Abstract
Controversy regarding estrogen action in the brain remains at the forefront of basic, translational and clinical science for women’s health. Here, I provide an integrative analysis of estrogen-inducible plasticity and posit it as a strategy for predicting cognitive domains affected by estrogen in addition to sources of variability. Estrogen enhancement of plasticity is evidenced by increases in neurogenesis, neural network connectivity and synaptic transmission. In parallel, estrogen increases glucose transport, aerobic glycolysis and mitochondrial function to provide the ATP necessary to sustain increased energetic demand. The pattern of plasticity predicts that estrogen would preferentially affect cognitive tasks of greater complexity, temporal demand and associative challenge. Thus, estrogen deprivation should be associated with decrements in these functions. Estrogen regulation of plasticity and bioenergetics provides a framework for predicting estrogen-dependent cognitive functions while also identifying sources of variability and potential biomarkers for identifying women appropriate for hormone therapy.
Introduction
Estrogen regulation of memory function ranges from fairly consistent in basic science analyses to variable in the extreme in human studies [1–3]. Not surprisingly, the disparity between the basic and clinical science findings of estrogen regulation of cognitive function has been the topic of much debate [1] (Asthana, S. et al., unpublished). Estrogen potentiation of neural plasticity serves as a platform to address the plausibility of estrogen regulation of memory function and provides insights into potential sources of variability in human studies.
Plasticity, broadly defined, has been proposed to be the underlying foundation for learning and memory function (Box 1). In the nervous system, plasticity is manifested as dynamic responses in neurogenesis, morphogenesis and synaptic transmission. Remarkably, estrogen increases each of these plasticity domains in the adult brain typically within minutes to hours of exposure (Figure 1). If plasticity is related to operational capability, such dramatic changes in plasticity at the cellular, morphological and synaptic transmission levels should impact function within the systems wherein the plasticity occurs. As estrogen enhances plasticity in the hippocampus and prefrontal cortex (PFC), which are brain regions involved in cognitive functions, there should be corollary changes in cognition.
Box 1. Plasticity.
Neural plasticity refers to the ability of the brain to change in response to intrinsic (e.g. hormonal or growth factors) or extrinsic (e.g. experience and sensory stimulation) factors. At the cellular level, plasticity takes the form of alterations in the generation of new neurons and integration of newly formed cells into neural circuits. Cellular plasticity can also refer to the ability of a cell to change its fate, for example from a glial to neuronal phenotype [73]. At the morphological level, structural modifications in the neural circuitry of the adult brain occur at the synaptic level. These modifications can be presynaptic and/or postsynaptic. Presynaptic changes include an increase in the number of neurotransmitter vesicles and/or modifications in the number of presynaptic boutons. Postsynaptic morphological alterations include modifications in the shape of the postsynaptic membrane creating multiple synaptic boutons or changes in dendritic spine number and/or shape [20]. At the synaptic transmission level, plasticity takes multiple forms. Most notable among them are short- or long-term potentiation and depression. It should be kept in mind that plasticity is bidirectional and can be manifested as an increase or decrease in a response. Collectively, coordinated changes at the cellular, morphological and synaptic levels are the basis for the dynamic adaptive range of the brain to learn, remember, forget, adapt and modify response patterns. As such, neural plasticity can be an indicator of the vitality of the brain and functional capability.
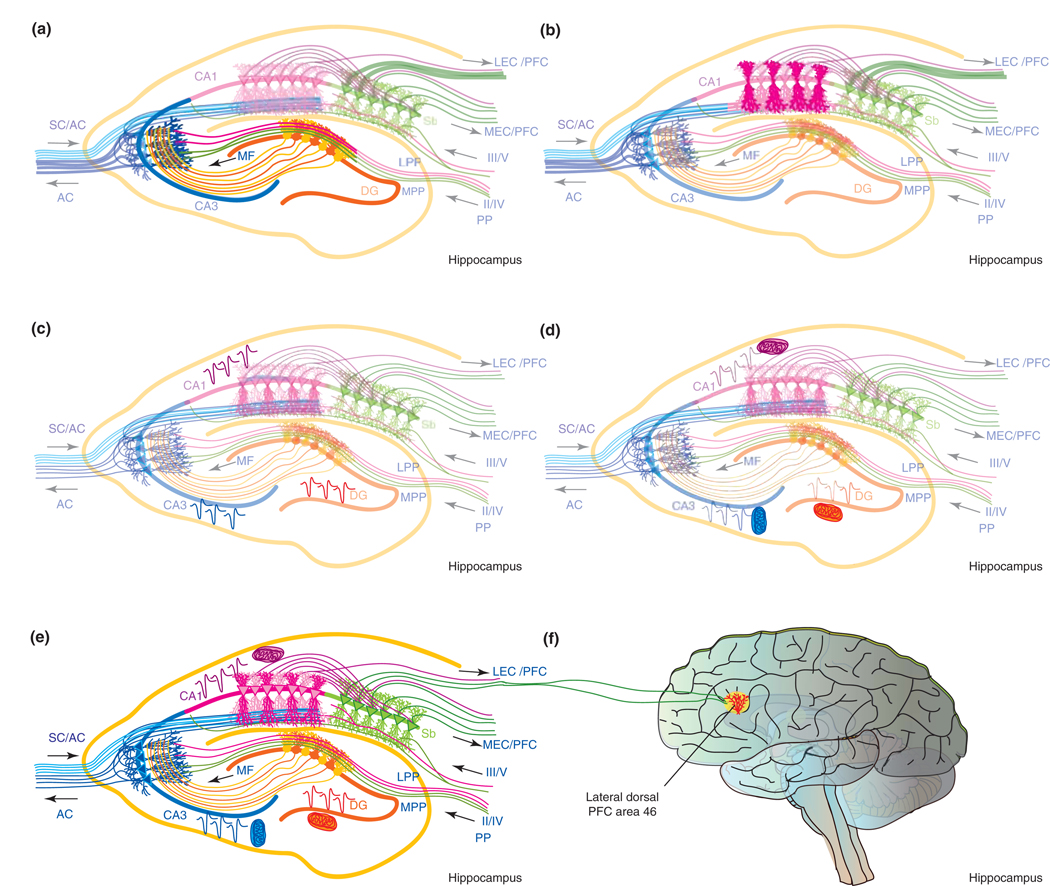
Figure 1.
