Abstract
Objectives
The goal of this study was to determine to what extent mechanical stability affects vascular repair during fracture healing.
Methods
Stabilized and non-stabilized tibia fractures were created in adult mice. Fracture tissues were collected at multiple time points during early fracture healing. Vasculature in fractured limbs was visualized by immunohistochemistry with an anti-PECAM-1 antibody on tissue sections and then quantified with stereology. Oxygen tension, vascular endothelial growth factor (VEGF) expression, and lactate accumulation at the fracture site were measured. Gene expression was compared between stabilized and non-stabilized fractures by micro-array analysis.
Results
We found that new blood vessel formation was robust by 3 days after fracture. Quantitative analysis showed that non-stabilized fractures had higher length density and surface density than stabilized fractures at 3 days after injury, suggesting that non-stabilized fractures were more vascularized. Oximetry analysis did not detect significant difference in oxygen tension at the fracture site between stabilized and non-stabilized fractures during the first 3 days after injury. Further micro-array analysis was performed to determine the effects of mechanical stability on the expression of angiogenic factors. No significant difference in the expression of VEGFs and other angiogenic factors was detected between stabilized and non-stabilized fractures.
Conclusions
Mechanical instability promotes angiogenesis during early fracture healing and further research is required to determine the underlying mechanisms.
Keywords: mechanical stability, fracture, angiogenesis, microarray, VEGF
Introduction
Mechanical stability plays an important role in fracture healing. Clinically, both inadequate fixation and immobilization that is too rigid may impair bone repair. Mechanical stability also regulates the mode of repair. In a mouse model, non-stabilized fractures heal through endochondral ossification, during which a cartilage template forms and is subsequently replaced by bone. In contrast, rigidly stabilized fractures heal through intramembranous ossification where bone forms directly without a cartilage intermediate [1]. Mechanical stimuli are involved in stem cell differentiation during fracture healing. Mechanical instability induces chondrocyte differentiation and mechanical stability leads to osteoblast differentiation. In vitro, cyclic, hydrostatic or static compression enhances formation of the cartilaginous matrix produced by bone marrow-derived mesenchymal cells [2]. Additionally, angiogenesis is required for normal bone formation and fracture healing and is regulated by mechanical signals. For example, shear stress was found to promote the formation and expansion of microvessel networks in collagen gel [3].
Stem cell differentiation and angiogenesis occur concurrently during early fracture healing. In our murine fracture model, early chondrocyte and osteoblast differentiation at the fracture site can be detected by 4 days after injury [4], and we have observed an increase in vascularity at day 3. However, the relationship between stem cell differentiation and angiogenesis has not been well determined. In the current study, we compared early tissue vascularization, tissue oxygenation, and expression of angiogenesis-related genes in stabilized and non-stabilized tibia fractures in a mouse model.
Materials and Methods
Creation of tibia fractures
All procedures in this work have been approved by the Institutional Animal Care and Use Committee (IACUC) at Dartmouth Medical School, Hanover, NH and at the University of California at San Francisco, San Francisco, CA. Non-stabilized fractures were created in the midshaft of tibiae of adult male 129J/B6 mice by three-point bending and were not stabilized. Stabilized fractures were created by applying an external fixator after induction of tibia fracture and manual examination confirmed that the fractures were rigidly stabilized [1]. All animals were allowed to ambulate freely after recovery.
Measuring oxygen tension in fracture callus
Stabilized (n=4) and non-stabilized (n=5) tibia fractures were created in adult male mice. Immediately after fracture, oxygen-sensitive lithium phthalocyanine (LiPc) crystals were implanted using a 25 gauge needle into the facture site. Oxygen levels at the fracture site were analyzed by Electron Paramagnetic Resonance (EPR) on the day of fracture, at days 1 and 3 after injury. This work was done at the EPR Center, Dartmouth Medical School (Hanover, NH). The mice were anesthetized with isoflurane (1.2–1.5%) in 21% oxygen and placed in the magnet. EPR oximetry was performed using a low frequency L-band (1.2 GHz) spectrometer that was developed specifically for in vivo studies [5, 6]. Spectra were detected using a surface loop resonator, which was positioned over the region of interest (fracture area). Body temperature was monitored by a rectal probe and maintained within the normal range (37-38 °C) using a heating pad. One-way ANOVA and LSD Post Hoc test were used to determine if there is significant difference in pO2 between stabilized and non-stabilized tibia fractures at each time point. Significance level is p=0.05.
