Abstract
Methylphenidate (MPD) is the most prescribed drug for attention deficit hyperactivity disorder. Licit and illicit use also occurs during pregnancy, however the effects from this use on offspring development is unknown. To model late gestational exposure, Sprague-Dawley litters were treated with 0, 5, 10, 20, or 30 mg/kg × 4/day every 2 h with MPD on postnatal days 11–20 (within-litter design; days chosen to be comparable to human third trimester brain development). During treatment, body weights were decreased in MPD-treated groups; weight recovery occurred in all but the MPD-30 group by start of testing. MPD-treated rats showed no changes in anxiety (elevated zero maze), swimming ability (straight channel swimming), or spatial learning/reference memory (Morris water maze). MPD does not appear to pose a risk to these CNS functions after exposure during a stage of rat development analogous to third trimester human brain development.
Keywords: Methylphenidate, Morris water maze, spatial learning, reference memory, developmental exposure, elevated zero maze, anxiety, rats
Introduction
Methylphenidate (MPD) is the most frequently prescribed treatment for attention deficit hyperactivity disorder (ADHD) in adults and children older than 6 years of age [17]. ADHD prevalence in the U.S. is 6–7% and MPD is prescribed in ~90% of cases [20, 21]. MPD is structurally related to amphetamine and causes similar acute effects including increased attention and energy, appetite suppression, and at higher doses, euphoria [16, 17]. Moreover, in recent years illicit MPD use has risen to be one of the top ten most frequently stolen prescriptions [17, 20].
Illicit use has also been reported among pregnant women, especially where other amphetamines are less available [15]. For adults with ADHD, treatment is usually continued during pregnancy [18]. Infants exhibit withdrawal symptoms after prenatal MPD exposure [15, 23]. While withdrawal symptoms are manageable [15], long-term effects remain largely unknown.
The mechanism of action for MPD is inhibition of dopamine (DA) reuptake by binding to the DA transporter (DAT) [17], a mechanism shared with cocaine, amphetamine, and methamphetamine. Given that the latter drugs have long-term effects on learning and memory [1, 19, 38], further investigation of MPD after developmental exposure is warranted.
Cross-species comparisons show that second and third trimester human brain development is analogous to rat development during the preweaning period [4, 12, 13]. We have shown that exposure of rats to methamphetamine on postnatal (P) days 11–20 results in impaired spatial learning and reference memory in the Morris water maze (MWM) when tested as adults [39]. These deficits appear as early as P30 [38, 40] and persist to at least P360 [40]. The purpose of this experiment was to determine whether MPD given on P11–20 in the same pattern as used previously with methamphetamine results in changes in anxiety and/or spatial learning and reference memory in rats [34]. Based on related mechanisms of action and previous results with other dopaminergic drugs (methamphetamine and 3,4-methylenedioxymethamphetamine), we hypothesized that spatial learning would be impaired in MPD-treated offspring.
Material and Methods
Subjects
Nulliparous female and male Sprague-Dawley CD IGS (Charles Rivers, Raleigh, NC) rats were bred in-house. Birth was designated P0. On P1, litters were culled to ten pups, five of each sex. Pups were separated from dams on P28 and offspring housed in same sex groups until P42 then rehoused two/cage of the same sex (cage: 46×24×20 cm). Animals were housed in a vivarium on a 14/10 h light/dark cycle (lights on at 600 h) at 21 ± 1°C with 50 ± 10% humidity. Food (Purina 5001) and water were available ad libitum. The vivarium was accredited by the Association for the Accreditation and Assessment of Laboratory Animal Care and the research protocol approved by the Institutional Animal Care and Use Committee.
Treatment
Within each litter, one male/female pair was assigned to one of five treatment groups as follows: 0 (saline: SAL), 5, 10, 20, or 30 mg/kg body weight of (±)-methylphenidate HCL expressed as the freebase (Mallinckrodt, St. Louis, MO) and designated SAL, MPD-5, MPD-10, MPD-20, and MPD-30, respectively. MPD was dissolved in isotonic saline and injected subcutaneously four times daily at 2 h intervals from P11–20. The dosing volume was 3 ml/kg and was delivered in the dorsum. Sixteen litters, for a total of 160 offspring (80/sex), were treated.
