Abstract
β-Galactoside-binding lectin 9 (galectin-9) is a tandem repeat-type member of the galectin family. It was initially characterized as an eosinophil chemoattractant and an inducer of apoptosis in thymocytes. Subsequently, galectin-9 was identified as a ligand for transmembrane immunoglobulin mucin domain 3 (Tim-3), a type I glycoprotein induced on T cells during chronic inflammation. Work in autoimmune diseases and chronic viral infections have led to the current hypothesis that the function of Tim-3 is to limit immune responses. However, it is still not known to what degree these effects are due to the galectin-9/Tim-3 interaction. In this study, we show that galectin-9 is not limited to the role of a pro-apoptotic agent, but that it can also induce the production of pro-inflammatory cytokines from T helper cells. This effect is dose-dependent and does not require Tim-3. These findings suggest that the effects of galectin-9 on T cells are more complex than previously thought and are mediated by additional receptors apart from Tim-3.
Keywords: apoptosis, cytokines, galectin, mucin, T helper cells
Introduction
β-Galactoside-binding lectin 9 (galectin-9) is a lectin composed of two nonidentical carbohydrate recognition domains separated by a linker peptide of variable lengths, depending on the isoform (Hirashima et al. 2004). Galectin-9 is secreted through a nonclassical route and can be found in either the cytoplasm or the extracellular matrix. Resting T cells express galectin-9 but can further up-regulate its expression when activated (Chabot et al. 2002). Although galectin-9 is able to bind to CD44, another surface glycoprotein expressed by lymphocytes (Katoh et al. 2007), its interaction with Tim-3 has been the most common focus in recent immunological studies.
Tim-3 is a type I glycoprotein whose expression on T cells is controlled in part by the Th1-transcription factor, T-bet (Anderson et al. 2010), consistent with the observation that both human and mouse Tim-3+ T cells have a Th1/Tc1 phenotype. Interestingly, Tim-3 has also been detected on Th17 and regulatory T cells (Tregs), T lymphocyte subsets whose polarization does not depend on T-bet (Seki et al. 2008; Hastings et al. 2009). In addition to galectin-9, Tim-3 has at least one another ligand, which remains to be identified. This putative ligand is expressed on activated T cells, macrophages and dendritic cells and is predicted to bind to Tim-3 at a site distinct from galectin-9 (Cao et al. 2007).
Due to the lack of agonistic antibodies to Tim-3, galectin-9 has been used in many studies focusing on the function of Tim-3. Administration of galectin-9 to mice chronically infected with Herpes simplex virus reduces the number of CD4+ T cells but increases the percentage of Tregs and myeloid suppressor cells (Sehrawat et al. 2009). In tumor-bearing mice, galectin-9 increases the percentage of Tim-3+ CD8+ T cells and dendritic cells (Nagahara et al. 2008). Thus, galectin-9 can have either positive or negative effects on different cell types or the same cell type in different inflammatory settings (Liu and Rabinovich 2010).
Another method commonly employed to study the function of Tim-3 is the blockade of Tim-3/Tim-3 ligand interactions. Antibodies that block Tim-3/Tim-3 ligand interactions restore the ability of exhausted T cells isolated from human subjects with HIV or multiple sclerosis to respond to anti-CD3 stimulation (Jones et al. 2008; Yang et al. 2008). Tim-3/immunoglobulin fusion proteins prevented the induction of tolerance in mice and hastened the onset of diabetes in NOD mice (Sabatos et al. 2003; Sanchez-Fueyo et al. 2003). Collectively, these studies support the current model that Tim-3 can negatively regulate immune responses. However, they do not specifically address whether the galectin-9/Tim-3 interaction is required for such effects. Thus, the target epitope for these blocking antibodies is still not known, and whether soluble Tim-3 proteins preferably disrupt the interaction of Tim-3 with galectin-9 or its other ligand(s) has not been established.
In this study, we show that galectin-9 cannot only instruct T helper cells to undergo apoptosis but it can also induce them to secrete pro-inflammatory cytokines. These effects are dependent on the concentration of galectin-9 but are independent of Tim-3. Thus, galectin-9 can have either positive or negative effects on T helper cells, both of which can occur independently of Tim-3.