Assessment of tissue vascularization
A second set of animals with stabilized (n=4) or non-stabilized (n=5) fractures were euthanized at 3 days after injury, and fractured tibiae with surrounding soft tissue were collected, fixed, decalcified, and prepared for frozen sectioning. Un-fractured normal tibiae (n=5) were collected as control. Vertical uniform random (VUR) sections (10μm) were prepared through each whole tissue block. Ten-15 VUR sections were analyzed using systemic random selection from each sample. Endothelium was labeled by immunohistochemistry with an anti-PECAM antibody [7]. Quantification of tissue vascularization using stereology was performed as described [8]. The length and the area of the outer surface of blood vessels within calluses were estimated by analyzing the length density (the length of blood vessels per unit volume of the reference space, Lv) and surface density (the area of the outer surface of blood vessels per unit volume of the reference space, Sv). One way ANOVA and LSD Post Hoc test were performed to determine the effect of stability on tissue vascularization at each time point. Significance level is p=0.05.
Measuring tissue lactate and VEGF levels
To quantify tissue lactate and VEGF levels, a third set of fracture samples were collected at 3 and 10 days after injury (n=5-6/group/time). Normal un-fractured legs (n=6) served as controls. Tissues were snap-frozen on dry ice, weighed, and homogenized in 6 volumes of distilled water plus 1/100 volume of protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) [9]. Tissue homogenates were centrifuged at 10,000 for 5 minutes. Supernatant was collected and kept at -20°C. Levels of lactate were measured using an YSI 1500 Sport Lactate Analyzer (YSI Life Sciences, Yellow Springs, Ohio). VEGF levels were determined using a Quantikine Mouse VEGF ELISA kit (R&D Systems, Minneapolis, MN), following manufacturer’s instruction. Levels of lactate and VEGF were normalized with the weight of the tissue. One way ANOVA and LSD Post Hoc test were performed to determine the effect of stability on tissue lactate or VEGF levels at each time point. Significance level is p=0.05.
Microarray analysis
To determine the effect of mechanical stability on the expression of genes that are related to angiogenesis, cDNA micro-array was performed on fracture tissues collected from a new set of un-fractured legs and non-stabilized or stabilized fractures at 2 and 7 days after injury (n=4/group/time). The rationale to choose these two time points for micro-array analysis and later time points (days 3 and 10) for histology and protein analyses was that changes in gene expression occurs earlier than proteins and histology. Total RNA was prepared from the entire lower leg from the knee joint to the ankle after removing the skin. This reduces confounding results due to the different modes of healing that occur in each mechanical environment and allows us to examine gene expression in the entire limb in response to the different mechanical environments. For gene expression analysis, Agilent whole mouse genome 4x44K Ink-jet array was used. Arrays were scanned using the Agilent microarray scanner and raw signal intensities were extracted with Feature Extraction v9.1 software (Agilent). This data set was normalized using quantile normalization to make the distribution of probe intensities to be the same across all arrays [10]. Differentially expressed genes are identified by fitting a 2X2 ANOVA model to the array data within each day using the limma package in Bioconductor. Genes that have 2-fold up- or down-regulation and B value > 0 were selected for functional annotation clustering using Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.7 (http://david.abcc.ncifcrf.gov/). In addition, the differential expression of more than 30 important angiogenesis-related genes was examined individually.
Results
Mechanical environment does not affect tissue pO2 at the fracture site
Our first goal was to assess the effect that mechanical stability had on tissue oxygen levels during early stages of facture healing. Our previous work has shown that the oxygen tension in uninjured tibia is at about 45.5 ± 15.3mmHg[11]. In the current study, tissue oxygen levels dropped to hypoxic levels immediately after fracture. By one day after injury oxygen levels increased in both stabilized and non-stabilized fractures. At three days after injury the oxygen level appeared the same as at day 1. We did not observe a statistically significant difference in oxygen levels between stabilized and non-stabilized fractures at any time point examined (Table 1).
Table 1.