Doses were chosen to model late gestational exposure in humans, i.e., to an adult women during pregnancy. Using pharmacologically-based interspecies scaling methods and the formula Dosehuman = Doserat × (Weighthuman/Weightrat)0.7, a dose of 10 mg/kg (MPD-10) in a 25 g rat (~P11) approximates a 58 mg absolute dose in an adult human [26, 33]. Accordingly, our MPD-5 group would be equivalent to a human non-weight adjusted individual dose of 29 mg, MPD-10 to 58 mg, MPD-20 group to 116 mg, and the MPD-30 group to 174 mg. Using the 60 kg body mass to represent an average adult women during pregnancy, these doses on a mg/kg basis would translate as follows: rat 5 mg/kg ≈ human ~0.5 mg/kg, rat 10 mg/kg ≈ human ~1 mg/kg, rat 20 mg/kg ≈ human ~2 mg/kg, and rat 30 mg/kg ≈ human ~3 mg/kg. Although human dosing regimens vary, the typical therapeutic range in a recent meta-analysis was 41.2–82 mg/day [11]; MPD abusers take much higher doses. While our doses are given 4 times/day, rats also metabolize/clear the drug more rapidly than humans making the present doses reasonable approximations of those used by a pregnant human adult.
Elevated Zero-Maze
The elevated zero-maze (EZM) is a circular runway 105 cm in diameter with a 10 cm path width made of black Kydex and divided into four equal quadrants. Two opposite quadrants have black acrylic sidewalls (28 cm high) and two are ‘open’ except for 1.3 cm clear acrylic curbs. The runway is elevated 72 cm above the floor [7]. Rats (~P50) were placed in one of the closed quadrants and behavior recorded for 5 min with an overhead camera connected to a video recorder. The maze was illuminated by a single lamp (~12 lux) and between animals the maze was cleaned with 70% ethanol. Dependent measures were: stretch-attends, head dips, and time-in-open areas.
Straight Channel
The day following EZM, animals were tested in a 15 × 244 cm straight swimming channel with an escape ladder at one end. This test serves three functions: (a) acclimation to swimming, (b) assess swimming speed/motivation to escape and ensure the absence of motor impairment, and (c) teach that escape is possible. Rats received four trials with a limit of 2 min/trial. Animals were placed in the channel facing one end and timed until they reached the exit.
Morris Water Maze (MWM)
This is test of allocentric/spatial learning and reference memory [6, 14, 27, 28]. The apparatus is 210 cm diameter made of stainless steel painted flat black. The tank was surrounded by curtains that could be closed or opened to conceal or reveal room cues. In addition, the three walls nearest the maze (designated arbitrarily as N, E, and W) had geometric figures mounted on the walls. A camera was mounted above the maze and attached to a computer and performance tracked (Polytrack software, San Diego Instruments, San Diego, CA) [32]. Testing was conducted in three phases: acquisition, reversal, and cued. The first two phases consisted of 4 trials/day for 5 days with the curtains open and the platform submerged; this was followed by an additional day when a single probe trial was given with the platform removed. The time limit was 2 min/trial with an intertrial interval (ITI) of 15 s. If the animal did not find the platform it was placed on it. The platform (5 × 5 cm) was 1–2 cm below the surface and was camouflaged by being transparent against a black background. Water temperature was 21±1°C.
The acquisition phase began three days after straight channel testing. During acquisition, the platform was located either in the SW for half the animals or NE quadrant for the other half and positioned midway between the center and the side. Rats were started at one of four positions located distal to the quadrant containing the platform in a quasi-random order with the restraint that they received one trial from each starting position/day [41]. Start positions for the SW goal were: NW, N, E, and SE (S and W were not used). Start positions for the NE goal were S, W, NW, and SE. The day after the last acquisition trial, each rat was given a 30 s probe trial with the platform removed. Rats were started from a position they had never been started from before (NE or SW). Latency, path length, and cumulative distance to the platform were recorded. For probe trials, first bearing, average distance, site crossovers, and percent distance and time in the target quadrant were analyzed.
During reversal, rats had to find the platform in the opposite quadrant for another 5 days, 4 trials/day. Following reversal, a second probe trial was given. 24 h later cued MWM testing commenced. Cued trials tested the animals’ ability to see and use proximal cues. The platform remained submerged but its location was marked with a plastic ball attached to a rod that protruded above the surface of the water by 10 cm. The curtains were closed to minimize extramaze cues and animals were tested for one trial per day for five days with the start and platform positions randomly positioned each day.
