SUMMARY
The ability of fluorine in a C-F bond to act as a hydrogen bond acceptor is controversial. To test such ability in complex RNA macromolecules, we have replaced native 2′-OH groups with 2′-F and 2′-H groups in two related systems, the Tetrahymena group I ribozyme and the ΔC209 P4-P6 RNA domain. In three cases the introduced 2′-F mimics the native 2′-OH group, suggesting that the fluorine atom can accept a hydrogen bond. In each of these cases the native hydroxyl group interacts with a purine exocyclic amine. Our results give new insight about the properties of organofluorine and suggest a possible general biochemical signature for tertiary interactions between 2′-hydroxyl groups and exocyclic amino groups within RNA.
INTRODUCTION
The use of chemical modifications has become a powerful tool to explore structure-function relationships in biologically important macromolecules (Chatterjee and Muir, 2010; Das et al., 2005; Hahn and Muir, 2005). Hydroxyl groups are ubiquitous in Nature and can act as hydrogen bond donors, hydrogen bond acceptors, or both. Replacement of a hydroxyl group attached to a carbon with a fluorine atom removes the hydrogen-bond donor capability in the newly introduced group and, because of the high electronegativity of fluorine relative to carbon (Pauling, 1932), fluorine would seem suited to act as a hydrogen-bond acceptor. However, the ability of fluorine in a C-F bond to act as a hydrogen bond acceptor is controversial.
Results from several computational studies and crystallographic surveys (Carosati et al., 2004; Dunitz, 2004; Dunitz and Taylor, 1997; Howard et al., 1996; Mehta and Sen, 2010; Muller et al., 2007; Murray-Rust et al., 1983; O’Hagan and Rzepa, 1997; Offen et al., 2009) suggest that hydrogen bonds to fluorine atoms are possible. However, it is often proposed that these hydrogen bonds are not as strong as hydrogen bonds to hydroxyls and that constraints within the system prevent the hydrogen bond donor from gaining access to better hydrogen bond acceptors than fluorine (Dunitz, 2004; Dunitz and Taylor, 1997). Much of the functional data pertains to fluorinated sugars in the context of sugar-modifying enzymes, but complexities associated with these systems frequently confound the analysis, including uncertainty about the influcence of stereoelectronic efficts on binding energies and about the rate-limiting step in the reaction (Martin et al., 1990; McCarter et al., 1992; Namchuk and Withers, 1995; Percival and Withers, 1992; Persson et al., 2001; Street et al., 1986; Street et al., 1989). To our knowledge, rigorous integration of structural and functional data has been possible for only one case, xylanase, which allowed Withers and colleagues to conclude that organofluorine can accept a hydrogen bond (Wicki et al., 2007).
Here, we have extended this rigorous analysis to the behavior of organofluorine in RNA systems. Using two well-studied RNA systems, the Tetrahymena group I ribozyme and the ΔC209 P4-P6 RNA domain derived from this ribozyme, we have determined the effects arising from replacement of a native 2′-OH group by a 2′-F versus a 2′-H atom.
RESULTS AND DISCUSSION
A 2′-F atom acts as hydrogen-bond acceptor in the Tetrahymena group I ribozyme
The Tetrahymena group I ribozyme catalyzes the site-specific attack of an exogenous guanosine molecule (G) on the phosphoryl group of an oligonucleotide substrate (S) [Equation 1] (Hougland et al., 2006).
| (1) |
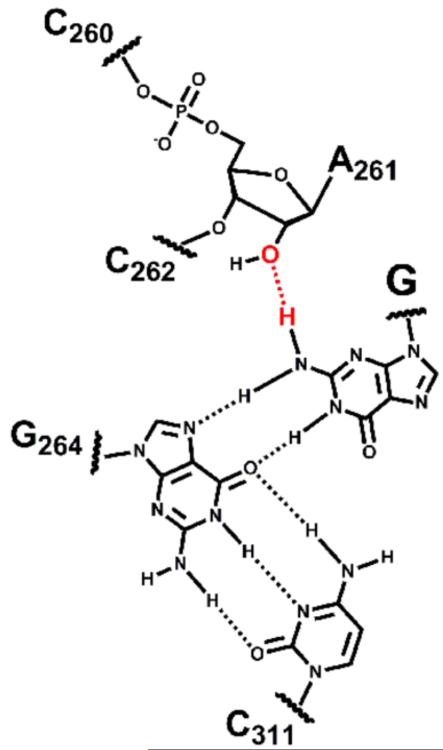
The ribozyme exists in at least two different conformations along the reaction coordinate, referred to as the ‘open complex’ and the ‘closed complex’ (Bevilacqua and Turner, 1991; Herschlag, 1992). Functional (Forconi et al., 2009; Forconi et al., 2010) and structural (Lipchock and Strobel, 2008) data strongly suggest that the exocyclic amino group of the guanosine nucleophile donates a hydrogen bond to the 2′-OH group of residue A261 in the closed complex (Figure 1) but that this hydrogen bond is not formed in the open complex.