Estrogen induces cellular, morphological and synaptic plasticity. (a) Estrogen (17β-estradiol; E2) enhances cellular plasticity via increased proliferation of neural progenitor cells within the subgranular zone, which are then integrated into the granule cell layer of the dentate gyrus (DG) (E2-induced newly generated cells are depicted as dark orange). Functionally new dentate granule cells contribute to the association of stimuli that are separated in time, a function that is a hippocampal-dependent type of associative learning and memory [69]. (b) Morphological plasticity is enhanced by E2 within the dentate and CA1 regions. E2 increased dendritic spines in the outer molecular layer of the dentate gyrus [6] (represented by the dark orange dendritic tree). Within the CA1 region, E2 increased spines of apical dendrites (dark pink dendritic tree) within the stratum radiatum, which receives excitatory input from CA3 neurons via the Schaffer collaterals [9] (blue pathway). The E2-induced increase in multiple synaptic boutons arising from CA3 Schaffer collateral axons synapsing onto CA1 apical dendrite spines [28] would predict an increase of either efficiency and/or capacity for information processing. Furthermore, the clustering of E2-inducible multisynaptic boutons [28] would indicate selective enhancement of input from particular CA3 cells. (c) E2 increased the field excitatory postsynaptic potential (fEPSP) within each subfield of the trisynaptic pathway (depicted in dark EPSPs in each subfield) and the dentate gyrus (represented by dark orange dendritic tree and fEPSP, respectively). E2 enhanced mossy-fiber input to CA3 while more robustly potentiating the AC fiber system of CA3 [34] (shown in blue). Potentiating both mossy-fiber and AC systems would be predicted to enhance CA3 associative memory to enhance the retrieval function of CA3 such that fewer elements (i.e. a partial representation) of a memory would be required for the whole memory to be retrieved [34]. In the CA1 subfield E2 potentiated both fEPSP and long-term potentiation [34], which would predict increased output to the subiculum (depicted in green). (d) E2 significantly increases aerobic glycolysis and mitochondrial function in the brain (depicted by dark mitochondria associated with fEPSP) [46,70,71]. The increase in glucose metabolism and mitochondrial function provide the energetic fuel and increased ATP to sustain increases in cellular, morphological and synaptic plasticity. (e) Collectively, E2 promotes cellular, morphological and synaptic plasticity in the hippocampus while simultaneously increasing the ATP necessary for enhanced plasticity. E2-induced increases in hippocampal plasticity impacts the output from subiculum to multiple brain regions including the PFC. (f) The lateral subiculum can project to area 46 of the lateral dorsal PFC [72], thereby providing a neuroanatomical connection between the information processing of the hippocampus and the temporal organization of behavior and reasoning governed by PFC. E2 significantly increased spine density of basal and apical dendrites of PFC neurons [6]. Estrogen-inducible increases in hippocampal and PFC plasticity predict that estrogen would preferentially affect cognitive tasks of greater complexity, temporal demand and associative challenge. Abbreviations: LEC, lateral entorhinal cortex; LPP, lateral perforant path; MEC, medial entorhinal cortex; MF, mossy fiber; MPP, medial perforant path; PP, perforant path; Sb, subiculum; SC, Schaffer collaterals. III/V and II/IV refer to the entorhinal cortex layers that feed into the pathways projecting to the hippocampus. Hippocampal scheme modified from www.bristol.ac.uk/synaptic/info/pathway/hippocampal.htm.
Here, I examine the plasticity induced by estrogen in the hippocampus and cortex to extrapolate from these responses a prediction of functional impact. Reviewed herein are analyses of estrogen regulation of cellular, morphological and electrophysiological plasticity. The implications of these plasticities are considered in light of their predicted impact on neural system function and, ultimately, for cognition. Both the magnitude and localization of estrogen-induced plasticity predict that estrogen would preferentially affect cognitive tasks of greater complexity, temporal demand and associative challenge. A corollary to this prediction is that estrogen-induced plasticity would not be required for less demanding learning and memory tasks. Therefore, the impact of estrogen deprivation should be apparent with increasing cognitive, temporal and associative demand. Data principally derived from analyses of the endogenous estrogen, 17β-estradiol (E2), are considered with exceptions noted in the text (Box 2).
Box 2. Estrogen receptor α, β and GPR30: cellular and subcellular localization.
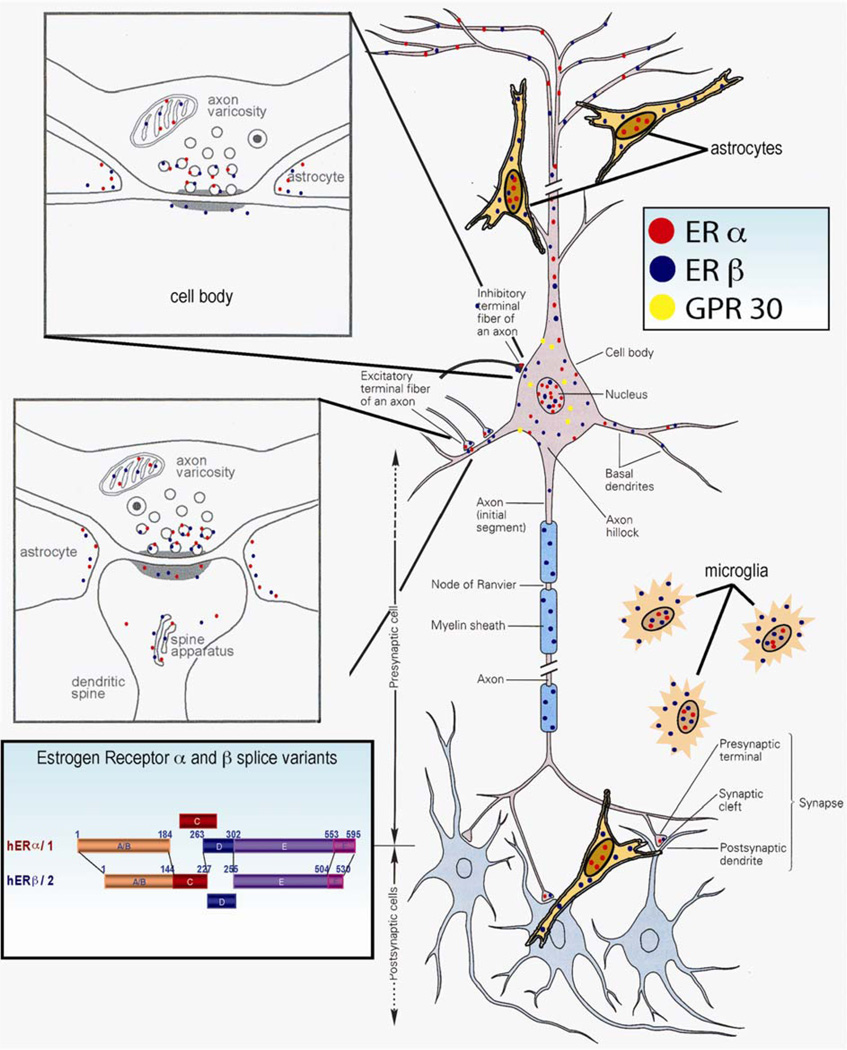
Two estrogen receptor (ER) subtypes, ERα and ERβ, have been identified in multiple vertebrate species (Figure I). Although encoded from separate genes, they share features common to the nuclear receptor superfamily (see estrogen receptor splice variant insert). These features include an N-terminal domain (termed the ‘A/B domain’), a highly conserved DNA-binding domain comprising two Cys4 zinc fingers (termed the ‘C domain’), a hinge region (termed the ‘D domain’), a less conserved C-terminal ligand-binding domain (termed the ‘E domain’) and a caudal C-terminal ‘F domain’. ERα and ERβ are highly conserved at the DNA-binding domain and the ligand-binding domain with 96% and 53% homology, respectively. ER expression occurs in every major cell group in the brain including neurons, astrocytes, oligodendrocytes and microglia (Figure I neuron, glia panel). In neural tissue, ERα and ERβ are detected in nuclear, cytoplasmic and membrane compartments [74–76]. Subcellularly, ERs are associated with organelles including mitochondria, synaptic vesicles and the spine apparatus. The expression and localization of ERs varies depending on brain region, cell type, hormonal status and neurological condition. Adding to the complexity is the expression of numerous ERα and ERβ splice variants, which have been detected in multiple brain regions including the hippocampus. ERα splice variants have been detected in multiple brain regions including the hippocampus [77]. The ERβ1δ4 splice variant, which lacks exon 4 containing the nuclear translocation signal and part of ligand-binding domain, is highly expressed in the hippocampus [78]. Not surprisingly, ERβ1δ4, which lacks the nuclear translocation signal, is localized within the cytoplasm. The role(s) of splice variants in the brain has yet to be identified, but their expression is regulated by age and diseases such as Alzheimer’s [77]. Increasing evidence links the rapid effects of estrogen to a class of G-protein-coupled receptors (called GPR30), which bind to E2 with high affinity [79]. GPR30 has a wide distribution in brain, including the hippocampus, with a cytoplasmic localization often associated with the endoplasmic reticulum where it regulates calcium signaling [79,80]. Other estrogen-binding proteins, of yet unknown function, have been detected in the brain [81].