Statistical Procedures
Data were analyzed using mixed linear analyses of variance (ANOVAs) (SAS Proc Mixed, SAS Institute, v.9.2, Cary, NC). Litter was used as a random (block) factor in a completely randomized block ANOVA. Treatment group and sex were factors within blocks. Covariance matrices were checked using fit statistics; in most cases the autoregressive-1 structure showed best fit. Kenward-Roger adjusted degrees of freedom were used. Measures taken repetitively on the same animal were repeated measure factors in the model. Significant interactions were analyzed using slice-effect ANOVAs. Data are presented as least square (LS) means ± LS SEM.
Results
Body Weight
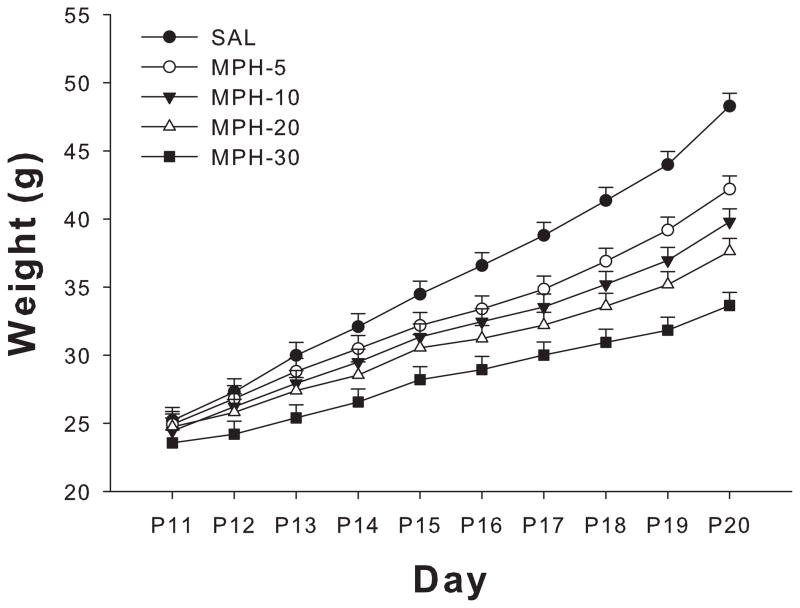
During MPD administration, there was an effect of treatment (F(4,134) = 72.23, p<0.001) and a treatment × day interaction (F(36,1327) = 10.37, p<0.0001) (Figure 1). The interaction showed that MPD-treated pups were significantly lighter than SAL controls. This weight difference continued from P14 and throughout the treatment period to P20.
Figure 1.
Offspring body weight during MPD treatment. All MPD treated groups differed significantly from SAL after P14, with pups exhibiting lower body weights and decreased weight gain. This persisted throughout dosing (see text for significance) but recovered in all MPD group after the end of dosing except in the MPD-30 group.
Weekly body weights were obtained after treatment and a significant treatment main effect was observed (F(4,134) = 7.88, p<0.0001). All MPD-treated groups showed body weight reductions that progressively recovered. The MPD-5, 10, and 20 groups recovered completely by P49; however the MPD-30 group weighed less than SAL controls (Table 1). There was a significant main effect of sex; females weighed less than males during treatment (F(4,134) = 3.29, p<0.002). After treatment on P77, there was also a main effect of treatment, F(1,134) = 2024.45, p<0.0001). As can be seen in Table 1, only the MPD-30 group differed significantly from the SAL group at P77.
Table 1.
Offspring Weight After Weaning. All MPD treated pups had recovered to SAL weights by start of behavior with the exception of the MPD-30 group.
| MPD (mg/kg) | P28 | P35 | P42 | P49 | P56 | P63 | P70 | P77 |
|---|---|---|---|---|---|---|---|---|
| SAL | 90.14 | 140.63 | 192.03 | 238.91 | 275.97 | 305.28 | 317.69 | 341.16 |
| MPD-5 | 85.90 | 138.17 | 192.47 | 237.06 | 271.50 | 305.25 | 334.13 | 338.13 |
| MPD-10 | 82.60 | 133.56 | 184.88 | 231.22 | 268.94 | 295.81 | 317.88 | 338.75 |
| MPD-20 | 81.02 | 132.43 | 183.56 | 228.03 | 271.06 | 298.98 | 318.88 | 340.72 |
| MPD-30 | 74.47 | 122.18 | 175.74 | 218.04 | 260.56 | 284.06 | 308.88 | 327.67 |
Elevated Zero-Maze
MPD treatment had no effect on time-in-open (s): SAL: 30.83 ± 9.63, MPD-5: 33.21 ± 9.62, MPD-10: 35.47 ± 9.63, MPD-20: 25.18 ± 9.63, MPD-30: 31.71 ± 9.63), stretch-attends, head dips, or zone entries compared with SAL animals. During the EZM test, females exhibited an increase in stretch-attends (F(1,147) = 5.23, p<0.03), head dips (F(1,147) = 22.12, p<0.0001), and zone entries (F(1,147) = 36.13, p<0.0001), as well as time-in-open (F(1,147) = 36.45, p<0.0001) compared with males. MPD did not interact with sex.