Figure 1.
Schematic representation of the interactions made by the base of the guanosine nucleophile in the closed complex of the Tetrahymena group I ribozyme. The hydrogen bond between G and residue A261 is shown in red.
If fluorine cannot replace oxygen as a hydrogen-bond acceptor partner for the exocyclic amino group of G, then G would be expected to bind to the closed complex of a ribozyme containing a 2′-F atom at position 261 with reduced affinity relative to the wild-type ribozyme. Conversely, if fluorine flawlessly replaces oxygen as a hydrogen-bond acceptor, then the affinity of G for the closed complex would be unaffected by the introduction of a 2′-F atom at position 261.
To distinguish these possibilities, we used a semi-synthetic protocol (Moore and Sharp, 1992) to generate a ribozyme containing a single 2′-F or 2′-H substitution at position A261 (herein referred to the A261F and the A261H ribozymes, respectively), and we measured their reactivity using pre-steady state kinetics. To ensure that the introduction of a fluorine atom does not unexpectedly alter the properties of the ribozyme, we measured the affinity of a guanosine analog, AUCG, for the open complexes of the wild-type (A261OH) and the modified ribozymes; as noted above the hydrogen bond between the exocyclic amino group of G and the 2′-OH of A261 is not formed in the open complex (Forconi et al., 2010). AUCG was used instead of G because it binds tighter without altering the reaction mechanism (Moran et al., 1993; Russell and Herschlag, 1999), thereby allowing precise determination of the nucleophile affinity for the open complex without incurring problems related to the poor solubility of G. The affinity of AUCG for the open complexes of the three ribozymes is the same within two-fold (Table 1), suggesting that there is no general effect on the guanosine-binding site from these single-atom substitutions.
Table 1.
Binding and reactivity of AUCG, measured at pH 6.9 and 50 mM Mg2+. Values in parentheses represent values relative to the A261OH ribozyme. See also Figure S1 and Table S1.
| ribozyme | kc (min−1) | |||
|---|---|---|---|---|
| Open | Closed | |||
|
| ||||
| A261OH |
6.5 ± 0.9 (1.0) |
0.58 ± 0.10 (1.0) |
0.076 ± 0.003 (1.0) |
1.2 × 105 (1.0) |
|
| ||||
| A261F |
10 ± 2 (1.5) |
0.64 ± 0.06 (1.1) |
0.017 ± 0.001 (0.22) |
2.3 × 104 (0.20) |
|
| ||||
| A261H |
5.0 ± 2.0 (0.77) |
3.2 ± 0.4 (5.5) |
0.0040 ± 0.0001 (0.053) |
1.1 × 103 (0.0092) |
We then measured AUCG affinity for the closed complexes of the three ribozymes. As noted above, the exocyclic amino group of the nucleophilic guanosine donates a hydrogen bond to the 2′-OH of residue A261 in this complex (Figure 1; Forconi et al., 2009; Forconi et al., 2010). Consistent with formation of this hydrogen bond, the affinity of AUCG for a ribozyme containing a 2′-H at position A261 is reduced by ~6-fold compared to the A261OH ribozyme (Table 1 and ref. 20). In contrast, we found identical AUCG affinity for the A261OH and the A261F ribozymes (Table 1; Figure S1 and Table S1).