Results
Galectin-9 modulates T helper cell function and viability in a dose-dependent manner
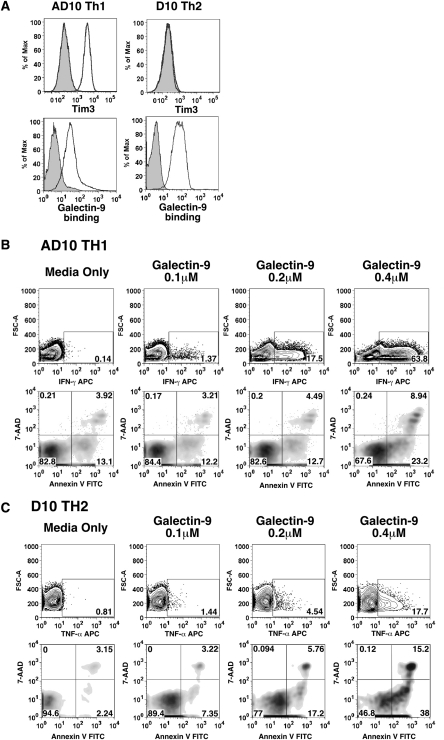
In the first description of the galectin-9/Tim-3 interaction, Th1 cells were shown to undergo apoptosis when treated with galectin-9 (Zhu et al. 2005). This report contributed significantly to the current model that Tim-3 regulates the immune system by terminating Th1 responses. Because galectins can have pleiotropic effects on immune cells (Liu and Rabinovich 2010), we wanted to determine if galectin-9 could mediate other effects on T helper cells. We treated a Th1 clone (AD10) and a Th2 clone (D10) with various concentrations of galectin-9, then assayed for cell viability and secretion of cytokines. AD10 Th1 cells are Tim-3+, whereas D10 Th2 cells are Tim-3− (Figure 1A, upper panels). Both cell lines, however, stained with galectin-9 (Figure 1A, lower panels). In agreement with Zhu et al., AD10 cells treated with galectin-9 underwent apoptosis (Figure 1B, lower panels). We also found that galectin-9 could induce the production of interferon (IFN)-γ by AD10 cells (Figure 1B, upper panels). Higher concentrations of galectin-9 were required to induce apoptosis, whereas the production of IFN-γ was still observed in AD10 cells treated with nonlethal concentrations of galectin-9. The same dose range of galectin-9 was also sufficient to induce Tim-3 negative D10 Th2 cells to secrete tumor necrosis factor (TNF)-α and undergo apoptosis (Figure 1C). Therefore, in addition to being a pro-apoptotic factor, galectin-9 can also induce T helper cells to secrete pro-inflammatory cytokines.
Fig. 1.
Galectin-9 induces cytokine secretion and apoptosis in Th1 and Th2T cells. (A) Staining of AD10 Th1 and D10 Th2 cells with (upper panel) isotype control (filled histogram) and Tim-3 pAb (empty histogram) and (lower panel) streptavidin (filled histogram) and biotin-galectin-9 (empty histogram). (B) AD10 Th1 cells were treated with galectin-9 for 6 h. Cells were then either fixed and permeablized for staining with anti-mouse IFN-γ APC (top panel) or washed and stained with 7-AAD/annexin V (bottom panel). Data are representative of three independent experiments. (C) D10 Th2 were treated with galectin-9 for 5 h and stained as described in (B), with anti-mouse TNF-α (upper panel) and 7-AAD/annexin V (bottom panel). Data are representative of two independent experiments. GolgiPlug was added to both AD10 and D10 cultures 1 h post-stimulation to facilitate retention and detection of cytokines.
Galectin-9 modulation of T helper cell function and viability is carbohydrate-dependent
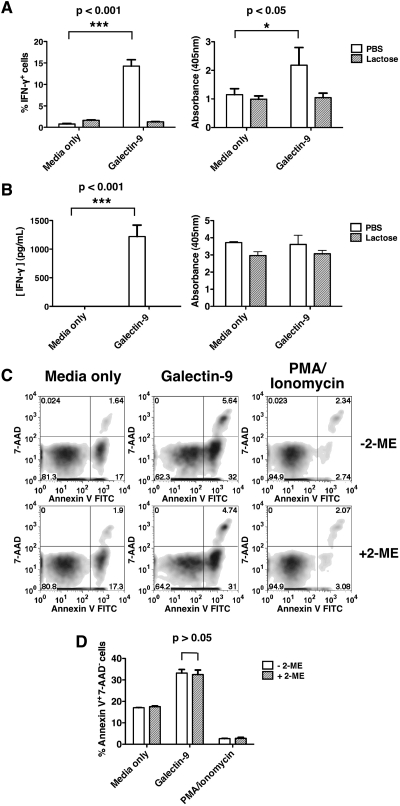
Carbohydrate-dependent binding of galectin-9 to its binding partners can be competitively inhibited by lactose (Leffler and Barondes 1986). To confirm that the effects of galectin-9 described in Figure 1 were carbohydrate-dependent, we compared the ability of galectin-9-treated AD10 Th1 cells to undergo apoptosis and secrete IFN-γ in the presence of phosphate-buffered saline (PBS) or lactose. We confirmed that galectin-9 treatment induces IFN-γ which was detectable intracellularly by 6 h (Figure 2A, left panel) and in cell culture supernatant by 20 h (Figure 2B, left panel). The addition of lactose prevented the secretion of IFN-γ by AD10 Th1 cells treated with galectin-9 (Figure 2A and B, left panels). By 6 h, galectin-9-treated AD10 cells displayed more internucleosomal DNA fragmentation than control cultures (Figure 2A, right panel), which confirms that galectin-9 induces apoptosis and not just PS exposure. However, by 20 h, AD10 cells cultured in media only or with galectin-9 were similarly enriched with nucleosomes (Figure 2B, right panel). This suggests that the pro-apoptotic effects of galectin-9 become less dominant when AD10 cells begin to succumb to other apoptotis-inducing conditions such as interleukin (IL)-2 withdrawal, between 6 and 20 h. The apoptosis observed after 6 h of galectin-9 treatment was not dependent on IFN-γ, since these experiments were performed in the presence of brefeldin A, which prevents cytokine secretion. Furthermore, the addition of blocking antibodies to IFN-γ did not diminish the capacity of galectin-9 to induce apoptosis (data not shown).