Multiple estrogenic molecules can bind to ERs. The molecules range from endogenous estrogens 17α- and 17β-estradiol to exogenous synthetic estrogenic molecules including conjugated equine estrogens (a frequently used ET extracted from the urine of pregnant mares [18]), estradiol acetate and ethinylestradiol (common in oral contraceptives), plant-derived phytoestrogens (e.g. isoflavones, lignans and coumestans [82]) and endocrine disruptors or xenoestrogens found in the environment as pollutants such as bisphenol A [83].
Estrogen regulation of cellular plasticity: neurogenesis
The regenerative capacity of the brain is most dramatically evident in the daily generation of new neurons in the two proliferative zones of the brain: the subgranular zone and the subventricular zone [4]. While the number of factors that regulate cellular plasticity grows (Box 1), ovarian hormones, in particular E2, are among those factors for which there is substantial evidence documenting their neurogenic efficacy and functional relevance.
In vivo, E2 increased the proliferation of neural progenitor cells in the dentate gyrus subgranular layer zone of ovariectomized rats (Figure 1a,e). Neurogenesis was greatest in the intact rat during proestrus, when ovarian hormone levels are highest, compared with estrus and diestrus [5]. In rats, the neurogenic effect of E2 is vulnerable to the duration of ovarian hormone deprivation and regimen of hormone replacement. Prolonged absence of ovarian hormones was associated with a loss of neurogenic response to E2, whether administered chronically or cyclically, and a decrease in the number of new cells expressing a neuronal phenotype [5]. These data indicate that prolonged deprivation of E2 leads to diminished responsiveness to E2 and a concomitant decline in neuron production [5].
These findings in a rodent model would indicate disturbing implications for neurogenesis in women deprived of ovarian hormones for an extended period of time. However, evidence from the nonhuman primate brain [6] indicates that the nonhuman primate brain, and by extension the human brain, has an extended period of responsivity to E2 relative to the rodent brain (see section on morphogenesis later). It remains to be determined whether the same extended window of estrogen responsivity in morphogenesis applies to neurogenesis.
The question immediately arises as to the biological importance of generating new granule cells in the dentate gyrus. While the role of neurogenesis in learning and memory continues to be debated, increasing evidence indicates that newly generated neurons in the dentate gyrus contribute to the association of stimuli that are separated in time, a function that is a hippocampal-dependent type of associative learning and memory [7,8]. As the dentate gyrus is the first region of the hippocampus that receives and integrates sensory information via the perforant path [9], expanding the temporal window of associating information from multiple inputs should result in greater integration of information over time. Consistent with this postulate, neural circuits generated by neurogenesis are proposed to lead to the formation of temporal clusters of long-term episodic memories [8].
E2-induced neurogenesis is also potentially relevant to disease states. In a mouse model of ischemia, E2 enhanced neurogenesis, in which both estrogen receptors α and β (ERα and ERβ; Box 2) contributed to the generation on new neurons; however, ERα contributed to a greater extent than ERβ [10]. The greater contribution of ERα to E2-induced neural proliferation observed in the ischemic rodents [10] is in contrast to the near-equal contribution in normal rats [11]. The ERα preference could be due to the higher level of ERα expression in the ischemia model, in which there is a 2–3-fold increase in ERα, whereas there is a decrease in ERβ expression [12]. In a different disease model, E2 restored neurogenesis in the subgranular and subventricular zones of the chronic diabetic mouse brain [13].
To pursue the relevance of E2-induced neurogenesis into the human realm, we investigated E2-regulated proliferation of human neural progenitor cells (hNPCs) [14]. E2 induced a significant increase in hNPC proliferation in a time- and dose-dependent manner. E2-induced hNPC DNA replication was paralleled by elevated cell-cycle protein expression and centrosome amplification, which was associated with augmentation of the total cell number [14]. The proliferative effect of E2 in hNPCs was mediated by ERβ activation of the mitogen-activated protein kinase signaling pathway [14]. Our findings of ERβ-mediated proliferation of human cortical neural progenitor cells are relevant to findings in ERβ knockout mice, in which brain size was smaller and fewer neurons were observed [15]. Expression of ERβ in human embryonic brain cells indicates a comparable role for ERβ in human brain development.
Estrogen regulation of morphological plasticity: spinogenesis
Estrogen, most notably E2 [16,17] but also other estrogens [18,19], are potent and rapid inducers of morphological plasticity (Box 1). In the adult hippocampus and cortex, morphological plasticity is best exemplified by increasing dendritc spine number or dendritic spine contacts via multiple synapse boutons [20]. Substantial evidence indicates that an increase in spine density in the hippocampus is associated with learning and memory whereas decreases are associated with decrements in these cognitive functions [20–23]. Moreover, decreased synapse density and synaptic dysfunction precede Alzheimer’s disease pathology and are the strongest neuropathological correlates of dementia severity [24,25].
Regionally, E2 induction of increased spines extends to multiple sites within the hippocampus, including the CA1 region and dentate gyrus, and to multiple brain regions including the medial amygdala and hypothalamus (Table 1; Figure 1a,b,e). Estrogen-induced increases in spine density on both the apical and basal dendrites of CA1 pyramidal cells receive excitatory input from CA3 pyramidal cells to form glutamatergic synapses [22,26,27] (Figure 1). Consistent with the glutamatergic innervation to these spines, E2-induced morphological enhancements are an NMDA (N-methyl-d-aspartate)-dependent process [16]). Electrophysiological analyses indicate that the estrogen-induced increase in spine density is paralleled by enhanced sensitivity of CA1 pyramidal cells to excitatory synaptic input through both AMPA (α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate) and NMDA receptors [26]. From a neural circuit perspective, E2 increased the number of multiple synapse boutons; this lead to more synaptic contacts to different postsynaptic neurons [28]. Thus, in addition to increasing the density of excitatory synaptic input to individual CA1 pyramidal cells, E2 increases the complexity of the neural circuit to include a greater number of postsynaptic CA1 pyramidal cells [28].
Table 1.
Estrogen-inducible changes in neuronal morphology
| Model | Brain region | Morphological complexity |
Spine number | Spine shape | Related outcomes | Refs |
|---|---|---|---|---|---|---|
| Cultured neurons | Hippocampus | Increased | Increased | NDa | ND | [16] |
| Cultured neurons | Cortex | Increased | ND | ND | ND | [16] |
| Cultured neurons | Basal Forebrain | Increased | ND | ND | ND | [16] |
| Mouseb | CA 1 | ND | No change | Increased number of mushroom-shaped spines | Increased PSD95, spinophilin and syntaxin | [66,67] |
| Ratb | CA1 | Increase in multiple synaptic boutons | Increased on apical and basal dendrites | ND | Improved spatial working memory correlated with an increase in spine density | [23,26] |
| Ratb | Medial amygdala | ND | Increased | ND | ND | [23] |
| Ratb | Hypothalamus | ND | Increased | ND | [23] | |
| Ratb: estrus cycle | CA1 (apical dendrites) | ND | Increased in proestrus | ND | Spine number declined in estrus | [23,26] |
| Ratb: aged | CA1 (apical and basal dendrites) | ND | No change | ND | ND | [6] |
| Monkeyb: young African green (vervet) | CA1 striatum radiatum Dorsolateral PFC (layer I) | ND | Increased | ND | ND | [6,68] |
| Monkeyb: young and Aged Rhesus | CA1 striatum radiatum | ND | Increased | ND | Increased hippocampal-dependent memory task | [6] |
| Monkeyb: young Rhesus | Dorsolateral PFC (area 46; layer III) | ND | Increased | ND | No effect on delayed response | [68] |
| Monkeyb: aged (perimenopausal) Rhesus | Dorsolateral PFC (area 46; layer III) | No effect on age-associated decline in dendritic length | Increased | Partial reversal of age-associated loss of long-neck, small-spine head spines | Reversed age-associated decline in delayed response | [68] |
ND, not determined.