Straight Channel
All groups showed reduced latency to the goal over the course of the four trials (main effect of trial: F (3, 359) = 163.78, p<0.0001). No significant main effect of treatment was found for straight channel swimming (SAL: 30.8 ± 9.6 s, MPD-5: 33.2 ± 9.6 s, MPD-10: 35.5 ± 9.6 s, MPD-20: 25.2 ± 9.6 s, MPD-30: 31.7 ± 9.6 s). There were no sex differences and no interactions between MPD and sex or trial.
Morris Water Maze
Acquisition
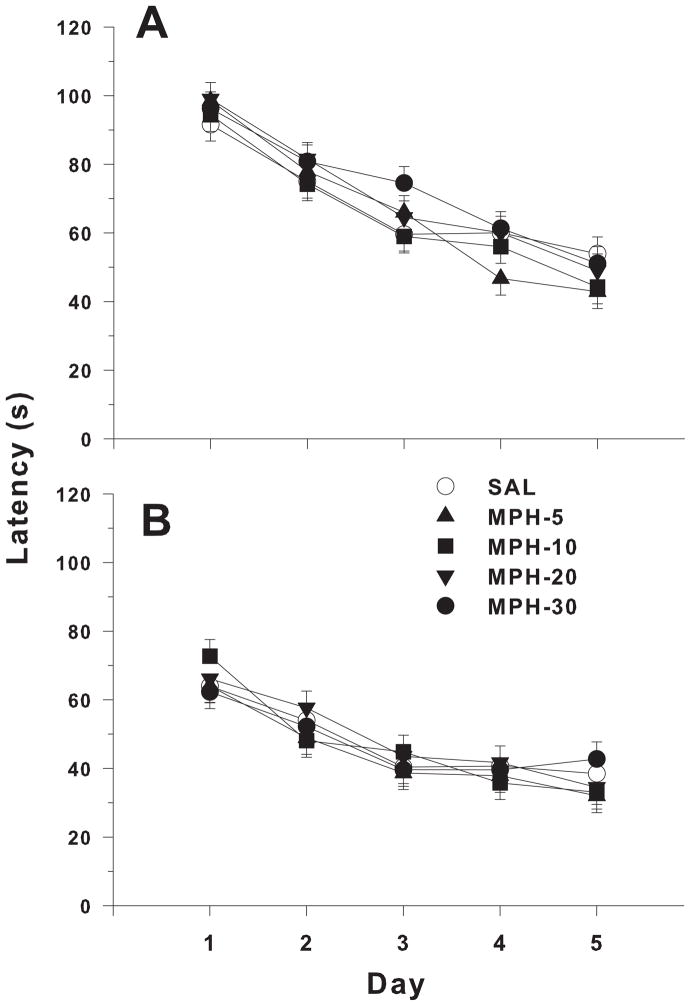
All groups showed learning as evidenced by decreased latencies over days (main effect of day F (4, 516) = 98.01, p<0.0001). However, no significant main effect or treatment-related interaction with MPD was observed (Figure 2A). A sex effect was found; males performed better than females (latency, F(1,118) = 32.66, P<0.0001). Platform placement affected performance during acquisition; animals learning the SW platform position found the goal more quickly than those learning the NE platform position (F(1,13.1) = 6.74, p<0.03) but goal location did not interact with treatment.
Figure 2.
Morris water maze latency. A) Acquisition Phase. B) Reversal Phase. Both panels highlight the ability of both MPD-treated and SAL control to master the MWM. There was no significant effect of treatment between groups.
Acquisition Probe
For probe trials, first bearing, average distance, crossovers, percent distance in target quadrant, and time in target quadrant were analyzed. Average distance was representative. No treatment effect or treatment-related interaction was found (SAL: 81.7 ± 3.2, MPD-5: 78.1 ± 3.2, MPD-10: 84.22 ± 3.2, MPD-20: 77.1 ± 3.2, MPD-30: 82.0 ± 3.2 cm). Sex effects were found for average distance (F(1,129) = 21.27, p<0.0001), crossovers (F(1,129) = 7.83, p<0.01), percent distance in target quadrant (F(1,129) = 8.38, p<0.005), and time in target quadrant (F(4,129) = 8.69, p<.005); males performed better in each index compared with females.