Oxygen levels in unstable and stabilized fractures.
| Mechanical Environment | O2 (mmHg) at the time of injury | O2 (mmHg) at Day 1 | O2 (mmHg) at Day 3 |
|---|---|---|---|
| Non-stabilized | 5.7±1.7 | 48.2±25.6 | 45.3±20.9 |
| Stabilized | 10.4±5.7 | 27.0±11.1 | 41.8±19.2 |
Non-stabilized fractures are more vascularized
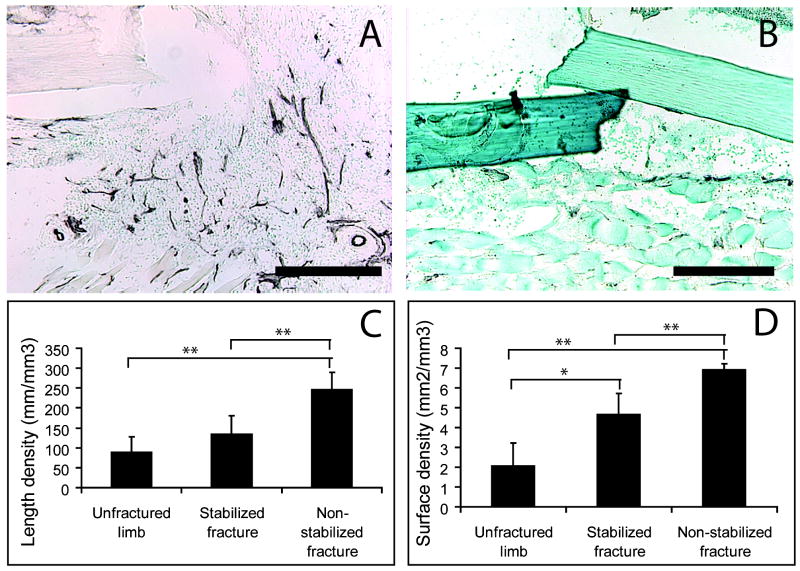
Our second goal was to assess the extent to which the mechanical environment influenced vascularity at the fracture site during the early stages of repair. At 3 days after injury, visual inspection of PECAM staining suggested that there were more blood vessels at the fracture site in non-stabilized fractures compared to stabilized fractures (Fig. 1A,B). This observation was confirmed quantitatively by stereology. Compared to stabilized fractures, non-stabilized fractures had significantly higher length density, and surface density (Fig. 1C,D), suggesting that mechanical instability could lead to a more robust angiogenic response after fracture. In addition, stereology analysis also demonstrated that fracture induces a robust angiogenic response. Compared to normal tibiae, stabilized fractures exhibited a significant higher surface density of vasculature (Fig. 1D). Non-stabilized fractures also showed significantly higher length density and surface density compared to normal tibiae (Fig. 1C, D).
Fig. 1.
Non-stabilized tibia fractures were more vascularized at 3 days after injury. (A) PECAM immunohistochemistry showed blood vessels (black color) in the fracture site of non-stabilized fractures. (B) Less blood vessels were detected at the fracture site of stabilized fractures. (C) Length density and (D) surface density of blood vessels in the fractured tibiae. Scale bars in A and B = 500um. In C and D, * p<0.05, ** p<0.01.
Effect of mechanical environment on tissue VEGF and lactate levels
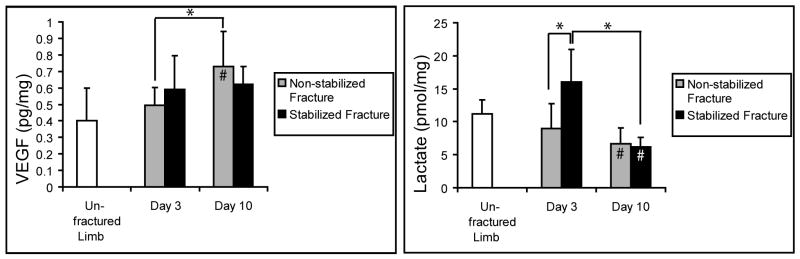
Next, we wanted to assess whether VEGF protein or lactate were significantly different in non-stabilized and stabilized fractures. Both of these molecules promote angiogenesis. Lactate acts as an intrinsic mediator of oxidant signaling by stabilizing Hif1a which leads directly to upregulation of VEGF [9, 12, 13]. VEGF and lactate were measured at 3 and 10 days after injury (Fig. 2). Non-stabilized fractures had higher VEGF levels at 10 days after injury compared to levels at day 3 and in control unfractured legs. We did not detect significant differences in VEGF expression between the two types of fractures. Stabilized fractures showed higher lactate levels at 3 days after injury compared to non-stabilized fractures. At 10 days, lactate levels in both stabilized and non-stabilized fractures were higher compared to controls. However, no difference was detected between the two types of fractures.