Ovariectomized and treated with either vehicle or E2.
A correlational analysis of spine density with behavioral function revealed two important findings [29]. First, E2-inducible spines were closely associated with memory performance. Second, E2 did not enhance spatial working memory at the shortest delay period between training and test trials (10 min) but was only effective at the longer more demanding delay periods of 30–100 min. At the longer delay points, the E2-high spine group sustained spatial working memory performance that mirrored their asymptotic performance at the 10 min delay test, whereas the performance of the E2-low spine groups declined precipitously with increasing temporal demand [29]. Behaviorally, E2-inducible spines did not enhance the minimal performance that was achievable by all groups but rather was required to sustain performance capability during increasing temporal demand.
Collectively, these findings from the rat brain indicate that, in addition to increasing synaptic strength, E2 is expanding the distribution of information across and communication between neural networks while substantially increasing the temporal window during which information can be effectively accessed and used to achieve goal-directed behavior.
E2 induction of spines is not limited to the rodent brain. In both young and aged monkeys, E2 generates a 35% increase in total spines in the striatum radiatum of CA1, which corresponds to an increase of 1.3 billion new spines in younger animals and 1.1 billion spines in the aged animals [6]. Surprisingly, aged female macaques remained responsive to E2 induction of spines, whereas the aged rat hippocampus does not [6]. Functionally, an E2-induced increase in dendritic spines was associated with enhanced performance on a hippocampal-dependent memory task in the same monkeys [30]. Important from a therapeutic perspective, long-term (2–3 years) cyclical estrogen therapy (ET) induced the same increase in spines within the CA1 of aged rhesus monkeys as short-term ET [6]. The continued responsiveness to E2 by aged macaques is particularly relevant to women because there are substantial similarities in endocrine senescence between humans and macaques.
E2 regulation of spines and spine shape is not limited to the hippocampus (Table 1). E2 significantly increased spine density of both apical and basal dendrites within the dorsolateral PFC of the nonhuman primate brain [6] (Table 1). In addition, spine shape was shifted towards smaller head size, which is associated with enhanced plasticity [31]. Mushroom-shaped spines and spines with a large head and thick neck are stable relative to small head spines, contain the greatest number of AMPA receptors and have been suggested to act as ‘memory spines’. In contrast, thin spines with small heads seem to be more transient in nature, contain less AMPA receptors, are highly plastic and are suggested to function as ‘learning spines’ [32]. All prefrontal regions receive projections from the hippocampus, either directly or indirectly [33]. The lateral region, which is maximally developed in humans, is crucial for temporal organization of behavior, speech and reasoning [33]. Routine, automatic or over-learned behavioral sequences do not engage the PFC [33]; this indicates that E2-induced spine changes in the dorsolateral PFC would not affect simple or routine cognitive functions.
If changes in spine density and shape within brain regions involved in cognition are relevant to function, it should be possible to predict cognitive outcomes in the presence or absence of E2. Beginning with spine changes in the entry point for the trisynaptic circuit, E2 preferentially increased spines in the outer molecular layer of the dentate gyrus [31]. Because the outer molecular layer of the dentate gyrus receives input from the entorhinal cortex via the perforant path [9], one could posit that, as E2 increases dendritic spines in the outer molecular layer of the dentate gyrus, a commensurate increase in either the efficiency and/or the capacity for processing input from the neocortex would occur. The increased processing of information from the neocortex by the dentate gyrus would be transmitted to CA3 neurons via mossy fibers. In turn, CA3 Schaffer collateral axons synapsing onto E2-induced CA1 apical dendrite spines [28] would predict an increase of either efficiency and/or capacity for information processing. In tandem, the clustering of E2-inducible multisynaptic boutons on CA1 apical dendrites [28] indicates selective enhancement of input from particular CA3 cells.
In the nonhuman primate hippocampus, the impact of increasing synaptic circuitry by >1 billion new spines should lead to a substantial increase in the number of neurons that are synaptically connected. If E2-inducible spines and neural network complexity is related to information-processing capability, the enriched hippocampal circuitry induced by E2 should be capable of enhanced processing, associating and encoding of more complex patterns of information over time. If this hypothesis is true, estrogen deprivation should preferentially affect cognitive tasks of greater complexity, temporal demand and associative challenge (i.e. linking information across time), whereas less demanding learning and memory tasks should not be effected. Furthermore, if the spine changes in the dorsal lateral PFC occur in concert with functional outcomes in the hippocampus, there should be a relationship between magnitude of information processing, associative learning and the temporal organization of working memory, behavioral outcome and reasoning.
Estrogen regulation of synaptic plasticity: circuit genesis
E2 is a potent and efficacious potentiator of synaptic transmission in the CA1 region of the hippocampus via potentiation of both glutamate AMPA and NMDA receptors [26,34] (Figure 1c,e). Enhancement of synaptic transmission is also associated with E2 attenuation of GABA-mediated inhibition of CA1 pyramidal neurons [26]. E2 enhancement of long-term potentiation is mediated by the Src/ERK (extracellular signal-regulated kinase) pathway via phosphorylation of the NR2 subunit of the NMDA receptor [35,36].
The distribution of both ERα and ERβ throughout the trisynaptic circuit of the hippocampus indicated that E2 could regulate synaptic transmission throughout the hippocampus. To address this issue, we used multielectrode arrays that enabled simultaneous in vitro recording of synaptic activity from multiple sites within the hippocampal subfields (dentate gyrus, CA3 and CA1) [34]. Results of those analyses showed that E2 potentiation of synaptic transmission was not unique to CA1 but was evident in each subfield of the trisynaptic hippocampal system (Figure 1e). Surprisingly, E2 potentiation of synaptic transmission was greatest in CA3 with a significant increase in the amplitude and slope of CA3 associational commissural (AC) fibers that innervate pyramidal neurons in the CA3 regions both ipsilaterally and contralaterally. AC fibers integrate information along the long axis of the hippocampus and unify hippocampi function [34].
Within the hippocampus, CA3 pyramidal neurons express the highest density of ERβ, receive input from mossy fibers and AC fibers, and express both l-type calcium channels and NMDA channels [34]. These two calcium channels participate in different phases of memory function with NMDA-channel-associated memory acquisition, whereas l-type calcium channels are associated with memory retention [34]. Consistent with these functional analyses, we have shown that E2 induces calcium influx through l-type calcium channels, which activates the Src/ERK signaling cascade; this leads to potentiation of calcium conductance through NMDA receptor channels [6]. Increasing evidence indicates that CA3 can serve as an associative-memory network owing to the sparse connectivity of mossy fibers and its denser connectivity of associational fibers [34]. This model proposes that entire memory patterns can be retrieved from partial representations of the memory and is manifested as pattern completion [34]. Local potentiation of synaptic transmission within each of the nodes of the trisynaptic pathway coupled with the morphogenic effects of E2 could transform local nodes of potentiation to a global network of potentiation with the AC fibers in CA3, enhancing memory retrieval through auto-associative memory and pattern completion. Functionally, E2-induced potentiation of each component of the trisynaptic pathway should result in an increase in the absolute number of items that can be stored in the memory network, whereas selective enhancement of the AC fiber system of CA3 by E2 should enhance the retrieval function of CA3 such that fewer elements of a memory would be required for the whole memory to be retrieved. If this hypothesis is correct then the corollary should be true: that a deficiency in estrogen would lead to a requirement for a greater number of the elements (larger representation) of a memory to retrieve the entire memory.