Fig. 2.
Carbohydrate dependence of galectin-9 effects on T cells. IFN-γ secretion was assayed at 6 h with intracellular cytokine staining (A, left panel) and 20 h with ELISA (B, left panel). Nucleosome enrichment was determined at 6 h (A, right panel) and 20 h (B, right panel). Data are representative of two independent experiments. (C and D) Apoptosis was examined in primary Th1 cells treated with galectin-9 in the absence (upper panel) and the presence (lower panel) of 2-mercaptoethanol (2-ME). Data are representative of three independent experiments. Error bars indicate SD from replicate cultures. P-values were calculated using two-way ANOVA.
Galectin-1, another member of the galectin family, has also been shown to induce apoptosis and cytokine secretion in Th2 cells (Toscano et al. 2007). However, galectin-1 has been reported to induce only PS exposure and not apoptosis, in the absence of reducing agents (Stowell et al. 2009). Therefore, we compared the ability of galectin-9 to induce apoptosis in primary T cells cultured in Th1 conditions, in the absence or the presence of 2-mercaptoethanol, which is usually present in our cell culture medium. In Figure 2C and D, we show that galectin-9 is able to induce apoptosis in one-round polarized primary Th1 cells to the same extent, in the absence and the presence of 2-mercaptoethanol. Therefore, unlike galectin-1, galectin-9 can induce the apoptosis of T helper cells independently of reducing agents.
Galectin-9 modulates T helper cell function and viability in the absence of Tim-3
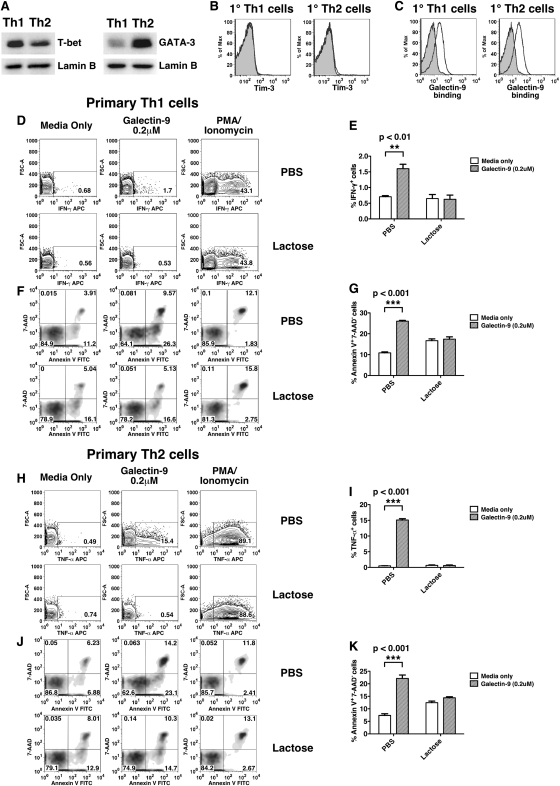
The results shown in Figure 2 suggest that Tim-3 may not be the major mediator for galectin-9 on T helper cells. To address this more directly, we assessed the effects of galectin-9 on CD4+ T cells stimulated only once under Th1 and Th2 polarizing conditions. As expected, T-bet and GATA-3 were up-regulated in Th1 and Th2 cells, respectively (Figure 3A). In agreement with previous reports, we found that these cells do not express Tim-3 on their surface (Figure 3B). However, they stained positively with galectin-9, which suggests that other binding partner(s) of galectin-9 are expressed on the surface of T helper cells after only one round of polarization under either Th1 or Th2 conditions (Figure 3C). Despite the absence of Tim-3 on the cell surface, galectin-9 treatment induced IFN-γ secretion (Figure 3D and E) and apoptosis (Figure 3F and G) in Th1 cells. Likewise, Th2 cells secreted TNF-α (Figure 3H and I) and underwent apoptosis (Figure 3J and K) in the presence of galectin-9. These effects were abrogated in the presence of lactose (Figure 3D, F, H and J), consistent with our findings in the AD10 Th1 and D10 Th2 clones.