Fig. 2.
Tissue levels of VEGF and lactate in stabilized and non-stabilized fractures. * p<0.05. # p<0.05 compared to unfractured controls.
Mechanical environment regulates gene expression
Finally, we expanded our analysis of the effect that the mechanical environment has on angiogenic gene expression by examining a microarray data set that we have generated. We first compared overall expression patterns of genes in fracture limbs to uninjured limbs. Compared to uninjured limbs, non-stabilized fractures had 972 genes that were down-regulated by at least two folds, and 1166 genes that were up-regulated at least two folds at 2 days after injury (B>0). At 7 days after injury, non-stabilized fractures had 1717 down-regulated genes and 1597 up-regulated genes (DE>2X, B>0). Similar patterns were observed in stabilized fractures, which had 1435 down-regulated and 1351 up-regulated genes at 2 days compared to uninjured limbs (DE>2X, B>0). At 7 days, stabilized fractures had 1597 down-regulated and 1663 up-regulated genes (DE>2X, B>0). DAVID analysis showed enrichment of genes associated with angiogenesis or vasculature development.
Fewer genes were differentially expressed between non-stabilized and stabilized fractures. At day 2, non-stabilized fractures had 127 genes that were down-regulated two folds and 136 genes that were up-regulated two folds compared to stabilized fractures (B>0). At day 7, 166 genes were down-regulated and 273 genes were up-regulated in non-stabilized fractures compared to stabilized fractures (B>0). Among these genes of differential expression, few were associated with angiogenesis or vascular development and there was a low enrichment score.
To better understand the biological significance of the microarray data, a list of more than 30 angiogenesis-related genes was selected and their fold change was examined (Table 2). We found that both non-stabilized and stabilized fractures had increased expression of VEGF-d, hif-1a, npn-2, and a set of MMPs by 1-2 folds and slightly decreased the expression of VEGFa and VEGFb at the fracture site at both 2 and 7 days after fracture. However, the expression levels of these genes were not significantly different between non-stabilized and stabilized fractures.
Table 2.
A list of important angiogenesis-related genes and their differential expression. Un2d: non-stabilized fractures at day 2. S2d: stabilized fractures at day 2. Un7d: non-stabilized fractures at day 7. S7d: stabilized fractures at day 7.Ctrl: unfractured legs. DE: differential expression.