Fueling the energy demands of enhanced plasticity
Estrogen-induced increases in plasticity at all levels have metabolic-demand consequences [37]. The increase in synaptic transmission requires substantial energy because the largest energy-consuming process of the brain is the maintenance of ion gradients across the plasma membrane [38]. Maintenance of these gradients is fueled by the ATP-dependent Na+,K+-ATPase, which is localized in neurons and glia. Activity of these pumps accounts for 50% of ATP utilization in the central nervous system [38].
E2 increases expression of glucose-transporter subunits Glut3 and Glut4 in frontal cortex neurons in the nonhuman primate brain [39] while also increasing glucose transport in the blood–brain barrier endothelium [40]. An increase in glucose-transporter protein would also require a concomitant change in factors regulating glucose metabolism such as insulin growth factor-1 (IGF-1) and its cognate receptor. In the nonhuman primate frontal cortex, E2 induced a significant increase in IGF-1 mRNA [39]. The synergistic coupling between ERs and IGF-1 receptors [41–44] link the IGF-1, PI3K/Akt signaling and ER pathways in estrogen-inducible neuroprotection [37]. Increases in glucose transport into neurons should be accompanied by increased glycolysis. Evidence derived from the rat brain indicate that in vivo E2 significantly increased glycolytic enzyme activity of hexokinase (soluble and membrane bound), phosphofructokinase and pyruvate kinase within 4 hours [37]. Hexokinases HKI and HKII bind to the mitochondrial voltage-dependent anion channel (also known as VDAC or porin) to directly couple intramitochondrial ATP synthesis to glucose metabolism (for review, see Ref. [37]).
If increased glucose uptake and glycolytic enzymes result in increased ATP to sustain the metabolic demand of increased synaptic transmission and cellular growth, pyruvate must be converted to acetyl-CoA for processing in the citric acid cycle (Figure 2). Conversion of pyruvate to acetyl-CoA is mediated by pyruvate dehydrogenase (PDH), which is a key regulatory enzyme complex linking the glycolytic metabolism to the citric acid cycle by transforming pyruvate into acetyl-CoA. In the brain, PDH is further responsible for directing acetyl-CoA to either the citric acid cycle or to acetylcholine synthesis [45]. E2 induced a twofold increase in the expression of multiple subunits of the PDH enzyme complex, which was mirrored by a commensurate increase in PDH activity [46]. Furthermore, E2 increased the expression and activity of proteins required for oxidative phosphorylation electron transfer, a result that was consistent with a coordinated response that optimizes glucose metabolism in the brain [46]. Estrogen significantly increased both protein expression and activity of complex IV subunits I–IV [46–48]. An increase in complex IV activity by E2 is particularly relevant given that a reduction in complex IV is an early marker of Alzheimer’s disease [49]. E2 also increased expression of ATP synthase F1α and β [46], which is consistent with the increase in proteins required for mitochondrial respiration and with our previous report of estrogen-induced increase in neuronal ATP [18]. E2-induced enhancement of energetic efficiency was paralleled by an increase in free radical defense systems [46,50]. Collectively, these data indicate that E2 increases each aspect of glucose availability, metabolism and conversion into fuel to drive the mitochondrial electron transport chain required for increased ATP generation necessary to fuel the energetic demands of cellular, morphological and synaptic plasticity (for reviews, see Refs [37,51,52]).
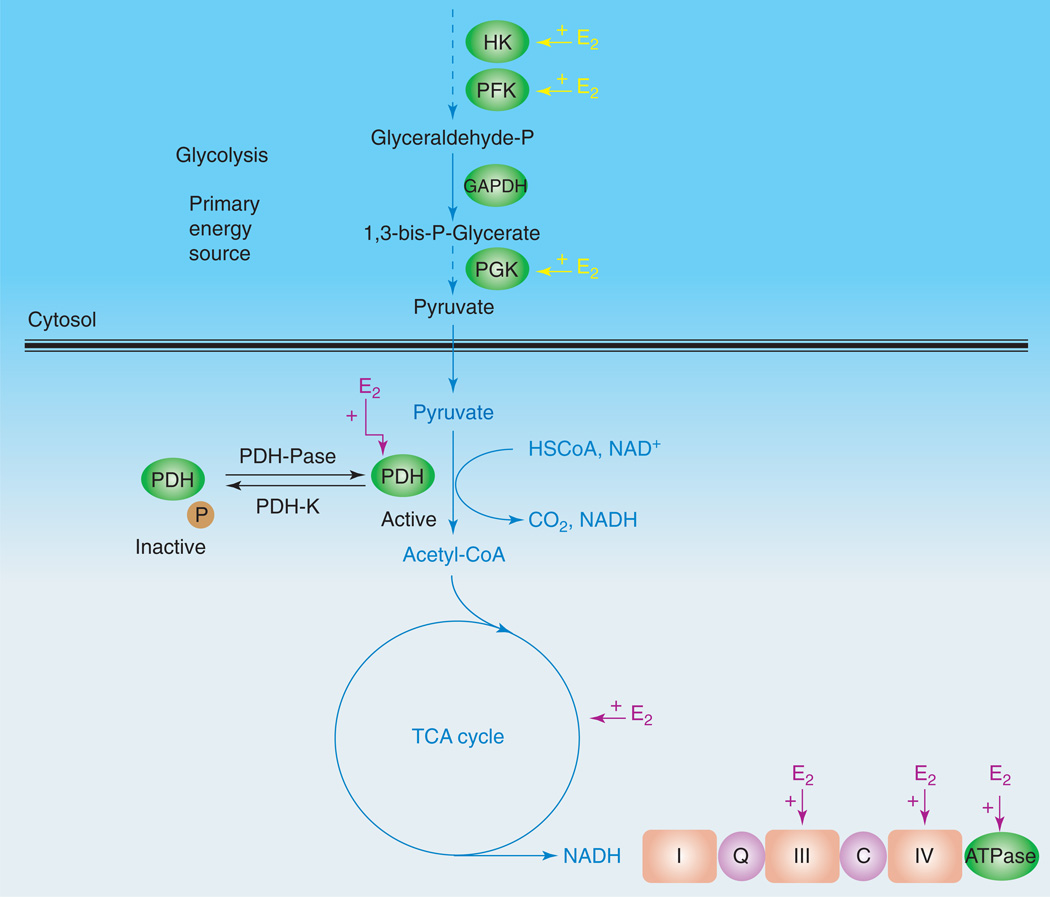
Figure 2.
E2 promotes glycolysis and glycolytic coupled tricarboxylic acid cycle (TCA) function, mitochondrial respiration and ATP generation. E2 increases key enzymes in the glycolytic pathway to promote the generation of pyruvate and its conversion by PDH to acetyl-CoA to initiate and sustain the TCA cycle. Estrogen enhances glucose uptake into the brain and the glycolytic/pyruvate/acetyl-CoA pathway to generate electrons required for oxidative phosphorylation and ATP generation. Collectively, estrogen enhancement of glucose metabolism and aerobic glycolysis promotes and sustains utilization of glucose as the primary fuel source of the brain, thereby preventing the shift to alternative fuels such as ketone bodies, which are characteristic of Alzheimer’s disease [37]. Abbreviations: C, cytochrome c; HSCoA, coenzyme A; I, complex I of the electron transport chain; III, complex III of the electron transport chain; IV, complex IV of the electron transport chain; PDH-K, pyruvate dehydrogenase kinase; PDH-Pase, pyruvate dehydrogenase phosphatase; PFK, phosphofructokinase; PGK, phosphoglycerate kinase; Q, coenzyme Q, also known as ubiquinol (reduced) or ubiquinone (oxidized).