The simplest explanation for this result is that a hydrogen bond is formed between the exocyclic amino group of AUCG and the 2′-F of the A261F ribozyme. A strong prediction from this model is that an oligonucleotide lacking the exocyclic amino group would bind the closed complexes of the A261OH, A261F and A261H ribozymes with the same affinity. In agreement with this prediction, the affinities for AUCI, which lacks the exocyclic amino group on the nucleophilic base, are within two-fold for these three ribozymes (Table 2).
Table 2.
Binding affinities of AUCI for the closed complexes of different ribozymes, measured at pH 8.1 and in 50 mM Mg2+.
| ribozyme | |
|---|---|
| A261OH | 360 ± 40 |
| A261F | 290 ± 10 |
| A261H | 390 ± 100 |
An alternative explanation is that the 2′-F substitution introduces a binding pocket for a localized water molecule and that this water molecule accepts a hydrogen bond from the guanosine nucleophile. However, this model is less likely as a rearrangement would be required that is not needed in the case of a direct interaction. Regardless, the fluorine atom would contribute to localization of the water molecule near the exocyclic amino group of AUCG in this model, again suggesting that the fluorine atom can accept a hydrogen bond.
Another possibility is that the preferred nucleoside conformation of the analogs could contribute to the observed effects. The 2′-F, 2′-OH, and 2′-H nucleotides have been reported to have fractional occupancies of the 3′-endo conformation of 0.81, 0.49, and 0.35, respectively (Guschlbauer and Jankowski, 1980). Indeed, at residues populating the 2′-endo conformation, substitution of 2′-F nucleotides is known to inhibit RNA activity (Ortoleva-Donnelly et al., 1998; Ryder and Strobel, 1999). In the group I structural models most relevant to the catalytic structure of the ribozyme (Lipchock and Strobel, 2008) the sugar ring of residue A261 adopts a 2′-endo conformation. On the basis of conformational preferences alone, the A261F substitution would be expected to impart 2.3-fold weaker rather than the observed 5-fold stronger binding of AUCG relative to A261H (Table 1). Thus, the simplest explanation to account for our observations posits that the 2′-F atom mimics the hydrogen-bond acceptor properties of the hydroxyl group.
We also measured the reactivity of the closed complexes of the three ribozymes noted above with bound AUCG (kc, Table 1). The A261H ribozyme reacts ~20-fold slower than wild type with saturating AUCG, whereas the A261F ribozyme reacts only ~4-fold slower (Table 1 and Figure 2). As the 2′-fluorine at position 261 in the A261F ribozyme cannot donate a hydrogen bond, a possible explanation is that a hydrogen bond donation from the 2′-OH group of A261 to the ribozyme is also important for the chemical step. Inspection of published group I ribozymes crystal structures (Golden et al., 2005; Lipchock and Strobel, 2008; Stahley and Strobel, 2005) suggests the pro-RP phosphoryl oxygen and the 5′-bridging oxygen of residue C262 as putative partners in such a hydrogen bond. A role of the pro-RP phosphoryl oxygen of residue C262 in the chemical step is supported by the ~10-fold reduced rate constant for the chemical step when this phosphoryl oxygen is substituted by a sulfur atom (Hougland et al., 2005). Alternatively, subtle conformational rearrangements upon fluorine substitution might affect the chemical step, presumably by altering the relative positioning within the active site (Forconi et al., 2010; Forconi et al., 2011).
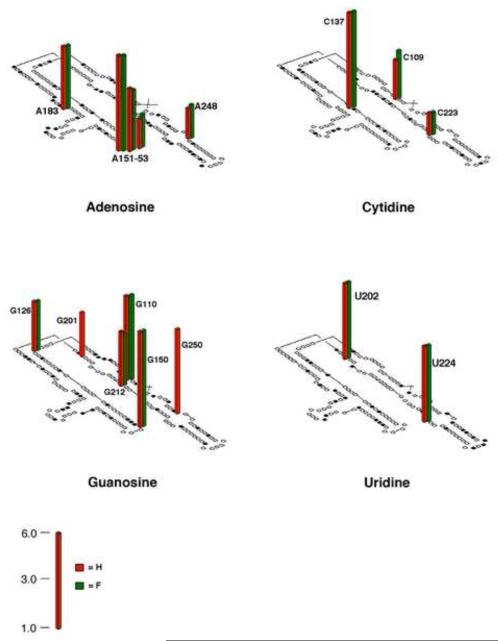
Figure 2.