Reversal
Latencies for the reversal phase showed no significant effect of treatment or treatment-related interaction. All groups improved across days (day main effect F(4, 372) = 56.58, p < .0001) (Figure 2B). The sex effect seen during acquisition continued during reversal (F(1,116) = 30.29, p<0.0001), with males performing better than females; however no effect of platform position was observed.
Reversal Probe
The results were similar to acquisition probe: there were no treatment main effects or treatment-related interactions. The means for average distance from the platform were: SAL: 96.7 ± 10.3, MPD-5: 95.2 ± 10.3, MPD-10: 99.6 ± 10.3, MPD-20: 101.9 ± 10.3, MPD-30: 104.7 ± 10.3 cm). There was a sex effect on average distance (F(1,128) = 28.44, p<0.0001) and crossovers (F(1,128) = 10.38, p<0.0016). Males performed better than females.
Cued
No significant effect of treatment or treatment-related interaction was found during cued trial learning. The means for latency (s) are as follows: SAL: 21.4 ± 3.7, MPD-5: 24.0 ± 3.7, MPD-10: 28.8 ± 3.7, MPD-20: 26.9 ± 3.7, MPD-30: 27.1 ± 3.7. Sex was again significant (F(1,117) = 35.85, p<0.0001) with males performing better than females.
Discussion
The potential long-term effect of third trimester MPD exposure in humans has received little attention. In a P11–20 rat model (analogous to the end of second trimester for cortical regions and third trimester for limbic regions, especially the hippocampus [12, 13, 34]), rats were treated with MPD 4 times per day at 2 h intervals and the long-term effects on anxiety-related behavior and spatial learning and reference memory were evaluated in the offspring as adults. We found no long-term effects of higher doses of MPD on EZM performance, swimming ability, or spatial learning and reference memory in the MWM even though the doses given caused significant decreases in body weights during and after MPD treatment. For body weight, the data show the drug had dose-dependent effects that gradually diminished after the termination of treatment. Body weight differences completely disappeared in the three lower dose groups by the time behavioral testing commenced, but a residual deficit remained in the MPD-30 group. Despite these reductions in growth, there were no effects on the behavioral tests used herein.
To our knowledge, a model of late in utero MPD exposure (which is postnatal in rodents) has not been previously studied in rats. There are data in mice. Pregnant mice were given a 5 mg/kg/day dose over a three day period during one of three treatment intervals: E8–10, E12–14, or E16–18. Offspring showed decreased anxiety in the elevated plus maze, but no other significant behavioral changes in a test battery which included a 3 day water maze task (visible platform) and a 4th day hidden platform probe trial [25]. The decreased anxiety may be explained by their use of short-term doses during gestation, whereas our regimen used longer exposure during later stages of brain development in order to model late second to third trimester development.
Most studies examining MPD expose juvenile animals to model ADHD treatment in children. As a result, rats are generally dosed from P20 onward. These studies have mixed results based on exposure length, but in general there are no effects on hippocampally-dependent learning [5, 10], something we also found in this study. Behaviors in adult animals that are affected after juvenile MPD exposure include the forced swim test and place conditioning [3, 10]. In another study, a longer dosing period (P15–45) produced negative effects on MWM performance, including increased latencies in both spatial and working memory versions of the task [31]. It should be noted that in this study, behavioral testing began immediately after the end of MPD exposure. In addition, Bethancourt et al. noted acute effects of MPD on fear conditioning; effects which resolved 48 h later [5]. In juvenile rats, MPD effects are mostly seen shortly after the end of treatment. Our study includes a significantly longer off-drug period before testing.
Results in animal experiments must be extrapolated to humans and this is complicated by the fact that animal models of ADHD were not used in these studies. Clinical studies in ADHD-afflicted children show improvement in a spatial memory task, but no improvement when distracting interference is introduced[30]. Others have found effects of developmental cocaine exposure on non-spatial learning ability during fMRI in clinical subjects [22], and using novel object recognition and delayed non-matching-to-sample in rats [29].