If E2 is increasing glucose uptake, utilization and mitochondrial function in the brain then there should be evidence of increased metabolic activity in the brain after estrogen administration. As part of a 9-year study in the Baltimore Longitudinal Study of Aging (www.grc.nia.nih.gov/branches/blsa/blsanew.htm), ET users showed better performance on neuropsychological tests of figural and verbal memory and on some aspects of the PET activation tests [53]. In a follow-up longitudinal study from the same cohort of healthy menopausal women, regional cerebral blood flow was increased in ET users relative to nonusers in the hippocampus, parahippocampal gyrus and temporal lobe (regions that form a memory circuit and that are sensitive to preclinical Alzheimer’s disease) [53]. Furthermore, the increase in regional cerebral blood flow was associated with higher scores on cognitive tests [53]. In a separate study, a significant decrease in metabolism of the posterior cingulate cortex was detected in non-ET women at 2-year follow-up, whereas ET users did not exhibit significant metabolic change in the posterior cingulate [54]. The findings that ET use preserves regional cerebral metabolism and protects against metabolic decline in postmenopausal women, especially in posterior cingulate cortex, are particularly important given that metabolism in this region of the brain declines in the earliest stages of Alzheimer’s disease [37].
Overall, E2 promotes the energetic capacity of brain mitochondria by maximizing aerobic glycolysis (oxidative phosphorylation coupled to pyruvate metabolism). Estrogen enhancement of aerobic glycolysis in the aging brain would be predicted to prevent conversion of the brain to using alternative sources of fuel such as the ketone body pathway characteristic of Alzheimer’s disease (for review, see Ref. [37]).
Estrogen regulation of memory function from rodents to humans
Analyses of estrogen regulation of memory function span basic science investigations in rodents to translational studies in nonhuman primates to clinical trials in humans [3]. Two common findings emerge from these analyses. First, the loss of estrogen via removal of the ovaries in either animals or humans is associated with decreased memory function [2,3,55]. Second, the variability in estrogen regulation of cognitive function increases as progression up the phylogenetic tree occurs; for example, studies in rodents are less variable in estrogen regulation of memory function relative to the high degree of variability in women.
Studies in rodents are typically controlled experiments conducted in ovariectomized animals or in animals whose stage of reproductive cycle is a controlled variable and are conducted when animals are still reproductively competent [3,55]. Removal of the ovaries leads to ovariectomy-induced decrements in memory (and cognitive) function, which is prevented or reversed with E2 treatment [3,55]. The delayed match-to-sample task provides a good comparison between the effects in rodents and nonhuman primates. In a delayed match-to-position T-maze task, ovariectomy significantly impaired acquisition of the working memory component of the task; this was prevented by E2 replacement. These E2-inducible memory effects are evident in other spatial learning tasks including T-maze alternation and radial arm maze tasks, water maze tasks and place-learning tasks [3,55]. E2-inducible responses are also evident in non-spatial tasks such as visual object recognition and contextual and cued fear conditioning.
As in the rodent, analyses in nonhuman primates typically have been conducted in ovariectomized females but several studies were conducted in the intact animal [56]. Ovariectomy in nonhuman primates led to robust deficits in discrimination learning, the magnitude of which substantially exceeded the mild impairment of intact aged monkeys [30]. E2 treatment reversed the ovariectomy-induced impairment on a delayed-response test of hippocampal-dependent spatial working memory and PFC-dependent memory task. Replacement with E2 restored working memory function to that of young ovary-intact females [30]. (In contrast to these findings, in aged rhesus monkeys no effect of ovariectomy or E2 treatment on cognitive tasks after 2 months of ovarian hormone deprivation was found in young premenopausal monkeys [6]). These findings, although representative of a larger body of literature [56], are indicative of the importance of age and the duration of ovarian hormone deprivation.
In neurologically healthy women, the most consistent effect of E2 replacement on cognition is exemplified by the randomized clinical trials [3]. In a paradigm that most closely resembled the animal studies, women (average age of 45 while still neurologically healthy) had their uterus and ovaries removed for medical reasons and were tested for cognitive function before surgery, after surgery and during ET intervention [3]. As in rodents and nonhuman primates, loss of gonadal hormones resulted in a significant decrement in memory function that was prevented by ET (for review, see Ref. [3]). Memory functions most consistently regulated by ovariectomy were short-term verbal memory (a hippocampal function) and working memory (a PFC-mediated function). In perimenopausal and early postmenopausal women (mean age was 51 years), those who randomly received transdermal E2 exhibited significant improvement on a frontal-lobe-mediated test of executive function compared with women who received placebo [3]. By contrast, four randomized clinical trials found no effect of ET on memory function [57]. Three of these trials did not evaluate verbal or working memory functions. Furthermore, ET was not initiated until years after the removal of the ovaries. Lastly, these clinical trials used oral administration of a complex ET (conjugated equine estrogens), whereas the trials indicating E2 regulation of memory function were administered by intramuscular injection or transdermal patch [57].
In observational investigations, the majority of studies found that estrogen-users performed significantly better than nonusers on tests of verbal fluency, verbal memory, and verbal and spatial working memory [3]. Longitudinal analyses indicate that over time there is a divergence of ET users and nonusers with ET users performing better on cognitive tests and experiencing less deterioration in aspects of cognition with increasing age compared with the nonusers [3].
The variability in human studies was addressed in a meta-analysis of hormone therapy and cognitive function in postmenopausal women [2]. Results of these analyses indicated that 21 out of 44 tests of memory (48%) and 24 of 51 non-memorial tests (47%) (speed of information processing, visuospatial skills, abstract reasoning, etc.) were positively affected by hormone therapy [2]. Abstract reasoning was enhanced in four of six studies. However, even for verbal memory, which showed the highest proportion of estrogen response, >50% of this type of test showed no effect of hormone therapy. Moreover, no tests yielded unanimously positive results except for paragraph recall, which, more than other verbal memory tests, depends upon on contextual processing. Furthermore, like the delay time of the rodent paradigm [29], the paragraph recall test has a temporal component with an immediate and 30 min delay in recall.
Concluding remarks
Collectively, the data from rodents to nonhuman primates to humans consistently indicate that loss of ovarian hormones can have a deleterious effect upon cognitive functions that include both hippocampal- and PFC-mediated behaviors. Ovariectomized women show a deficit relative to preoperative baseline in short-term memory, long-term memory and logical reasoning (cognitive functions that require the hippocampus and PFC). Although a loss of ovarian hormones is not always associated with deficits in cognitive function particularly in younger nonhuman primates and women, disturbing evidence indicates that a loss of ovarian hormones has long-term deleterious neurological consequences. Ovariectomy before natural menopause is associated with increased risk of cognitive impairment or dementia and Parkinson’s disease [58,59]. Moreover, dementia in women was preceded by weight loss 10 years before diagnosis, which is consistent with dysfunction in glucose metabolism and mitochondrial function and a switch to ketone bodies as an alternative fuel [37,60].