ΔC209 P4-P6 secondary structure with histogram indicating the 2′-F/2′-H interference profile at 0.45 mM MgCl2. Sites of 2′-F and 2′-H interference are represented by the green and red bars, respectively. Sites of unique 2′-F interference correlate with residues that adopt a 2′-endo conformation in the structural models, as previously observed for the hairpin ribozyme (Ryder and Strobel, 1999), and are omitted for clarity. Interference values were calculated as described by Ryder et al. (Ryder et al., 2000). Only sites with interference values >1.5 are shown to take into account the experimental errors. See also Table S2 and Figure S2.
The overall effect of the 2′-H substitution at position A261 is 110-fold decrease in reactivity (Table 1, ), whereas the 2′-F substitution gives only a 5-fold decrease. The difference in reactivity between the A261F and A261H ribozymes is larger than expected for a differential sugar puckering preference (see also above) and suggests that there can be significant energetic consequences from hydrogen bonding to this fluorine.
Additional support for the ability of 2′-F atoms to act as hydrogen-bond acceptors
To more broadly evaluate whether 2′-F can supplant important 2′-OH groups within structured RNA, we used 2′-deoxy- and 2′-deoxy-2′-fluoronucleotide α-thiotriphosphates to perform NAIM experiments (Ryder et al., 2000) in the context of ΔC209 P4-P6 RNA. This RNA, derived from the Tetrahymena ribozyme, consists of two sets of coaxially-stacked helices that pack against one another through tertiary interactions to form a compact architecture even in the absence of the remainder of the ribozyme. Crystallographic analyses have defined the P4-P6 structure at atomic resolution (Cate et al., 1996; Juneau et al., 2001; Ye et al., 2008), and biochemical analyses have defined its folding thermodynamics and kinetics (Battle and Doudna, 2002; Matsumura et al., 2003; Sattin et al., 2008; Schlatterer et al., 2008; Takamoto et al., 2004; Young and Silverman, 2002). Changes in global compaction of the domain upon folding allow separation of folded and unfolded molecules by non-denaturing gel electrophoresis (Murphy et al., 1994), which provides a convenient assay for folding interference (Basu and Strobel, 1999; Schwans et al., 2003).
Previous work has demonstrated that 15 sites of 2′-deoxynucleotide interference within this domain identify residues bearing important 2′-OH groups, and that these 15 sites coincide with all of the crystallographically inferred 2′-OH interactions (Schwans et al., 2003; Schwans et al., 2004). An additional site, A151, shows weak 2′-H interference, although structural models provide no evidence for a hydrogen bond.
To examine the effect of 2′-fluoro substitution at residues involved in hydrogen bonds, we compared the 2′-fluoro and 2′-deoxynucleotide interference maps to generate a 2′-fluoro/2′-deoxynucleotide interference profile for every residue (Figure 2). We determined the sites of 2′-fluoro/2′-deoxynucleotide interference using non-denaturing gel electrophoresis to separate folded from unfolded molecules as previously described (Basu and Strobel, 1999; Schwans et al., 2003). To achieve maximal sensitivity to folding interference without compromising signal we conducted the folding reactions and non-denaturing gel electrophoresis at the [Mg2+]½ for folding of ΔC209 P4-P6 (0.45 mM MgCl2). At this Mg2+ concentration, the 2′-fluoronucleotides interfered with folding at 13 of the 15 2′-deoxynucleotide interference sites, indicating that the 2′-F atom cannot replace the 2′-OH at these sites without energetic penalty. These sites of 2′-fluoro/2′-deoxynucleotide interference coincide with residues that bear hydroxyl groups implicated as hydrogen bond donors (ribose zipper motifs and residues where the 2′-hydroxyl lies within hydrogen bond distance of N1 or N3 of purines; see Table S2), consistent with the loss of hydrogen bond donor ability expected for both 2′-fluoro and 2′-deoxynucleotides. However, two of the 15 sites can accommodate the 2′-F atom without inducing folding interference, indicating that a fluorine atom can supplant the role of the hydroxyl group at those sites. The same interference pattern was obtained at 2.0 mM MgCl2 (data not shown).