Fig. 3.
Effects of galectin-9 on primary Th1 and Th2 cells. (A) T-bet and GATA-3 expression in nuclear lysates of primary Th1 or Th2 cells (upper panels). Lamin B expression is shown as a loading control (lower panels). (B) Primary Th1 and Th2 cells were stained with isotype control (filled histogram) and Tim-3 pAb (empty histogram), or (C) streptavidin (filled histogram) and biotin-galectin-9 (empty histogram). Primary Th1 cells treated with galectin-9 for 6 h in the presence of PBS or lactose were either stained for intracellular IFN-γ (D) or 7-AAD/annexin V (F). Results are summarized in (E) and (G). Primary Th2 cells treated with galectin-9 for 6 h in the presence of PBS or lactose were either stained for intracellular TNF-α (H) or 7-AAD/annexin V (J). Results are summarized in (I) and (K). Error bars indicate SD from replicate cultures. Data are representative of two independent experiments. P-values were calculated using two-way ANOVA.
Tim-3 is not the major mediator of galectin-9 effects on fully differentiated Th1 cells
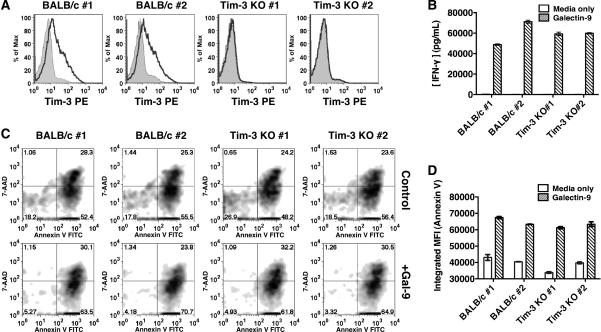
To further confirm that galectin-9 does not require Tim-3 to modulate T helper function and viability, we stimulated CD4+ T cells from wild-type and Tim-3 KO mice under Th1-polarizing conditions for three rounds to induce the surface expression of Tim-3. We confirmed that only Th1 cells from wild-type mice and not from Tim-3 KO mice express Tim-3 (Figure 4A). After 20 h of treatment with galectin-9, we were able to detect significant amounts of IFN-γ in the supernatant of both wild-type and Tim-3 KO Th1 cells (Figure 4B). We also found that wild-type and Tim-3 KO Th1 cells were equally susceptible to galectin-9-induced apoptosis as determined by 7-AAD/annexin V staining (Figure 4C and D). Therefore, galectin-9 does not require Tim-3 to induce cytokine secretion and apoptosis in fully differentiated Th1 cells.
Fig. 4.
Effects of galectin-9 on wild-type and Tim-3 KO fully differentiated Th1 cells. (A) CD4+ T cells from spleens of wild-type and Tim-3 KO mice were subjected to three rounds of Th1 polarization and stained with either rat immunoglobulin G1 (filled histogram) or anti-Tim-3 (empty histogram). (B) Th1 cells from both BALB/c and Tim-3 KO mice were stimulated in duplicate sets for 20 h. Supernatants were analyzed by ELISA for IFN-γ. (C) Th1 cells from (B) were stained with 7-AAD/annexin V. (D) Results from (C) are quantified as integrated MFI (% of annexin V+ cells X MFI annexin V+ cells) to reflect cells undergoing both early and late apoptosis. Increases in IFN-γ and apoptosis due to galectin-9 treatment were found to be significant in both wild-type and Tim-3 KO Th1 cultures (P < 0.001, two-way ANOVA).
Discussion
In this study, we show for the first time that in addition to inducing apoptosis, galectin-9 can promote the production of pro-inflammatory cytokines by both Th1 and Th2 cells. These effects occur in a dose-dependent fashion and do not require Tim-3. The ability of galectin-9 to bind and modulate both T helper subsets is noteworthy, as Th1 and Th2 cells have distinct glycophenotypes (Toscano et al. 2007). These findings clearly highlight the complex nature of the biological effects of galectin-9, and call into question the centrality of the galectin-9/Tim-3 interaction for such effects.