Previously, we have has shown deficits in the MWM following methamphetamine and MDMA exposure during the same time period as used in the present experiment [34, 38, 40]. Both methamphetamine and MDMA affect reuptake of monoamines via inhibiting DAT and/or SERT and cause these transporters to reverse direction cause DA and 5-HT efflux rather than normal reuptake. Both drugs also inhibit VMAT2-regulated vesicular reuptake and MAO metabolism. In combination, these effects lead to increases of monoamines in the presynaptic terminal and extracellularly in the synaptic cleft [35]. The molecular mechanism of MPD differs in that it, similarly to cocaine, it inhibits DAT but does not reverse the direction of DAT flux, or inhibit VMAT2 or MAO [35]. Interestingly, cocaine, while addictive, has a similar lack of effects on spatial learning in the MWM when animals are exposed during the same or nearly the same developmental period as in the present study [19, 36, 37]. The MPD mechanism of action has been speculated to include increased catecholaminergic signaling in the prefrontal cortex in addition to increased DA and 5-HT at the synaptic cleft [9]. During juvenile periods of exposure, increases in CREB, an important transcription factor, can also be seen after MPD exposure [3]. Because of its integral role in synaptogenesis and maintenance [2, 24] regulating cellular processes and gene transcription[8], CREB may be part of a neuroadaptive developmental mechanism activated during developmental MPD exposure. This may partially explain a lack of detrimental effects on learning and memory, although more research into mechanisms and behavioral outcomes are clearly needed.
Finally, treatment in previous studies has been 2–5 mg/kg dose, which is considered clinically relevant to children on a mg/kg basis. Our study differs in that a broader dose-response range was included. Although the 5 mg/kg dose is included here, the response curve was increased to include higher doses which model the upper end of the clinical extending into the abuse range. The higher doses, putatively in the maternal MPD abuse range, could cause detrimental effects on the offspring but apparently not on spatial learning and reference memory. Results in this study indicate that MPD use during late pregnancy causes presumptive evidence of harm to the extent tested in the elevated zero and Morris water mazes.
Limitations to the present study include evaluating behavior using only one test of learning and memory. Also, only one exposure period was employed although it was specifically chosen to match previous experiments with other stimulants, i.e., the exposure was during granule cell development in the rat hippocampus and dentate gyrus and the MWM assesses predominantly hippocampally-dependent learning and memory and this test is sensitive to exposure at these ages to other psychostimulants. We used a range of doses; therefore, it is unlikely that effects were missed because of an inadequate dose range but effects on other forms of learning and memory or other behaviors cannot be ruled out.
Conclusion
Using P11–20 exposure in rats, MPD-treatment caused decreases in body weight compared with SAL-treated controls across a broad range of doses. This effect gradually dissipated after treatment ended. MPD had no effects on anxiety-regulated behavior in the EZM, swimming ability or motivation in a simple-to-learn straight swimming channel, or in spatial acquisition or reversal learning in the MWM, or reference memory during probe trials suggesting that MPD does not pose a risk to these functions after late gestational-trimester equivalent exposure in rats on P11–20.
Research Highlights.
Developmental exposure to methylphenidate in rats
Elevated zero-maze test of anxiety
Morris water maze test of spatial learning and reference memory
No effects of methylphenidate on elevated zero-maze or Morris maze were found
Acknowledgments
Supported by NIH R01 DA006733 (CVV) and T32 07051 (RMAK)
Abbreviations
- ADHD
Attention Deficit Hyperactivity Disorder
- MPD
Methylphenidate
- DA
Dopamine
- DAT
Dopamine Transporter
- MDMA
Methylenedioxymethamphetamine
- SERT
Serotonin Transporter
- VMAT2
Vesicular monoamine transporter 2
- 5-HT
Serotonin
- MAO
Monoamine oxidase
- SAL
Saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acuff-Smith KD, Schilling MA, Fisher JE, Vorhees CV. Stage-specific effects of prenatal d-methamphetamine exposure on behavioral and eye development in rats. Neurotoxicol Teratol. 1996;18:199–215. doi: 10.1016/0892-0362(95)02015-2. [DOI] [PubMed] [Google Scholar]