In the crystal structure the hydroxyl groups at the two sites showing 2′-H but not 2′-F interference have two features in common: (1) both reside within range to accept a hydrogen bond from an exocyclic amine, and (2) neither appears to donate a hydrogen bond, as no heteroatoms bearing nonbonding electron pairs reside nearby (Figure S2). The latter feature may allow fluorine to accept a hydrogen bond from the exocyclic amine without incurring unfavorable interactions with local atoms.
In the RNA folding reaction hydrogen bond formation at an individual residue is invariably coupled to the formation of other interactions. Nevertheless, the ability of 2′-analogs to mimic an interaction mediated by a specific 2′-OH group can be assessed by the differential effect of 2′-H and 2′-F substitution at that residue. In the simplest model, if a hydrogen bond is made in the folded structure, a more stable folded structure is expected compared to a molecule lacking this interaction. Thus, if the 2′-substitution disrupts this interaction, a less stable folded structure is expected, and if the 2′-substution does not disrupt this interaction the stability of the folded structure is expected to be similar to the 2′-OH containing molecule. The observation that two sites with 2′-F substitution in P4-P6 RNA do not affect folding provides additional evidence that a 2′-F atom can mimic the hydrogen-bond acceptor properties of the native hydroxyl group.
SIGNIFICANCE
In this work we identified three different contexts where a 2′-F, but not a 2′-H, can effectively substitute for the native 2′-OH, suggesting that a hydrogen bond is maintained with fluorine acting as a hydrogen bond acceptor. In each case the hydroxyl group interacts with a purine exocyclic amine. These results are consistent with the suggestion from analysis of crystal structures that the ability of a 2′-F atom to substitute for a 2′-OH group may be limited to interactions with protons attached to a nitrogen atom (Murray-Rust et al., 1983). If so, deleterious effects caused by 2′-deoxynucleotides but not 2′-fluoronucleotides may represent a general biochemical signature for tertiary interactions between 2′-hydroxyl groups and exocyclic amino groups within RNA. Additionally, the ability of fluorine to substitute at these positions appears to depend upon the absence of hydrogen bond acceptors to the native hydroxyl group. Additional analyses, akin to the study herein, in which structural and functional data are integrated, will be required to probe potential hydrogen bonds to fluorine from other groups and thereby reveal whether our findings can be generalized to other hydrogen bond partners and other RNA systems.
EXPERIMENTAL PROCEDURES
Ribozymes preparations and kinetic assays were performed essentially as previously described (Forconi et al., 2009; Forconi et al., 2010). Full details are given in the Supplemental Information. 2′-Fluorocytidine and 2′-fluorouridine were purchased from ChemGenes and 2′-fluoroguanosine was synthesized as described by Kawaskai et al. (Kawasaki et al., 1993) The nucleosides were converted to the α-thiotriphosphates and purified as previously described for 2′-mercaptonucleoside-α-thiotriphosphates (Schwans et al., 2003). Interference mapping experiments were conducted as previously described by Schwans et al. and references therein (Schwans et al., 2003).
Supplementary Material
HIGHLIGHTS.
We tested the ability of organofluorine to accept hydrogen bonds in two RNA systems.
In three cases a 2′-F, but not a 2′-H, can effectively substitute the native 2′-OH.
In these cases the native 2′-OH accepts a hydrogen bond from a purine amino group
We suggest that a 2′-F can accept a hydrogen bond from an RNA purine amino group.