| Gene | Full Name | un2d/ctrl | un7d/ctrl | s2d/ctrl | s7d/ctrl | un2d/s2d | un7d/s7d |
|---|---|---|---|---|---|---|---|
| Angpt1 | Angiopoietin-1 | No DE | No DE | No DE | -0.82 | No DE | No DE |
| Angpt2 | Angiopoietin-2 | No DE | No DE | No DE | No DE | No DE | No DE |
|
| |||||||
| Col18a1 | Collagen, type XVIII, alpha 1 | 1.4 | 2.89 | 1.12 | 2.49 | No DE | No DE |
| Ctgf | Connective tissue growth factor | No DE | 2.36 | 2.69 | 2.09 | No DE | No DE |
|
| |||||||
| Ecgf1 | Endothelial cell growth factor-1 | No DE | 0.57 | No DE | 0.64 | No DE | No DE |
| Edg5 | Endothelial differentiation gene-5 | 1.18 | 1.19 | 1 | 0.98 | No DE | No DE |
| Egf | Epidermal growth factor | No DE | -2.16 | No DE | -1.51 | No DE | No DE |
|
| |||||||
| Fgf2 | Fibroblast growth factor-2 | No DE | No DE | No DE | No DE | No DE | No DE |
| Fgf21 | Fibroblast growth factor-21 | No DE | 2.2 | No DE | 3.64 | No DE | No DE |
| Fgfr1 | Fibroblast growth factor receptor-1 | No DE | 1.38 | No DE | No DE | No DE | No DE |
| Fgfr2 | Fibroblast growth factor receptor-2 | No DE | 1.38 | No DE | No DE | -0.01 | 0.6 |
| Fgfr3 | Fibroblast growth factor receptor-3 | No DE | 2.3 | No DE | No DE | -0.04 | 1.43 |
| Fgfr4 | Fibroblast growth factor receptor-4 | No DE | 2.25 | No DE | 1.55 | No DE | No DE |
| Flt1 | VEGFR1 | No DE | -1.08 | No DE | -1 | No DE | No DE |
| Flk-1 | VEGFR2 | -1.03 | No DE | No DE | No DE | No DE | No DE |
| fzd1 | Frizzled-1 | 0.86 | 2.14 | No DE | 1.46 | No DE | No DE |
|
| |||||||
| Hgf | Hepatocyte growth factor | 0.78 | 0.81 | 0.63 | 1.08 | No DE | No DE |
| Hif1a | Hypoxia inducible factor-1, alpha unit | 1.87 | 2.08 | 1.7 | 1.61 | No DE | No DE |
| Hif1an | Hif-1a inhibitor | -0.78 | -0.81 | -1.14 | No DE | No DE | No DE |
|
| |||||||
| Mmp2 | Matrix metallopeptidase 2 | No DE | 1.85 | No DE | No DE | 0.18 | 1.05 |
| Timp1 | Tissue inhibitor of metalloproteinase 1 | 4.19 | 4.24 | 4.27 | 4.22 | No DE | No DE |
|
| |||||||
| Nrp1 | Neuropilin1 | 1.58 | No DE | No DE | No DE | No DE | No DE |
| Nrp2 | Neuropilin2 | 1.46 | 1.51 | 0.96 | 1.13 | No DE | No DE |
|
| |||||||
| Pdgfa | Platelet derived growth factor a | No DE | No DE | No DE | No DE | No DE | No DE |
| Pdgfc | Platelet derived growth factor b | No DE | 1.51 | No DE | No DE | No DE | No DE |
|
| |||||||
| Tgfb1 | Transforming growth factor, beta 1 | No DE | No DE | No DE | No DE | No DE | No DE |
| Tgfb2 | Transforming growth factor, beta 2 | -1.14 | No DE | No DE | No DE | No DE | No DE |
| Tgfb3 | Transforming growth factor, beta 3 | No DE | 0.92 | -1.38 | 0.79 | No DE | No DE |
| Tie-1 | TIE tyrosine kinase | No DE | No DE | No DE | No DE | No DE | No DE |
| Tie-2 | The TEK receptor tyrosine kinase | No DE | No DE | No DE | No DE | No DE | No DE |
|
| |||||||
| Vegfa | Vascular endothelial growth factor, a | -1.02 | -1.01 | -1.05 | -1.04 | No DE | No DE |
| Vegfb | Vascular endothelial growth factor, b | -1.37 | -1.02 | -1.84 | -1.11 | No DE | No DE |
| Vegfc | Vascular endothelial growth factor, c | No DE | No DE | No DE | No DE | No DE | No DE |
| Vegfd | Vascular endothelial growth factor, d | 1.76 | 1.07 | 1.39 | 1.27 | No DE | No DE |
Discussion
Mechanical environment affects angiogenesis
Our previous studies have shown that non-stabilized fractures heal through endochondral ossification and stabilized fractures heal via direct bone formation [1, 14]. Here, we found that non-stabilized fractures had more vascularization at 3 days after injury compared to stabilized fractures, suggesting mechanical instability may promote angiogenesis during early stages of fracture healing. This finding is consistent with what has been reported in the literature. In an ovine tibia osteotomy model, larger interfragmentary movement is associated with increased cortico-medullary blood supply at 2 weeks after injury [15]. In a sheep osteotomy model, larger degrees of instability increases tissue vascularization at 2 weeks after injury [16]. However, at later stages of fracture healing mechanical instability may have different effects on tissue vascularization. Lienau et al. showed that there were fewer blood vessels in the less stable fractures at 6 weeks after osteotomy[16]. In another study, Claes et al. found that larger interfragmentary movement in a sheep metatarsal osteotomy model led to more fibrocartilage formation and fewer blood vessels at 9 weeks after injury [17]. The decreased vasculature at these later time points may be the result of increased amounts of avascular cartilage in the callus.