- 2.Aguado F, Diaz-Ruiz C, Parlato R, Martinez A, Carmona MA, Bleckmann S, Urena JM, Burgaya F, del Rio JA, Schutz G, Soriano E. The CREB/CREM transcription factors negatively regulate early synaptogenesis and spontaneous network activity. J Neurosci. 2009;29:328–333. doi: 10.1523/JNEUROSCI.5252-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA., Jr Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- 4.Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- 5.Bethancourt JA, Camarena ZZ, Britton GB. Exposure to oral methylphenidate from adolescence through young adulthood produces transient effects on hippocampal-sensitive memory in rats. Behav Brain Res. 2009;202:50–57. doi: 10.1016/j.bbr.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Brandeis R, Brandys Y, Yehuda S. The Use of the Morris Water Maze in the Study of Memory and Learning. International Journal of Neuroscience. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- 7.Braun AA, Skelton MR, Vorhees CV, Williams MT. Comparison of the elevated plus and elevated zero mazes in treated and untreated male Sprague-Dawley rats: Effects of anxiolytic and anxiogenic agents. Pharmacol Biochem Behav. 2010 doi: 10.1016/j.pbb.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Britton GB. Cognitive and emotional behavioural changes associated with methylphenidate treatment: a review of preclinical studies. Int J Neuropsychopharmacol. 2011:1–13. doi: 10.1017/S1461145711000472. [DOI] [PubMed] [Google Scholar]
- 9.Britton GB, Bethancourt JA. Characterization of anxiety-related responses in male rats following prolonged exposure to therapeutic doses of oral methylphenidate. Pharmacol Biochem Behav. 2009;93:451–459. doi: 10.1016/j.pbb.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Carlezon WA, Jr, Mague SD, Andersen SL. Enduring behavioral effects of early exposure to methylphenidate in rats. Biol Psychiatry. 2003;54:1330–1337. doi: 10.1016/j.biopsych.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Castells X, Ramos-Quiroga JA, Rigau D, Bosch R, Nogueira M, Vidal X, Casas M. Efficacy of methylphenidate for adults with attention-deficit hyperactivity disorder: a meta-regression analysis. CNS Drugs. 2011;25:157–169. doi: 10.2165/11539440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- 14.D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 15.Debooy VD, Seshia MM, Tenenbein M, Casiro OG. Intravenous pentazocine and methylphenidate abuse during pregnancy. Maternal lifestyle and infant outcome. Am J Dis Child. 1993;147:1062–1065. doi: 10.1001/archpedi.1993.02160340048012. [DOI] [PubMed] [Google Scholar]
- 16.Fang TY, Tan SE. The effects of methylphenidate and maturation on exploratory activity in rats. Chin J Physiol. 1998;41:53–58. [PubMed] [Google Scholar]
- 17.Golub M, Costa L, Crofton K, Frank D, Fried P, Gladen B, Henderson R, Liebelt E, Lusskin S, Marty S, Rowland A, Scialli J, Vore M. NTP-CERHR Expert Panel Report on the reproductive and developmental toxicity of methylphenidate. Birth Defects Res B Dev Reprod Toxicol. 2005;74:300–381. doi: 10.1002/bdrb.20049. [DOI] [PubMed] [Google Scholar]
- 18.Humphreys C, Garcia-Bournissen F, Ito S, Koren G. Exposure to attention deficit hyperactivity disorder medications during pregnancy. Can Fam Physician. 2007;53:1153–1155. [PMC free article] [PubMed] [Google Scholar]
- 19.Inman-Wood SL, Williams MT, Morford LL, Vorhees CV. Effects of prenatal cocaine on Morris and Barnes maze tests of spatial learning and memory in the offspring of C57BL/6J mice. Neurotoxicol Teratol. 2000;22:547–557. doi: 10.1016/s0892-0362(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 20.Kollins SH, MacDonald EK, Rush CR. Assessing the abuse potential of methylphenidate in nonhuman and human subjects: a review. Pharmacol Biochem Behav. 2001;68:611–627. doi: 10.1016/s0091-3057(01)00464-6. [DOI] [PubMed] [Google Scholar]
- 21.LeFever GB, Arcona AP. ADHD among American Schoolchildren: Evidence of Overdiagnosis and Overuse of Medication. The Scientific Review of Mental Health Practice. 2003;2 [Google Scholar]
- 22.Li Z, Coles CD, Lynch ME, Hamann S, Peltier S, LaConte S, Hu X. Prenatal cocaine exposure alters emotional arousal regulation and its effects on working memory. Neurotoxicol Teratol. 2009;31:342–348. doi: 10.1016/j.ntt.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundquest DE, Young WK, Edland JF. Maternal death associated with intravenous methylphenidate (Ritalin) and pentazocine (Talwin) abuse. J Forensic Sci. 1987;32:798–801. [PubMed] [Google Scholar]
