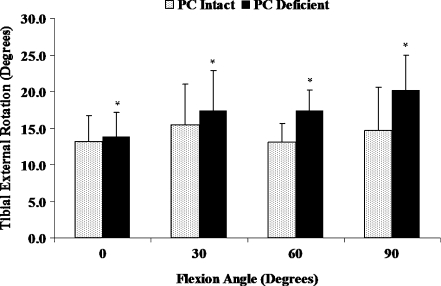
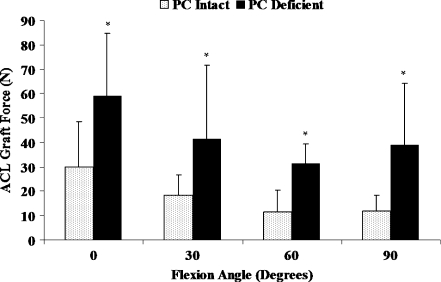
External tibial rotations were significantly increased at all selected flexion angles due to isolated sectioning of the PC compared to the intact PC knee condition (Fig. 4, P < 0.05). Increases in the amount of external tibial rotations after isolated sectioning of the PC were significantly larger at high flexion angles (≥60°) than at low flexion angles (≤30°). The maximum increase in external rotation of 5.6 ± 2.6° occurred at 90° of flexion. Further, isolated sectioning of the PC induced a significant coupled posterior tibial translation and significantly increased the coupled varus tibial rotation at 60 and 90° of flexion (Table 2, P < 0.05). The ACL graft forces were significantly increased at all selected flexion angles after isolated sectioning of the PC compared to the intact PC knee condition (Fig. 5, P < 0.05). The increase in the ACL graft forces ranged from 20 ± 16 N at 60° of flexion to 29 ± 26 N at 0° of flexion.
Fig. 4.
External tibial rotations of PC intact and PC deficient knee under external tibial torque. *Significant difference compared to the intact PC knee condition (P < 0.05)
Table 2.
Kinematics (average ± standard deviation) of tibia with respect to femur under external tibial torque (5 N m)
| (-)Anterior/(+)posterior (mm) | (-)Medial/(+)lateral (mm) | (-)Valgus/(+)varus (°) | ||||
|---|---|---|---|---|---|---|
| Intact PC | PC deficient | Intact PC | PC deficient | Intact PC | PC deficient | |
| 0° | −2.1 ± 1.5 | −2.7 ± 1.6* | −0.1 ± 1.5 | −0.2 ± 1.7 | 0.4 ± 1.8 | 0.5 ± 1.8* |
| 30° | −2.5 ± 2.0 | −2.3 ± 2.0 | 0.5 ± 1.5 | 0.4 ± 1.7 | 0.5 ± 1.3 | 0.7 ± 1.5 |
| 60° | −0.8 ± 0.8 | 0.2 ± 1.4* | 0.4 ± 1.6 | 0.6 ± 2.1 | 0.5 ± 1.0 | 0.9 ± 1.4* |
| 90° | −0.4 ± 0.9 | 0.9 ± 1.7* | 0.9 ± 2.1 | 1.5 ± 2.7 | 1.2 ± 1.6 | 2.2 ± 2.3* |
*Significant difference compared to the intact PC knee condition (P < 0.05)
Fig. 5.
ACL graft forces in PC intact and PC deficient knee under external tibial torque. *Significant difference compared to the intact PC knee condition (P < 0.05)
Discussion
Incidences of isolated injuries to the PC are believed to be more common than they are generally diagnosed. Undiagnosed and hence untreated injuries to the PC could result in subsequent failure of ACL reconstructions. In this investigation, we observed minimal changes in the varus tibial rotation due to isolated PC injury in an ACL reconstructed knee under varus tibial torque. Consequently, no significant increase in the ACL graft force was observed due to isolated sectioning of the PC under varus tibial torque. On the contrary, isolated sectioning of the PC significantly increased the external tibial rotations at all selected flexion angles compared to the intact PC knee condition under external tibial torque. These changes in kinematics under external tibial torque due to isolated sectioning of the PC were manifested as elevated ACL graft forces at all selected flexion angles.