What is the role of angiogenesis in tissue differentiation during early fracture healing? Differentiation of mesenchymal stem cells into chondrocytes or osteoblasts at the fracture site occurs early after bone injury. In mice, chondrocyte/osteoblast differentiation in fracture callus is detected by 4 days after injury [4]. In the current study, we observed robust blood vessel formation in non-stabilized fractures by 3 days after injury. These findings show that mechanical instability favors chondrocyte differentiation, while at the same time promotes angiogenesis. We may speculate that increased angiogenesis leads to chondrocyte differentiation or vice versa, but there is lack of experimental evidence demonstrating this point. In fact, published results in the literature regarding the relationship between angiogenesis and chondrogenesis remain controversial. On one hand, inhibiting angiogenesis [18] or disrupting the blood supply [19] leads to decreased chondrogenesis in fracture healing. On the other hand, treatments that improve tissue vascularization are also associated with less cartilage formation. For example, a mutation in the thrombospondin-2 (Tsp2) gene in mice leads to a two-fold increase in tissue vascularization and an accompanying decrease in chondrogenesis during early fracture healing [20]. As another example, VEGF treatment improves tissue vascularization and promotes bone formation during early fracture healing [21]. Therefore, an alternative hypothesis is that mechanical instability increases angiogenesis but may constrain the blood supply thus the function of blood vessels due to constant disruption of new vasculature. Our EPR oximetry data may support this hypothesis, which showed that oxygen tension in non-stabilized fractures was not significantly improved compared to stabilized fractures during the first 3 days after injury. However, our data on lactate levels at the fracture site is not consistent. At 3 days after fracture, non-stabilized fractures had less lactate compared to stabilized ones. Since lactate is accumulated in anaerobic metabolism as well as aerobic conditions, the significance of increased lactate in stabilized fractures need to be further determined. In conclusion, results from current study are not sufficient to conclude on the relationship between angiogenesis and tissue oxygenation and stem cell fate decision.
It is worth mentioning that factors affect angiogenesis may have direct effects on osteoblasts and chondrocytes. Receptors for VEGF are found on osteoblast progenitors [22] and can stimulate gene expression in osteoblasts [23, 24]. Tsp2, as described above, is anti-angiogenic, but also has direct effect on skeletal progenitor cells and may be expressed in prechondrogenic and preosteogenic cells during fracture repair [20].
Mechanisms that underlie the effects of mechanical environment on angiogenesis
Post-injury angiogenesis is a complicated process, which involves activity of many growth factors and enzymes, such as VEGFs, Hif-1, and angiopoeitins. We used microarray and ELISA to determine the effects of mechanical stability on the expression of important angiogenesis-related factors. Hif-1 is a dimeric transcription factor comprised of Hif-1a and Hif1b sub-units and controls the expression of VEGF[25]. Hif-1a was up-regulated in fractured legs at both 2 and 7 days after bone injury. VEGF is a family of proteins that regulate angiogenesis and lymphoangiogenesis [26]. Among them VEGFa and VEGFb are regulators of vascular repair. VEGFc and VEGFd are also involved in lymphoangiogeneis. Our ELISA showed that total VEGF protein was high in non-stabilized fractures at 10 days after injury. Further micro-array analysis found that mRNA of VEGFd, but not of VEGFa, VEGFb, and VEGFc, was mildly up-regulated after bone injury. The increased expression of VEGFd in fractured limbs suggests that lymphoangiogenesis could play a role in bone repair or occurs concomitantly. Among VEGF receptors, the expression of neuropilin-2 was increased after fracture. VEGFR1 (flk-1) was decreased at day 7 after fracture and VEGFR2 (flt-1) remained unchanged during early fracture healing. Angiopoietins are another family of angiogenesis-related growth factors. However, we did not detect expression of angiopoietin 1 and 2, neither their receptors (Tie1 and Tie2) in this work. Levels of lactate, another regulator of angiogenesis, were also changed during the process of fracture healing, however, the changes were not correlated with changes in VEGF expression.