The pro-apoptotic activity of galectin-9 was first identified using thymocytes, cells that are not known to express Tim-3 (Wada et al. 1997). Following the identification of galectin-9 as a ligand for Tim-3, numerous biological effects have been attributed to the galectin-9/Tim-3 interaction. For example, galectin-9 is thought to increase viral burden or ameliorate autoimmune disease in mice by inducing the apoptosis of Tim-3 expressing, antigen-specific, T cells (Seki et al. 2008; Sehrawat et al. 2009). However, the loss of Tim-3+ cells in vivo due to galectin-9-induced apoptosis has yet to be demonstrated directly. The enhanced susceptibility of Tim-3 expressing cells to galectin-9-induced apoptosis has been addressed in vitro. Th1 cells polarized from Tim-3 KO mice displayed less nucleosomal enrichment than those from wild-type mice following galectin-9 treatment, up to 12 h, the last time point analyzed (Zhu et al. 2005). Here, we show that 20 h after galectin-9 treatment, there were no significant differences in the ability of Th1 cells from wild-type and Tim-3 KO mice to undergo apoptosis. However, there are several differences between our approaches. Zhu et al. used CD4+ T cells polarized under Th1 conditions with peptide-loaded splenocytes, whereas we employed plate-bound anti-CD3 and anti-CD28. Additionally, the previous study treated Th1 cells with mouse galectin-9 (0.5 μM), whereas we used human galectin-9 (0.2 μM). Lastly, Zhu et al. detected apoptosis using the nucleosome enrichment assay, which we have found to be not as sensitive as 7-AAD/annexin V staining for detection of small changes in apoptosis (Figure 4C).
Tim-3 is up-regulated in vitro with repeated rounds of Th1 polarization and in vivo under chronic inflammatory conditions (Golden-Mason et al. 2009; Sehrawat et al. 2009). Tim-3 expression on CD4+ and CD8+ T cells in chronic viral infections is associated with immune “exhaustion” (Jones et al. 2008; Golden-Mason et al. 2009). In such settings, Tim-3+ cells only respond to T cell receptor for antigen (TCR) ligation when the interaction between Tim-3 and its ligand(s) is disrupted. This suggests that when Tim-3 is occupied by a ligand, it can antagonize signals emanating from the T-cell receptor without causing cell death. The ligand(s) that elicit this effect from Tim-3 remains unknown but galectin-9 is an unlikely candidate at this point, as it has not been shown to negatively regulate effector T cell function in a manner that is independent of cell death or apoptosis.
Our findings raise the question of how galectin-9 is able to induce the bimodal effects that we have observed. We propose that for the induction of apoptosis, sufficient galectin-9/receptor interactions must be formed on the surface of effector T cells, to reach a certain threshold for signaling. This could be achieved through the formation of high-order lattice structures that allow galectin-9 to cross-link multiple receptors in close proximity. Another possibility is that galectin-9 binds to multiple distinct receptors with varying affinities to induce apoptosis or cytokine production. Thus, the bimodal effects we observe in Th1 cells may be the result of galectin-9 having a greater affinity for the receptor required for cytokine production than that required for apoptosis.
Although biochemical studies have validated the galectin-9/Tim-3 interaction, the exact biological effects of this ligand/receptor pair have not been firmly established (Zhu et al. 2005). One of the main challenges stems from the fact that galectin-9 can have a wide range of effects on immune cells (Liu and Rabinovich 2010). In addition, both T cells and antigen presenting cells can up-regulate the expression of galectin-9 and Tim-3 under similar conditions (Hirashima et al. 2004; Hastings et al. 2009; Dardalhon et al. 2010). The ability of galectins to bind to more than one cell surface receptor to modulate T helper cells is not unusual (Pace et al. 1999; Stillman et al. 2006). Galectin-1, a prototype member of the galectin family, can induce the apoptosis of T cells through CD7 and CD45 (Earl et al., Pace et al. 2000). Therefore, when galectin-9 is administered to wild-type mice undergoing chronic inflammation, it is difficult to distinguish between the Tim-3-dependent and the Tim-3-independent effects of galectin-9 on T cells and antigen-presenting cells. Further in vivo validation of our findings will require mice deficient for both Tim-3 and galectin-9, or a panel of blocking antibodies to both Tim-3 and galectin-9 with well-characterized epitopes.