ACKNOWLEDGEMENTS
This work was supported by grants from the NIH to D.H. (GM 49243) and J.A.P. (AI081987), by NIH Grant Number P20 RR-016461 from the National Center for Research Resources (to M.F.), and by a grant to the College of Charleston from the Howard Hughes Medical Institute through their Undergraduate Education program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Basu S, Strobel SA. Thiophilic metal ion rescue of phosphorothioate interference within the Tetrahymena ribozyme P4-P6 domain. RNA. 1999;5:1399–1407. doi: 10.1017/s135583829999115x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battle DJ, Doudna JA. Specificity of RNA-RNA helix recognition. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11676–11681. doi: 10.1073/pnas.182221799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua PC, Turner DH. Comparison of binding of mixed ribose deoxyribose analogs of CUCU to a ribozyme and to GGAGAA by equilibrium dialysis: Evidence for ribozyme specific interactions with 2′-OH groups. Biochemistry. 1991;30:10632–10640. doi: 10.1021/bi00108a005. [DOI] [PubMed] [Google Scholar]
- Carosati E, Sciabola S, Cruciani G. Hydrogen bonding interactions of covalently bonded fluorine atoms: From crystallographic data to a new angular function in the GRID force field. J. Med. Chem. 2004;47:5114–5125. doi: 10.1021/jm0498349. [DOI] [PubMed] [Google Scholar]
- Cate JH, Gooding AR, Podell E, Zhou K, Golden BL, Kundrot CE, Cech TR, Doudna JA. Crystal structure of a group I ribozyme domain: principles of RNA packing. Science. 1996;273:1678–1685. doi: 10.1126/science.273.5282.1678. [DOI] [PubMed] [Google Scholar]
- Chatterjee C, Muir TW. Chemical approaches for studying histone modifications. J. Biol. Chem. 2010;285:11045–11050. doi: 10.1074/jbc.R109.080291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SR, Fong R, Piccirilli JA. Nucleotide analogues to investigate RNA structure and function. Curr. Opin. Chem. Biol. 2005;9:585–593. doi: 10.1016/j.cbpa.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Dunitz JD. Organic fluorine: Odd man out. Chembiochem. 2004;5:614–621. doi: 10.1002/cbic.200300801. [DOI] [PubMed] [Google Scholar]
- Dunitz JD, Taylor R. Organic fluorine hardly ever accepts hydrogen bonds. Chem. Eur. J. 1997;3:89–98. [Google Scholar]
- Forconi M, Sengupta RN, Liu MC, Sartorelli AC, Piccirilli JA, Herschlag D. Structure and function converge to identify a hydrogen bond in a group I ribozyme active site. Angew. Chem., Int. Ed. 2009;48:7171–7175. doi: 10.1002/anie.200903006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forconi M, Sengupta RN, Piccirilli JA, Herschlag D. A rearrangement of the guanosine-binding site establishes an extended network of functional interactions in the Tetrahymena group I ribozyme active site. Biochemistry. 2010;49:2753–2762. doi: 10.1021/bi902200n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forconi M, Porecha RH, Piccirilli JA, Herschlag D. Tightening of active site interactions en route to the transition state revealed by single-atom substitution in the guanosine-binding site of the Tetrahymena group I ribozyme. J. Am. Chem. Soc. 2011;133:7791–7800. doi: 10.1021/ja111316y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden BL, Kim H, Chase E. Crystal structure of a phage Twort group I ribozyme-product complex. Nat. Struct. Mol. Biol. 2005;12:82–89. doi: 10.1038/nsmb868. [DOI] [PubMed] [Google Scholar]
- Guschlbauer W, Jankowski K. Nucleoside conformation is determined by the electronegativity of the sugar substituent. Nucleic Acids Res. 1980;8:1421–1433. doi: 10.1093/nar/8.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn ME, Muir TW. Manipulating proteins with chemistry: a cross-section of chemical biology. Trends Biochem. Sci. 2005;30:26–34. doi: 10.1016/j.tibs.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Herschlag D. Evidence for processivity and two-step binding of the RNA substrate from studies of J1/2 mutants of the Tetrahymena ribozyme. Biochemistry. 1992;31:1386–1399. doi: 10.1021/bi00120a015. [DOI] [PubMed] [Google Scholar]