Consistent with the literature, the results of this study demonstrated that the PC is not the primary restraint to varus rotation [3, 17]. Isolated sectioning of the PC in an ACL reconstructed knee did not significantly affect either the varus tibial rotation or the ACL graft force under varus tibial torque. However, secondary sectioning of the PC following other ligamentous structures such as the LCL, PCL, PFL and/or posterolateral capsule have been reported to significantly increase the varus tibial rotations [11, 17]. Further, this secondary increase in the varus tibial rotations has been shown to elevate the ACL graft forces [11]. These results demonstrate the complexity in the function of the PLS as knee stabilisers. While sequential sectioning studies have provided significant knowledge about the stabilising function of the PLS, some critical stabilising roles of these structures may have been masked by the sequence in which they were sectioned. Therefore, it is imperative to accurately diagnose injuries to all of the structures and hence provide an appropriate treatment to restore the normal joint function.
Several studies have demonstrated that the PC is an important stabiliser of tibial external rotation [3, 17, 23]. The results of this study are in agreement with the previous literature. We found that the external tibial rotations were significantly increased at all selected flexion angles due to isolated sectioning of the PC compared to the intact PC knee condition in ACL reconstructed knees. The maximum increase in external rotation of 5.6 ± 2.6° occurred at 90° of flexion. Similarly, Pasque et al. [17] reported an increase of 5–6° in external rotation following isolated sectioning of the PC under 5 N m external tibial torque from 30 to 120° of flexion. Further, our results demonstrated a significantly greater increase in the external rotation due to isolated sectioning of the PC at high flexion angles (≥60°) than at low flexion angles (≤30°). Based on the results of their study, LaPrade et al. [3] proposed that the LCL and PC had a complementary function in resisting external tibial rotations, with a greater role of the LCL at low flexion angles and the PC at high flexion angles. Greater increases in external rotations at high compared to low flexion angles, following isolated sectioning of the PC, observed in this study, further reinforce the concept of complementary functions of the LCL and PC in resisting external tibial rotations.
ACL grafts in our study experienced elevated forces at all selected flexion angles under the external tibial torque due to PC deficiency. The percentage of increase in the ACL graft forces ranges from 98% at 0° of flexion to 229% at 90° of flexion. However, in contrast to our results, LaPrade et al. [11] and Markolf et al. [10] found that the ACL and ACL graft were unloaded (decrease in ACL and ACL graft forces) under external tibial torque, when the LCL and PC or LCL and posterolateral capsular structures were sectioned. While there is no obvious explanation for the discrepancy observed between these studies and our results, critical factors such as the differences in the loading conditions, sequence of sectioning the PLS and coupled motions under external tibial torque may have contributed. Numerous studies have explored the mechanics of noncontact ACL injuries in athletes to identify the precise kinematics that predispose the ACL to extreme tensile loads leading to its subsequent failure [24–28]. The evidence from these studies has associated excessive tibial combined valgus and external/internal rotations to be the most detrimental motion pattern to the ACL. Further, excessive external rotation has been shown to result in ACL impingement against the intercondylar notch causing an increase in ACL strain [29]. This prior knowledge about the increased risk of ACL injury due to external tibial rotation, integrated with the results of this study, elucidates the importance of accurate diagnoses of PC injury and its subsequent treatment to avoid future complications.
Prior to conclusive interpretation of the results of this study, limitations of this study need to be considered. Combined loading (valgus and external) is proposed to be the more detrimental and common occurrence than pure external torque at the time of ACL injury. However, since our goal was to estimate the forces experienced by the ACL graft due to isolated injury to the PC, we chose to apply a pure external torque. Secondly, in this study we did not evaluate the ACL graft forces under weight-bearing conditions. It has been reported that external torque under weight-bearing conditions may have a greater effect on the ACL strain than under non-weight-bearing conditions [30, 31]. However, we believe that our results represent a conservative estimate of the elevated forces in the PC under external tibial torque and these forces may further increase under weight-bearing or combined loading conditions. Further, we did not evaluate the effect of graft source and the tunnel positions on the forces experienced by the ACL graft. Future investigations are needed to study the effect of these parameters and the effect of isolated PC injury on the medial collateral ligament.
Conclusion
Our study demonstrated that isolated injury to the PC has minimal effect on the varus stability under varus tibial load but can significantly increase the external tibial rotation and ACL graft forces under external tibial torque. Prompt diagnosis of isolated PC injury and its treatment are warranted to prevent potential failure of ACL reconstruction.
Acknowledgement
We gratefully acknowledge the support of the National Institutes of Health (R01AR055612) and the Department of Orthopaedic Surgery of Massachusetts General Hospital.
Conflict of interest The authors have no conflicts of interests.