Materials and methods
Antibodies and reagents
The antibodies used are: anti-mouse CD3ε (clone 500.A2), anti-FLAG (M2, Invitrogen, Carlsbad, CA), anti-mouse CD3ε (clone 2C11), anti-mouse CD28 (clone 37.51), anti-mouse IL-12p40 (clone 11B11) and anti-mouse IL-4 (clone C17.8, Biolegend, San Diego, CA), anti-mouse Tim-3 (Clone 8B.2C12, eBioscience, San Diego, CA), recombinant protease-resistant human galectin-9 (Dr. Mitsuomi Hirashima, Kagawa University, Japan), biotin-human galectin-9 (Dr. Linda Baum, UCLA, LA), anti-mouse IFN-γ (clone XMG1.2) and recombinant mouse IL-12p70, GolgiPlug (BD Pharmingen, San Diego, CA) and anti-mouse Tim3 polyclonal Ab and recombinant mouse IL-4 (R&D Systems, MN). T-bet (4B10) and GATA-3 (HG3-31) antibodies were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA).
Cell lines
AD10 Th1 clone were restimulated with mitomycin-C (50 μg/mL, Sigma Aldrich, St. Louis, MO) treated B10.A splenocytes and PCC (30 μg/mL, Sigma Aldrich) every 21 days. AD10 cells were maintained continually in the presence of recombinant human IL-2 (50 IU/mL). A fast-growing variant of the D10 Th2 clone was obtained from M. Krummel (University of California, San Francisco, CA) and maintained as described (Kane et al. 2004).
Cell surface staining
AD10 Th1, D10 Th2, primary Th1 and Th2 cells were washed once with staining buffer (1% fetal bovine serum (FBS), 0.01% sodium azide in PBS) before incubation with either isotype controls, anti-Tim-3 or biotin-human galectin-9 for 30 min on ice. Cells were then washed once with FACS buffer and then incubated for another 10 min with fluorescently conjugated secondary antibodies. Cells were then washed three times, before being resuspended in FACS buffer and analyzed on the BD LSR II Flow Cytometer. Entire procedure was performed at 4°C.
Measurement of cytokine secretion
Intracellular cytokine staining
AD10 Th1, D10 Th2 cells, primary Th1 and Th2 cells were treated with various concentrations of galectin-9 (Nishi et al. 2005) in cell culture media containing 50 μM 2-mercaptoethanol unless noted otherwise. GolgiPlug was added 1 h post-treatment. At the end of the stimulation period, cells were divided into stain for intracellular cytokines (Szabo et al. 2000) or cell viability.
Enzyme-linked immunosorbent assay (ELISA)
AD10 Th1 cells and primary Th1 cells were stimulated in 96-well plates (0.2 × 106 cells/well) for 20 h. Supernatants were then collected and frozen at −80°C. Before analysis, supernatants were spun down at 1800 rpm to pellet cell debris. When necessary, supernatants were diluted with culture media before being analyzed with a mouse IFN-γ ELISA kit (BD Bioscience, San Jose, CA).
Measurement of apoptosis
To measure internucleosomal DNA fragmentation, the manufacturer's protocol for Cell Death Detection ELISAPLUS was followed (Roche Diagnostics, Indianapolis, IN). The manufacturer's protocol was followed for 7-AAD/annexin V staining (BD Pharmingen).
Th1 and Th2 polarization
Spleens harvested from C57Bl/6 mice or Tim-3 KO mice and wild-type littermates (Sanchez-Fueyo et al. 2003) were mechanically disrupted to liberate resident cells. CD4+ T cells were then isolated from splenocytes using magnetic bead isolation (CD4+ T Cell Isolation Kit II, Miltenyi Biotec, Auburn, CA). CD4+ T cells were then stimulated in 24-well plates coated with anti-CD3 (1 μg/mL) and anti-CD28 (5 μg/mL) antibodies, under either Th1 polarizing conditions, anti-mouse IL-4 (10 μg/mL) and recombinant IL-12 p70 (5 ng/mL), or Th2 polarizing conditions, anti-mouse IL-12p40 (10 μg/mL) and recombinant IL-4 (10 ng/mL), for 3 days. On day 4, CD4+ T cells were removed 24-well plates and expanded until day 10 in culture media containing recombinant human IL-2 (50 IU/mL). For secondary and tertiary stimulations, the same process was adopted except that anti-CD3 and anti-CD28 were plated at 2 μg/mL. Functional assays were performed either 7 days after first round of polarization or 11 days after third-round polarization.
Western blotting
CD4+ T cells (10 × 106 cells) were subjected to nuclear fractionation after one round of polarization under Th1 or Th2 conditions using the NE-PER kit (Thermo Scientific, Rockford, IL). Nuclear lysates were divided, run on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS–PAGE) gel, and western blotted for either T-bet or GATA-3, after transfer to polyvinylidene fluoride (PVDF) membrane. Equal loading was confirmed by blotting for lamin B.