- 24.Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, Martin Villalba A, Tronche F, Kellendonk C, Gau D, Kapfhammer J, Otto C, Schmid W, Schutz G. Disruption of CREB function in brain leads to neurodegeneration. Nat Genet. 2002;31:47–54. doi: 10.1038/ng882. [DOI] [PubMed] [Google Scholar]
- 25.McFadyen-Leussis MP, Lewis SP, Bond TL, Carrey N, Brown RE. Prenatal exposure to methylphenidate hydrochloride decreases anxiety and increases exploration in mice. Pharmacol Biochem Behav. 2004;77:491–500. doi: 10.1016/j.pbb.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Mordenti J, Chappell W. The use of inter-species scaling in toxicokinetics. In: Yacobi A, Kelly J, Batra V, editors. Toxicokinetics in New Drug Development. Pergamon Press; New York: 1989. pp. 42–96. [Google Scholar]
- 27.Morris R. Developments of a Water-Maze Procedure for Studying Spatial-Learning in the Rat. J Neurosci Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 28.Morris RGM. Spatial Localization Does Not Require the Presence of Local Cues. Learn Motiv. 1981;12:239–260. [Google Scholar]
- 29.Morrow BA, Elsworth JD, Roth RH. Prenatal cocaine exposure disrupts non-spatial, short-term memory in adolescent and adult male rats. Behav Brain Res. 2002;129:217–223. doi: 10.1016/s0166-4328(01)00338-2. [DOI] [PubMed] [Google Scholar]
- 30.Prehn-Kristensen A, Krauel K, Hinrichs H, Fischer J, Malecki U, Schuetze H, Wolff S, Jansen O, Duezel E, Baving L. Methylphenidate does not improve interference control during a working memory task in young patients with attention-deficit hyperactivity disorder. Brain Res. 2011;1388:56–68. doi: 10.1016/j.brainres.2011.02.075. [DOI] [PubMed] [Google Scholar]
- 31.Scherer EB, da Cunha MJ, Matte C, Schmitz F, Netto CA, Wyse AT. Methylphenidate affects memory, brain-derived neurotrophic factor immunocontent and brain acetylcholinesterase activity in the rat. Neurobiol Learn Mem. 2010;94:247–253. doi: 10.1016/j.nlm.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Skelton MR, Able JA, Grace CE, Herring NR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT. (+/−)-3,4-Methylenedioxymethamphetamine treatment in adult rats impairs path integration learning: a comparison of single vs once per week treatment for 5 weeks. Neuropharmacology. 2008;55:1121–1130. doi: 10.1016/j.neuropharm.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skelton MR, Williams MT, Vorhees CV. Developmental effects of 3,4-methylenedioxymethamphetamine: a review. Behav Pharmacol. 2008;19:91–111. doi: 10.1097/FBP.0b013e3282f62c76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skelton MR, Williams MT, Vorhees CV. Treatment with MDMA from P11–20 disrupts spatial learning and path integration learning in adolescent rats but only spatial learning in older rats. Psychopharmacology (Berl) 2006;189:307–318. doi: 10.1007/s00213-006-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Trksak GH, Glatt SJ, Mortazavi F, Jackson D. A meta-analysis of animal studies on disruption of spatial navigation by prenatal cocaine exposure. Neurotoxicol Teratol. 2007;29:570–577. doi: 10.1016/j.ntt.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vorhees CV, Inman-Wood SL, Morford LL, Reed TM, Moran MS, Pu C, Cappon GD. Evaluation of neonatal exposure to cocaine on learning, activity, startle, scent marking, immobility, and plasma cocaine concentrations. Neurotoxicol Teratol. 2000;22:255–265. doi: 10.1016/s0892-0362(99)00071-9. [DOI] [PubMed] [Google Scholar]
- 38.Vorhees CV, Reed TM, Morford LL, Fukumura M, Wood SL, Brown CA, Skelton MR, McCrea AE, Rock SL, Williams MT. Periadolescent rats (P41–50) exhibit increased susceptibility to D-methamphetamine-induced long-term spatial and sequential learning deficits compared to juvenile (P21–30 or P31–40) or adult rats (P51–60) Neurotoxicol Teratol. 2005;27:117–134. doi: 10.1016/j.ntt.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Vorhees CV, Skelton MR, Grace CE, Schaefer TL, Graham DL, Braun AA, Williams MT. Effects of (+)-methamphetamine on path integration and spatial learning, but not locomotor activity or acoustic startle, align with the stress hyporesponsive period in rats. Int J Dev Neurosci. 2009;27:289–298. doi: 10.1016/j.ijdevneu.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vorhees CV, Skelton MR, Williams MT. Age-dependent effects of neonatal methamphetamine exposure on spatial learning. Behav Pharmacol. 2007;18:549–562. doi: 10.1097/FBP.0b013e3282ee2abe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]