References
- 1.Gollehon DL, Torzilli PA, Warren RF. The role of the posterolateral and cruciate ligaments in the stability of the human knee. A biomechanical study. J Bone Joint Surg Am. 1987;69(2):233–242. [PubMed] [Google Scholar]
- 2.Grood ES, Stowers SF, Noyes FR. Limits of movement in the human knee. Effect of sectioning the posterior cruciate ligament and posterolateral structures. J Bone Joint Surg Am. 1988;70(1):88–97. [PubMed] [Google Scholar]
- 3.LaPrade RF, Tso A, Wentorf FA. Force measurements on the fibular collateral ligament, popliteofibular ligament, and popliteus tendon to applied loads. Am J Sports Med. 2004;32(7):1695–1701. doi: 10.1177/0363546503262694. [DOI] [PubMed] [Google Scholar]
- 4.Hughston JC, Jacobson KE. Chronic posterolateral rotatory instability of the knee. J Bone Joint Surg Am. 1985;67(3):351–359. [PubMed] [Google Scholar]
- 5.LaPrade RF, Terry GC. Injuries to the posterolateral aspect of the knee. Association of anatomic injury patterns with clinical instability. Am J Sports Med. 1997;25(4):433–438. doi: 10.1177/036354659702500403. [DOI] [PubMed] [Google Scholar]
- 6.Juhng SK, Lee JK, Choi SS, Yoon KH, Roh BS, Won JJ. MR evaluation of the “arcuate” sign of posterolateral knee instability. AJR Am J Roentgenol. 2002;178(3):583–588. doi: 10.2214/ajr.178.3.1780583. [DOI] [PubMed] [Google Scholar]
- 7.Theodorou DJ, Theodorou SJ, Fithian DC, Paxton L, Garelick DH, Resnick D. Posterolateral complex knee injuries: magnetic resonance imaging with surgical correlation. Acta Radiol. 2005;46(3):297–305. doi: 10.1080/02841850510021067. [DOI] [PubMed] [Google Scholar]
- 8.Baker CL, Jr, Norwood LA, Hughston JC. Acute combined posterior cruciate and posterolateral instability of the knee. Am J Sports Med. 1984;12(3):204–208. doi: 10.1177/036354658401200307. [DOI] [PubMed] [Google Scholar]
- 9.DeLee JC, Riley MB, Rockwood CA., Jr Acute posterolateral rotatory instability of the knee. Am J Sports Med. 1983;11(4):199–207. doi: 10.1177/036354658301100403. [DOI] [PubMed] [Google Scholar]
- 10.Markolf KL, Wascher DC, Finerman GA. Direct in vitro measurement of forces in the cruciate ligaments. Part II: the effect of section of the posterolateral structures. J Bone Joint Surg Am. 1993;75(3):387–394. doi: 10.2106/00004623-199303000-00010. [DOI] [PubMed] [Google Scholar]
- 11.LaPrade RF, Resig S, Wentorf F, Lewis JL. The effects of grade III posterolateral knee complex injuries on anterior cruciate ligament graft force. A biomechanical analysis. Am J Sports Med. 1999;27(4):469–475. doi: 10.1177/03635465990270041101. [DOI] [PubMed] [Google Scholar]
- 12.Baker CL, Jr, Norwood LA, Hughston JC. Acute posterolateral rotatory instability of the knee. J Bone Joint Surg Am. 1983;65(5):614–618. [PubMed] [Google Scholar]
- 13.Burstein DB, Fischer DA. Isolated rupture of the popliteus tendon in a professional athlete. Arthroscopy. 1990;6(3):238–241. doi: 10.1016/0749-8063(90)90081-N. [DOI] [PubMed] [Google Scholar]
- 14.McConkey JP. Avulsion of the popliteus tendon. J Pediatr Orthop. 1991;11(2):230–233. doi: 10.1097/01241398-199103000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Nakhostine M, Perko M, Cross M. Isolated avulsion of the popliteus tendon. J Bone Joint Surg Br. 1995;77(2):242–244. [PubMed] [Google Scholar]
- 16.Rose DJ, Parisien JS. Popliteus tendon rupture. Case report and review of the literature. Clin Orthop Relat Res. 1988;226:113–117. [PubMed] [Google Scholar]
- 17.Pasque C, Noyes FR, Gibbons M, Levy M, Grood E. The role of the popliteofibular ligament and the tendon of popliteus in providing stability in the human knee. J Bone Joint Surg Br. 2003;85(2):292–298. doi: 10.1302/0301-620X.85B2.12857. [DOI] [PubMed] [Google Scholar]