Funding
This work was supported by grants from the NIH (AI067544 and AI073748) to L.P.K.
Conflict of interest
None declared.
Abbreviations
IFN, Interferon; IL, interleukin; Tim-3, transmembrane immunoglobulin and mucin protein 3; PBS, phosphate-buffered saline; TNF, tumor necrosis factor; Tregs, regulatory T cell; galectin-9, β-galactoside-binding lectin 9.
Acknowledgements
We thank Dr. Linda Baum (UCLA, CA, USA) for providing reagents, critically reviewing this manuscript and for helpful advice. We also thank Stephanie Poe and Dr. Anuradha Ray for providing reagents.
References
- Anderson AC, Lord GM, Dardalhon V., Lee D.H., Sabatos-Peyton C.A., Glimcher L.H., Kuchroo V.K. T-bet, a Th1 transcription factor regulates the expression of Tim-3. Eur J Immunol. 2010;40:859–866. doi: 10.1002/eji.200939842. doi:10.1002/eji.200939842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao E, Zang X, Ramagopal U.A., Mukhopadhaya A., Fedorov A., Fedorov E., Zencheck W.D., Lary J.W., Cole J.L., Deng H., et al. T cell immunoglobulin mucin-3 crystal structure reveals a galectin-9-independent ligand-binding surface. Immunity. 2007;26:311–321. doi: 10.1016/j.immuni.2007.01.016. doi:10.1016/j.immuni.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Chabot S, Kashio Y, Seki M., Shirato Y., Nakamura K., Nishi N., Nakamura T., Matsumoto R., Hirashima M. Regulation of galectin-9 expression and release in Jurkat T cell line cells. Glycobiology. 2002;12:111–118. doi: 10.1093/glycob/12.2.111. doi:10.1093/glycob/12.2.111. [DOI] [PubMed] [Google Scholar]
- Dardalhon V, Anderson AC, Karman J., Apetoh L., Chandwaskar R., Lee D.H., Cornejo M., Nishi N., Yamauchi A., Quintana F.J., et al. Tim-3/galectin-9 pathway: Regulation of Th1 immunity through promotion of CD11b+Ly-6G+ myeloid cells. J Immunol. 2010;185:1383–1392. doi: 10.4049/jimmunol.0903275. doi:10.4049/jimmunol.0903275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl LA, Bi S, Baum LG. N- and O-glycans modulate galectin-1 binding, CD45 signaling, and T cell death. J Biol Chem. 2010;285:2232–2244. doi: 10.1074/jbc.M109.066191. doi:10.1074/jbc.M109.066191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden-Mason L, Palmer BE, Kassam N., Townshend-Bulson L., Livingston S., McMahon B.J., Castelblanco N., Kuchroo V., Gretch D.R., Rosen H.R. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. doi:10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings WD, Anderson DE, Kassam N., Koguchi K., Greenfield E.A., Kent S.C., Zheng X.X., Strom T.B., Hafler D.A., Kuchroo V.K. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol. 2009;39:2492–2501. doi: 10.1002/eji.200939274. doi:10.1002/eji.200939274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima M, Kashio Y, Nishi N., Yamauchi A., Imaizumi T.A., Kageshita T., Saita N., Nakamura T. Galectin-9 in physiological and pathological conditions. Glycoconj J. 2004;19:593–600. doi: 10.1023/B:GLYC.0000014090.63206.2f. doi:10.1023/B:GLYC.0000014090.63206.2f. [DOI] [PubMed] [Google Scholar]
- Jones RB, Ndhlovu LC, Barbour J.D., Sheth P.M., Jha A.R., Long B.R., Wong J.C., Satkunarajah M., Schweneker M., Chapman J.M., et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. doi:10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane LP, Mollenauer MN, Weiss A. A proline-rich motif in the C terminus of Akt contributes to its localization in the immunological synapse. J Immunol. 2004;172:5441–5449. doi: 10.4049/jimmunol.172.9.5441. [DOI] [PubMed] [Google Scholar]
- Katoh S, Ishii N, Nobumoto A., Takeshita K., Dai S.Y., Shinonaga R., Niki T., Nishi N., Tominaga A., Yamauchi A. Galectin-9 inhibits CD44-hyaluronan interaction and suppresses a murine model of allergic asthma. Am J Respir Crit Care Med. 2007;176:27–35. doi: 10.1164/rccm.200608-1243OC. doi:10.1164/rccm.200608-1243OC. [DOI] [PubMed] [Google Scholar]
- Leffler H, Barondes SH. Specificity of binding of three soluble rat lung lectins to substituted and unsubstituted mammalian beta-galactosides. J Biol Chem. 1986;261:10119–10126. [PubMed] [Google Scholar]