- Hougland JL, Kravchuk AV, Herschlag D, Piccirilli JA. Functional identification of catalytic metal ion binding sites within RNA. PLoS Biol. 2005;3:1536–1548. doi: 10.1371/journal.pbio.0030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougland JL, Piccirilli JA, Forconi M, Lee J, Herschlag D. How the group I intron works: A case study of RNA structure and function. In: Gesteland RF, Cech TR, Atkins JF, editors. The RNA World. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: pp. 133–205. [Google Scholar]
- Howard JAK, Hoy VJ, OHagan D, Smith GT. How good is fluorine as a hydrogen bond acceptor? Tetrahedron. 1996;52:12613–12622. [Google Scholar]
- Juneau K, Podell E, Harrington DJ, Cech TR. Structural basis of the enhanced stability of a mutant ribozyme domain and a detailed view of RNA- solvent interactions. Structure. 2001;9:221–231. doi: 10.1016/s0969-2126(01)00579-2. [DOI] [PubMed] [Google Scholar]
- Kawasaki AM, Casper MD, Freier SM, Lesnik EA, Zounes MC, Cummins LL, Gonzalez C, Cook PD. Uniformly modified 2′-deoxy-2′-fluoro phosphorothioate oligonucleotides as nuclease-resistant antisense compounds with high affinity and specificity for RNA targets. J. Med. Chem. 1993;36:831–841. doi: 10.1021/jm00059a007. [DOI] [PubMed] [Google Scholar]
- Lipchock SV, Strobel SA. A relaxed active site after exon ligation by the group I intron. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5699–5704. doi: 10.1073/pnas.0712016105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JL, Johnson LN, Withers SG. Comparison of the binding of glucose and glucose 1-phosphate derivatives to T-state glycogen phosphorylase b. Biochemistry. 1990;29:10745–10757. doi: 10.1021/bi00500a005. [DOI] [PubMed] [Google Scholar]
- Matsumura S, Ikawa Y, Inoue T. Biochemical characterization of the kink-turn RNA motif. Nucleic Acids Res. 2003;31:5544–5551. doi: 10.1093/nar/gkg760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta G, Sen S. Probing Fluorine Interactions in a Polyhydroxylated Environment: Conservation of a C-F⋯··H-C Recognition Motif in Presence of O-H⋯··O Hydrogen Bonds. Eur. J. Org. Chem. 2010:3387–3394. [Google Scholar]
- Moore MJ, Sharp PA. Site-specific modification of pre-mRNA - The 2′-hydroxyl groups at the splice sites. Science. 1992;256:992–997. doi: 10.1126/science.1589782. [DOI] [PubMed] [Google Scholar]
- Moran S, Kierzek R, Turner DH. Binding of guanosine and 3′ splice site analogues to a group I ribozyme: Interactions with functional groups of guanosine and with additional nucleotides. Biochemistry. 1993;32:5247–5256. doi: 10.1021/bi00070a037. [DOI] [PubMed] [Google Scholar]
- Muller K, Faeh C, Diederich F. Fluorine in pharmaceuticals: looking beyond intuition. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- Murphy FL, Wang YH, Griffith JD, Cech TR. Coaxially stacked RNA helices in the catalytic center of the Tetrahymena ribozyme. Science. 1994;265:1709–1712. doi: 10.1126/science.8085157. [DOI] [PubMed] [Google Scholar]
- Murray-Rust P, Stallings WC, Monti CT, Preston RK, Glusker JP. Intermolecular Interactions of the C-F Bond - the Crystallographic Environment of Fluorinated Carboxylic-Acids and Related Structures. J. Am. Chem. Soc. 1983;105:3206–3214. [Google Scholar]
- Offen WA, Zechel DL, Withers SG, Gilbert HJ, Davies GJ. Structure of the Michaelis complex of beta-mannosidase, Man2A, provides insight into the conformational itinerary of mannoside hydrolysis. Chem. Comm. 2009:2484–2486. doi: 10.1039/b902240f. [DOI] [PubMed] [Google Scholar]
- O’Hagan D, Rzepa HS. Some influences of fluorine in bioorganic chemistry. Chem. Comm. 1997:645–652. [Google Scholar]
- Ortoleva-Donnelly L, Szewczak AA, Gutell RR, Strobel SA. The chemical basis of adenosine conservation throughout the Tetrahymena ribozyme. RNA. 1998;4:498–519. doi: 10.1017/s1355838298980086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauling L. The nature of chemical bonds. IV. The energy of single bonds and the relative electronegativity of atoms. J. Am. Chem. Soc. 1932;54:3570. [Google Scholar]