The question of whether estrogen regulates memory and other cognitive functions in peri- to postmenopausal women is still a topic of debate. In humans, the effect of estrogen or hormone therapy is highly variable and seems to be dependent upon multiple factors including neurological health, age, the type of menopause (surgical versus natural) and the type of cognitive test, among others [1]. The data from basic science analyses would indicate that estrogen regulates cognitive functions that require the following: (i) efficient transfer of information, (ii) distribution of information to multiple neural circuits, (iii) association of information across time and (iv) a higher order of information complexity (i.e. information across multiple sensory modalities or from multiple experiences to generate a higher executive function). Testing the validity of this prediction would require selecting not only the appropriate cognitive instruments but also the correct population of women. The variability between women in the timing, hormonal status and severity of climacteric symptoms are crucial factors that will impact the variability of estrogen or hormone therapy regulation of cognitive function. The multifactorial nature of estrogen responsivity in women leads to the issue of biomarkers for determining women appropriate for estrogen or hormone therapy, the dose, regimen, combination and duration of therapy. Essentially, there are, to date, no reliable biomarkers to predict a priori those women who will experience estrogen-deprivation-associated cognitive deficits. One biomarker that has potential validity is the number of objectively determined, not self reported, hot flashes [61]. The frequency of objectively determined hot flashes was significantly related to deficits in verbal memory performance [61]. Lastly, remaining challenge is the development of estrogen alternatives that are both safe and efficacious to sustain estrogen-dependent function in the brain to sustain neurological health and cognitive function while preventing neurodegenerative diseases such as Alzheimer’s [62–65].
Figure I.
Diversity and complexity of estrogen receptor expression and localization in neurons and glia of the central nervous system. Abbreviations: hERα1, human estrogen receptor α, also known as estrogen receptor 1; hERβ2, human estrogen receptor β, also known as estrogen receptor 2.
Acknowledgements
The contributions of the Brinton laboratory estrogen research team, especially Jon Nilsen, Ronald Irwin, Shuhua Chen, Liqin Zhao, Ryan Hamilton and Jia Yao, and the graphic design by Kathy Cho are very gratefully acknowledged. I am indebted to the many important contributions generated by scientists working in the field and regret that much of the primary work could not be referenced owing to space limitations. Research and preparation of this review were supported by grants from the National Institute on Aging (www.nia.nih.gov; P01 AG026572; P01-AG014751; Project 2), National Institute of Mental Health (www.nimh.nih.gov; 1R01 MH67159), Kenneth T. and Eileen L. Norris Foundation (www.ktn.org) and Bensussen Translational Research Fund to R.D.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann. N. Y. Acad. Sci. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- 2.Hogervorst E, et al. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: a meta-analysis. Neuroscience. 2000;101:485–512. doi: 10.1016/s0306-4522(00)00410-3. [DOI] [PubMed] [Google Scholar]
- 3.Sherwin BB, Henry JF. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front. Neuroendocrinol. 2008;29:88–113. doi: 10.1016/j.yfrne.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Zhao C, et al. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 5.Tanapat P, et al. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J. Comp. Neurol. 2005;481:252–265. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- 6.Morrison JH, et al. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J. Neurosci. 2006;26:10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sisti HM, et al. Neurogenesis and the spacing effect: learning over time enhances memory and the survival of new neurons. Learn. Mem. 2007;14:368–375. doi: 10.1101/lm.488707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aimone JB, et al. Potential role for adult neurogenesis in the encoding of time in new memories. Nat. Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 9.Andersen P, et al., editors. The Hippocampus Book. Oxford University Press; 2007. [Google Scholar]
- 10.Suzuki S, et al. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors α and β. J. Comp. Neurol. 2007;500:1064–1075. doi: 10.1002/cne.21240. [DOI] [PubMed] [Google Scholar]
- 11.Mazzucco CA, et al. Both estrogen receptor α and estrogen receptor β agonists enhance cell proliferation in the dentate gyrus of adult female rats. Neuroscience. 2006;141:1793–1800. doi: 10.1016/j.neuroscience.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 12.Wise PM. Estrogen therapy: does it help or hurt the adult and aging brain? Insights derived from animal models. Neuroscience. 2006;138:831–835. doi: 10.1016/j.neuroscience.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 13.Saravia F, et al. Oestradiol restores cell proliferation in dentate gyrus and subventricular zone of streptozotocin-diabetic mice. J. Neuroendocrinol. 2004;16:704–710. doi: 10.1111/j.1365-2826.2004.01223.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang JM, et al. Estradiol-17β-induced human neural progenitor cell proliferation is mediated by an estrogen receptor β-phosphorylated extracellularly regulated kinase pathway. Endocrinology. 2008;149:208–218. doi: 10.1210/en.2007-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, et al. Estrogen receptor (ER)β knockout mice reveal a role for ERβ in migration of cortical neurons in the developing brain. Proc. Natl. Acad. Sci. U. S. A. 2003;100:703–708. doi: 10.1073/pnas.242735799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinton RD. Cellular and molecular mechanisms of estrogen regulation of memory function and neuroprotection against Alzheimer's disease: recent insights and remaining challenges. Learn. Mem. 2001;8:121–133. doi: 10.1101/lm.39601. [DOI] [PubMed] [Google Scholar]
- 17.Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm. Behav. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- 18.Brinton RD, et al. The women's health initiative estrogen replacement therapy is neurotrophic and neuroprotective. Neurobiol. Aging. 2000;21:475–496. doi: 10.1016/s0197-4580(00)00109-3. [DOI] [PubMed] [Google Scholar]
- 19.MacLusky NJ, et al. The 17α and 17β isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- 20.Lamprecht R, LeDoux J. Structural plasticity and memory. Nat. Rev. Neurosci. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- 21.Leuner B, et al. Associative memory formation increases the observation of dendritic spines in the hippocampus. J. Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leuner B, Shors TJ. New spines, new memories. Mol. Neurobiol. 2004;29:117–130. doi: 10.1385/MN:29:2:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J. Neurobiol. 2005;64:34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- 24.Shankar GM, et al. Amyloid-β protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat. Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oddo S, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 26.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu. Rev. Pharmacol. Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 27.Shors TJ, et al. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yankova M, et al. Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: a serial electron-microscopic study. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3525–3530. doi: 10.1073/pnas.051624598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav. Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- 30.Rapp PR, et al. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J. Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao J, et al. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J. Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasai H, et al. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- 33.Fuster JM. The prefrontal cortex–an update: time is of the essence. Neuron. 2001;30:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 34.Kim MT, et al. 17β-Estradiol potentiates field excitatory postsynaptic potentials within each subfield of the hippocampus with greatest potentiation of the associational/commissural afferents of CA3. Neuroscience. 2006;141:391–406. doi: 10.1016/j.neuroscience.2006.03.075. [DOI] [PubMed] [Google Scholar]