- Liu FT, Rabinovich GA. Galectins: Regulators of acute and chronic inflammation. Ann N Y Acad Sci. 2010;1183:158–182. doi: 10.1111/j.1749-6632.2009.05131.x. doi:10.1111/j.1749-6632.2009.05131.x. [DOI] [PubMed] [Google Scholar]
- Nagahara K, Arikawa T, Oomizu S., Kontani K., Nobumoto A., Tateno H., Watanabe K., Niki T., Katoh S., Miyake M., et al. Galectin-9 increases Tim-3+ dendritic cells and CD8+ T cells and enhances antitumor immunity via galectin-9-Tim-3 interactions. J Immunol. 2008;181:7660–7669. doi: 10.4049/jimmunol.181.11.7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi N, Itoh A, Fujiyama A., Yoshida N., Araya S., Hirashima M., Shoji H., Nakamura T. Development of highly stable galectins: Truncation of the linker peptide confers protease-resistance on tandem-repeat type galectins. FEBS Lett. 2005;579:2058–2064. doi: 10.1016/j.febslet.2005.02.054. doi:10.1016/j.febslet.2005.02.054. [DOI] [PubMed] [Google Scholar]
- Pace KE, Hahn HP, Pang M., Nguyen J.T., Baum L.G. Cutting edge: CD7 delivers a pro-apoptotic signal during galectin-1-induced T cell death. J Immunol. 2000;165:2331–2334. doi: 10.4049/jimmunol.165.5.2331. [DOI] [PubMed] [Google Scholar]
- Pace KE, Lee C, Stewart P.L., Baum L.G. Restricted receptor segregation into membrane microdomains occurs on human T cells during apoptosis induced by galectin-1. J Immunol. 1999;163:3801–3811. [PubMed] [Google Scholar]
- Sabatos CA, Chakravarti S, Cha E., Schubart A., Sanchez-Fueyo A., Zheng X.X., Coyle A.J., Strom T.B., Freeman G.J., Kuchroo V.K. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4:1102–1110. doi: 10.1038/ni988. doi:10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- Sanchez-Fueyo A, Tian J, Picarella D., Domenig C., Zheng X.X., Sabatos C.A., Manlongat N., Bender O., Kamradt T., Kuchroo V.K., et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. doi:10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- Sehrawat S, Suryawanshi A, Hirashima M., Rouse B.T. Role of Tim-3/galectin-9 inhibitory interaction in viral-induced immunopathology: Shifting the balance toward regulators. J Immunol. 2009;182:3191–3201. doi: 10.4049/jimmunol.0803673. doi:10.4049/jimmunol.0803673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Oomizu S, Sakata K.M., Sakata A., Arikawa T., Watanabe K., Ito K., Takeshita K., Niki T., Saita N., et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008;127:78–88. doi: 10.1016/j.clim.2008.01.006. doi:10.1016/j.clim.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Stillman BN, Hsu DK, Pang M., Brewer C.F., Johnson P., Liu F.T., Baum L.G. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol. 2006;176:778–789. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- Stowell SR, Karmakar S, Arthur C.M., Ju T., Rodrigues L.C., Riul T.B., Dias-Baruffi M., Miner J., McEver R.P., Cummings R.D. Galectin-1 induces reversible phosphatidylserine exposure at the plasma membrane. Mol Biol Cell. 2009;20:1408–1418. doi: 10.1091/mbc.E08-07-0786. doi:10.1091/mbc.E08-07-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa G.L., Zhang X., Fathman C.G., Glimcher L.H. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. doi:10.1016/S0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Toscano MA, Bianco GA, Ilarregui J.M., Croci D.O., Correale J., Hernandez J.D., Zwirner N.W., Poirier F., Riley E.M., Baum L.G., et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–834. doi: 10.1038/ni1482. doi:10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- Wada J, Ota K, Kumar A., Wallner E.I., Kanwar Y.S. Developmental regulation, expression, and apoptotic potential of galectin-9, a beta-galactoside binding lectin. J Clin Invest. 1997;99:2452–2461. doi: 10.1172/JCI119429. doi:10.1172/JCI119429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Anderson DE, Kuchroo J., Hafler D.A. Lack of TIM-3 immunoregulation in multiple sclerosis. J Immunol. 2008;180:4409–4414. doi: 10.4049/jimmunol.180.7.4409. [DOI] [PubMed] [Google Scholar]
- Zhu C, Anderson AC, Schubart A., Xiong H., Imitola J., Khoury S.J., Zheng X.X., Strom T.B., Kuchroo V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. doi:10.1038/ni1271. [DOI] [PubMed] [Google Scholar]