The mechanical environment did not significantly alter the major signaling pathways of angiogenesis. Our analyses did not reveal any significant differences in angiogenic gene expression profiles between stabilized and non-stabilized fractures. However, mechanical stimuli may have direct effects on endothelial cells and angiogenesis. There are reports showing that endothelial cells exposed to shear stress align with the direction of mechanical loading [27] and the endothelial cytoskeleton may undergo spatial reorganization [28]. Further, expression of manganese super-oxide dismutase and cyclooxygenase-2 in cultured endothelial cells is up-regulated by steady laminar shear stress [29], which supports a model whereby mechanical stimuli directly affect vascularity.
In summary, the mechanical environment affects tissue vascularization during fracture healing. Mechanical instability promotes angiogenesis at early stages of bone repair. The significance and role of the vasculature and oxygen supply in stem cell differentiation and later callus formation and the underlying mechanisms need to be further analyzed.
Acknowledgments
This work was funded by National Institutes of Health-NIAMS (KO8-AR002164 and RO1-AR053645 to TM.), Orthopaedic Trauma Association (a research grant to CL.), Foundation for Orthopaedic Trauma (a grant to CL), and Zimmer Inc. We would like to thank Drs. Yuanyuan Xiao and Jeffery Lange for their expertise in microarray data analysis, and the UCSF Lung Biology Microarray Core Facility.
Source of Funding: Theodore Miclau is a consultant for Amgen Inc. He is serving as a committee for AO Experimental Research Board; Osteosynthesis and Trauma Care Foundation; as a board member/officer/committee chair for Orthopaedic Trauma Association; San Francisco General Hospital Foundation; and Orthopaedic Research Society.
Footnotes
Conflicts of Interest For the remaining authors none were declared.
This study was presented in part at the Annual Meeting of the Orthopaedic Trauma Association, Baltimore, Maryland, 2010.
References
- 1.Thompson Z, Miclau T, Hu D, et al. A model for intramembranous ossification during fracture healing. J Orthop Res. 2002;20(5):1091–8. doi: 10.1016/S0736-0266(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 2.Angele P, Schumann D, Angele M, et al. Cyclic, mechanical compression enhances chondrogenesis of mesenchymal progenitor cells in tissue engineering scaffolds. Biorheology. 2004;41(3-4):335–46. [PubMed] [Google Scholar]
- 3.Ueda A, Koga M, Ikeda M, et al. Effect of shear stress on microvessel network formation of endothelial cells with in vitro three-dimensional model. Am J Physiol Heart Circ Physiol. 2004;287(3):H994–1002. doi: 10.1152/ajpheart.00400.2003. [DOI] [PubMed] [Google Scholar]
- 4.Le AX, Miclau T, Hu D, et al. Molecular aspects of healing in stabilized and non-stabilized fractures. J Orthop Res. 2001;19(1):78–84. doi: 10.1016/S0736-0266(00)00006-1. [DOI] [PubMed] [Google Scholar]
- 5.Swartz HM, Clarkson RB. The measurement of oxygen in vivo using EPR techniques. Physics in medicine and biology. 1998;43(7):1957–75. doi: 10.1088/0031-9155/43/7/017. [DOI] [PubMed] [Google Scholar]
- 6.Swartz HM, Khan N, Buckey J, et al. Clinical applications of EPR: overview and perspectives. NMR in biomedicine. 2004;17(5):335–51. doi: 10.1002/nbm.911. [DOI] [PubMed] [Google Scholar]
- 7.Lu C, Marcucio R, Miclau T. Assessing angiogenesis during fracture healing. Iowa Orthop J. 2006;26:17–26. [PMC free article] [PubMed] [Google Scholar]
- 8.Lu C, Hansen E, Sapozhnikova A, et al. Effect of age on vascularization during fracture repair. J Orthop Res. 2008;26(10):1384–9. doi: 10.1002/jor.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunt TK, Aslam RS, Beckert S, et al. Aerobically derived lactate stimulates revascularization and tissue repair via redox mechanisms. Antioxid Redox Signal. 2007;9(8):1115–24. doi: 10.1089/ars.2007.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolstad BM, Irizarry RA, Astrand M, et al. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19(2):185–93. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 11.Lu C, Rollins M, Hou H, et al. Tibial fracture decreases oxygen levels at the site of injury. The Iowa orthopaedic journal. 2008;28:14–21. [PMC free article] [PubMed] [Google Scholar]