- 35.Bi R, et al. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc. Natl. Acad. Sci. U. S. A. 2000;97:3602–3607. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bi R, et al. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc. Natl. Acad. Sci. U. S. A. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magistretti P. Brain energy metabolism. In: Squire L, et al., editors. Fundamental Neuroscience. Academic Press; 2008. pp. 271–296. [Google Scholar]
- 39.Cheng CM, et al. Estrogen augments glucose transporter and IGF1 expression in primate cerebral cortex. FASEB J. 2001;15:907–915. doi: 10.1096/fj.00-0398com. [DOI] [PubMed] [Google Scholar]
- 40.Shi J, Simpkins JW. 17 β-Estradiol modulation of glucose transporter 1 expression in blood-brain barrier. Am. J. Physiol. 1997;272:E1016–E1022. doi: 10.1152/ajpendo.1997.272.6.E1016. [DOI] [PubMed] [Google Scholar]
- 41.Mendez P, et al. Cross-talk between estrogen receptors and insulin-like growth factor-I receptor in the brain: cellular and molecular mechanisms. Front. Neuroendocrinol. 2006;27:391–403. doi: 10.1016/j.yfrne.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Mendez P, Garcia-Segura LM. Phosphatidylinositol 3-kinase and glycogen synthase kinase 3 regulate estrogen receptor-mediated transcription in neuronal cells. Endocrinology. 2006;147:3027–3039. doi: 10.1210/en.2005-1224. [DOI] [PubMed] [Google Scholar]
- 43.Cardona-Gomez GP, et al. Interactions of estrogen and insulin-like growth factor-I in the brain: molecular mechanisms and functional implications. J. Steroid Biochem. Mol. Biol. 2002;83:211–217. doi: 10.1016/s0960-0760(02)00261-3. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Segura LM, et al. Insulin-like growth factor-I receptors and estrogen receptors interact in the promotion of neuronal survival and neuroprotection. J. Neurocytol. 2000;29:425–437. doi: 10.1023/a:1007125626308. [DOI] [PubMed] [Google Scholar]
- 45.Holmquist L, et al. Lipoic acid as a novel treatment for Alzheimer's disease and related dementias. Pharmacol. Ther. 2007;113:154–164. doi: 10.1016/j.pharmthera.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Nilsen J, et al. Estradiol in vivo regulation of brain mitochondrial proteome. J. Neurosci. 2007;27:14069–14077. doi: 10.1523/JNEUROSCI.4391-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bettini E, Maggi A. Estrogen induction of cytochrome c oxidase subunit III in rat hippocampus. J. Neurochem. 1992;58:1923–1929. doi: 10.1111/j.1471-4159.1992.tb10070.x. [DOI] [PubMed] [Google Scholar]
- 48.Stirone C, et al. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol. Pharmacol. 2005;68:959–965. doi: 10.1124/mol.105.014662. [DOI] [PubMed] [Google Scholar]
- 49.Lin M, Beal M. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 50.Nilsen J, Brinton RD. Mitochondria as therapeutic targets of estrogen action in the central nervous system. Curr. Drug Target. CNS Neurol. Disord. 2004;3:297–313. doi: 10.2174/1568007043337193. [DOI] [PubMed] [Google Scholar]
- 51.Nilsen J, et al. Estrogen protects neuronal cells from amyloid β-induced apoptosis via regulation of mitochondrial proteins and function. BMC Neurosci. 2006;7:74. doi: 10.1186/1471-2202-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simpkins JW, Dykens JA. Mitochondrial mechanisms of estrogen neuroprotection. Brain Res. Brain Res. Rev. 2008;57:421–430. doi: 10.1016/j.brainresrev.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Maki PM, Resnick SM. Effects of estrogen on patterns of brain activity at rest and during cognitive activity: a review of neuroimaging studies. Neuroimage. 2001;14:789–801. doi: 10.1006/nimg.2001.0887. [DOI] [PubMed] [Google Scholar]
- 54.Rasgon NL, et al. Estrogen use and brain metabolic change in postmenopausal women. Neurobiol. Aging. 2005;26:229–235. doi: 10.1016/j.neurobiolaging.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 55.Gibbs RB, Johnson DA. Sex specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague-Dawley rats. Endocrinology. 2008;149:3176–3183. doi: 10.1210/en.2007-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lacreuse A. Effects of ovarian hormones on cognitive function in nonhuman primates. Neuroscience. 2006;138:859–867. doi: 10.1016/j.neuroscience.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 57.Sherwin BB. Estrogen and cognitive aging in women. Trends Pharmacol. Sci. 2002;23:527–534. doi: 10.1016/s0165-6147(02)02093-x. [DOI] [PubMed] [Google Scholar]
- 58.Rocca WA, et al. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 59.Rocca WA, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70:200–209. doi: 10.1212/01.wnl.0000280573.30975.6a. [DOI] [PubMed] [Google Scholar]
- 60.Knopman DS, et al. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007;69:739–746. doi: 10.1212/01.wnl.0000267661.65586.33. [DOI] [PubMed] [Google Scholar]
- 61.Maki PM, et al. Objective hot flashes are negatively related to verbal memory performance in midlife women. Menopause. 2008;15:848–856. doi: 10.1097/gme.0b013e31816d815e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brinton RD. Requirements of a brain selective estrogen: advances and remaining challenges for developing a NeuroSERM. J. Alzheimers Dis. 2004;6:S27–S35. doi: 10.3233/jad-2004-6s607. [DOI] [PubMed] [Google Scholar]
- 63.Zhao L, Brinton RD. Estrogen receptor β as a therapeutic target for promoting neurogenesis and preventing neurodegeneration. Drug Dev. Res. 2006;66:103–117. [Google Scholar]
- 64.Zhao L, et al. Selective estrogen receptor modulators SERMs) for the brain: current status and remaining challenges for developing NeuroSERMs. Brain Res. Brain Res. Rev. 2005;49:472–493. doi: 10.1016/j.brainresrev.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 65.Zhao L, et al. Design, synthesis, and estrogenic activity of a novel estrogen receptor modulator–a hybrid structure of 17β-estradiol and vitamin E in hippocampal neurons. J. Med. Chem. 2007;50:4471–4481. doi: 10.1021/jm070546x. [DOI] [PubMed] [Google Scholar]
- 66.Li C, et al. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc. Natl. Acad. Sci. U. S. A. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu F. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nat. Neurosci. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 68.Hao J, et al. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 2007;104:11465–11470. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shors TJ. Memory traces of trace memories: neurogenesis, synaptogenesis and awareness. Trends Neurosci. 2004;27:250–256. doi: 10.1016/j.tins.2004.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31:529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brinton RD. Estrogen regulation of glucose metabolism and mitochondrial function: therapeutic implications for prevention of Alzheimer's disease. Adv. Drug Deliv. Rev. 2008;60:1504–1511. doi: 10.1016/j.addr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nat. Rev. Neurosci. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- 73.Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 74.Brann DW, et al. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McEwen B, et al. Tracking the estrogen receptor in neurons: implications for estrogen-induced synapse formation. Proc. Natl. Acad. Sci. U. S. A. 2001;98:7093–7100. doi: 10.1073/pnas.121146898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spencer JL, et al. Uncovering the mechanisms of estrogen effects on hippocampal function. Front. Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ishunina TA, Swaab DF. Hippocampal estrogen receptor-α splice variant TADDI in the human brain in aging and Alzheimer's Disease. Neuroendocrinology. 2008 doi: 10.1159/000158573. http://content.karger.com 10.1159/000158573. [DOI] [PubMed] [Google Scholar]
- 78.Price RH, Jr, et al. Differential expression of estrogen receptor β splice variants in rat brain: identification and characterization of a novel variant missing exon 4. Brain Res. Mol. Brain Res. 2000;80:260–268. doi: 10.1016/s0169-328x(00)00135-2. [DOI] [PubMed] [Google Scholar]
- 79.Prossnitz ER, et al. GPR30: a novel therapeutic target in estrogen-related disease. Trends Pharmacol. Sci. 2008;29:116–123. doi: 10.1016/j.tips.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 80.Brailoiu E, et al. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J. Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- 81.Toran-Allerand CD, et al. Minireview: a plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- 82.Zhao L, Brinton RD. WHI and WHIMS follow-up and human studies of soy isoflavones on cognition. Expert Rev. Neurother. 2007;7:1549–1564. doi: 10.1586/14737175.7.11.1549. [DOI] [PubMed] [Google Scholar]
- 83.Petrovic M, Barcelo D. Determination of phenolic xenoestrogens in environmental samples by liquid chromatography with mass spectrometric detection. J. AOAC Int. 2001;84:1074–1085. [PubMed] [Google Scholar]