- Percival MD, Withers SG. Binding energy and catalysis: deoxyfluoro sugars as probes of hydrogen bonding in phosphoglucomutase. Biochemistry. 1992;31:498–505. doi: 10.1021/bi00117a028. [DOI] [PubMed] [Google Scholar]
- Persson K, Ly HD, Dieckelmann M, Wakarchuk WW, Withers SG, Strynadka NC. Crystal structure of the retaining galactosyltransferase LgtC from Neisseria meningitidis in complex with donor and acceptor sugar analogs. Nat. Struct. Biol. 2001;8:166–175. doi: 10.1038/84168. [DOI] [PubMed] [Google Scholar]
- Russell R, Herschlag D. Specifity from steric restrictions in the guanosine binding pocket of a group I ribozyme. RNA. 1999;5:158–166. doi: 10.1017/s1355838299981839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder SP, Ortoleva-Donnelly L, Kosek AB, Strobel SA. Chemical probing of RNA by nucleotide analog interference mapping. Methods Enzymol. 2000;317:92–109. doi: 10.1016/s0076-6879(00)17008-9. [DOI] [PubMed] [Google Scholar]
- Ryder SP, Strobel SA. Nucleotide analog interference mapping of the hairpin ribozyme: implications for secondary and tertiary structure formation. J. Mol. Biol. 1999;291:295–311. doi: 10.1006/jmbi.1999.2959. [DOI] [PubMed] [Google Scholar]
- Sattin BD, Zhao W, Travers K, Chu S, Herschlag D. Direct measurement of tertiary contact cooperativity in RNA folding. J. Am. Chem. Soc. 2008;130:6085–6087. doi: 10.1021/ja800919q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatterer JC, Kwok LW, Lamb JS, Park HY, Andresen K, Brenowitz M, Pollack L. Hinge stiffness is a barrier to RNA folding. J. Mol. Biol. 2008;379:859–870. doi: 10.1016/j.jmb.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwans JP, Cortez CN, Olvera JM, Piccirilli JA. 2′-Mercaptonucleotide interference reveals regions of close packing within folded RNA molecules. J. Am. Chem. Soc. 2003;125:10012–10018. doi: 10.1021/ja035175y. [DOI] [PubMed] [Google Scholar]
- Schwans JP, Li NS, Piccirilli JA. A packing-density metric for exploring the interior of folded RNA molecules. Angew. Chem., Int. Ed. 2004;43:3033–3037. doi: 10.1002/anie.200353575. [DOI] [PubMed] [Google Scholar]
- Stahley MR, Strobel SA. Structural evidence for a two-metal-ion mechanism of group I intron splicing. Science. 2005;309:1587–1590. doi: 10.1126/science.1114994. [DOI] [PubMed] [Google Scholar]
- Street IP, Armstrong CR, Withers SG. Hydrogen bonding and specificity. Fluorodeoxy sugars as probes of hydrogen bonding in the glycogen phosphorylase-glucose complex. Biochemistry. 1986;25:6021–6027. doi: 10.1021/bi00368a028. [DOI] [PubMed] [Google Scholar]
- Street IP, Rupitz K, Withers SG. Fluorinated and deoxygenated substrates as probes of transition-state structure in glycogen phosphorylase. Biochemistry. 1989;28:1581–1587. doi: 10.1021/bi00430a024. [DOI] [PubMed] [Google Scholar]
- Takamoto K, Das R, He Q, Doniach S, Brenowitz M, Herschlag D, Chance MR. Principles of RNA compaction: insights from the equilibrium folding pathway of the P4-P6 RNA domain in monovalent cations. J. Mol. Biol. 2004;343:1195–1206. doi: 10.1016/j.jmb.2004.08.080. [DOI] [PubMed] [Google Scholar]
- Wicki J, Schloegl J, Tarling CA, Withers SG. Recruitment of both uniform and differential binding energy in enzymatic catalysis: Xylanases from families 10 and 11. Biochemistry. 2007;46:6996–7005. doi: 10.1021/bi700359e. [DOI] [PubMed] [Google Scholar]
- Ye JD, Tereshko V, Frederiksen JK, Koide A, Fellouse FA, Sidhu SS, Koide S, Kossiakoff AA, Piccirilli JA. Synthetic antibodies for specific recognition and crystallization of structured RNA. Proc. Natl. Acad. Sci. U. S. A. 2008;105:82–87. doi: 10.1073/pnas.0709082105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BT, Silverman SK. The GAAA tetraloop-receptor interaction contributes differentially to folding thermodynamics and kinetics for the P4-P6 RNA domain. Biochemistry. 2002;41:12271–12276. doi: 10.1021/bi0264869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.