- 12.Komatsu DE, Hadjiargyrou M. Activation of the transcription factor HIF-1 and its target genes, VEGF, HO-1, iNOS, during fracture repair. Bone. 2004;34(4):680–8. doi: 10.1016/j.bone.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Schipani E, Maes C, Carmeliet G, et al. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24(8):1347–53. doi: 10.1359/jbmr.090602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colnot C, Thompson Z, Miclau T, et al. Altered fracture repair in the absence of MMP9. Development. 2003;130(17):4123–33. doi: 10.1242/dev.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallace AL, Draper ER, Strachan RK, et al. The vascular response to fracture micromovement. Clin Orthop Relat Res. 1994;301:281–90. [PubMed] [Google Scholar]
- 16.Lienau J, Schell H, Duda GN, et al. Initial vascularization and tissue differentiation are influenced by fixation stability. J Orthop Res. 2005;23(3):639–45. doi: 10.1016/j.orthres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Claes L, Eckert-Hubner K, Augat P. The effect of mechanical stability on local vascularization and tissue differentiation in callus healing. J Orthop Res. 2002;20(5):1099–105. doi: 10.1016/S0736-0266(02)00044-X. [DOI] [PubMed] [Google Scholar]
- 18.Hausman MR, Schaffler MB, Majeska RJ. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone. 2001;29(6):560–4. doi: 10.1016/s8756-3282(01)00608-1. [DOI] [PubMed] [Google Scholar]
- 19.Lu C, Miclau T, Hu D, et al. Ischemia leads to delayed union during fracture healing: a mouse model. J Orthop Res. 2007;25(1):51–61. doi: 10.1002/jor.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor DK, Meganck JA, Terkhorn S, et al. Thrombospondin-2 influences the proportion of cartilage and bone during fracture healing. J Bone Miner Res. 2009;24(6):1043–54. doi: 10.1359/jbmr.090101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Street J, Bao M, deGuzman L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99(15):9656–61. [Google Scholar]
- 22.Leung K. [123I]Vascular endothelial growth factor. Bethesda (MD): National Center for Biotechnology Information (US); 2010. [PubMed] [Google Scholar]
- 23.Athanasopoulos AN, Schneider D, Keiper T, et al. Vascular endothelial growth factor (VEGF)-induced up-regulation of CCN1 in osteoblasts mediates proangiogenic activities in endothelial cells and promotes fracture healing. The Journal of biological chemistry. 2007;282(37):26746–53. doi: 10.1074/jbc.M705200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genetos DC, Wong A, Watari S, et al. Hypoxia increases Annexin A2 expression in osteoblastic cells via VEGF and ERK. Bone. 2010;47(6):1013–9. doi: 10.1016/j.bone.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Wan C, Gilbert SR, et al. Oxygen sensing and osteogenesis. Annals of the New York Academy of Sciences. 2007;1117:1–11. doi: 10.1196/annals.1402.049. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Eriksson U. Novel VEGF family members: VEGF-B, VEGF-C and VEGF-D. Int J Biochem Cell Biol. 2001;33(4):421–6. doi: 10.1016/s1357-2725(01)00027-9. [DOI] [PubMed] [Google Scholar]
- 27.Levesque MJ, Nerem RM. The study of rheological effects on vascular endothelial cells in culture. Biorheology. 1989;26(2):345–57. doi: 10.3233/bir-1989-26218. [DOI] [PubMed] [Google Scholar]
- 28.Galbraith CG, Skalak R, Chien S. Shear stress induces spatial reorganization of the endothelial cell cytoskeleton. Cell Motil Cytoskeleton. 1998;40(4):317–30. doi: 10.1002/(SICI)1097-0169(1998)40:4<317::AID-CM1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Topper JN, Cai J, Falb D, et al. Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci U S A. 1996;93(19):10417–22. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]