- 18.Amis AA, Bull AM, Gupte CM, Hijazi I, Race A, Robinson JR. Biomechanics of the PCL and related structures: posterolateral, posteromedial and meniscofemoral ligaments. Knee Surg Sports Traumatol Arthrosc. 2003;11(5):271–281. doi: 10.1007/s00167-003-0410-7. [DOI] [PubMed] [Google Scholar]
- 19.Yoo JD, Papannagari R, Park SE, DeFrate LE, Gill TJ, Li G. The effect of anterior cruciate ligament reconstruction on knee joint kinematics under simulated muscle loads. Am J Sports Med. 2005;33(2):240–246. doi: 10.1177/0363546504267806. [DOI] [PubMed] [Google Scholar]
- 20.Gadikota HR, Seon JK, Kozanek M, Oh LS, Gill TJ, Montgomery KD, Li G. Biomechanical comparison of single-tunnel-double-bundle and single-bundle anterior cruciate ligament reconstructions. Am J Sports Med. 2009;37(5):962–969. doi: 10.1177/0363546508330145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakane M, Fox RJ, Woo SL, Livesay GA, Li G, Fu FH. In situ forces in the anterior cruciate ligament and its bundles in response to anterior tibial loads. J Orthop Res. 1997;15(2):285–293. doi: 10.1002/jor.1100150219. [DOI] [PubMed] [Google Scholar]
- 22.Wu JL, Seon JK, Gadikota HR, Hosseini A, Sutton KM, Gill TJ, Li G. In situ forces in the anteromedial and posterolateral bundles of the anterior cruciate ligament under simulated functional loading conditions. Am J Sports Med. 2010;38(3):558–563. doi: 10.1177/0363546509350110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shahane SA, Ibbotson C, Strachan R, Bickerstaff DR. The popliteofibular ligament. An anatomical study of the posterolateral corner of the knee. J Bone Joint Surg Br. 1999;81(4):636–642. doi: 10.1302/0301-620X.81B4.9501. [DOI] [PubMed] [Google Scholar]
- 24.Krosshaug T, Nakamae A, Boden BP, Engebretsen L, Smith G, Slauterbeck JR, Hewett TE, Bahr R. Mechanisms of anterior cruciate ligament injury in basketball: video analysis of 39 cases. Am J Sports Med. 2007;35(3):359–367. doi: 10.1177/0363546506293899. [DOI] [PubMed] [Google Scholar]
- 25.Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002–1012. doi: 10.1177/0363546503261724. [DOI] [PubMed] [Google Scholar]
- 26.Griffin LY, Albohm MJ, Arendt EA, Bahr R, Beynnon BD, Demaio M, Dick RW, Engebretsen L, Garrett WE, Jr, Hannafin JA, Hewett TE, Huston LJ, Ireland ML, Johnson RJ, Lephart S, Mandelbaum BR, Mann BJ, Marks PH, Marshall SW, Myklebust G, Noyes FR, Powers C, Shields C, Jr, Shultz SJ, Silvers H, Slauterbeck J, Taylor DC, Teitz CC, Wojtys EM, Yu B. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006;34(9):1512–1532. doi: 10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- 27.Shimokochi Y, Shultz SJ. Mechanisms of noncontact anterior cruciate ligament injury. J Athl Train. 2008;43(4):396–408. doi: 10.4085/1062-6050-43.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quatman CE, Hewett TE. The anterior cruciate ligament injury controversy: is “valgus collapse” a sex-specific mechanism? Br J Sports Med. 2009;43(5):328–335. doi: 10.1136/bjsm.2009.059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fung DT, Zhang LQ. Modeling of ACL impingement against the intercondylar notch. Clin Biomech (Bristol, Avon) 2003;18(10):933–941. doi: 10.1016/S0268-0033(03)00174-8. [DOI] [PubMed] [Google Scholar]
- 30.Beynnon B, Howe JG, Pope MH, Johnson RJ, Fleming BC. The measurement of anterior cruciate ligament strain in vivo. Int Orthop. 1992;16(1):1–12. doi: 10.1007/BF00182976. [DOI] [PubMed] [Google Scholar]
- 31.Fleming BC, Renstrom PA, Beynnon BD, Engstrom B, Peura GD, Badger GJ, Johnson RJ. The effect of weightbearing and external loading on anterior cruciate ligament strain. J Biomech. 2001;34(2):163–170. doi: 10.1016/S0021-9290(00)00154-8. [DOI] [PubMed] [Google Scholar]